Conspectus
Stimuli-responsive surfaces have sparked considerable interest in recent years, especially in view of their biomimetic nature and widespread biomedical applications. Significant efforts are continuously being directed at developing functional surfaces exhibiting specific property changes triggered by variations in electrical potential, temperature, pH and concentration, irradiation with light, or exposure to a magnetic field. In this respect, electrical stimulus offers several attractive features, including a high level of spatial and temporal controllability, rapid and reverse inducement, and noninvasiveness. In this Account, we discuss how surfaces can be designed and methodologies developed to produce electrically switchable systems, based on research by our groups. We aim to provide fundamental mechanistic and structural features of these dynamic systems, while highlighting their capabilities and potential applications. We begin by briefly describing the current state-of-the-art in integrating electroactive species on surfaces to control the immobilization of diverse biological entities. This premise leads us to portray our electrically switchable surfaces, capable of controlling nonspecific and specific biological interactions by exploiting molecular motions of surface-bound electroswitchable molecules. We demonstrate that our self-assembled monolayer-based electrically switchable surfaces can modulate the interactions of surfaces with proteins, mammalian and bacterial cells. We emphasize how these systems are ubiquitous in both switching biomolecular interactions in highly complex biological conditions while still offering antifouling properties. We also introduce how novel characterization techniques, such as surface sensitive vibrational sum-frequency generation (SFG) spectroscopy, can be used for probing the electrically switchable molecular surfaces in situ. SFG spectroscopy is a technique that not only allowed determining the structural orientation of the surface-tethered molecules under electroinduced switching, but also provided an in-depth characterization of the system reversibility. Furthermore, the unique support from molecular dynamics (MD) simulations is highlighted. MD simulations with polarizable force fields (FFs), which could give proper description of the charge polarization caused by electrical stimulus, have helped not only back many of the experimental observations, but also to rationalize the mechanism of switching behavior. More importantly, this polarizable FF-based approach can efficiently be extended to light or pH stimulated surfaces when integrated with reactive FF methods. The interplay between experimental and theoretical studies has led to a higher level of understanding of the switchable surfaces, and to a more precise interpretation and rationalization of the observed data. The perspectives on the challenges and opportunities for future progress on stimuli-responsive surfaces are also presented.
1. Introduction
Surfaces with stimuli-responsive properties have emerged as a fascinating class of biomedical and biotechnological materials for a broad spectrum of applications, ranging from cell biology research to drug delivery, tissue engineering, and regenerative medicine.1−6 From a biological perspective, the ability to respond to stimuli is inherently present in living systems. From the leaves of Mimosa pudica that collapse suddenly when touched, to humans that raise their body core temperature through fever to fight off invading bacteria or viruses, all living systems have evolved a variety of responsive mechanisms to preserve their integrity or well-being. At their most fundamental level, the stimuli-triggered responses of natural systems are molecular level processes that can ultimately manifest itself at the microscopic or macroscopic scale.7,8
With the natural inspiration of responsive systems all around us, coupled with the increasing capability to understand and manipulate structures of matter at molecular level,9 the time has come for us to embrace the design and construction of materials and surfaces with specific chemical and physical attributes that change in response to various stimuli. In this context, molecular-based stimuli-responsive surfaces are rapidly emerging as a powerful tool to modulate the availability of specific chemical groups on surfaces.1,2 The changes in the surface chemical properties of a material are determined by the interplay between parameters such as the chemical composition, the spatial arrangement of chemical groups and topography of the surface, and the location and type of stimulus. In turn, the selection of a stimulus is driven by the material characteristics, the capability to induce a particular change in the material surface chemistry and the application requirements. In a biomedical application context, electrically triggered activation is particularly attractive as it provides fast response times, allows for easy creation of multiple individually addressable switchable regions on the same surface, and uses low drive voltages and fields that are compatible with biological systems.10
In this Account, we describe our recent progress on the development of self-assembled monolayers (SAMs) on gold substrates that respond to electrical potentials with altered molecular conformations. We examine how these stimuli-responsive monolayers can be tailored at the molecular level to modulate the interactions of surfaces with proteins,11,12 mammalian13 and bacterial cells.14 We describe how surface sensitive vibrational sum-frequency generation (SFG) spectroscopy can be used to gain insights into the mechanistic principles underpinning electrically driven surfaces.15 It follows by outlining how molecular dynamics (MD) simulations can most successfully be applied to elucidate dynamic molecular-level events occurring on the surface in response to stimuli, being it an electrical stimulus16,17 or other stimulus such as light18−20 and pH.21
2. Molecular-Based Design and Synthesis of Electrically Responsive Surfaces
Developing the relationship between the molecular structure of the surface and its tunable electrically responsive behavior requires designing and synthesizing structurally well-defined organic surfaces. The molecular precursors of such organic surfaces have to exhibit electrically responsive properties and the capability to undergo conformational and/or chemical changes on receiving the stimulus. An effective and reproducible way of tailoring such chemically well-defined surfaces is to use SAMs.9 SAMs possess important properties of self-organization and adaptability on a number of technologically relevant surface substrates. The structure of the SAM molecular precursors can be divided into three components, headgroup, backbone and terminal group. While the headgroup provides SAM anchored to a specific surface substrate, the backbone and terminal group can be modulated to produce a wide variety of molecularly tailored surfaces with unique responsive properties. The above flexible molecular architecture has led to the development of a range of electroswitchable SAMs that have been used in showcasing their potential for a variety of biomedical applications. By creating SAMs comprising electroactive end groups (e.g., nitro and hydroquinone moieties), which can undergo oxidation or reduction upon application of an electrical potential, control over the immobilization of different biological entities, including peptides,22 DNA,23 proteins,24 and cells,25 on surfaces has been established. A specific example developed by us24 involved SAMs of 4-nitrothiophenol on gold surfaces, in which the nitro-terminated groups could be reduced electrochemically and selectively to amino-terminated groups by applying a negative voltage. By employing a homobifunctional activated ester linker, proteins were immobilized with high affinity and selectivity onto the amino regions, after activation.
