Abstract
Social behavioral deficits have been observed in patients diagnosed with alternating hemiplegia of childhood (AHC), rapid-onset dystonia-parkinsonism and CAPOS syndrome, in which specific missense mutations in ATP1A3, encoding the Na+, K+-ATPase α3 subunit, have been identified. To test the hypothesis that social behavioral deficits represent part of the phenotype of Na+, K+-ATPase α3 mutations, we assessed the social behavior of the Myshkin mouse model of AHC, which has an I810N mutation identical to that found in an AHC patient with co-morbid autism. Myshkin mice displayed deficits in three tests of social behavior: nest building, pup retrieval and the three-chamber social approach test. Chronic treatment with the mood stabilizer lithium enhanced nest building in wild-type but not Myshkin mice. In light of previous studies revealing a broad profile of neurobehavioral deficits in the Myshkin model – consistent with the complex clinical profile of AHC – our results suggest that Na+, K+-ATPase α3 dysfunction has a deleterious, but nonspecific, effect on social behavior. By better defining the behavioral profile of Myshkin mice, we identify additional ATP1A3-related symptoms for which the Myshkin model could be used as a tool to advance understanding of the underlying neural mechanisms and develop novel therapeutic strategies.
Keywords: Alternating hemiplegia of childhood, ATP1A3; mouse model, social behavior
Introduction
Alternating hemiplegia of childhood (AHC; OMIM: 614820) is a rare neurodevelopmental disorder that manifests as episodic hemiplegia starting in the first 18 months of life, with a spectrum of persistent motor, movement and cognitive deficits that become progressively more apparent with age (Sweney et al. 2009, Panagiotakaki et al. 2010). Children with AHC are prone to a wide range of behavioural and psychiatric disorders, including impulsivity, lack of attention control, difficulties in acquiring speech, obsessionality and short-temperedness (Neville and Ninan 2007). Published findings of comprehensive assessments of neuropsychological functioning in children with AHC are limited to two case studies, which both report deficits in language, memory, attention and information processing, as well as difficulty with impulsivity (Shafer et al. 2005, Muriel et al. 2015).
Heterozygous missense mutations of the ATP1A3 gene, encoding the Na+,K+-ATPase α3 subunit, have been identified as the primary cause of AHC (Heinzen et al. 2014). Na+,K+-ATPases are membrane-bound transporters that harness the energy of ATP hydrolysis to pump three Na+ ions out of the cell in exchange for two K+ ions moving inwards. Na+,K+-ATPase α3-expressing neurons are present throughout the nervous system (Benarroch 2011). Most ATP1A3 mutations in AHC patients are clustered in or near transmembrane α-helix TM6, including recurrent mutations of the isoleucine at position 810: I810F, I810N and I810S (Heinzen et al. 2012, Rosewich et al. 2014a, Yang et al. 2014, Panagiotakaki et al. 2015). To date, three cases of AHC with mutation I810N have been reported, including a 22-year-old man from Belgium with autism (Yang et al. 2014, Panagiotakaki et al. 2015, Yang et al. 2015, Weckhuysen S 2015, Personal communication). All of the AHC mutations studied to date result in a catalytically inactive Na+,K+-ATPase α3 (Clapcote et al. 2009, Weigand et al. 2014).
Other missense mutations in Na+,K+-ATPase α3 have been identified in patients with a phenotypic continuum of ATP1A3-related encephalopathy: (1) rapid-onset dystonia-parkinsonism (RDP; DYT12; OMIM: 128235; Heinzen et al. 2014); (2) an intermediate AHC/RDP presentation (Roubergue et al. 2013, Boelman et al. 2014, Heinzen et al. 2014, Rosewich et al. 2014a, Sasaki et al. 2014, Termsarasab et al. 2015); (3) cerebellar ataxia with areflexia, pes cavus, optic atrophy and sensorineural deafness (CAPOS; OMIM: 601338; Demos et al. 2014, Heimer et al. 2015); (4) an intermediate CAPOS/AHC presentation (Rosewich et al. 2014b); (5) early infantile epileptic encephalopathy (EIEE; Paciorkowski et al. 2015); (6) relapsing encephalopathy with cerebellar ataxia (RECA; Dard et al. 2015). Five RDP patients with mutation T613M from an Irish family are reported to have profound difficulties socializing and maintaining relationships, resulting in a diagnosis of social anxiety disorder in one individual (Pittock et al. 2000, McKeon et al. 2007). Two CAPOS patients with mutation E818K from a British family have been diagnosed with autism spectrum disorder (Demos et al. 2014). The activity of Na+,K+-ATPase α3 is also impaired by its aberrant association with misfolded and aggregated proteins implicated in Alzheimer’s disease (β-amyloid; Ohnishi et al. 2015), Parkinson’s disease (α-synuclein; Shrivastava et al. 2015) and amyotrophic lateral sclerosis (SOD1; Ruegsegger et al. 2016).
Heterozygous Myshkin (Atp1a3Myk /+; Myk/+) mutant mice have an I810N mutation identical to that present in AHC, which reduces total Na+,K+-ATPase activity (α1 + α2 + α3) in the whole brain by 42% (Clapcote et al. 2009). Myk/+ mice move with a paretic, tremulous gait that becomes transiently more severe after stress (Kirshenbaum et al. 2013, 2016). Other phenotypic abnormalities include neuronal hyperexcitability, and increased susceptibility to epileptic seizures in mixed 129/B6 and FVB/B6 genetic backgrounds (Clapcote et al. 2009). Myk/+ mice have, however, shown intact audioception in the acoustic startle test (Kirshenbaum et al. 2013), ophthalmoception in the head tracking test (Kirshenbaum et al. 2011a), and gustaoception in the sucrose preference test (Kirshenbaum et al. 2011a). To test the hypothesis that social behavioral deficits represent part of the phenotype of Na+,K+-ATPase α3 mutations, we assessed the social behavior of the Myshkin mouse model of AHC.
