Abstract
Background
The study aim was to assess the prevalence and co-occurrence of alcohol and sexual risk behaviors among emergency department (ED) patients in community hospitals.
Methods
Systematic screening of ED patients (N = 6,486; 56.5% female) was conducted in 2 community hospitals in the northeast during times with high patient volume, generally between the hours of 10 AM to 8 PM, Monday through Saturday. Screening occurred from May 2011 through November 2013. Assessment included validated measures of alcohol use and sexual risk behavior.
Results
Overall results identified high rates of alcohol use, sexual risk behaviors, and their co-occurrence in this sample of ED patients. Specifically, ED patients in between the ages of 18 and 35 were consistently highest in hazardous alcohol use (positive on the Alcohol Use Disorders Identification Test or endorsing heavy episodic drinking [HED]), sexual risk behaviors, and the co-occurrence of alcohol and sex-risk behaviors.
Conclusions
Findings show a high co-occurrence of hazardous drinking and unprotected sex among ED patients and highlight the role of HED as a factor associated with sexual risk behavior. Efforts to integrate universal screening for the co-occurrence of alcohol and sexual risk behavior in ED settings are warranted; brief interventions delivered to ED patients addressing the co-occurrence of alcohol and sexual risk behaviors have the potential to decrease the risk of sexually transmitted infections and HIV among a large number of patients.
Keywords: Alcohol, Sex Risk, Emergency Department
Alcohol-Related Emergency Department (ED) visits have increased significantly over the past 15 years (Cherpitel and Ye, 2012), and individuals with alcohol-related problems are typically overrepresented among ED patients relative to the general population (Cherpitel, 1994). In addition, EDs very commonly treat patients with alcohol-use problems for issues related or unrelated to their alcohol use (Cohen et al., 2007). Significant associations between alcohol use and sexual risk have been observed in multiple groups (Bolton et al., 1992; Cook and Clark, 2005; Halpern-Felsher et al., 1996; Hendershot and George, 2007; Kalichman et al., 2007; Leigh, 2002; Weinhardt and Carey, 2000). Indeed, alcohol use has been associated with multiple forms of sex-risk behavior, including having unprotected sex, multiple sex partners, sex outside of marriage, coercive sex, and sex in exchange for money or other goods (Shillington et al., 1995; Weinhardt and Carey, 2000). It is these sex-risk behaviors that are also linked to the risk of acquiring HIV; a recent meta-analysis identified individuals who consumed alcohol at a 77% higher risk of HIV-incident infection. Further, individuals consuming alcohol prior to or at the time of sex were at a 87% increased risk, and individuals engaging in binge drinking (5 or more drinks in 1 sitting for men, 4 for women) had twice the risk of nonbinge drinkers for HIV-incident infection (Baliunas et al., 2010). Specific to ED populations, 1 investigation found evidence of a significant positive relationship between reported sexual risk of HIV and alcohol use among men and women patients, with women who reported binge drinking more likely to have higher reported sexual risk of HIV compared to women who did not report binge drinking (Trillo et al., 2013). In addition, Fenton (2007) reports that more new HIV cases are diagnosed among ED patients compared to patients in any other clinic setting.
EDs are often the only source of medical care for many patients, particularly among the uninsured and underinsured (Tang et al., 2010). EDs may serve as a primary site of health care for individuals at risk of HIV (Rothman, 2004), and the Centers for Disease Control and Prevention (CDC) and other national organizations have identified EDs as strategic settings for delivering HIV prevention interventions including HIV testing and counseling (Branson et al., 2006; Dowdy et al., 2011; Haukoos et al., 2011; Trillo et al., 2013). Thus, hazardous alcohol use and sex-risk behaviors have been established as 2 prevalent behavioral risks among ED patients. While studies have found the brief interventions for alcohol conducted in the ED are efficacious (Mello and Longabaugh, 2013; Monti et al., 2007), others have not demonstrated efficacy in comparison to control/usual care (Maio et al., 2005). There is promise in ED-based intervention but the co-occurrence of heavy alcohol use and risky sexual behaviors among patients in the ED setting, and whether there is evidence to prioritize integrating alcohol–HIV intervention protocols for patients and health providers in ED settings has not been established.