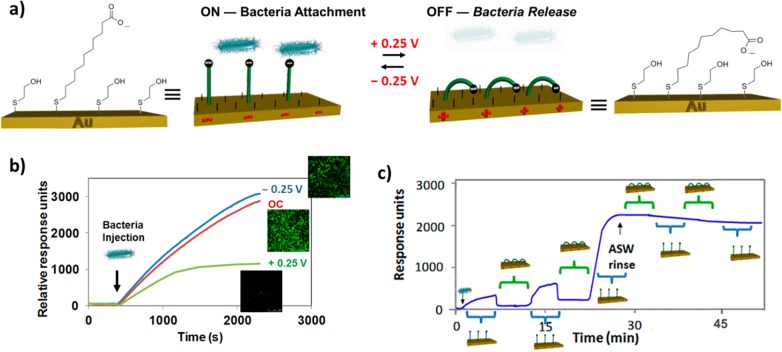
While the systems above show some capabilities in dynamically interacting with biological entities, considering Nature’s principles as inspiration, there is clearly scope for the development of more advanced surfaces with intriguing electrically responsive properties.26,27 With this proviso in mind, we have designed and created a number of monolayer architectures that respond to electrical potentials with altered conformations and, as a result, different, reversible surface functional properties. The monolayer structures themselves are based on two-component SAMs, wherein one of the molecular components acts as the functional and switchable entity and the other one as a lateral spacer such that conformational transitions of the former are not affected by steric constraints. Starting from this premise, we have constructed an electroswitchable SAM which is able to control the early stages of bacterial adhesion by switching between an attractive and a repellent state.14 Studies were initially conducted28 employing several one-component SAMs that possessed not only different backbones (hydrophilic and hydrophobic), but also different terminal groups (hydrophilic, hydrophobic, neutral, positively and negatively charged) to study in real-time, using surface plasmon resonance (SPR), the initial stages of bacterial adhesion to surfaces. The hydrophobic marine bacterium Marinobacter hydrocarbonoclasticus (Mh) exhibited the lowest adhesion on the most hydrophobic surface, while readily and firmly attaching to both positively and negatively charged surfaces. On the basis of this different, a two-component SAM of 11-mercaptoundecanoic-acid (MUA) and mercaptoethanol (MET) on gold was devised that could expose either negatively charged or hydrophobic moieties in response to an applied electrical potential (Figure 1a). The MUA acted as our functional and switchable entity, whereby the MUA-containing SAM undergoes conformational changes upon attraction/repulsion of the carboxylic acid charged end group to/from the substrate surface by an applied positive or negative electrical potential. It results in either straight chains with carboxylate anions exposed at the surface (negatively charged surface) or bent chains with alkyl chains exposed at the surface (hydrophobic surface).
Figure 1.
(a) Schematic representation of the switching of MUA:MET SAMs for controlling bacterial adhesion. (b) SPR sensorgram traces and confocal microscopy images showing adhesion of Mh to the MUA:MET SAM at −0.25 V, OC and +0.25 V. (c) SPR monitoring of bacterial adhesion by alternating the potential from −0.25 V to +0.25 V every 5 min. Reproduced with permission from ref (14). Copyright 2013 WILEY-VCH Verlag GmbH & Co.
The fabrication of a homogeneous two-component SAM was achieved by using a bulky terminal group (dendron) which establishes the space (dendritic effect) that each MUA occupies on the SAM.29 Following SAM preparation, the dendron is removed by hydrolysis allowing the insertion of the shorter backfiller, MET. The preparation of well-mixed binary monolayers without domains and phase separation is paramount to maximize the switching efficiency of the SAM.
The adhesion of the bacteria to the switchable SAMs was performed at open circuit (OC) conditions and applied negative (−0.25 V) and positive (+0.25 V) potentials using electrochemical SPR (eSPR). eSPR allows monitoring of surface binding while an electrical potential is applied to the surface using a three-electrode electrochemical cell and a potentiostat. The gold surfaces serve as the working electrode, a Pt wire as the counter electrode, and a standard calomel electrode as the reference electrode. High bacterial adhesion was observed for OC and an applied negative potential, while its inhibition was detected for an applied positive potential (Figure 1b). This behavior was not observed in one-component, well-packed monolayers of MUA. By taking advantage of the fast switching capability of the system, we were able to perform ON/OFF switching cycles with different intervals of time (3, 5, 10, and 20 min) between stimulations and simultaneously monitor how temporary surface charge exposure or concealment can influence the initial process of bacterial adhesion. For 5 min switching (Figure 1c), the initial presence of the surface charge (ON) promotes bacterial adhesion which can be reversed by concealing the charge (OFF). Following another ON/OFF cycle, the possibility to remove the cells attached is reduced. In the next cycle, the attachment of bacteria became irreversible. With this novel dynamic platform we are able to monitor in real-time the passage between reversible and nonreversible cell adhesion. This dynamic surface platform is highly desirable for a number of biomedical applications which range from diagnostic, genetic expression and biomaterials fouling control.
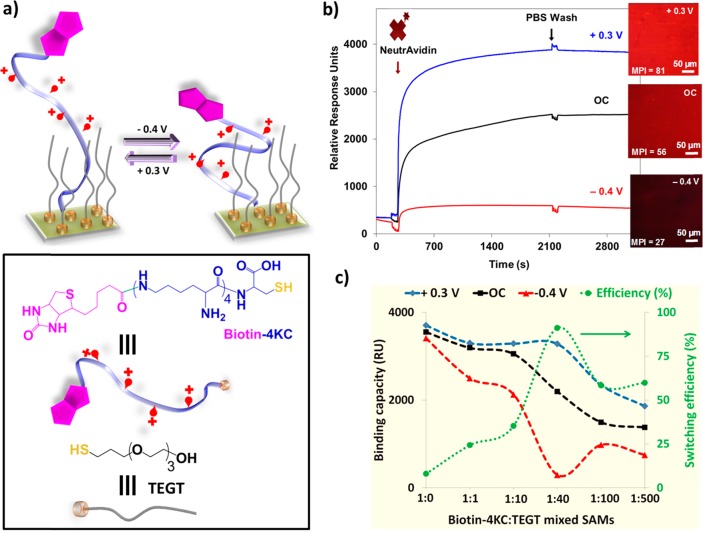
The question then arises: apart from nonspecific interactions, can we also control specific biomolecular interactions by electrically induced transitions between conformational states in surface-immobilized flexible molecules containing charged groups? Addressing this challenge, we have built11 an electroswitchable surface based on the response of a charged molecular backbone on the structure of a mixed SAM that dramatically alter the binding activity of a ligand (biotin) to a protein (neutravidin) (Figure 2a). The mixed SAM was composed of a two molecular components, (i) a positively charged 4-mer of lysine (K) that is functionalized at one end with biotin, which recognizes the neutravidin, and at the other end with a cysteine (C), for binding to gold substrates (biotin-4KC), and (ii) an ethylene glycol-terminated thiol (i.e., (3-mercaptopropyl)tri(ethylene glycol) - TEGT) to space out the oligolysines and preventing nonspecific interaction from the protein. When a + 0.3 V was applied, high neutravidin binding was observed, while the application of a negative potential (−0.4 V) resulted in minimal protein binding (Figure 2b). OC conditions provided intermediate protein binding capability. The bio-inactive state leads to more than 90% reduction in protein binding.