Materials and methods
Mice
Myk/+ males that had been backcrossed for 20 generations to the C57BL/6NCr strain were mated with C57BL/6NCrl (Charles River, Margate, UK) females to yield wild-type (+/+) and Myk/+ littermates. Myk/+ mice at N20 C57BL/6NCr were previously reported to be free of stress-induced seizure activity during electrocorticography (Kirshenbaum et al. 2011a). Myk/+ mice were genotyped by the presence of an EcoO109I (New England BioLabs, Hitchin, UK) restriction site using polymerase chain reaction primers F, 5′-CTG CCG GAA ATA CAA TAC TGA-3′ and R, 5′-ATA AAT ACC CCA CCA CTG AGC-3′. Mice were weaned at four weeks of age and grouped housed (2–5 mice/cage) with same-sex littermates. Mice were tested at 8–14 weeks of age. Males and females were included in balanced numbers, apart from the pup retrieval test. Subjects were handled daily for 5 min/day for seven days prior to testing, which was conducted during the light phase (0900–1700 h). Prior to experiments, subjects were left undisturbed in the testing environment for 30 min to allow for acclimation. All procedures involving animals were conducted in accordance with the Animals (Scientific Procedures) Act 1986, and were approved by the University of Leeds Ethical Review Committee.
Nest building and utilization
Mice were individually housed in a clean cage with nesting material comprising a 3 g 5 cm2 square of compressed cotton (“nestlet”; Lillico, Horley, UK). During 5 min of observation, the duration and frequency of exploration and shredding of the nestlet were recorded, and then the cage was placed onto the cage rack. At 30 min, 60 min, 90 min, 3 h and 24 h, the percentage of the nestlet that was shredded was recorded, and the quality of the nest was scored as follows (Moretti et al. 2005): 0 = nesting material unmodified; 1 = flat nest with partially shredded nesting material; 2 = shallow nest with shredded material, but lacking fully formed walls; 3 = nest with well-developed walls; 4 = nest in a shape of a cocoon with partial or complete roof. At 3 h, utilization of the nest was assessed by recording whether the mouse was positioned inside or outside the nest. At 24 h, the height of the nest in cm was recorded.
Pup retrieval behavior
Several pairs of female littermates – one Myk/+ and one +/+ – were housed together from weaning to about 60 days of age, when an experienced C57BL/6NCrl stud male was introduced to each cage. Females were checked for vaginal plugs each morning to determine if mating had occurred. When both females had been mated, the male was removed and the cage was checked daily for litters. In four cages where both females had given birth within 12 h, pup retrieval behavior was assessed. On postnatal day 5, between 09:00 and 12:00 h, one of the females (counterbalanced for genotype) was temporarily removed and put into a clean cage. Next, a healthy pup with milk in its stomach was taken from the communal nest and placed at the far end of the cage. The experimenter was blind to the genotype of the pups. Latency for the remaining female to retrieve the pup was recorded. The next day (postnatal day 6), the other female was removed and the experiment was repeated.
Social approach test
Social approach was assessed using a three-chambered apparatus (60 × 40 cm), which had two doors to allow the mouse to access left and right chambers from a central compartment (each chamber being 40 × 20 cm). Following a 15-min habituation period, two cylindrical enclosures (10 × 10.5 cm, comprising vertical metal bars 9 mm apart) were placed into the left and right chambers, into one of which an unfamiliar adult male C57BL/6 mouse (age 10 weeks; “stranger 1”) was placed. Left/right placement was counterbalanced across groups. Time spent exploring each enclosure was measured for 10 min. A second unfamiliar adult male C57BL/6 mouse (“stranger 2”) was then placed into the empty cylinder and time spent exploring each enclosure was measured for 10 min. The time spent exploring stranger 1 or the empty cylinder in the first phase, and time spent exploring either stranger 1 or 2 in phase two were recorded. A solution of 70% ethanol was used to clean surfaces and equipment between subjects.
Drug treatment
Lithium carbonate (Li2CO3) was administered in the diet at 0.4% for 28 d before the assessment of nest building, and the control group received an identical drug-free diet (CRM-P; Special Diets Services, Witham, UK). To prevent ion imbalances from lithium, all mice were provided with an additional water bottle containing 0.9% saline. Serum lithium levels were measured by a spectrophotometry kit (Roche Diagnostics, Burgess Hill, UK), and therapeutic serum lithium levels (0.75–0.95 mmol/L; Gelenberg et al. 1989) were reached in +/+ and Myk/+ mice, as previously described (Kirshenbaum et al. 2011a).
Data analysis
All statistics were calculated by STATISTICA (StatSoft, Tulsa, USA). Data were subjected to analysis of variance (ANOVA) with genotype, sex and drug as between-subjects factors, and time as a within-subject factor. When ANOVA detected statistically significant main effects, pairwise differences were evaluated using the Tukey–Kramer post hoc multiple comparison test, with significance set at p < 0.05. All values reported in the figures are expressed as mean ± standard error of the mean (SEM).