Summary and Objective
Recent reviews have called attention to the co-occurrence and reciprocal interactions of alcohol and HIV risk behaviors in various high-risk communities (Bryant, 2006; Fritz et al., 2010; Rehm et al., 2012; Woolf-King and Maisto, 2011). Independent studies have identified ED patients as being at relatively high risk of both heavy alcohol use (Cohen et al., 2007) and HIV infection (Alpert et al., 1996; Trillo et al., 2013). With approximately 56,000 new HIV cases per year (CDC, 2012), it is important to identify the associations between alcohol use and increased risks of HIV infection. As these risk factors are found in disproportionately high rates of individuals from lower socioeconomic backgrounds, for whom the ED is often their only form of health care, a determination of rates of alcohol use, sex-risk behaviors, and their intersection among ED patients is an essential first step in determining needs for screening, HIV testing, and intervention approaches to reduce sex-risk behaviors and heavy alcohol use. The objective of the current study was to identify the prevalence of hazardous alcohol users (as identified through positive Alcohol Use Disorders Identification Test [AUDIT] scores or endorsing heavy episodic drinking [HED]), sex-risk behaviors, and their co-occurrence among adult ED patients in 2 community hospital settings. Should this co-occurrence be common, adjustments to the current Screening and Brief Intervention and Referral to Treatment (SBIRT) model may be needed to incorporate the broader risk profile of ED patients. Should the data show low or no co-occurrence, this would offer support for the continued use of current SBIRT models with ED patients that focus solely on alcohol use.
Materials and Methods
Screening Methods
Anonymous screening of adult patients 18 years or older (N = 24,918) in 2 community hospital EDs was conducted between May 2011 and November 2013. Site 1 treats an average of 24,000 adult patients (ages 18 to 64) with approximately 54% women, and racial distribution of 77.8% Caucasian, 15.8% African-American, 0.2% Asian, 0.05% American Indian/Alaska Native, 1.1% multiracial, and 3.95% other or unknown. Hispanic ethnicity is 20%. Site 2 treats an average of 47,443 adult patients (ages 18 to 64) annually, 54% of whom are women. Racial distribution at Site 2 is 92.5% Caucasian, 2.2% African-American, 0.5% Asian, 0.2% American Indian/Alaska Native, and 0.6% multiracial, 4.0% other, unknown, or refused to answer. Hispanic ethnicity is 3%. At the time of the study, both EDs offered HIV testing via blood sample when requested or when it was medically indicated by the patient. For this study, screening was conducted from Monday through Saturday from 10 am to 8 pm (with occasional screening also occurring between 8 and 10 am). The decision to screen during these times was determined by hospital records indicating the interval with highest patient volume. Patient recruitment and research activities took place during breaks in patient medical care to minimize disruption of services. Adult male and female patients were approached and screened by trained masters or doctoral-level interventionists after gaining medical provider approval. All patients between the ages of 18 and 65 were approached for screening, except in instances where the patient was (i) non-English-speaking; (ii) being treated for an intentional self-inflicted injury (i.e., suicide attempt) or suicidal ideation; (iii) in police custody; or (iv) advised not to screen by ED clinical staff due to medical or treatment condition. Patients were not required to complete written consent as no identifying information was collected beyond gender and age. Patients did not receive reimbursement for study participation. All procedures were approved by the appropriate university and hospital institutional review boards (IRBs). The IRB procedures required all assessments meet literacy levels for an 8th grade reading level; therefore, patients who stated they were unable to read or had difficulty reading were excluded.
To ensure privacy, family or other individuals accompanying the patient were asked to leave the area immediately prior to the screening procedures. Patients completed the screening battery independently on a tablet computer. In cases when the patient was unable to self-administer the survey due to illness or injury, the researcher orally administered the questions and patients used written response cards to indicate their responses by pointing to the response (e.g., “1 = yes” or “0 = no”) on the card. This procedure was used to provide the maximum amount of privacy. A total of 24 screening items were administered over 5 minutes.
Measures
Demographics
Gender and age were collected prior to screening.
Alcohol Use Disorders Identification Test (Saunders et al., 1993)
This 10-item questionnaire was developed by the World Health Organization to identify patients whose alcohol consumption has become harmful. Questions are scored from 0 to 4 with a cumulative score range of 0 to 40. AUDIT scores of 8 or higher have historically reflected risky use (Conigrave et al., 1995), but more recent research has identified an alternative cut-point of 6 or higher for females (Reinert and Allen, 2002). For the current study, men scoring 8 or above and women scoring 6 or higher were considered as AUDIT positive (AUDIT+).