Figure 2.
(a) Schematic illustration and chemical structures of the biotin-4KC:TEGT SAM and (b) its switching properties as followed by SPR and fluorescence microscopy. (c) Binding capacity under OC, +0.3 and −0.4 V as well as switching efficiency of the biotin-4KC:TEGT SAMs at different solution ratios. Reproduced with permission from refs (11 and 17). Copyright 2010 WILEY-VCH Verlag GmbH & Co. and 2014 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Experimental studies were also conducted using shorter (biotin-2KC) and longer (biotin-6KC) oligopeptides.17 SPR results indicated that there is a relationship between the length of the lysine switching unit and switching efficiency, wherein the long and flexible nature of the biotin-6KC limits its capability to expose and conceal the biotin and regulate its binding to neutravidin in response to an applied electrical potential. Investigations on the effect of oligopeptide:TEGT ratio on the surface also illustrated that the oligopeptide should be presented at optimum surface ratio such that binding capacity and switching efficiency can be maximized (Figure 2c).
3. Performance under Complex Biological Conditions
The experiments described hitherto were conducted under limited biological conditions: artificial seawater for controlling bacterial adhesion and phosphate buffer saline (PBS) solution for regulating specific biomolecular interactions. Having established very high switching efficiencies in such media, we were interested to investigate whether the switchable surfaces that rely on electrically induced conformational changes can be used in more complex biological conditions. Different media commonly used for cell and tissue culture were investigated,12 namely, Dulbecco’s modified Eagle’s medium (DMEM), DMEM containing 10% fetal bovine serum (DMEM-FBS), and DMEM-FBS with (4-(2-hydroxyethyl)-1-piperazineethanessulfonic acid) HEPES buffer (DMEM-FBS-HEPES). DMEM contains a mixture of inorganic salts, amino acids, glucose, and vitamins. The switching efficiency in these different media was studied using a mixed SAM on gold composed from the biotin-4KC and an 11-carbon TEG-terminated thiol (C11TEG) and following its binding to neutravidin by e-SPR. In contrast with a TEGT SAM, the more ordered C11TEG SAM has shown a high resistance to serum adsorption, thus providing the required nonfouling and specific binding properties to the mixed SAM. While rendering the surface more resistant to nonspecific binding, the biotin-4KC:C11TEG SAM performs in PBS at a lower switching efficiency (SE) when compared with the biotin-4KC:TEGT SAM. SE, which is calculated as the percent difference between the binding capacity at OC conditions (BCOC) and the BC at −0.4 V divided by BCOC, stands at approximately 90% and 70% for the biotin-4KC:TEGT SAM and biotin-4KC:C11TEG SAM, respectively. The lower SE for the biotin-4KC:C11TEG SAM might be attributed to the formation of a more organized EG matrix, restricting to some extent conformational changes from taking place. With that said, the biotin-4KC:C11TEG SAM nonetheless exhibits a high SE, making them well suited for integration into systems involving complex biological solutions.
The biotin-4KC:C11TEG SAM exhibited similar switching performance in PBS and DMEM (i.e., 70%). DMEM-FBS and DMEM-FBS-HEPES induced a drop in the SE of the SAM to values close to 45%. Further studies of the role of FBS and HEPES in the switching process indicated that their interaction with the oligopeptide is likely to be responsible for the observed SE reduction. FBS is proposed to nonspecifically interact with oligopeptide, sitting on the surface and inhibiting the oligopeptide ability to change conformations. On the other hand, the decrease in SE using the zwitterionic HEPES has been attributed to their ability to form stable intermolecular interactions with the peptide, restricting it from electrostatically interacting with the negatively charged gold surface and changing its conformation. Interestingly, while FBS in PBS medium interfered significantly with the switching ability of the biotin-4KC:C11TEG SAM (SE dropped to 15%), the presence of DMEM or HEPES in the medium considerably mitigates this adverse effect. Dilution of the different media (DMEM, DMEM-FBS, DMEM-FBS-HEPES) in PBS have also a strong positive impact on the SE. These findings highlight that maximum switching performance can be achieved in oligopeptide-based switchable surfaces, or in any other switchable surface system that bases its switching mechanism on a charged molecular backbone or end group, by carefully controlling the complex biological medium on which they need to operate.