Results and discussion
Nest building and utilization are impaired in Myk/+ mice
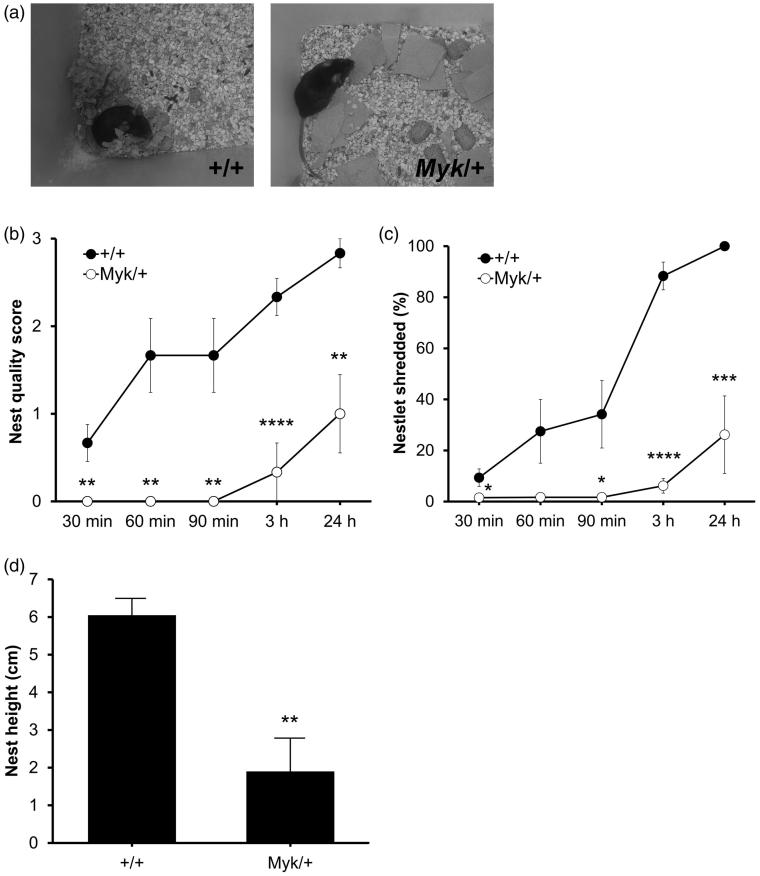
Mice build a nest when provided with suitable material and are typically found lying in it during the daytime. A previous study (of circadian rhythms) provided anecdotal evidence of deficient nest building by Myk/+ mice given a ripped up paper towel (Kimberly–Clark) as nesting material (Kirshenbaum et al. 2011a; Figure 1(a)). As a home cage activity related to maternal care and social behavior (Peripato and Cheverud 2002, Moretti et al. 2005), we studied nest building in mice provided with a nestlet of compressed cotton, which requires shredding. At 30 min, 60 min, 90 min, 3 h and 24 h after mice were placed into a clean cage, the percentage of the nestlet that was shredded was recorded, and the quality of the nest was scored on a 1–4 scale. The nests of Myk/+ mice were of consistently lower quality than those of +/+ littermates (Figure 1(b)). The difference in nest quality was paralleled by a greater propensity of +/+ mice to shred the nesting material compared with Myk/+ mice, whose nestlets remained largely untouched after 24 h (Figure 1(c)). The height of nests built by +/+ mice was greater at 24 h than the nests of Myk/+ mice (Figure 1(d)). The difference between genotypes did not appear to be related to slower building of the nest, as it persisted even for up to one week (data not shown).
Figure 1.
Nest building behavior. (a) Representative examples of nest building by singly-housed Myk/+ (right) and +/+ (left) mice provided with a ripped up paper towel in a previous circadian rhythm study. (b) Nest quality score (0–4 scale) at 30 min, 60 min, 90 min, 3 h and 24 h after placement into a clean cage. Main effects of genotype (F(1, 44) = 73.86, p < 0.0001) and time (F(4, 44) = 8.66, p < 0.0001) were observed. (c) Percentage of nestlet shredded at 30 min, 60 min, 90 min, 3 h and 24 h after placement into a clean cage. Main effects of genotype (F(1, 44) = 74.12, p < 0.0001), time (F(4, 44) = 17.87, p < 0.0001) and genotype × time interaction (F(4, 44) = 7.73, p < 0.0001) were observed. (d) Height of nests at 24 h after placement into a clean cage. A main effect of genotype (F(1, 8) = 15.82, p < 0.01) was observed. Myk/+ mice (n = 6); +/+ mice (n = 6). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 versus +/+ mice.
Utilization of the nest was assessed by recording the position of the mouse during rest/sleep compared with the location of the nest in the light phase of the 12:12 light:dark cycle. Upon observation at 24 h, 100% (6 of 6) of +/+ mice versus 16.7% (1 of 6) of Myk/+ mice were found to be resting. Myk/+ mice are known to exhibit less REM and non-REM sleep (Kirshenbaum et al. 2011a, 2014). All of the +/+ mice were resting inside the nest, whereas the single resting Myk/+ mouse was found outside the nest.
Most behavioral tests in experimental animals depend on the measurement of motor output and may be influenced by underlying motor dysfunction. Myk/+ mice have a tremor (Kirshenbaum et al. 2013), which could conceivably disrupt fine motor skills, so we cannot formally exclude the possibility that tremor may have impaired physical manipulation of the nestlets. However, our finding that nest utilization and building time were both reduced in Myk/+ mice suggests that the difference in nesting behavior is more likely due to decreased interest in building the nest than simple impairment in execution of a motor task. Indeed, deficient nest building was first observed in Myk/+ mice given nesting material – a ripped up paper towel – that does not require extensive shredding. Moreover, similar abnormalities in nest building and utilization have been exhibited by two mouse models of Rett syndrome, an autistic spectrum disorder, independent of whether gross body tremor was present (Mecp2 tm1Hzo; Moretti et al. 2005) or absent (Mecp2 tm1.1Bird; Samaco et al. 2008) in the mice.
