Recently, two Japanese clinical studies of antihypertensive drugs, supported by pharmaceutical companies and published in the Lancet by groups of Japanese investigators, were later withdrawn (Editors of the Lancet, 2009, 2013a). Four other studies of one of these drugs (Editors of the Lancet, 2013b; Uzu et al., 2008; Muramatsu et al., 2012; Narumi, Hakano, and Shindo, 2011), all funded by the Swiss company Novartis that supported one of the studies, but conducted by different Japanese research groups, were later found to be tainted by misconduct unacceptable by scientific standards (Review Committee for the Recent Cases of Clinical Study on an Antihypertensive Drug, 2013). In this article, we discuss deeply-rooted ties between clinical investigators and pharmaceutical companies that underlie these recently surfaced examples of impropriety in patient-oriented research in Japan, which have been banned in most major U.S. medical centers for some time (Conflict of Interest Policies at Academic Medical Centers, 2014). Typically, companies finance many clinical studies, then pick their preferred research data, and advertise the less-than-scientific conclusions through popular professors.
Incidents in Japan
Novartis Japan, a subsidiary of Novartis, a global pharmaceutical company based in Basel, Switzerland, funded five separate clinical studies, with the participation of 15 university medical centers, on the organ-protective effect of their blood pressure pill Valsartan in the early 2000s. The company provided circa $11 million to fund those studies (Review Committee for the Recent Cases of Clinical Study on an Antihypertensive Drug, 2013). Subsequently, internal and external reviews triggered by critiques (Yui, 2012) revealed multiple problems in all of these studies (Alleged Data Fabrications in The Clinical Studies on Diovan (Valsartan) from Novartis, 2013).
It was further revealed that a Novartis Japan employee participated in and played a critical role in all these studies, but while listed as an author, was not identified as employed by Novartis in the articles describing those studies (Novartis Pharmaceuticals Corporation, 2013). In some, falsification of data was identified (Kyoto Prefectural University of Medicine, 2013; Jikei University of Medicine, 2013). Moreover, the informed consent form signed by participating patients listed the financial source as simply “internal” or “self-funding.” Most notably, misrepresenting or obscuring the source of study funding of this kind has not been considered to deviate from ethical norms in Japan. The widely scrutinized and publicized flaws in this series of Novartis-supported studies are not isolated events but, we would argue, representative of the serious problems that continue to undermine the research process in clinical investigations, and remain unwelcome for discussion in the clinical research arena in Japan (Watanabe, 2015; Workshop for Research, 2015).
Multiple layers of a fundamental problem
Strings attached
It is common in Japan for marketing–-not research and development—divisions of pharmaceutical companies to provide doctors at leading medical centers with money officially termed “scholarship donations” or “research funds,” and, in business slang, “social expenses” or “admission fees.” The funding is based on a time-limited contract, is renewable, and comes with an implicit expectation that the recipients will support the companies’ products. As a result, researchers have a strong incentive to design studies or publish data that are favorable to the sponsoring companies (Novartis Pharmaceuticals Corporation, 2013).
Pharmaceutical companies also often maintain a strong hand in deciding which results are published. This may contribute to the long-standing observation that data from research funded by companies tend to give results that are more favorable to their products when compared to those funded by other means (Ichikawa and Motojima, 2012; Friedberg et al., 1999).
The presence of a wide evidence--practice gap
In many fields of clinical medicine, well-designed and well-executed studies are performed at academic medical centers, and the results support evidence-based medicine, the foundation of good medical practice. To achieve the best visibility for their work and to build their evidence base, researchers seek to have their work published in respected journals, for which they must meet high scientific and ethical standards (Figure 1, top right). But while the data thus published may have a significant impact on the practice of doctors in academic centers, the vast majority of patients who should benefit from the data are under the care of private or local practicing doctors, who are often unfamiliar with the latest research data, thus creating an “evidence--practice gap.” The gap is due in part to the highly sophisticated and specialized language used in those research articles that, while possibly filling the goal of satisfying the critical views of reviewers, remain absolutely foreign to general practicing physicians in Japan. For many of these physicians, the English language is already quite foreign: Japanese remains the predominant language in medical communications in Japan, e.g., in medical school classes, for medical records, and in discussions at conferences.
Figure 1.