4. Modulation of Specific Cellular Interactions
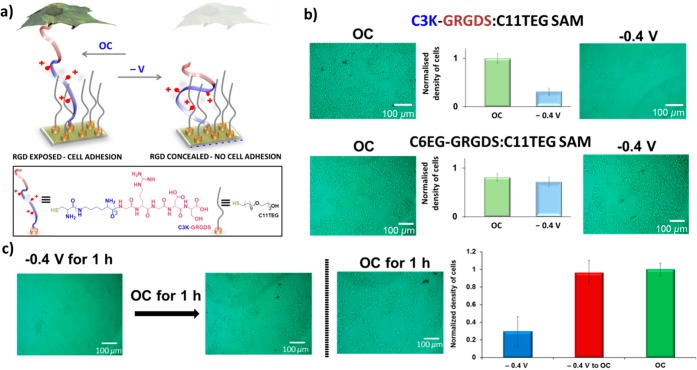
The possibility to control cell adhesion can have an important impact on tissue engineering and regenerative medicine and provide a means to further understand cell function. By incorporating all we learned from our previous results, a surface based on an arginine-glycine-aspartate (RGD) oligopeptide was designed that is able to modulate cell adhesion.13 RGD is present in most of the adhesive extracellular matrix proteins and it is specific for integrin-mediated cell adhesion. The switching unit was composed of three lysine units and a glycine-arginine-glycine-aspartate-serine (GRGDS) recognition motif peptide to create a C3K-GRGDS:C11TEG mixed SAM on gold (Figure 3a). In order to demonstrate that the C3K-GRGDS:C11TEG SAMs can support or resist cell adhesion on demand, macrophage cells were cultured on the C3K-GRGDS:C11TEG SAM in DMEM medium under OC conditions and −0.4 V for a period of 1 h. The C3K-GRGDS:C11TEG SAM supported cell adhesion under OC, while under −0.4 V a few cells adhered to the surface (Figure 3b). Thus, small changes in the conformation/orientation of the RGD peptide on the surface induced by application of an electrical potential can modulate the availability and potency of the RGD sites for cell surface receptors. Control mixed SAMs comprising C11TEG and a peptide where the three lysine residues as the switching unit were replaced by six EG units: C6EG-GRGDS demonstrated that the presence of the lysines on the oligopeptide are critical for RGD switching and regulate the adhesion of cells (Figure 3b). Further investigations into the switching between different cell adhesive states (cell-resistant and cell-adhesive) revealed that cells are not able to be detached upon application of −0.4 V due to the adhesion being mediated by multiple RGD–integrin bonds in parallel. On the other hand, a reversal of the switching sequence demonstrated that the C3K-GRGDS:C11TEG SAM is highly effective at switching from a nonadhesive to cell-adhesive state (Figure 3c).
Figure 3.
(a) Schematic and chemical structures of the C3K-GRGDS:C11TEG SAM utilized for controlling specific cellular interactions. Microscopic images and normalized density of adhered cells on (b) C3K-GRGDS:C11TEG SAMS (top), C6EG-GRGDS:C11TEG SAMs (bottom) and (c) C3K-GRGDS:C11TEG SAMs that were incubated with cells for 1 h while applying −0.4 V and subsequently in OC conditions for 1 h. The third image corresponds to SAMs that were incubated with cells in OC conditions for 1 h. Reproduced from ref (13). Published by The Royal Society of Chemistry.
5. Experimental Insights into the Switching Mechanism
A key to understand the molecular motions that occur in electro-switchable SAMs is the ability to observe these reorganizations in situ. This not only implies to measure at the solid–liquid interface, but also to specifically address molecules residing on the surface and not in the respective bulk phases. Additionally, the technique should have real-time capabilities to investigate changes during the switching process, being nondestructive in order to investigate the switching reversible nature, and should be specific to certain parts of the molecules on the surface. With this in mind, in a recent study, the switching mechanism of electro-switchable SAMs was studied in situ by using SFG spectroscopy.15 In these experiments, IR and visible laser pulses are overlapped in time and space at an interface and spectra are recorded as a function of IR frequency. When the IR light is exciting vibrational states that are both IR and Raman active, SFG signals are resonantly enhanced.30
SFG allows the observation of biotin orientations in the biotin-4KC:TEGT SAM in response to an applied potential by taking advantages of even-order nonlinear optical selection rules that precludes signals from isotropic environments or molecular arrangements that possess inversion symmetry.31 A first attempt to study molecular changes in the biotin-4KC:TEGT SAM was performed by using a purpose built electrochemical cell that allows to measurement of SFG spectra while applying an electrical potential. It turned out that the SFG intensity from the SAM was extremely small due to a low surface coverage of charged biomolecules and their greater degree of conformational freedom, which do not support strong SFG signals. However, by focusing on changes rather than on static conditions, it was possible to identify a molecular vibration associated with biotin in the biotin-4KC:TEGT SAM. Although the specifics of the spectral trace are rather complex and a result of nonresonant contributions from the gold electrode and broadband vibrational contributions of water, a narrow resonant peak from the biotin heterocyclic imidazole moiety was detected. When a positive potential is applied, in the bioactive state, the peptide chains adopted an extended conformation, resulting in an anisotropic upright orientation of the biotin group pointing away from the substrate. This scenario is in accordance to a dip of the biotin vibration within the broad SFG spectrum (Figure 4a). When a negative potential is applied, the peptide chain collapsed with a rather isotropic biotin orientation which may point slightly toward the substrate. This scenario leads to a very weak peak in the SFG spectrum. SFG also provides insights into the reversible nature of the switching (Figure 4b), as it is a nondestructive probing technique. These studies demonstrate that, by switching from +0.3 to −0.4 V, and back to +0.3 V, it was possible to turn on and off the upward orientation of the biotin and reversibly monitor the molecular reorientation from isotropic to anisotropic in situ.
Figure 4.
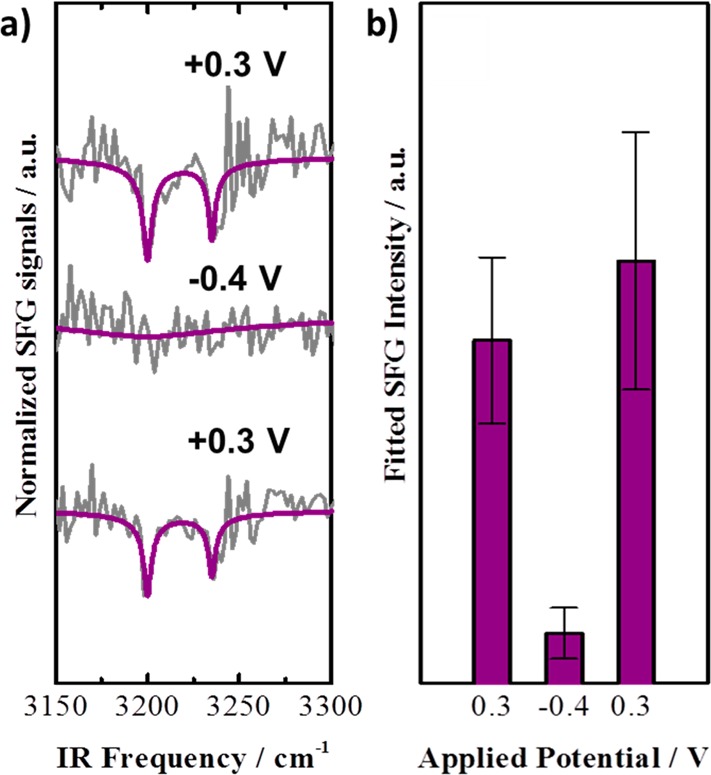
(a) Normalized and baseline corrected SFG spectra of the biotin-4KC:TEGT SAM (gray lines) and their corresponding fits (purple lines) for +0.3 V, −0.4 V, and returning to +0.3 V. (b) Sum of the fitted resonant SFG intensity at switching surface potentials. Reproduced from ref (15). Copyright 2014 The Authors. Published by WILEY-VCH Verlag GmbH & Co.