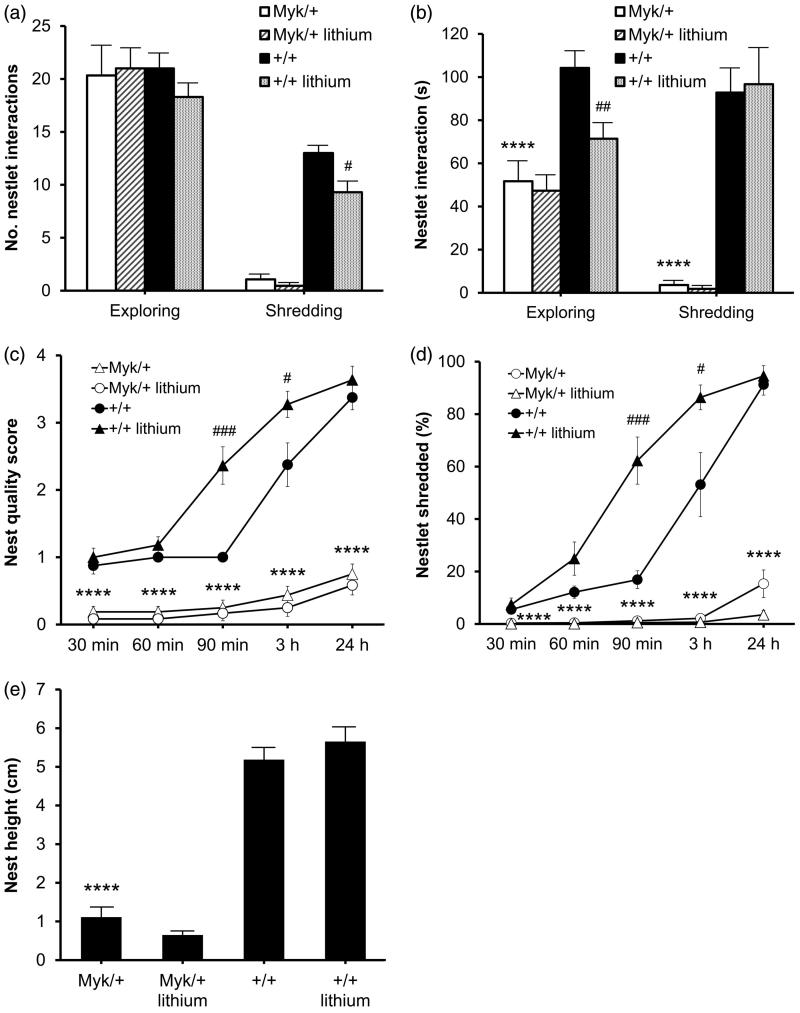
Chronic treatment with the mood stabilizer lithium is reported to promote sociability in the Fmr1 knock-out mouse model of Fragile X syndrome (Mines et al. 2010), a developmental disorder that often includes symptoms of autism (Kaufmann et al. 2004). Lithium has also been shown to reduce the hyperambulation and risk-taking behavior of Myk/+ mice (Kirshenbaum et al. 2011a), so we subjected a separate cohort of mice to chronic dietary treatment with Li2CO3 and observed their nest building behavior in a clean cage. Over 5 min of observation, Myk/+ mice engaged in fewer bouts of shredding, and spent less time exploring and shredding the nesting material than +/+ littermates, but there was no genotypic difference in the number of exploratory approaches (Figure 2(a,b)). Wild-type mice spent 30.9 ± 3.8% of the time building the nest, whereas Myk/+ mice spent only 1.2 ± 0.7% of the time actively manipulating the same material. We found that Li2CO3 treatment had no effect on any nest building and utilization parameter in Myk/+ mice (Figure 2(a–e)), but it did enhance the nest building behavior of +/+ mice at 90 min and 3 h after placement into a clean cage (Figure 2(c,d)).
Figure 2.
Effects of chronic lithium treatment on nesting building. (a) Bouts of exploration and shredding of the nesting material. Main effects of genotype (F(1, 37) = 233.31, p < 0.0001), drug (F(1, 37) = 9.80, p < 0.01) and genotype × drug interaction (F(1, 37) = 5.39, p < 0.05) on bouts of shredding were observed. (b) Time spent exploring and shredding the nesting material over 5 min of observation in a clean cage. Main effects of genotype (F(1, 37) = 18.06, p < 0.0001) and drug (F(1, 37) = 4.39, p < 0.05) on time spent exploring, and a main effect of genotype (F(1, 37) = 101.27, p < 0.0001) on time spent shredding, were observed. (c) Nest quality score (0–4 scale) at 30 min, 60 min, 90 min, 3 h and 24 h after placement into a clean cage. Main effects of genotype (F(1, 212) = 595.26, p < 0.0001), drug (F(1, 212) = 9.87, p < 0.0001), time (F(4, 212) = 74.68, p < 0.0001), genotype × drug interaction (F(1, 212) = 24.82, p < 0.0001) and genotype × time interaction (F(4, 212) = 33.99, p < 0.0001) were observed. (d) Percentage of nestlet shredded at 30 min, 60 min, 90 min, 3 h and 24 h after placement into a clean cage. Main effects of genotype (F(1, 212) = 579.50, p < 0.0001), drug (F(1, 212) = 21.25, p < 0.0001), time (F(4, 212) = 100.05, p < 0.0001), genotype × drug interaction (F(1, 212) = 38.89, p < 0.0001), genotype × time interaction (F(4, 212) = 67.39, p < 0.0001), sex × drug interaction (F(1, 212) = 5.39, p < 0.05) and drug × time interaction (F(4, 212) = 5.69, p < 0.0001) were observed. (e) Height of nests at 24 h after placement into a clean cage. A main effect of genotype (F(1, 40) = 238.88, p < 0.0001) was observed. Myk/+ mice on standard diet (n = 15); Myk/+ mice on lithium diet (n = 11); +/+ mice on standard diet (n = 8); +/+ mice on lithium diet (n = 10). *p < 0.05; **p < 0.01; ****p < 0.0001 Myk/+ mice on standard diet versus +/+ mice on standard diet. # p < 0.05; ### p < 0.001 +/+ mice on lithium diet versus +/+ mice on standard diet.
Myk/+ dams display deficit in pup retrieval
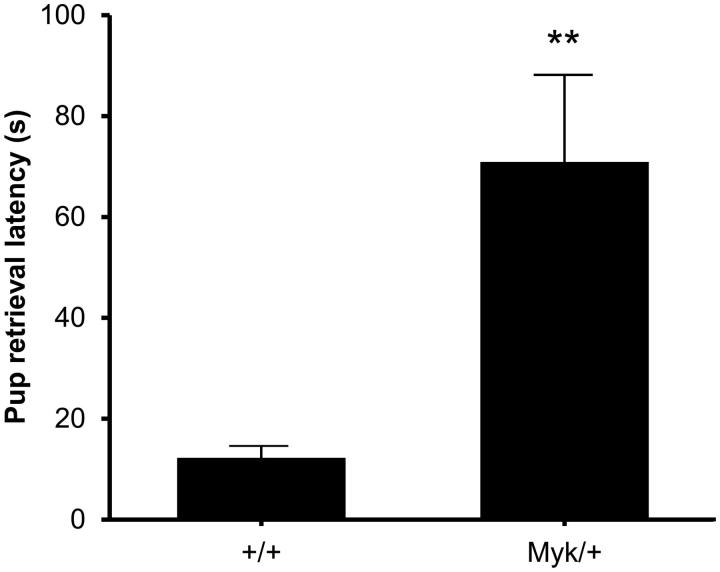
Ultrasonic vocalizations (USV) emitted by pups removed from the nest will elicit search-and-retrieval behavior in lactating dams (Young et al. 2010). As a measure of maternal care, we compared the pup retrieval latencies of Myk/+ and +/+ dams. Myk/+ dams exhibited deficient maternal behavior by taking longer than +/+ dams to initiate pup retrieval (Figure 3). CAPOS patients with ATP1A3 mutations exhibit optic atrophy and sensorineural deafness (Demos et al. 2014), but the intact audioception of Myk/+ mice (Kirshenbaum et al. 2013) suggests that the reduced maternal retrieval behavior of Myk/+ dams is unlikely to be a simple consequence of hearing loss. While it is possible that Myk/+ pups may have emitted fewer USV than +/+ pups, this is unlikely to be responsible for the reduced pup retrieval of Myk/+ dams because all pups were removed by the experimenter from communal nests containing on average 75% +/+ pups and 25% Myk/+ pups.