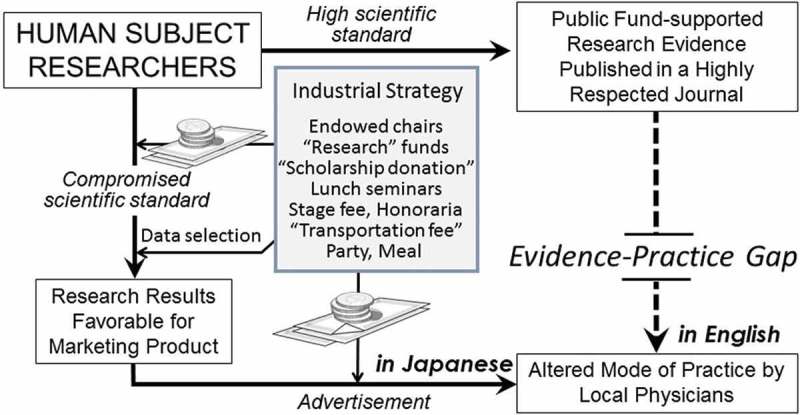
The two contrasting pathways of research through which Japanese clinical studies with human subjects influence the behavior of Japanese practicing physicians, who are more exposed to the compromised scientific standards of industry than the higher scientific standards possible without such pressure.
Research data information controlled by companies
Pharmaceutical companies operating in Japan take many actions to promote their products (Figure 1, center, “Industrial Strategy”). As described above, their marketing divisions give research doctors financial incentives to conduct studies on their products, via either a research fund or a “scholarship donation” (Figure 1, top left money pile). For these doctors, this money does not require high scientific, ethical standards, or voluminous paperwork, nor does it require conformity to strict rules for spending, as it is the case with public funds. The results of this research may be published in the less prestigious, Japanese language journals, which make them more accessible to local physicians. Assuming, perhaps correctly, that many clinicians do not routinely read many medical journals, the companies then, no matter what data are published, pick the research results most favorable to them (Figure 1, bottom left arrow “Data selection”) and finance a variety of advertising opportunities, including workshops of various scale, lunch seminars at academic conferences with medical experts, where the audience is always treated to meals and speakers, and chairpersons with hefty honoraria (Figure 1, center money pile). Medical school professors are the favored speakers (Watanabe, 2015). For research doctors, the opportunity to give a speech to a large audience has financial and professional benefits of gaining broader recognition of their work, as they do not publish in the more widely-read journals: Companies often advertise this work in non-peer-reviewed commercial journals and brochures. For most practicing doctors in Japan, opportunities to learn new data in their native language may be more palatable than those available in more internationally respected journals in English. Japanese medical schools continue to mostly teach from textbooks written in Japanese, not English, unlike in many other Asian countries.
A few Japanese scholars argue that “scholarship donations” from pharmaceutical companies are an indispensable resource for research in the country as public funding for clinical studies is scarce (Kawai, 2013). Indeed, company-funded research is claimed to be an important component of the residency or fellowship training, for which not much public funding is available. It is ethically unacceptable, however, for research doctors to serve as advertisers for such research data (Yasushi, 2013). Biased drug information causes unjustified over-prescription of new drugs, protected by the patent system in Japan, which eventually becomes an unfair burden to individual patients and the national welfare system.
Company representatives from drug companies in Japan even have their own euphemism. Called MRs (for “medical representatives”), they number more than 60,000 in Japan (MR Education, 2015), roughly comparable to the number in the United States, in a population less than half its size (Hays Japan; Sufrin and Ross, 2008). According to the code of conduct established for MRs by the Japan Pharmaceutical Manufacturers Association, they have a duty not only of “promoting their merchandise,” but also of “sharing responsibilities in healthcare services” (Japan Pharmaceutical Manufacturers Association, 2013). The phenomena described earlier, however, indicate that the former has come to clearly outweigh the latter.
Japanese doctors, in both the research and practice arenas, are steeped in an environment of wining and dining by pharmaceutical companies. “Abenomics,” (Abenomics refers to the economic policies advocated by Prime Minister Abe) the new Japanese government strategy invoked to energize the national economy, includes drastic reductions in the taxation on entertainment and social expenses for large corporations. The new rules may well encourage these pharmaceutical companies to cajole doctors even more through more “scholarship donations,” employing more MRs, and more wining and dining.