6. Theoretical Simulations of Switching Process
With the rapid development of electronic structure theory, classical molecular dynamics, and coarse-grained simulations, theoretical computation has become a vital tool in the study of stimuli-responsive surfaces (Figure 5a). MD simulation can provide vivid snapshots (Figure 5b) that show the dynamic conformational transition process, which are not attainable by experiments in a direct and visual way. The critical electric field strength that triggers the switching can also be predicted in theory. Moreover, the effect of ratio between different functional species on a surface, as well as their density on the switching efficiency, can be systematically investigated to provide preliminary predictions, which will suggest promising candidates and screen out highly impossible ones. In addition, the interplay between the stimuli and the responsive groups in the terminal group or backbone, as well as the binding mode between the recognition site and the target probe, can be explored to reveal the underlying switching mechanisms, and to further guide experimental fabrication of bioactive SAMs in a predictive and controllable way.
Figure 5.
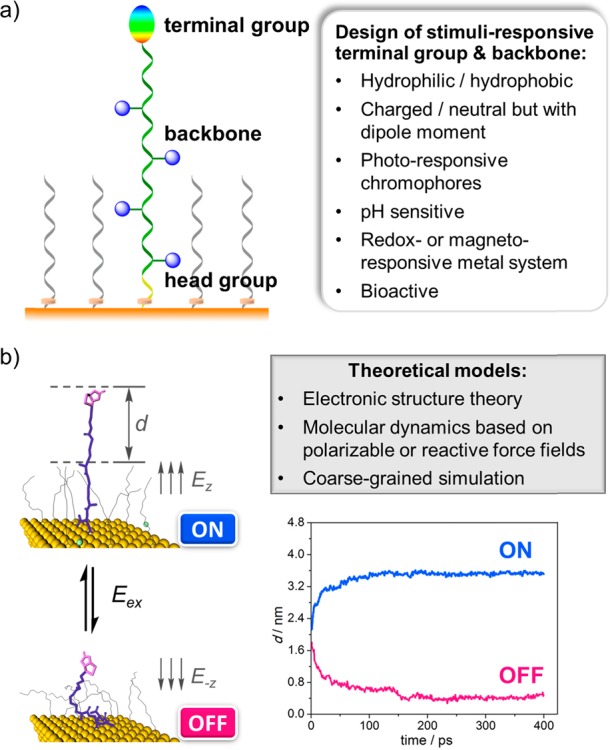
(a) Composition of stimuli-responsive SAM molecular precursors. (b) MD snapshots showing the switching under an electric field. Reproduced from ref (17). Copyright 2014 The Authors. Published by WILEY-VCH Verlag GmbH & Co.
The electric field effect plays a crucial role in the research of electrically induced conformational changes of SAMs and accordingly a variety of theoretical models have been developed and implemented over the past two decades.32−39 However, it still remains a difficult task to address this effect properly. By incorporating a uniform electric field into MD simulations, the electric field driven switching behavior of mixed long carboxyalkyl and short alkyl chains on a H–Si(111) surface was predicted.40 The terminal part of the carboxyalkyl chain is negatively charged, which serves as the switching and hydrophilic unit, while the backbone is neutral and hydrophobic. In such kind of SAMs, only the terminal group responds to external electric fields, thus the polarization effect may not be significant. In contrast to this system with only one single switching unit in each molecule, it becomes complicated when multiple switching units are introduced in the backbone such as the above-mentioned biotin-4KC:TEGT SAMs (Figure 2a). Since each biotin-4KC backbone involves 4 positively charged side groups–lysine residues and a polarizable biotin terminal group, failure in reproducing experiments for ON state of biotin-4KC:TEGT SAMs under the upward electric field (Eex in Figure 5b) was demonstrated by simply using a conventional force field, in which the atomic partial charges for the biotin-4KC were fixed all the time, no matter how its conformation changed and whether the electric field is applied.16
It is a great challenge to model the polarization effect with the classic force fields (FFs),41 especially for the charged groups under electric fields. One effective solution is to implement a charge-variable polarizable (Q-POL) FF, in which the partial charges were updated from electronic structure calculations to reflect the electrostatic polarization under electric fields and conformational changes. The MD simulation with Q-POL demonstrated that the distance, d, between the biotin terminal of biotin-4KC and the space-filling short TEGT chain increased to 3.6 nm in the ON state under the upward electric field. When the electric filed was switched to the opposite direction, the biotin-4KC chain bent down toward the gold surface with the decreased value of d, in good agreement with experiments. To save the computational costs, the fragment-based multilayer coarse-graining polarization model, which utilizes fragment-centered dipole moments to evaluate dipole–dipole interactions, was also implemented to calculate the electrostatic energy in biotin-4KC.16 When treating SAMs with complex components consisting of hundreds or even thousands of atoms, the Q-POL model can also be integrated with the generalized energy-based fragmentation (GEBF) approach,42 which is a simple linear-scaling strategy of treating large-sized systems by cutting the whole system into various small “electrostatically embedded” subsystems.