Figure 3.
Pup retrieval. Latency of Myk/+ (n = 8) and +/+ (n = 8) dams to return pup to the nest location. A main effect of genotype (F(1, 14) = 11.20, p < 0.01) was observed. **p < 0.01 Myk/+ mice versus +/+ mice.
Myk/+ mice show reduced social interaction
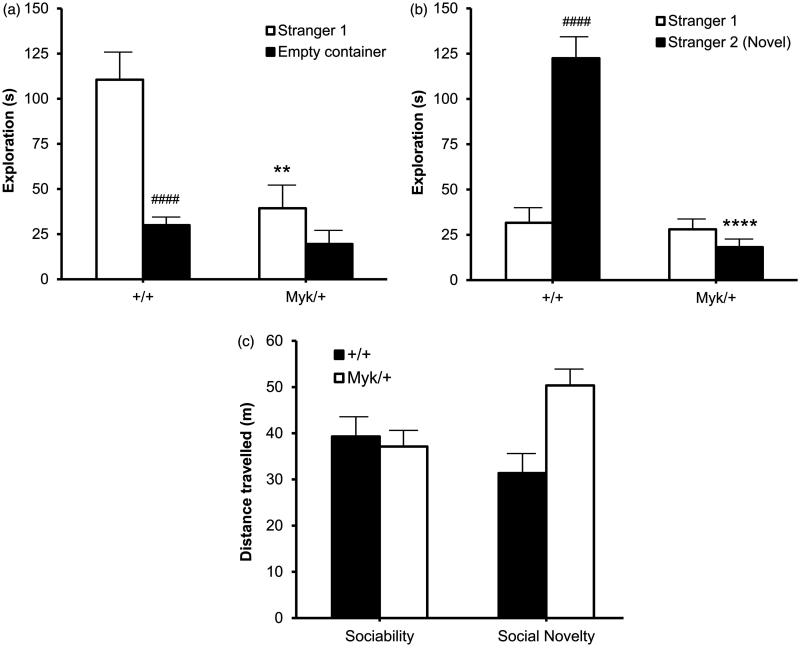
Laboratory mice are naturally social animals (Bailey and Crawley 2009). To assess the social interaction of Myk/+ mice, we utilized the three-chamber social approach test, which has been extensively used in studies of sociality in a variety of mouse lines (Yang et al. 2011). The sociability phase of the test measures the preference of the subject to explore either a novel adult male conspecific enclosed in a ventilated container (Stranger 1) or an identical but otherwise empty container. This task has face validity to the tendency of autistic children to spend more time playing with an inanimate toy than engaged in social interactions with other children (Ryan et al. 2008). By contrast with +/+ mice, Myk/+ mice spent less time exploring the novel mouse, and did not show a preference for exploring the novel mouse versus the empty container (Figure 4(a)).
Figure 4.
Three-chamber social approach test. (a) Sociability phase: time spent by the subject exploring a novel adult male mouse (Stranger 1) or an empty container. A main effect of genotype (F(1, 14) = 5.98, p < 0.05) on time in contact with the novel mouse was observed. (b) Social Novelty phase: time spent by the subject exploring the mouse previously explored (Stranger 1) and a second novel adult male mouse (Stranger 2). A main effect of genotype (F(1, 14) = 26.71, p < 0.0001) on time in contact with Stranger 2 was observed. (c) Distance travelled (m) in the Sociability and Social Novelty phases of the test. There was no main effect of genotype or sex. Myk/+ mice (n = 8); +/+ mice (n = 10). **p < 0.01; ****p < 0.0001 versus +/+ mice. #### p < 0.0001 versus Stranger 1.
In the social novelty phase of the test, the subject encountered a second novel adult male mouse in the previously empty container (Stranger 2), as well as the now familiar conspecific. By contrast with +/+ mice, Myk/+ mice spent less time exploring the novel mouse, and did not demonstrate a preference for exploring the novel mouse versus the previously introduced mouse (Figure 4(b)). This apparent inability to discriminate between Stranger 1 and Stranger 2 is consistent with the reported social recognition deficiency of Myk/+ mice; mutants showed reduced recognition of a juvenile male C57BL/6NCrl mouse 24 h after being exposed to it for 2 min, but not for 10 min (Kirshenbaum et al. 2015). The reduced social interaction of Myk/+ mice was not due to deficient ambulation within the arena, as there were no genotypic differences in distance travelled during either phase of the test (Figure 4(c)).
Impairments in social approach and nest building have previously been exhibited by mice deficient in the CNTNAP2 and SHANK2 genes implicated in autism (Peñagarikano et al. 2011, Won et al. 2012); SHANK2-deficient mice also show a deficit in pup retrieval (Won et al. 2012). Mice rely heavily upon olfaction during typical social interactions (Otmakhova et al. 1992). Social approach by male C57BL/6J mice in the three-chamber test is driven primarily by social olfactory cues (Ryan et al. 2008). CNTNAP2 and SHANK2 deficient mice were shown to have intact olfactory function (Peñagarikano et al. 2011, Won et al. 2012), so their abnormal social behavior cannot be attributed to olfactory deficits. Olfaction usually implies detection of compounds in gaseous or airborne form (remote chemoreception), whereas gustation involves direct contact with a substrate (contact chemoreception). The demonstration of sucrose preference and conditioned taste aversion by Myk/+ mice (Kirshenbaum et al. 2011a, 2013) suggests that their gustatory perception is intact, but we cannot formally exclude the presence of a specific olfaction defect in Myk/+ mice.