The pervasiveness of pharmaceutical companies in funding research and influencing prescribing practice stands in stark contrast to the very strict code of conduct imposed upon Japanese government officials in any area, who are forbidden from receiving gifts due to concerns about conflict of interest (National Public Service Ethics Board). Physicians and investigators are quite susceptible to these practices, despite their protests to the contrary (Bero, 2013; Dwan et al., 2013). This lack of recognition of the industry’s influence is what connects and underlies the multiple flaws that have recently surfaced in Japan’s research field.
Inertia in education
Education, we believe, is the key to breaking through the desensitization and denial. Japan, however, might be handicapped in this regard. First, the level of English language knowledge required at the graduate level is superficial at best. As a result, according to a recent data collected during the 2007–2014 period, Japan ranks 30th in adult English proficiency, after Vietnam (Newswire, 2015). Thus the access that Japanese physicians practicing in Japan have to the current streams of international research and research ethics is limited.
It may be argued that even in the United States, the emphasis on ethics education for research trainees has only recently become a trend. It was not until 2009, after all, that the National Institutes of Health (NIH) recommended that individuals who are supported by its training grant take at least eight hours of “Responsible Conduct of Research” training every four years (National Institutes of Health, 2009). However, in Japan, only an hour or two of attending lectures on laws and guidelines regarding human rights protection and use of public funds is the current predominant mode of what is defined as ethics. Such a lightweight obligation is welcomed by researchers in the short run, and it is hard to expect that such a cursory focus on regulation alone will have the necessary impact on doctors who are daily cajoled by the pharmaceutical industry in a less than robust economy.
Nine years ago, the Science Council of Japan formed a task force to analyze research misconduct in Japan. Of interest, the final report recommended, “as Japanese researchers do not have the kinds of autonomy comparable to their Euro-American peers, they should not be subjected to penalties for misconduct comparable to that imposed upon European and American researchers” (A Standing Committee for Science and the Society, the Science Council of Japan, 2005). This approach of exempting Japanese researchers is a direct shirking of responsibility that we feel must be rejected for Japan to gain credibility in her research accomplishments. We feel the country should step up and embrace principles of responsible research conduct.
Government and professionals’ reluctance to regulate behavior
Historically, even more so than in other developed countries, and the United States in particular, Japanese professional groups tend to take little initiative in policy making, leaving this important task to bureaucrats and politicians at the national government level. Thus, instead of pooling their expertise to regulate their own behavior (the sine qua non of professionalism) or to propose or to create effective national policies, members of professional societies function primarily to lobby for government funding in their respective research fields. The Japanese people, however, seek to limit government power as a result of their collective fear that historically, totalitarian ideological education has led to many excesses, including war (Nishikawa, 2014). The current government has been hesitant to implement ethics education on these grounds.
A need to increase social stigma around research misconduct
Physicians in Japan who conduct research for private companies may have little incentive to avoid research misconduct. If they are found to have engaged in it, they can always turn to the clinical care professions for work, where they can make a comfortable living, with patient demand virtually assured by universal health care coverage and an aging population, many of whom have the time to seek care. Thus, a critical issue may be to increase the stigma surrounding misconduct.
A lack of buy-in by leadership
Japan is still a society where seniority is given heavy weight. The governance of universities and medical centers is commonly run by senior professors, who are often unfamiliar with and/or resistant to the recent trend of emphasizing ethics education from abroad. Oftentimes not fully appreciating the current concerns of global research ethics, senior professors dominating the review committee for misconduct cases make a serious error, which afterwards invites public outcry (Waseda University Ph.D. Review Committee, 2014).
New trends and questions
The influence of pharmaceutical funding looms particularly large because government funding of patient-oriented research remains small due in part to the financial burden of the national health care system. Also, in Japan, private foundations for such research are small in number and scale. The environment is such that researchers must seek money, with strings attached, in practice if not explicitly, from the medical industry, which is keenly interested in the research results.
The recent publicized cases in Japan with the flawed results, however, finally led its government and the country’s private sectors to take the initiative to tackle ethics education (Ichikawa and Motojima, 2012; The Science Council of Japan, 2013). Thus, it is becoming mandatory for the recipients of the government’s various research funds, to take an ethics training course that includes discussions of conflict of interest (Asahi Shinbun Editors, 2013). While greater buy-in by leadership and greater availability of public and foundation funding are also necessary, we believe that educating current and future investigators about the importance of research ethics and integrity will enhance the quality of research in Japan and shore up its reputation around the world. Through such education, we hope that investigators come to recognize the manipulation techniques that surround them and are prevalent in our commercial society.