The SAMs’ function can be further tuned by adding other photo-, pH-, redox-, magneto-, and enzyme-responsive units into the electrically triggered system. In those multiresponsive SAMs, theoretical models need some modifications, because those complex systems may consist of thousands of atoms and the switching is triggered by quantum-mechanical events under stimuli. A reactive molecular dynamics (RMD) method is well suited to deal with the light-driven switching of azobenzene-based SAMs, where the reactive potential energy curve for conformational transition, such as cis/trans conversion, is fitted from electronic structure calculations.18−20 The MD simulations were also carried out on the novel pH/voltage/photoresponsive SAMs, whose functions depend on the formation of multiple salt bridges and guest release upon protonolysis modulated by the surface potential.21
In face of the challenges in simulation of responsive SAMs to a variety of triggers, there are some major problems to be solved. In MD simulations, the surface voltage is considered as an electrostatic field and no current was involved in the system. In the real experiment, however, a circuit was formed with constant current. Furthermore, the predicted critical triggering electric field strength is usually 1–2 orders of magnitude higher than the experimental measurement, which may result from the oversimplified models of the electrode and the electric field screening effect. Moreover, the charge transport between SAMs and the substrate, as well as the relaxation of the surface are not currently considered. In addition, most current MD simulations are based on the internal energy profile. In experiments, however, only the change in binding free energies for the ON/OFF states can be measured, which often takes place between recognition units and protein probes. With the rapid development of more powerful theoretical tools, we can expect more valuable predictions to guide laboratory preparation, reduce research cost, or even offer new physical pictures for these fascinating smart surfaces.
7. Conclusion and Outlook
Through synergetic theoretical and experimental efforts, electrically responsive surfaces, which base their switching mechanism on a charged molecular backbone or end group, have been demonstrated to enable the control of nonspecific and specific biomolecular interactions. Indeed, by exploring various dynamic molecular architectures on surfaces, electrically responsive surfaces have arose as powerful tools for modulating interactions of surfaces with proteins, bacterial and mammalian cells. Oligopeptides, which undergo conformational changes between collapsed (“OFF” state) and fully extended (“ON” state) structures on surfaces, allow for reversible biomolecule switching and control over biomolecular interactions under complex biological matrixes. Stimuli-responsive SAMs are robust under many biological conditions and when compared with polymer systems, they offer higher precision of distribution of surface functional entities and faster changes in surface properties.
The described electrically responsive surfaces provide new opportunities for mechanistic studies of the pathways by which cells sense, integrate, and respond to changes in their environments. Using the developed stimuli-responsive surfaces, unprecedented reversibility and spatial and temporal precision is achievable, thus offering new experimental approaches to studying the regulation and dynamics of cell signaling. Electrically responsive surfaces, and indeed other stimuli-responsive systems, can also open new interesting prospects in the fields of drug delivery, bioimaging, tissue engineering, and regenerative medicine. The smart surfaces may be used to influence, lead, and accelerate various biological processes such as cell adhesion and growth, providing a means to deliver molecularly guided tissue regeneration. Biosensors, which transduce a biorecognition event into measurable electronic or optoelectronic signals, have a crucial role in a wide range of application, including clinical diagnosis, environmental monitoring, forensic analysis, and antiterrorism. Surfaces with highly efficient binding capacities for target species and with ability to modulate biomolecule activity on surfaces can be useful as a way to develop highly specific, ultrasensitive biosensors for early diagnosis of critical diseases. Thus, future research should be directed toward exploring such emerging opportunities. This journey will clearly bring new challenges and stimulate further efforts and contributions to this exciting field of stimuli-responsive surfaces. With nature as a source of inspiration and creativity, future efforts are expected to yield surfaces with superior reversibility characteristics, multiple biomolecule switching and control over the activity of larger biomolecules such as proteins.
Biographies
Eleonora Cantini received her BSc (2007) in Chemistry and MSc (2010) in Chemistry of Biological Molecules from the University of Florence, Italy. She is currently a PhD student in Prof. Mendes’ group, and her research is focused on the development of novel switchable surfaces for biomedical applications.
Xingyong Wang received his PhD in Chemistry from Nanjing University (China) in 2013. He is currently a Vice-Chancellor’s Postdoctoral Research Fellow in School of Chemistry at the University of Wollongong, Australia. His research interests include theoretical modeling of molecular switches and excited-state reactions in biomolecular systems.
Patrick Koelsch received his Diploma (2003) and PhD (2005) in Physics from the University of Potsdam, Germany. After postdoctoral studies at the University of Leipzig, he joined the University of Heidelberg in 2006, where he became a Privatdozent in 2010. In 2011, he assumed a position as a Research Assistant Professor of Bioengineering at the University of Washington. Since 2016 he is a Senior Research Scientist for Synrad, a manufacturer for industrial CO2 laser.
Jon Preece received his BSc (1990) from Loughborough University and PhD (1994) from the University of Birmingham, UK. He undertook postdoctoral research at the Jonannes-Gutenberg Universitat in Germany (1995–1996). In 1997, he returned to the UK and became Professor of Nanoscale Chemistry in 2004 and Head of School in 2011. His research interests lies in the area of functional nanomaterials and their potential applications.
Jing Ma received her PhD (1998) in Theoretical Chemistry from Nanjing University, China. She was a JSPS fellow (1998–2000) at the Gifu University, Japan. She began to work at Nanjing University as an Associated Professor (2000–2005) and then Professor of Chemistry since 2005. Her research focused on theoretical design and simulation of responsive materials with unique optical and electrical properties.
Paula Mendes received her MSc (1997) and PhD (2002) in Chemical Engineering from the University of Porto, Portugal. She undertook postdoctoral research at the University of Birmingham, UK (2002–2004) and University of California, Los Angeles (2004–2006). In 2013, she became a Professor of Advanced Materials and Nanotechnology and an EPSRC Leadership Fellow in the School of Chemical Engineering, University of Birmingham, UK. She is an ERC consolidator investigator and her research interests lies in the development of novel methods for controlling the structure and functionality of materials at the molecular and nanometer scales and their application in biology and medicine.
We acknowledge the support of EPSRC (EP/K027263/1), ERC (Consolidator Grant 614787), and the National Natural Science Foundation of China (21273102, 21290192).
The authors declare no competing financial interest.