Drinking Status
Two items were used to assess current drinking. Patients were asked whether they had consumed any alcohol (more than just a sip) over the past 30 days and were asked how many heavy drinking episodes (4+ drinks in 1 sitting for women and 5+ drinks for men; National Institute on Alcohol Abuse and Alcoholism, 2004) they had over the past 3 months. For the current, study patients endorsing, at minimum, 1 episode of heavy drinking (gender based) were considered positive for risky alcohol use.
Hazardous Drinking
A combined item was created to identify individuals considered positive for hazardous drinking. Patients who were AUDIT + or endorsed at least 1 heavy drinking episode were categorized as positive for hazardous drinking.
Sexual Behaviors
Sexual risk-taking behaviors were assessed using 5 items drawn from prior research (Kalichman et al., 1998; Millstein and Moscicki, 1995) to identify individuals at risk of HIV/sexually transmitted infection (STI) transmission. The first item assessed the patient's current relationship status (in a monogamous relationship or not; if yes, length of relationship). The next 4 items evaluated sexual behaviors over the past 3 months, including the following: (i) number of sex partners (open ended response); (ii) how often the patient engaged in unprotected sexual intercourse (vaginal or anal sex without a condom); (iii) frequency of consuming alcohol before or during sex; and (iv) frequency of any drug use to get high or intoxicated before or during sex. The final 3 questions were rated on a 4-point scale from (0) never, (1) sometimes, (2) usually, and (3) always, and responses were then dichotomized to no risk (never) or risk (sometimes, usually, or always). Each risk behavior was first identified independently, and then, an index of sex risk was created which reflected the endorsement of at least 1 of the 4 sex-risk questions, dichotomized as sex risk (more than 1 current sex partner, sex without a condom, sex under the influence of alcohol/drugs) or no sex risk.
Data Analysis
Descriptive analyses were conducted to identify the percentage of male and female patients completing screening and the average age of screened patients. Initial descriptive analyses were conducted on the AUDIT score, risky drinking, and engagement in sex-risk behaviors. We next examined associations between alcohol use and sex-risk behaviors using cross-tabulations within gender and age categories. Age was stratified into the following categories: 18 to 25, 26 to 35, 36 to 50, and 50 to 65. Finally, 1-way analyses of variance were conducted using Tukey honest significant difference follow-up tests to examine differences between drinking and sexual risk behavior outcomes by age group.
Results
Participants
Of 24,918 age-eligible adult patients on site during working shifts, 6,486 (26.0%) patients were screened: 3,665 (56.5%) female and 2,821 (43.5%) male patients. The average age of screened patients was 40.4 years (SD = 13.6). Of patients screened, 25.3% were between the ages of 18 and 25, 23.9% were between the ages of 26 and 35, and 31.7% were between the ages of 36 to 50, and the remaining 19.1% were between the ages of 51 and 65. The most common reasons for not screening were as follows: advised not to screen by clinician (20.5%), patient declined to screen (16.9%), discharged prior to approach (13.7%), inadequate privacy (3.5%), and under medical isolation (3.2%).
Alcohol Use
Of patients screened, 1,315 (20.3%) participants were AUDIT positive (score of 8 or higher for men, 6 or higher for women), and 3,092 (47.7%) reported 1 or more heavy episodic drinking (HED) occasions in the past 3 months. When individual risk factors were combined to create a single index of hazardous drinking (AUDIT+ or HED+), 46% of male and 55% of female patients screened met the criterion (see Table 1). Examination of differences in hazardous drinking status by age groups revealed significant differences between age groups when controlling for gender (F = 88.4; see Fig. 1). Patients in the 2 youngest age groups (18 to 25 and 26 to 35) had higher proportions of hazardous drinkers than the 36- to 50- and 51- to 65-year-old age groups (ps < 0.001).
Table 1. Three-Month Co-Occurrence of Alcohol and Sex-Risk Behaviors.
| AUDIT+ (N = 1,315) | HED+ (N = 3,092) | AUDIT- and HED- (N = 3,310) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Sex partner number/type | 473 (35.9) | 824 (26.6) | 644 (19.5) |
| Sex without a condom | 885 (67.3) | 2,121 (68.6) | 1,944 (58.7) |
| Alcohol with sex | 854 (64.9) | 1,682 (54.4) | 288 (8.7) |
| Drugs with sex | 408 (31.0) | 633 (20.5) | 251 (7.6) |
Total number of patients screened, N = 6,486. AUDIT, Alcohol Use Disorders Identification Test; HED, heavy episodic drinking. AUDIT+ were male patients scoring 8 or higher and female patients scoring 6 or higher on the AUDIT. HED+ patients were male patients endorsing 5 or more drinks in 1 sitting, female patients endorsing 4 or more drinks in 1 sitting. Sex partner number/type refers to patients having more than 1 sex partner or a single sex partner of unknown monogamy status.