References
- A Standing Committee for Science and the Society, the Science Council of Japan Current status of misconduct in science and preventive measures. 2005 http://www.scj.go.jp/ja/info/kohyo/pdf/kohyo-19-t1031-8.pdf July 21.
- Abenomics refers to the economic policies advocated by Prime Minister Abe It is based upon “three arrows” of fiscal stimulus, monetary easing and structural reforms. The Economist characterized the program as a “mix of reflation, government spending and a growth strategy designed to jolt the economy out of suspended animation that has gripped it for more than two decades.”. http://lexicon.ft.com/Term?term=abenomics from the Financial Times Lexicon. “Abe’s Master Plan,” The Economist, May 18, 2013.
- Alleged data fabrications in the clinical studies on Diovan (Valsartan) from Novartis 2013 http://diovan-novartis.blogspot.jp/
- Asahi Shinbun editors It is now mandatory for researchers to take ethics training, the Japan Science and Technology Agency has announced. Asahi Shinbun. 2013 http://www.asahi.com/shimen/articles/TKY201307270545.html
- Bero L. Industry sponsorship and research outcome a Cochrane review. JAMA Internal Medica. 2013;173(7):580–81. doi: 10.1001/jamainternmed.2013.4190. [DOI] [PubMed] [Google Scholar]
- Conflict of interest Policies at Academic Medical Centers. 2014. AMSA Scorecard American Medical Student Association. 2014 http://amsascorecard.org/
- Dwan K., Gamble C., Williamson P. R., Kirkham J. J., the Reporting Bias Group Systematic review of the empirical evidence of study publication bias and outcome reporting bias — An updated review. PLoS ONE. 2013;8(7):e66844. doi: 10.1371/journal.pone.0066844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editors of the Lancet Retraction-Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): A randomised controlled trial. The Lancet. 2009;374:1226. doi: 10.1016/S0140-6736(09)61768-2. [DOI] [PubMed] [Google Scholar]
- Editors of the Lancet Retraction: Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): A randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2013a;382:843. doi: 10.1016/S0140-6736(13)61847-4. [DOI] [PubMed] [Google Scholar]
- Editors of the Lancet Retraction of: Effect of valsartan on morbidity and mortality in uncontrolled hypertensive patients with high cardiovascular risks: KYOTO HEART Study. [(2009). Euro Heart J 30: 2461-2469] Euro Heart Journal. 2013b;34:1023. doi: 10.1093/eurheartj/eht030. [DOI] [PubMed] [Google Scholar]
- Friedberg M., Saffran B., Stingson T. J., Nelson W., Bennett C. L. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. Jama. 1999;282:1453–57. doi: 10.1001/jama.282.15.1453. [DOI] [PubMed] [Google Scholar]
- Hays Japan Life sciences jobs: MR (Medical Representative) hays.co.jp. http://www.hays.co.jp/en/jobs/life-sciences-jobs/HAYS_055099
- Ichikawa I., Motojima M. Creating the CITI-Japan program for web-based training: Where ethics, law and science experts meet. In: Mayer T., Steneck N., editors. Promoting Research Integrity in a Global Environment. Kanagawa, Japan: World Scientific Publishing Co; 2012. [Google Scholar]
- Japan Pharmaceutical Manufacturers Association JPMA Code of Practice. 2013 http://www.jpma.or.jp/english/isuues/pdf/code_practice.pdf January 16.