References
- Mendes P. M. Stimuli-Responsive Surfaces for Bio-Applications. Chem. Soc. Rev. 2008, 37, 2512–2529. 10.1039/b714635n. [DOI] [PubMed] [Google Scholar]
- Mendes P. M. Cellular nanotechnology: making biological interfaces smarter. Chem. Soc. Rev. 2013, 42, 9207–9218. 10.1039/c3cs60198f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard M.; Chaubey A.; Malhotra B. D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. 10.1016/S0956-5663(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Yin B. C.; Guan Y. M.; Ye B. C. An ultrasensitive electrochemical DNA sensor based on the ssDNA-assisted cascade of hybridization reaction. Chem. Commun. 2012, 48, 4208–4210. 10.1039/c2cc30997a. [DOI] [PubMed] [Google Scholar]
- Takahashi H.; Matsuzaka N.; Nakayama M.; Kikuchi A.; Yamato M.; Okano T. Terminally functionalized thermoresponsive polymer brushes for simultaneously promoting cell adhesion and cell sheet harvest. Biomacromolecules 2012, 13, 253–260. 10.1021/bm201545u. [DOI] [PubMed] [Google Scholar]
- Inaba R.; Khademhosseini A.; Suzuki H.; Fukuda J. Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials 2009, 30, 3573–3579. 10.1016/j.biomaterials.2009.03.045. [DOI] [PubMed] [Google Scholar]
- Alberts B.; Johnson A.; Lewis J.; Raff M.; Roberts K.; Walter P.. Molecular biology of the cell; Garland Science: New York, 2008. [Google Scholar]
- Nick P., Ed. Plant Microtubules: Development and Flexibility, 2nd ed.; Plant Cell Monographs; Springer-Verlag: Berlin, 2008; Vol. 11. [Google Scholar]
- Iqbal P.; Preece J. A.; Mendes P. M.. Nanotechnology: The “Top Down” and “Botttom Up” Approaches. In Supramolecular Chemistry: From Molecules to Nanomaterials; Steed J. W., Gale P. A., Eds.; John Wiley & Sons Ltd: Chichester, UK, 2012; Vol. 8; pp 3589–3602. [Google Scholar]
- Parthasarathy P.; Mendes P. M.; Schopf E.; Preece J. A.; Stoddart J. F.; Chen Y. Spatially Controlled Assembly of Nanomaterials at the Nanoscale. J. Nanosci. Nanotechnol. 2009, 9, 650–654. 10.1166/jnn.2009.J061. [DOI] [PubMed] [Google Scholar]
- Yeung C. L.; Iqbal P.; Allan M.; Lashkor M.; Preece J. A.; Mendes P. M. Tuning Specific Biomolecular Interactions Using Electro-Switchable Oligopeptide Surfaces. Adv. Funct. Mater. 2010, 20, 2657–2663. 10.1002/adfm.201000411. [DOI] [Google Scholar]
- Lashkor M.; Rawson F. J.; Preece J. A.; Mendes P. M. Switching Specific Biomolecular Interactions on Surfaces under Complex Biological Conditions. Analyst 2014, 139, 5400–5408. 10.1039/C4AN01225A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashkor M.; Rawson F. J.; Stephenson-Brown A.; Preece J. A.; Mendes P. M. Electrically-Driven Modulation of Surface-Grafted RGD Peptides for Manipulation of Cell Adhesion. Chem. Commun. 2014, 50, 15589–15592. 10.1039/C4CC06649A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzetti A.; Mieszkin S.; Iqbal P.; Rawson F. J.; Callow M. E.; Callow J. A.; Koelsch P.; Preece J. A.; Mendes P. M. An Electrically Reversible Switchable Surface to Control and Study Early Bacterial Adhesion Dynamics in Real-Time. Adv. Mater. 2013, 25, 2181–2185. 10.1002/adma.201204880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzetti A.; Davis M.; Yeung C. L.; Preece J. A.; Koelsch P.; Mendes P. M. Direct Observation of Reversible Biomolecule Switching Controlled By Electrical Stimulus. Adv. Mater. Interfaces 2014, 1, 1400026. 10.1002/admi.201400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.; Wang X. Y.; Jiang N.; Yan T. Y.; Kan Z. G.; Mendes P. M.; Ma J. Polarization Effect and Electric Potential Changes in the Stimuli-Responsive Molecular Monolayers Under an External Electric Field. J. Phys. Chem. C 2015, 119, 22866–22881. 10.1021/acs.jpcc.5b04805. [DOI] [Google Scholar]
- Yeung C. L.; Wang X.; Lashkor M.; Cantini E.; Rawson F. J.; Iqbal P.; Preece J. A.; Ma J.; Mendes P. M. Modulation of Biointeractions by Electrically Switchable Oligopeptide Surfaces: Structural Requirements and Mechanism. Adv. Mater. Interfaces 2014, 1, 1300085. 10.1002/admi.201300085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J.; Tian Z. Q.; Ma J. Light- and Electric-Field-Induced Switching of Thiolated Azobenzene Self-Assembled Monolayer. J. Phys. Chem. C 2013, 117, 19934–19944. 10.1021/jp404434r. [DOI] [Google Scholar]
- Tian Z. Q.; Wen J.; Ma J. Reactive molecular dynamics simulations of switching processes of azobenzene-based monolayer on surface. J. Chem. Phys. 2013, 139, 014706. 10.1063/1.4812379. [DOI] [PubMed] [Google Scholar]
- Tian Z. Q.; Wen J.; Ma J. Dynamic simulations of stimuli-responsive switching of azobenzene derivatives in self-assembled monolayers: reactive rotation potential and switching functions. Mol. Simul. 2015, 41, 28–42. 10.1080/08927022.2014.918974. [DOI] [Google Scholar]
- Chen S.; Itoh Y.; Masuda T.; Shimizu S.; Zhao J.; Ma J.; Nakamura S.; Okuro K.; Noguchi H.; Uosaki K.; Aida T. Subnanoscale hydrophobic modulation of salt bridges in aqueous media. Science 2015, 348, 555–559. 10.1126/science.aaa7532. [DOI] [PubMed] [Google Scholar]
- Yousaf M. N.; Houseman B. T.; Mrksich M. Turning on cell migration with electroactive substrates. Angew. Chem., Int. Ed. 2001, 40, 1093–1096. . [DOI] [PubMed] [Google Scholar]
