Abstract
Background
Alcohol is a significant component of the diet with dose-dependent risks and benefits. High doses of alcohol damage the liver and early symptoms of liver disease include changes in routinely assessed liver enzymes. Less is known regarding the mechanisms responsible for the benefits of moderate alcohol consumption, including their effects on the liver. The objectives of this study were to examine alcohol’s dose-dependent effects on markers of liver function (alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), and bilirubin), as well as to compare the different methods of assessing alcohol intake using NHANES 2001–2010 adult data (N = 24,807).
Methods
Three methods were used to estimate alcohol intake from all volunteers: 24-h recall; the National Cancer Institute (NCI) method of usual intake; and a specific alcohol intake questionnaire.
Results
Mean alcohol intake by 24-h recall, NCI method and questionnaire was 41.0 ± 0.8 g/d, 10.9 ± 0.2 g/d and 11.0 ± 0.2 g/d, respectively. Alcohol consumers had significantly lower levels of ALP and higher levels of AST, GGT and bilirubin compared to non-consumers (P < 0.01) and activities of ALT, AST, and GGT increased and of ALP decreased as alcohol intake increased, regardless of intake assessment method used. The most sensitive measure of alcohol consumption was GGT.
Conclusions
Since alcohol had a graded linear effect on several liver enzymes, including at low and moderate doses, benefits as well as risks of alcohol intake may be related to liver function. Since the NCI method and alcohol questionnaire yielded very similar alcohol intake estimates, this study cross-validated these methods and demonstrated the robustness of the NCI method for estimating intake of irregularly consumed foods.
Keywords: Alkaline phosphatase, Alanine aminotransferase, Aspartate aminotransferase, Gamma glutamyl transferase, Bilirubin, NCI method
Introduction
Alcohol is consumed by about 70 % of U.S. adults [1]. Consumption of this dietary component has both risks and benefits. Low to moderate doses of alcohol lower the risk of cardiovascular disease and all-cause mortality [2]. The Dietary Guidelines for Americans 2010 and American Heart Association’s dietary guidelines suggest that if alcohol is consumed, males consume no more than two alcoholic drinks per/day (28 g/day) and women no more than one per/day (14 g/day) [2, 3]. However, approximately 38 million adults in the United States report binge drinking an average of four times per month and consuming an average of eight drinks per episode [4]. Excessive alcohol intake was responsible for approximately 10 % of deaths among working age adults in the United States during 2006–2010 [5] and cost the United States $223.5 billion in 2006 [6]. It is estimated to be the fourth leading preventable cause of death in the United States [7]. Heavy drinking, including binge drinking, increases the risk of liver disease, hypertension, stroke, type II diabetes, gastrointestinal cancers, injuries and violence [2]. Alcohol abuse is the leading cause of liver-related morbidity and mortality [8]. In alcoholic patients increased levels of several liver-derived biomarkers are associated with excessive ethanol intake and alcoholic liver disease, and a number of studies have reported induction of liver enzyme function due to excessive alcohol consumption [9–17]. However, only a few small studies have investigated the effects of moderate alcohol consumption on liver enzymes [12, 13].
The National Health and Nutrition Examination Survey (NHANES), a large nationally representative survey of the U.S. population, is designed to monitor the health and nutritional status of adults and children [18]. The NHANES data are currently released every 2 years with data from approximately 5000 adult volunteers and include an in-home survey and physical examination conducted in a mobile facility. Food consumption, body composition, health status, multiple blood chemistry measures, and various other health-related parameters are assessed. Because the NHANES is conducted regularly using the same standardized procedures, very large data sets can be obtained by combining multiple years of data. This provides an opportunity to examine, in a large nationally-representative data set, self-reported alcohol use and its association with various markers of liver function. In addition, the NHANES employs two methods that can be used to independently estimate alcohol, collection of two days of 24-h recall data which then can be used to estimate usual intake and an alcohol intake questionnaire.
The primary objective of this study was to examine the effects of graded levels of alcohol intake on markers of liver function using the very large, nationally representative, NHANES data set. We also compared several methods for assessing alcohol intake: 1) single 24-h recall; 2) usual alcohol intake based on the two 24-h recalls collected by NHANES and adjusted using the National Cancer Institute (NCI) usual intake procedure; and 3) an alcohol consumption questionnaire.