Support for the involvement of Na+,K+-ATPase α3 in the regulation of social behavior is provided by heterozygous Atp1a3 tm1Ling/+ mice, which have a point mutation in Atp1a3 intron 4 that reduces hippocampal α3 protein expression by around 60% and whole brain Na+,K+-ATPase activity by around 16%, without visible neurological defects (Moseley et al. 2007, Kirshenbaum et al. 2011b). Atp1a3 tm1Ling/+ mice show robust gustaoception in the sucrose preference test (Kirshenbaum et al. 2011b) and exhibited deficits in motor coordination and balance only in females and only after exposure to restraint stress for five days (DeAndrade et al. 2011). Under standard husbandry conditions, Atp1a3 tm1Ling/+ mice show normal social approach in the three-chamber test, but both sexes showed deficient sociability and preference for social novelty, and a reduction in whole brain Na+,K+-ATPase activity of around 33%, after exposure to chronic variable stress, comprising single housing and one to two unpredictable mild stressors per day for six weeks (Kirshenbaum et al. 2011b).
Conclusion
The observation of social behavioral deficits in patients diagnosed with AHC (I810N; Panagiotakaki et al. 2015), RDP (T613M; Pittock et al. 2000, McKeon et al. 2007), and CAPOS syndrome (E818K; Demos et al. 2014) raised the hypothesis that social deficits represent part of the complex phenotype of Na+,K+-ATPase α3 mutations. Consequently, we assessed the social behavior of the Myshkin mouse model of AHC, which has an I810N mutation identical to that found in an AHC patient with co-morbid autism. Myk/+ mice displayed deficits in nest building, pup retrieval and the three-chamber social approach test, suggesting that Na+,K+-ATPase α3 dysfunction has a deleterious effect on social behavior. This finding supports the notion that social deficits are part of the complex phenotype of Na+,K+-ATPase α3 mutations.
Previous behavioral analyses have revealed novelty-induced hyperambulation, increased risk-taking behavior, motor dysfunction and cognitive impairment in Myk/+ mice (Kirshenbaum et al. 2011a, 2013, 2015). This broad profile of neurobehavioral deficits, to which the present study has added social behavioral deficits, is consistent with the complex clinical profile of AHC patients, which includes a wide range of behavioral and psychiatric disorders (Neville and Ninan 2007). These multiple behavioral deficits are likely to be interrelated, such that a deficit in attention, for example, could be manifested as impairments in several behavioral tests that require sustained attention.
Na+,K+-ATPase α3 is highly expressed in the hippocampal CA2 and amygdala (Grillo et al. 1997), brain regions that are important in the regulation of social behavior (Hitti and Siegelbaum 2014, Stevenson and Caldwell 2014, Mineur et al. 2016, Radke et al. 2015), but the phenotypic abnormalities of Myk/+ mice are not restricted to social behavioral deficits. It is, therefore, conceivable that the reduced outcomes in the three social behavioral tests reported herein may be the consequence of an underlying general behavioral abnormality, resulting from Na+,K+-ATPase α3 dysfunction; this could also be a possible explanation for the wide range of neurobehavioral deficits exhibited by AHC patients.
By better defining the behavioral profile of Myk/+ mice, the present study has identified additional ATP1A3-related symptoms for which the Myk/+ model could be used as a tool to advance understanding of the underlying neural mechanisms and develop novel therapeutic strategies. Future work could apply exploratory factor analysis to a balanced selection of variables that best characterize the behavioral variability of Myk/+ and +/+ mice (Valenti et al. 2001), to statistically dissect the involvement of factors that underlie deficits in various tests.
Acknowledgements
We thank the Centre for Modeling Human Disease (www.cmhd.ca) for producing the founder Myshkin mutant. This study was funded by the UK Medical Research Council (G0900625) and the Canadian Institutes of Health Research (MOP-94856). Greer S. Kirshenbaum was supported by an Ontario Mental Health Foundation studentship. James Dachtler was supported by a Wellcome Trust Junior Investigator Development Fellowship. John C. Roder held a Canada Research Chair in Learning and Memory.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bailey K.R., Crawley J.N.2009Anxiety-related behaviors in miceIn:Buccafusco J.J.Methods of Behavior Analysis in Neuroscience 2nd edBoca Raton: CRC Press [Google Scholar]
- Benarroch E.E. Na+,K+-ATPase: functions in the nervous system and involvement in neurologic disease. Neurology. 2011;76:287–293. doi: 10.1212/WNL.0b013e3182074c2f. [DOI] [PubMed] [Google Scholar]
- Boelman C., Lagman-Bartolome A.M., MacGregor D.L., McCabe J., Logan W.J., Minassian B.A. Identical ATP1A3 mutation causes alternating hemiplegia of childhood and rapid-onset dystonia parkinsonism phenotypes. Pediatric Neurology. 2014;51:850–853. doi: 10.1016/j.pediatrneurol.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Clapcote S.J., Duffy S., Xie G., Kirshenbaum G., Bechard A.R., Schack V., Roder J.C. Mutation I810N in the α3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proceedigs of the National Academy of Science USA. 2009;106:14085–14090. doi: 10.1073/pnas.0904817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dard R., Mignot C., Durr A., Lesca G., Sanlaville D., Roze E., Mochel F. Relapsing encephalopathy with cerebellar ataxia related to an ATP1A3 mutation. Developmental Medicine and Child Neurology. 2015;57:1183–1186. doi: 10.1111/dmcn.12927. [DOI] [PubMed] [Google Scholar]
- DeAndrade M.P., Yokoi F., Groen T., Lingrel J.B., Li Y. Characterization of Atp1a3 mutant mice as a model of rapid-onset dystonia with parkinsonism. Behavioral Brain Research. 2011;216:659–665. doi: 10.1016/j.bbr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos M.K., Karnebeek C.D., Ross C.J., Adam S., Shen Y., Zhan S.H. A novel recurrent mutation in ATP1A3 causes CAPOS syndrome. Orphanet Journal of Rare Disease. 2014;9:15. doi: 10.1186/1750-1172-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg A.J., Kane J.M., Keller M.B., Lavori P., Rosenbaum J.F., Cole K., Lavelle J. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. The New England Journal of Medicine. 1989;321:1489–1493. doi: 10.1056/NEJM198911303212201. [DOI] [PubMed] [Google Scholar]