- Lee C. S.; Baker S. E.; Marcus M. S.; Yang W. S.; Eriksson M. A.; Hamers R. J. Electrically addressable biomolecular functionalization of carbon nanotube and carbon nanofiber electrodes. Nano Lett. 2004, 4, 1713–1716. 10.1021/nl048995x. [DOI] [Google Scholar]
- Mendes P. M.; Christman K. L.; Parthasarathy P.; Schopf E.; Ouyang J.; Yang Y.; Preece J. A.; Maynard H. D.; Chen Y.; Stoddart J. F. Electrochemically Controllable Conjugation of Proteins on Surfaces. Bioconjugate Chem. 2007, 18, 1919–1923. 10.1021/bc7002296. [DOI] [PubMed] [Google Scholar]
- Yousaf M. N.; Houseman B. T.; Mrksich M. Using electroactive substrates to pattern the attachment of two different cell populations. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 5992–5996. 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahann J.; Mitragotri S.; Tran T. N.; Kaido H.; Sundaram J.; Choi I. S.; Hoffer S.; Somorjai G. A.; Langer R. A reversibly switching surface. Science 2003, 299, 371–374. 10.1126/science.1078933. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Mu L.; Liu B. H.; Zhang S.; Yang P. Y.; Kong J. L. Controlled protein assembly on a switchable surface. Chem. Commun. 2004, 1194–1195. 10.1039/b400776j. [DOI] [PubMed] [Google Scholar]
- Pranzetti A.; Salauen S.; Mieszkin S.; Callow M. E.; Callow J. A.; Preece J. A.; Mendes P. M. Model Organic Surfaces to Probe Marine Bacterial Adhesion Kinetics by Surface Plasmon Resonance. Adv. Funct. Mater. 2012, 22, 3672–3681. 10.1002/adfm.201103067. [DOI] [Google Scholar]
- Iqbal P.; Rawson F. J.; Ho W. K. W.; Lee S.-F.; Leung K. C.-F.; Wang X.; Beri A.; Preece J. A.; Ma J.; Mendes P. M. Surface Molecular Tailoring Using pH-Switchable Supramolecular Dendron-Ligand Assemblies. ACS Appl. Mater. Interfaces 2014, 6, 6264–6274. 10.1021/am501613c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M. J.; Koelsch P.. Introduction to Quantitative Data Analysis in Vibrational Sum-Frequency Generation Spectroscopy. In Soft Matter at Aqueous Interfaces; Lang P. R., Liu Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Vol. 917. [Google Scholar]
- Shen Y. R. Surface properties probed by second-harmonic and sum-frequency generation. Nature 1989, 337, 519–525. 10.1038/337519a0. [DOI] [Google Scholar]
- Vemparala S.; Kalia R. K.; Nakano A.; Vashishta P. Electric Field Induced Switching of Poly (ethylene glycol) Terminated Self-Assembled Monolayers: A Parallel Molecular Dynamics Simulation. J. Chem. Phys. 2004, 121, 5427–5433. 10.1063/1.1781120. [DOI] [PubMed] [Google Scholar]
- Rant U.; Arinaga K.; Tornow M.; Kim Y. W.; Netz R. R.; Fujita S.; Yokoyama N.; Abstreiter G. Dissimilar Kinetic Behavior of Electrically Manipulated Single- and Double-Stranded DNA Tethered to a Gold Surface. Biophys. J. 2006, 90, 3666–3671. 10.1529/biophysj.105.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C.; Paci I. Conformational Behavior of Chemisorbed Azobenzene Derivatives in External Electric Fields: A Theoretical Study. J. Phys. Chem. C 2010, 114, 20556–20563. 10.1021/jp104967e. [DOI] [Google Scholar]
- Kim H.; Jang S. S.; Kiehl R. A.; Goddard W. A. Negative Differential Resistance of Oligo(Phenylene Ethynylene) Self-Assembled Monolayer Systems: The Electric-Field-Induced Conformational Change Mechanism. J. Phys. Chem. C 2011, 115, 3722–3730. 10.1021/jp1114916. [DOI] [Google Scholar]
- Cao Q. Q.; Zuo C. C.; Li L. J.; Yan G. Effects of Chain Stiffness and Salt Concentration on Responses of Polyelectrolyte Brushes Under External Electric Field. Biomicrofluidics 2011, 5, 044119–12. 10.1063/1.3672190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Cruz-Chu E. R.; Woodard J. C.; Gartia M. R.; Schulten K.; Liu L. Electrically Induced Conformational Change of Peptides on Metallic Nanosurfaces. ACS Nano 2012, 6, 8847–8856. 10.1021/nn3027408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamanu J.; Borodin O.; Smith G. D. Molecular Dynamics Simulation Studies of the Structure of a Mixed Carbonate/LiPF6 Electrolyte near Graphite Surface as a Function of Electrode Potential. J. Phys. Chem. C 2012, 116, 1114–1121. 10.1021/jp2101539. [DOI] [Google Scholar]
- Langer A.; Kaiser W.; Svejda M.; Schwertler P.; Rant U. Molecular Dynamics of DNA–Protein Conjugates on Electrified Surfaces: Solutions to the Drift-Diffusion Equation. J. Phys. Chem. B 2014, 118, 597–607. 10.1021/jp410640z. [DOI] [PubMed] [Google Scholar]
- Pei Y.; Ma J. Electric Field Induced Switching Behaviors of Monolayer-Modified Silicon Surfaces: Surface Designs and Molecular Dynamics Simulations. J. Am. Chem. Soc. 2005, 127, 6802–6813. 10.1021/ja045506m. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yan T.; Ma J. Polarizable Force Fields Based on Physical Models and Quantum Chemical Calculations. Int. J. Quantum Chem. 2015, 115, 545–549. 10.1002/qua.24829. [DOI] [Google Scholar]
- Li S.; Li W.; Ma J. Generalized Energy-Based Fragmentation Approach and Its Applications to Macromolecules and Molecular Aggregates. Acc. Chem. Res. 2014, 47, 2712–2720. 10.1021/ar500038z. [DOI] [PubMed] [Google Scholar]