Fig. 1.
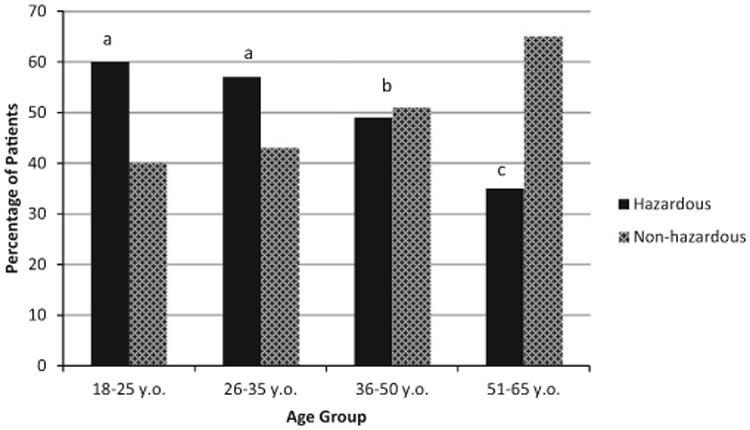
Past 3-month hazardous drinking status by age. Note. Hazardous drinking defined as either Alcohol Use Disorders Identification Test+ or heavy episodic drinking+. Analyses controlled for gender. Age groups with different superscripts differ significantly. The 18- to 25- and 26- to 35-year-old groups did not differ in the proportion of hazardous drinkers (signified by a), while the 36- to 50- and 51- to 65-year-old groups had significantly different proportions of hazardous drinkers than all other age groups (p < 0.001).
Sex-Risk Behaviors
Overall, during the past 3 months, 23% of screened patients identified having more than 1 sex partner or a single sex partner of unknown monogamy status, 63.5% engaged in sex without a condom, 30.8% engaged in sex under the influence of alcohol, and 13.8% engaged in sex under the influence of a drug other than alcohol. For patients who identified 2 or more sex partners, the average number of partners was 3.17 (SD = 2.67) with a range of 2 to 25 partners. When compared to male patients, a higher percentage of females aged 18 to 25 and 26 to 35 endorsed engaging in sex without a condom. Among men, patients in the 36 to 50 age group were more likely to report having sex without a condom during the past 3 months than men in other age categories (see Table 2). When controlling for gender, patients in the 18 to 25 age range more commonly endorsed having risk related to partner type (i.e., sex with a partner of unknown monogamy status) over the past 3 months (see Fig. 2). Men and women patients reported similar rates of sex under the influence of alcohol and sex under the influence of other drugs, during the past 3 months with no significant differences between age groups (see Table 2).
Table 2. Percentage of Alcohol and Sexual Risk Behavior by Age Group and Gender.
| Age group | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 18–25 | 26–35 | 36–50 | 51+ | |||||
|
|
|
|
|
|||||
| M | F | M | F | M | F | M | F | |
| Alcohol risk | ||||||||
| AUDIT+ | 29.4 | 25.1 | 25.6 | 20.6 | 23.7 | 18.3 | 15.5 | 11.9 |
| HED+ | 64.7 | 56.2 | 61.9 | 51.1 | 52.2 | 43.6 | 39.1 | 28.9 |
| Sexual risk behavior | ||||||||
| Sex partner number/type | 43.8 | 39.2 | 35.9 | 27.7 | 18.9 | 18.0 | 12.2 | 7.7 |
| Sex without a condom | 60.1 | 70.4 | 70.8 | 77.7 | 70.1 | 66.8 | 52.4 | 44.1 |
| Alcohol with sex | 43.3 | 38.0 | 47.6 | 35.9 | 36.8 | 27.5 | 20.2 | 12.6 |
| Drugs with sex | 27.8 | 17.8 | 25.0 | 13.7 | 15.5 | 10.4 | 7.6 | 4.8 |
Total number of patients screened, N = 6,486. AUDIT, Alcohol Use Disorders Identification Test; HED, heavy episodic drinking. AUDIT+ were male patients scoring 8 or higher and female patients scoring 6 or higher on the AUDIT. HED+ patients were male patients endorsing 5 or more drinks in 1 sitting, female patients endorsing 4 or more drinks in 1 sitting. Sex partner number/type refers to patients having more than 1 sex partner or a single sex partner of unknown monogamy status.