- Grillo C., Piroli G., Lima A., McEwen B.S., Nicola A.F. Aldosterone up-regulates mRNA for the alpha3 and beta1 isoforms of (Na,K)-ATPase in several brain regions from adrenalectomized rats. Brain Research. 1997;767:120–127. doi: 10.1016/s0006-8993(97)00541-6. [DOI] [PubMed] [Google Scholar]
- Heimer G., Sadaka Y., Israelian L., Feiglin A., Ruggieri A., Marshall C.R., Zeev B.B. CAOS-episodic cerebellar ataxia, areflexia, optic atrophy, and sensorineural hearing loss: a third allelic disorder of the ATP1A3 gene. Journal of Child Neurology. 2015;30:1749–1756. doi: 10.1177/0883073815579708. [DOI] [PubMed] [Google Scholar]
- Heinzen E.L., Swoboda K.J., Hitomi Y., Gurrieri F., Nicole S., Vries B., Goldstein D.B. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nature Genetics. 2012;44:1030–1034. doi: 10.1038/ng.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen E.L., Arzimanoglou A., Brashear A., Clapcote S.J., Gurrieri F., Goldstein D.B. Distinct neurological disorders with ATP1A3 mutations. Lancet Neurology. 2014;13:503–514. doi: 10.1016/S1474-4422(14)70011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti F.L., Siegelbaum S.A. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann W.E., Cortell R., Kau A.S., Bukelis I., Tierney E., Gray R.M., Stanard P. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medicine Genetics A. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum G.S., Clapcote S.J., Duffy S., Burgess C.R., Petersen J., Jarowek K.J., Roder J.C. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase α3 sodium pump. Proceedings of the National Academy of Science USA. 2011a;108:18144–18149. doi: 10.1073/pnas.1108416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum G.S., Saltzman K., Rose B., Petersen J., Vilsen B., Roder J.C. Decreased neuronal Na+,K+-ATPase activity in Atp1a3 heterozygous mice increases susceptibility to depression-like endophenotypes by chronic variable stress. Genes Brain Behavior. 2011b;10:542–550. doi: 10.1111/j.1601-183X.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum G.S., Dawson N., Mullins J.G., Johnston T.H., Drinkhill M.J., Edwards I.J., Clapcote S. Alternating hemiplegia of childhood-related neural and behavioural phenotypes in Na+,K+-ATPase α3 missense mutant mice. PLoS One. 2013;8:e60141. doi: 10.1371/journal.pone.0060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum G.S., Dachtler J., Roder J.C., Clapcote S.J. Attenuation of mania-like behavior in Na+,K+-ATPase α3 mutant mice by prospective therapies for bipolar disorder: melatonin and exercise. Neuroscience. 2014;260:195–204. doi: 10.1016/j.neuroscience.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum G.S., Dachtler J., Roder J.C., Clapcote S.J. Characterization of cognitive deficits in mice with an alternating hemiplegia-linked mutation. Behavioral Neuroscience. 2015;129:822–831. doi: 10.1037/bne0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum G.S., Dachtler J., Roder J.C., Clapcote S.J. Transgenic rescue of phenotypic deficits in a mouse model of alternating hemiplegia of childhood. Neurogenetics. 2016;17:57–63. doi: 10.1007/s10048-015-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon A., Ozelius L.J., Hardiman O., Greenway M.J., Pittock S.J. Heterogeneity of presentation and outcome in the Irish rapid-onset dystonia-parkinsonism kindred. Movement Disorders. 2007;22:1325–1327. doi: 10.1002/mds.21335. [DOI] [PubMed] [Google Scholar]
- Mines M.A., Yuskaitis C.J., King M.K., Beurel E., Jope R.S. GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS One. 2010;5:e9706. doi: 10.1371/journal.pone.0009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur Y.S., Fote G.M., Blakeman S., Cahuzac E.L., Newbold S.A., Picciotto M.R. Multiple nicotinic acetylcholine receptor subtypes in the mouse amygdala regulate affective behaviors and response to social stress. Neuropsychopharmacology. 2016;41:1579–1587. doi: 10.1038/npp.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P., Bouwknecht J.A., Teague R., Paylor R., Zoghbi H.Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Human Molecular Genetics. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Moseley A.E., Williams M.T., Schaefer T.L., Bohanan C.S., Neumann J.C., Behbehani M.M., Lingrel J.B. Deficiency in Na,K-ATPase α isoform genes alters spatial learning, motor activity, and anxiety in mice. Journal of Neuroscience. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriel V., Garcia-Molina A., Aparicio-Lopez C., Ensenat A., Roig-Rovira T. Neuropsychological deficits in alternating hemiplegia of childhood: a case study. Review Neurology. 2015;61:25–28. [PubMed] [Google Scholar]
- Neville B.G., Ninan M. The treatment and management of alternating hemiplegia of childhood. Developmental Medicine and Child Neurology. 2007;49:777–780. doi: 10.1111/j.1469-8749.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Yanazawa M., Sasahara T., Kitamura Y., Hiroaki H., Fukazawa Y., Hoshi M. Na, K-ATPase α3 is a death target of Alzheimer patient amyloid-β assembly. Proceedings of the National Academy of Science USA. 2015;112:E4465–E4474. doi: 10.1073/pnas.1421182112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova N.A., Gurevich E.V., Katkov Y.A., Nesterova I.V., Bobkova N.V. Dissociation of multiple behavioral effects between olfactory bulbectomized C57Bl/6J and DBA/2J mice. Physiology and Behavior. 1992;52:441–448. doi: 10.1016/0031-9384(92)90329-z. [DOI] [PubMed] [Google Scholar]