- Jikei University of Medicine Second Interim Report from “Jikei Heart Study” Investigation. 2013 http://www.jikei.ac.jp/news/20130730.html
- Kawai S. ‘Scholarship Donation’ is indispensable for the sake of research activities. In Repetitive incidence of research misconduct: Proposals from ten experts, aiming to restore public trust on research. 2013 http://cmad.nikkeibp.co.jp/?4_199013_56290_9
- Kyoto Prefectural University of Medicine An Investigation Report from the Clinical Study “Kyoto Heart Study”. 2013 http://www.kpu-m.ac.jp/doc/news/2013/ files/20130711press.pdf
- Muramatsu T., Matsushita K., Yamashita K., Kondo T., Maeda K., Shintani S., Ichimiya S., Ohno M., Sone T., Ikeda N., Wataraj M., Murohara T. Comparison between valsartan and amlodipine regarding cardiovascular morbidity and mortality in hypertensive patients with glucose intolerance: NAGOYA HEART Study. Hypertension. 2012;59:580–6. doi: 10.1161/HYPERTENSIONAHA.111.184226. [DOI] [PubMed] [Google Scholar]
- MR Education & Accreditation Center of Japan White paper on MR. mre.or.jp. 2015 www.mre.or.jp/whitep_guideline/pdf/2015hakusyo-01-11.pdf
- Narumi H., Hakano H., Shindo S. Effects of valsartan and amlodipine on cardiorenal protection in Japanese hypertensive patients: The Valsartan Amlodipine Randomized Trial. Hypertension Research : Official Journal of the Japanese Society of Hypertension. 2011;34:62–69. doi: 10.1038/hr.2010.186. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Update on the Requirement for Instruction in the Responsible Conduct of Research. 2009 Nov 24; http://grants.nih.gov/grants/guide/notice-files/NOT-OD-10-019.html
- National Public Service Ethics Board Q&A on the code of conduct for national government officials. http://www.jinji.go.jp/rinri/kaisetu/shitugi.pdf#search= ‘%E5%9B%BD%E5%AE%B6%E5%85%AC%E5%8B%99%E5%93%A1%E5%80%AB%E7%90%86%E8%A6%8F%E5%AE%9A
- Newswire P. R. Sweden at Top, Middle East at Bottom of EF’s Global Ranking of English Skills. 2015 http://www.prnewswire.com/news-releases/sweden-at-top-middle-east-at-bottom-of-efs-global-ranking-of-english-skills-539730692.html November 3.
- Nishikawa R. Issues involved in the special curriculum, “Morality”. Nhk.or.jp. 2014 http://www.nhk.or.jp/kaisetsu-blog/100/201572.html
- Novartis Pharmaceuticals Corporation A report on the role of Novartis Pharmaceuticals Corporation in the five investigator-initiated clinical studies. 2013 http://www.novartis.co.jp/valsartan/0729/pdf/novartis_report20130729_2.pdf
- Review Committee for the Recent Cases of Clinical Study on an Antihypertensive Drug Proposal for the management and preventive measures for misconducts based on the recent cases of clinical study on antihypertensive drugs: An Interim Report. 2013 http://www.mhlw.go.jp/file/05-Shingikai-10801000-Iseikyoku- Soumuka/0000026006.pdf
- Sufrin C., Ross J. Pharmaceutical industry marketing: Understanding its impact on women’s health. Obstetrical & Gynecological Survey. 2008 Sep;63(9):585–96. doi: 10.1097/OGX.0b013e31817f1585. [DOI] [PubMed] [Google Scholar]
- The Science Council of Japan Announcement of a science forum on promoting responsible conduct of research. 2013 http://www.scj.go.jp/ja/event/pdf2/166-s-0219.pdf
- Shiga Microalbuminuria Reduction Trial (SMART) Group. Uzu T., Sawaguchi M. Impact of renin-angiotensin system inhibition on microalbuminuria in type 2 diabetes: A post hoc analysis of the Shiga Microablbuminuria Reduction Trial (SMART) Hypertension Research : Official Journal of the Japanese Society of Hypertension. 2008;31:1171–76. doi: 10.1291/hypres.31.1171. [DOI] [PubMed] [Google Scholar]
- Waseda University PhD Review Committee Report from the Waseda University Review Committee on the PhD thesis of Haruko Obokata. 2014 http://www.waseda.jp/jp/news14/data/140717_committee_report.pdf
- Watanabe J. Professors reminding audience members of drug names for hefty honorariums from drug companies1. Asahi Shinbun. 2015 www.asahi.com/articles/ASH305GJFH30UUPI006.html
- Workshop for research ethics education general panel discussion. Jams.med.or.jp. 2015 http://jams.med.or.jp/researchethics/index.html
- Yasushi T. Researchers need to be more aware how contact with MRs may affect their research. In Repetitive incidence of research misconduct: Proposals from ten experts, aiming to restore public trust on research. 2013 http://cmad. nikkeibp.co.jp/?4_199013_56290_10
- Yui Y. Concerns about the Jikei Heart Study. The Lancet. 2012;379:e48. doi: 10.1016/S0140-6736(12)60599-6. [DOI] [PubMed] [Google Scholar]