Fig. 2.
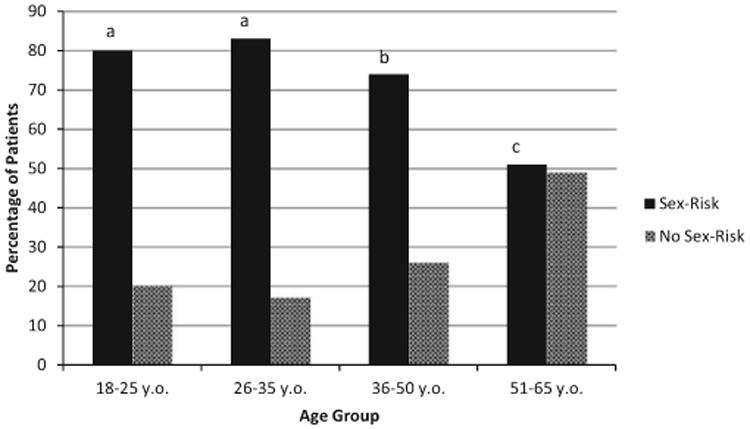
Past 3-month sex-risk behaviors by age. Note. Sex risk was defined as patients not in a monogamous relationship for 6 months or longer and endorsing at least 1 of the following risk factors (multiple sex partners, sex without a condom, sex under the influence of alcohol/drugs). Analyses controlled for gender. There were no significant differences between 18- to 25- and 26- to 35-year-old patients in proportion engaging in sex-risk behavior (signified by a), while the 36- to 50- and 51- to 65-year-old groups were significantly different than all other groups (p < 0.05).
When examining the combined index of sexual risk behaviors, patients in the 26- to 35-year-old group reported the highest rates (83%) with only a slightly lower proportion of patients between 18 and 25 years (80%) reporting sex risk. These groups were not significantly different from one another, but both groups had significantly higher rates of sexual risk behaviors compared to the 36- to 50- and 51- to 65-year-old groups (all p < 0.001) after controlling for gender (see Fig. 2).
Combined Alcohol and Sex Risk
Finally, we examined the proportion of patients who met criteria for both hazardous drinking and sexual risk-taking behaviors (see Table 1). Patients in the 18- to 25 (52%)- and 26- to 35-year-old (49%) age groups were significantly more likely to show both hazardous alcohol use and risky sexual behavior than the 2 older subcategories (37% and 20%, respectively) related to combined heavy alcohol use and sexual risk behaviors (p < 0.001). There were no statistical differences between the 2 younger age groups when controlling for gender in the analyses. Figure 3 identifies the percentage of patients endorsing both hazardous drinking and risky sexual behaviors.
Fig. 3.
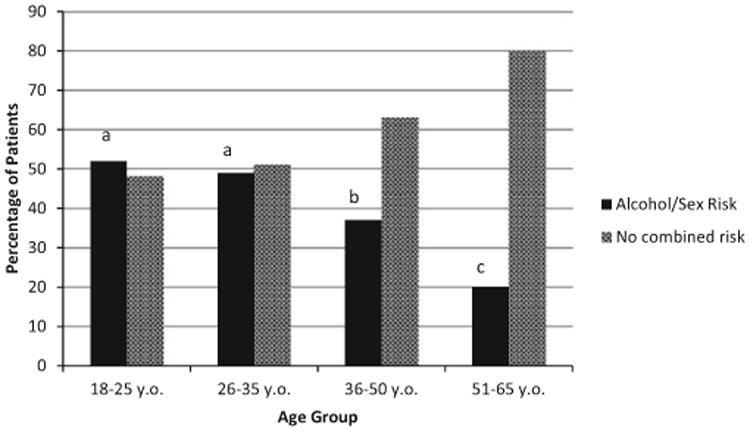
Combined alcohol and sex-risk behaviors past 3 months. Note. Analyses controlled for gender. There were no significant differences between 18- to 25-and 26- to 35-year-old patients (signified by a), while the 36- to 50- and 51- to 65-year-old groups were significantly different than all other groups (p < 0.05).