- Paciorkowski A.R., McDaniel S.S., Jansen L.A., Tully H., Tuttle E., Ghoneim D.H., Hahn S. Novel mutations in ATP1A3 associated with catastrophic early life epilepsy, episodic prolonged apnea, and postnatal microcephaly. Epilepsia. 2015;56:422–430. doi: 10.1111/epi.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakaki E., Gobbi G., Neville B., Ebinger F., Campistol J., Nevsímalová S., Arzimanoglou A. Evidence of a non-progressive course of alternating hemiplegia of childhood: study of a large cohort of children and adults. Brain. 2010;133:3598–3610. doi: 10.1093/brain/awq295. [DOI] [PubMed] [Google Scholar]
- Panagiotakaki E., De Grandis E., Stagnaro M., Heinzen E.L., Fons C., Sisodiya S. Clinical profile of patients with ATP1A3 mutations in Alternating Hemiplegia of Childhood – a study of 155 patients. Orphanet Journal of Rare Disease. 2015;10:123. doi: 10.1186/s13023-015-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O., Abrahams B.S., Herman E.I., Winden K.D., Gdalyahu A., Dong H., Sonnenblick L.I., Geschwind D.H. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peripato A.C., Cheverud J.M. Genetic influences on maternal care. American Naturalist. 2002;160:S173–S185. doi: 10.1086/342900. [DOI] [PubMed] [Google Scholar]
- Pittock S.J., Joyce C., Keane V., Hugle B., Hardiman M.O., Brett F., Webb D.W. Rapid-onset dystonia-parkinsonism: a clinical and genetic analysis of a new kindred. Neurology. 2000;55:991–995. doi: 10.1212/wnl.55.7.991. [DOI] [PubMed] [Google Scholar]
- Radke S., Volman I., Mehta P., Son V., Enter D., Sanfey A., Roelofs K. Testosterone biases the amygdala toward social threat approach. Science Advances. 2015;1:e1400074. doi: 10.1126/sciadv.1400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosewich H., Ohlenbusch A., Huppke P., Schlotawa L., Baethmann M., Carrilho I., Brockmann K. The expanding clinical and genetic spectrum of ATP1A3-related disorders. Neurology. 2014a;82:945–955. doi: 10.1212/WNL.0000000000000212. [DOI] [PubMed] [Google Scholar]
- Rosewich H., Weise D., Ohlenbusch A., Gärtner J., Brockmann K. Phenotypic overlap of alternating hemiplegia of childhood and CAPOS syndrome. Neurology. 2014b;83:861–863. doi: 10.1212/WNL.0000000000000735. [DOI] [PubMed] [Google Scholar]
- Roubergue A., Roze E., Vuillaumier-Barrot S., Fontenille M.J., M̀neret A., Vidailhet M., Nicole S. The multiple faces of the ATP1A3-related dystonic movement disorder. Movement Disorders. 2013;28:1457–1459. doi: 10.1002/mds.25396. [DOI] [PubMed] [Google Scholar]
- Ruegsegger C., Maharjan N., Goswami A., Etang A., Weis J., Troost D., Saxena S. Aberrant association of misfolded SOD1 with Na+/K+ATPase-α3 impairs its activity and contributes to motor neuron vulnerability in ALS. Acta Neuropathology. 2016;131:427–551. doi: 10.1007/s00401-015-1510-4. [DOI] [PubMed] [Google Scholar]
- Ryan B.C., Young N.B., Moy S.S., Crawley J.N. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behavioral Brain Research. 2008;193:235–242. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco R.C., Fryer J.D., Ren J., Fyffe S., Chao H.T., Sun Y., Neul J.L. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Human Molecular Genetics. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Ishii A., Saito Y., Morisada N., Iijima K., Takada S., Hirose S. Genotype–phenotype correlations in alternating hemiplegia of childhood. Neurology. 2014;82:482–490. doi: 10.1212/WNL.0000000000000102. [DOI] [PubMed] [Google Scholar]
- Shafer M.E., Mayfield J.W., McDonald F. Alternating hemiplegia of childhood: a study of neuropsychological functioning. Applied Neuropsychology. 2005;12:49–56. doi: 10.1207/s15324826an1201_8. [DOI] [PubMed] [Google Scholar]
- Shrivastava A.N., Redeker V., Fritz N., Pieri L., Almeida L., Spolidoro M., Triller A. α-synuclein assemblies sequester neuronal α3-Na+/K+-ATPase and impair Na+ gradient. EMBO Journal. 2015;34:2408–2423. doi: 10.15252/embj.201591397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson E.L., Caldwell H.K. Lesions to the CA2 region of the hippocampus impair social memory in mice. European Journal of Neuroscience. 2014;40:3294–3301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweney M.T., Silver K., Gerard-Blanluet M., Pedespan J.M., Renault F., Arzimanoglou A., Swoboda K.J. Alternating hemiplegia of childhood: early characteristics and evolution of a neurodevelopmental syndrome. Pediatrics. 2009;123:e534–e541. doi: 10.1542/peds.2008-2027. [DOI] [PubMed] [Google Scholar]
- Termsarasab P., Yang A.C., Frucht S.J. Intermediate phenotypes of ATP1A3 mutations: phenotype-genotype correlations. Tremor and Other Hyperkinetic Movements (NY) 2015;5:336. doi: 10.7916/D8MG7NS8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti P., Cozzio A., Nishida N., Wolfer D.P., Sakaguchi S., Lipp H.P. Similar target, different effects: late-onset ataxia and spatial learning in prion protein-deficient mouse lines. Neurogenetics. 2001;3:173–184. doi: 10.1007/s100480100117. [DOI] [PubMed] [Google Scholar]
- Weigand K.M., Messchaert M., Swarts H.G., Russel F.G., Koenderink J.B. Alternating hemiplegia of childhood mutations have a differential effect on Na+,K+-ATPase activity and ouabain binding. Biochimica Biophysica Acta. 2014;1842:1010–1016. doi: 10.1016/j.bbadis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Won H., Lee H.R., Gee H.Y., Mah W., Kim J.I., Lee J., Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Yang M., Silverman J.L., Crawley J.N. Automated three-chambered social approach task for mice. Current Protocols Neuroscience. 2011;8 doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Gao H., Zhang J., Xu X., Liu X., Wu X., Zhang Y. ATP1A3 mutations and genotype–phenotype correlation of alternating hemiplegia of childhood in Chinese patients. PLoS One. 2014;9:e97274. doi: 10.1371/journal.pone.0097274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Zhang Y., Yuan D., Xu X., Li S., Wei L., Wu X. ATP1A3 gene mutations in patients with alternating hemiplegia of childhood. Chinese Journal of Pediatrics. 2015;53:835–839. [PubMed] [Google Scholar]
- Young D.M., Schenk A.K., Yang S.B., Jan Y.N., Jan L.Y. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proceedings of the National Academy of Science USA. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]