Discussion
The current study sought to describe the prevalence of alcohol use, sex-risk behaviors, and their co-occurrence in a sample of ED patients from 2 community hospitals. Although past research has found high prevalence of hazardous alcohol use and (in different investigations) sexual risk behaviors among patients seeking ED services, findings reported here bring attention to the co-occurrence of the 2 risk behaviors and their likely association with risk of transmission of HIV and other STIs. Generally, there was a declining prevalence for both hazardous drinking and sexual risk with age. Possibly, the most important finding with relevance for prevention and intervention in EDs is that approximately 50% of patients under the age of 35 in our sample met criteria for both hazardous drinking and sexual risk behaviors. Indeed, there were many reports of both hazardous drinking and engaging in sex-risk behaviors among patients below the age of 50.
Individuals in this ED sample who engaged in HED reported a range of sexual risk behaviors, with the most common behavior being sex without a condom. This was especially true for patients in the sample under the age of 50, and women under 35 were less likely than men to use condoms during sex. Individually, 32.8% of men between the age of 36 and 50 reported both HED and no condom use, compared to only 21.4% for men in the 18 to 25 age group. For women, the risk was similar across age groups (excluding patients >51) where the higher risk was in the 36 to 50 age group (29.7%). As no other known studies in the ED have examined the combination of these risk behaviors, it is unclear how this may impact interventions focused on increased condom use. Although the data in the current study do not explore the reasons for a lack of condom use in the patients, there may be a need for different approaches to address these risk behaviors for men and women. Future research investigating the underlying reasons for condomless sex, specific to men and women, is needed to more clearly understand how to integrate this information into intervention approaches.
Strengths and Limitations
Strengths of this study included the use of systematic screening procedures, recruitment of a large sample of ED patients in 2 community hospitals, and use of valid measures of alcohol and sexual risk behavior. Patients were recruited from EDs in community hospital settings, which we consider a strength, as most ED research is conducted in urban teaching hospitals. However, the study was conducted in EDs in 2 community hospitals that generally treat mid- to lower income populations, thereby limiting generalizability of findings. To maintain the brevity of screening procedures, we assessed only a limited number of sociodemographic variables and did not collect additional co-factors known to be associated with alcohol use and HIV risk (e.g., income, mental health, race/ethnicity, incarceration). This may limit the ability to identify patients with the highest sexual risk behaviors. We did not collect any information on reasons for ED visit during the screening process; however, past studies have shown patients enrolled in alcohol-use reduction studies report a wide range of medical concerns reaching beyond issues related to alcohol and sexual risk behaviors (e.g., Mello and Longabaugh, 2013; Monti et al., 2014). Also, screening was conducted during limited hours which did not include overnight shifts, when the potential for alcohol-related issues is likely highest. Finally, only patients between the ages of 18 and 65 were screened.
Conclusions
Findings bring attention to the co-occurrence of high levels of hazardous drinking and condomless sex among patients in the ED setting. Given that heavy drinking increases the risk of STI and HIV transmission, findings also suggest the importance of developing standardized ED protocols for screening patients for co-occurring alcohol and sexual risk behaviors. Although there have been previous independent recommendations to screen ED patients for alcohol use and to test for HIV in ED settings (Alpert et al., 1996; Dowdy et al., 2011; Trillo et al., 2013), the current study suggests the integration of simple screening questions as a component of ED treatment may efficiently identify patients at risk of HIV/STIs and alcohol-related problems. ED patients reporting HED might suggest also screening for HIV and STI risk behavior and to conduct biological assessment of HIV or other STIs. As administration of the screening questionnaire used in this study required fewer than 5 minutes, this may be an efficient way to identify patients in need of further services or treatment options. Although the CDC recommends HIV opt-out screening for patients in all healthcare settings, including EDs, to increase HIV screening of patients, foster earlier detection of HIV infection, and to identify and counsel persons with unrecognized HIV infection and link them to clinical and prevention services (Branson et al., 2006), this has yet to be integrated into daily practice, making screening an important aspect of identifying those at risk. Given the results of this screening study, asking ED patients 1 question about HED (gender specific) and 1 about the number of sex partners/type (referring to patients having more than 1 sex partner or a single sex partner of unknown monogamy status) would identify a substantial number of individuals at risk of heavy alcohol use and STI/HIV risk. The current data showed only 18 total patients in the screening study were AUDIT+ and did not endorse HED. This offers additional confidence in the suggestion to use HED as the 1 screening question related to alcohol use. Reducing the number of questions by eliminating the AUDIT will make identifying hazardous drinkers in a busy ED environment more simplified and efficient.
A number of follow-up care options exist for the identified population, including the possibility of brief interventions that address the combination of alcohol and sexual risk behaviors for ED patients. This could be performed through the training of ED staff to provide confidential, effective, and safe brief interventions for reducing alcohol and sexual risk behaviors among patients. As Edelman and colleagues (2012) found, a brief alcohol and sexual risk behavior intervention combined with HIV screening successfully reduced risk behaviors in young drinking patients. The current study offers support to expand this approach to older patient populations given the indicated risk reaching beyond young adulthood. Further, as has been noted, providing condoms and HIV testing in ED settings may also reduce HIV risk among patients (Trillo et al., 2013).
References
- Alpert PL, Shuter J, Deshaw MG, Webber MP, Klein RS. Factors associated with unrecognized HIV-1 infection in an inner-city emergency department. Ann Emerg Med. 1996;28:159–164. doi: 10.1016/s0196-0644(96)70056-2. [DOI] [PubMed] [Google Scholar]
- Baliunas D, Rehm J, Irving H, Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: a meta-analysis. Int J Public Health. 2010;55:159–166. doi: 10.1007/s00038-009-0095-x. [DOI] [PubMed] [Google Scholar]
- Bolton R, Vincke J, Mak R, Dennehy E. Alcohol and risky sex: in search of an elusive connection. Med Anthropol. 1992;14:323–363. doi: 10.1080/01459740.1992.9966077. [DOI] [PubMed] [Google Scholar]
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, Clark JE. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. [Accessed January 15, 2015];2006 Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm. [PubMed]
- Bryant KJ. Expanding research on the role of alcohol consumption and related risks in the prevention and treatment of HIV/AIDS. Subst Use Misuse. 2006;41:1465–1507. doi: 10.1080/10826080600846250. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012;17(4) [Google Scholar]
- Cherpitel CJ. Alcohol consumption and injury in the general population: from a national sample. Drug Alcohol Depend. 1994;34:217–224. doi: 10.1016/0376-8716(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Cherpitel CJ, Ye Y. Trends in alcohol-and drug-related emergency department and primary care visits: data from four US national surveys (1995–2010) J Stud Alcohol Drugs. 2012;73:454–458. doi: 10.15288/jsad.2012.73.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86:214–221. doi: 10.1016/j.drugalcdep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349–1356. doi: 10.1046/j.1360-0443.1995.901013496.x. [DOI] [PubMed] [Google Scholar]
- Cook RL, Clark DB. Is there an association between alcohol consumption and sexually transmitted diseases? A systematic review. Sex Transm Dis. 2005;32:156–164. doi: 10.1097/01.olq.0000151418.03899.97. [DOI] [PubMed] [Google Scholar]
- Dowdy DW, Rodriguez RM, Hare CB, Kaplan B. Cost-effectiveness of targeted human immunodeficiency virus screening in an urban emergency department. Acad Emerg Med. 2011;18:745–753. doi: 10.1111/j.1553-2712.2011.01110.x. [DOI] [PubMed] [Google Scholar]
- Edelman EJ, Dinh A, Radulescu R, Lurie B, D'Onofrio G, Tetrault JM, Fiellin DA, Fiellin LE. Combining rapid HIV testing and a brief alcohol intervention in young unhealthy drinkers in the emergency department: a pilot study. Am J Drug Alcohol Abuse. 2012;38:539–543. doi: 10.3109/00952990.2012.701359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton KA. Changing epidemiology of HIV/AIDS in the United States: implications for enhancing and promoting HIV testing strategies. Clin Infect Dis. 2007;45(Suppl 4):S213–S220. doi: 10.1086/522615. [DOI] [PubMed] [Google Scholar]
- Fritz K, Morojele N, Kalichman S. Alcohol: the forgotten drug in HIV/AIDS. Lancet. 2010;376:398–400. doi: 10.1016/S0140-6736(10)60884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern-Felsher BL, Millstein SG, Ellen JM. Relationship of alcohol use and risky sexual behavior: a review and analysis of findings. J Adolesc Health. 1996;19:331–336. doi: 10.1016/S1054-139X(96)00024-9. [DOI] [PubMed] [Google Scholar]
- Haukoos JS, Hopkins E, Hull A, Dean C, Donahoe K, Ruzas CM, Bauerle JD, Terrien B, Forsyth J, Kalish B, Thrun M, Rothman R. HIV testing in emergency departments in the United States: a national survey. Ann Emerg Med. 2011;58(1 Suppl 1):S10-6.e1–8. doi: 10.1016/j.annemergmed.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, George WH. Alcohol and sexuality research in the AIDS era: trends in publication activity, target populations and research design. AIDS Behav. 2007;11:217–226. doi: 10.1007/s10461-006-9130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Nachimson D, Cherry C, Williams E. AIDS treatment advances and behavioral prevention setbacks: preliminary assessment of reduced perceived threat of HIV-AIDS. Health Psychol. 1998;17:546–550. doi: 10.1037//0278-6133.17.6.546. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8:141–151. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- Leigh BC. Alcohol and condom use: a meta-analysis of event-level studies. Sex Transm Dis. 2002;29:476–482. doi: 10.1097/00007435-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Maio RF, Shope JT, Blow FC, Gregor MA, Zakrajsek JS, Weber JE, Nypaver MM. A randomized controlled trial of an emergency department-based interactive computer program to prevent alcohol misuse among injured adolescents. Ann Emerg Med. 2005;45:420–429. doi: 10.1016/j.annemergmed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Mello MJ, Longabaugh R. A brief intervention by emergency department providers decreased 12 month alcohol use. Evid Based Med. 2013;18:e24. doi: 10.1136/eb-2012-101024. [DOI] [PubMed] [Google Scholar]
- Millstein SG, Moscicki AB. Sexually-transmitted disease in female adolescents: effects of psychosocial factors and high risk behaviors. J Adolesc Health. 1995;17:83–90. doi: 10.1016/1054-139X(95)00065-Z. [DOI] [PubMed] [Google Scholar]
- Monti PM, Barnett NP, Colby SM, Gwaltney CJ, Spirito A, Rohsenow DJ, Woolard R. Motivational interviewing versus feedback only in emergency care for young adult problem drinking. Addiction. 2007;102:1234–1243. doi: 10.1111/j.1360-0443.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, Colby SM, Mastroleo NR, Barnett NP, Gwaltney CJ, Apodaca TR, Rohsenow DJ, Magill M, Gogineni A, Mello MJ, Biffl WL, Cioffi WG. Individual versus significant-other-enhanced brief motivational intervention for alcohol in emergency care. J Consult Clin Psychol. 2014;82:936–948. doi: 10.1037/a0037658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA News. 2004;3 [Google Scholar]
- Rehm J, Shield KD, Joharchi N, Shuper PA. Alcohol consumption and the intention to engage in unprotected sex: systematic review and meta-analysis of experimental studies. Addiction. 2012;107:51–59. doi: 10.1111/j.1360-0443.2011.03621.x. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res. 2002;26:272–279. [PubMed] [Google Scholar]
- Rothman RE. Current Centers for Disease Control and Prevention guidelines for HIV counseling, testing, and referral: critical role of and a call to action for emergency physicians. Ann Emerg Med. 2004;44:31–42. doi: 10.1016/j.annemergmed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Shillington AM, Cottler LB, Compton WM, 3rd, Spitznagel EL. Is there a relationship between “heavy drinking” and HIV high risk sexual behaviors among general population subjects? Int J Addict. 1995;30:1453–1478. doi: 10.3109/10826089509055842. [DOI] [PubMed] [Google Scholar]
- Tang N, Stein J, Hsia RY, Maselli JH, Gonzales R. Trends and characteristics of US emergency department visits, 1997–2007. JAMA. 2010;304:664–670. doi: 10.1001/jama.2010.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillo AD, Merchant RC, Baird JR, Ladd GT, Liu T, Nirenberg TD. Interrelationship of alcohol misuse, HIV sexual risk and HIV screening uptake among emergency department patients. BMC Emerg Med. 2013;13:9. doi: 10.1186/1471-227X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhardt LS, Carey MP. Does alcohol lead to sexual risk behavior? Findings from event-level research. Annu Rev Sex Res. 2000;11:125–157. [PMC free article] [PubMed] [Google Scholar]
- Woolf-King SE, Maisto SA. Alcohol use and high-risk sexual behavior in Sub-Saharan Africa: a narrative review. Arch Sex Behav. 2011;40:17–42. doi: 10.1007/s10508-009-9516-4. [DOI] [PubMed] [Google Scholar]