Methods
Study population
NHANES data are collected by the National Center for Health Statistics (NCHS) of the Center for Disease Control and Prevention (CDC) on a nationally representative sample of non-institutionalized Americans [18]. All participants or proxies provided written informed consent and the Research Ethics Review Board at the NCHS approved the survey protocol. Data from NHANES 2001–2002, 2003–2004, 2005–2006, 2007–2008 and 2009–2010 were combined for the analysis. The combined sample included 24,807 adults age 19 years and older (12,561 males and 12,246 females) and excluded pregnant or lactating females, those with incomplete dietary records or missing liver enzyme data. Response rates for NHANES are typically quite good, but they are related to age; for example medical examination response rated typically exceed 80 % for persons under age 20 but tend to be less than 70 % for those age 70 or more [19].
Estimates of alcohol intake
Alcohol intake (g/day) was assessed three ways: 1) using a single 24-h recall, an estimate of an individual’s self-reported alcohol consumption on the day of recall; 2) by determining usual alcohol intakes with the NCI method, an estimate of long-term intake that employed data from 2 days of 24-h recalls; and 3) via an alcohol intake questionnaire quantifying annual consumption of alcohol. Participants completed an in-person 24-h dietary recall and health examination in a Mobile Examination Center and a second 24-h dietary recall was collected via telephone 3–10 day after the first recall using the United States Department of Agriculture’s automated multiple-pass method. A detailed description of the survey design and the data collection procedures are available elsewhere [18]. The usual intake analysis used the NCI method with a two part correlated model in which the probability of non-zero intake on a given day and the usual intake on consumption days were estimated and assumed to be correlated [20]. The two-part correlated model was used since unlike nutrient intake, alcohol is not consumed on most days by most people. The MIXTRAN and INDIVNT macros were used to estimate individual usual intakes with a recall day and weekday/weekend (Friday-Sunday) intake indicator in the model. Additionally, since an alcohol questionnaire was administered during the NHANES household interview it was also used to assess alcohol intake as average number of drinks per day over the last year [18]. The alcohol content of alcoholic drinks is defined as 12 oz of beer, 5 oz of wine or 1.5 oz of liquor and equals 14 g of alcohol [8, 21].
Outcome variables for markers of liver function
As part of the NHANES in-person health examination in the Mobile Examination Center, participants provided a blood specimen for laboratory analyses. Fasting prior to collection of the blood sample was not required. The activities of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma glutamyl transferase (GGT) were measured spectrophotometerically in U/L units using their respective kinetic enzymatic methods, and bilirubin levels in mg/dl were measured spectrophotometerically using a timed-endpoint Diazo method [18]. ALT and AST are aminotransferases that, when elevated, are indicative of reduced hepatocyte integrity. GGT and bilirubin are markers of cholestasis [18, 22–24] and elevated levels of bilirubin are associated with hemolytic jaundice [18, 22–24].
Statistical analysis
Analyses were conducted using SAS 9.2 (SAS Institute, Inc.; Cary, NC) and SUDAAN release 11.0 (Research Triangle Institute, Research Triangle Park, NC). Appropriate weighting factors were used to adjust for oversampling of selected groups, non-response to the survey by some individuals, and day of the week when the interview was conducted. For each alcohol assessment approach, subjects were grouped into deciles of intake as well as one of seven alcohol consumption groups in g/d: 0, 0 to ≤7, >7 to ≤14, >14 to ≤21, >21 to ≤28, >28 to ≤35, and >35 (the usual intake method did not have a 0 g/d group). These levels are approximately equivalent to 0, 0 to ≤0.5, >0.5 to ≤1.0, >1.0 to ≤1.5, >1.5 to ≤2.0, >2.0 to ≤2.5 and >2.5 drinks/d, respectively. Least square (LS) means and standard errors (SE) of enzyme activities were determined using PROC REGRESS of SUDAAN after adjustment for various covariates. Outcome variables were adjusted for race-ethnicity, age, physical activity (categorized as sedentary, moderate, or vigorous based on responses to questions on activity), poverty income ratio, smoking habits (yes/no), energy intake, and BMI. Laboratory test values for ALP, ALT, AST, GGT, and bilirubin were compared across deciles of alcohol intake as well as alcohol intake levels in g/day and effects of alcohol intake on liver enzymes were assessed to ascertain whether an increase in alcohol intake was associated with changes in enzyme activities and whether changes were linear or curvilinear as a function of alcohol. We used the Dunnett significant difference post-hoc test (DUNNETT test option within PROC GLM) to compare each category of alcohol intake to the non/low alcohol consumer group. These post-hoc tests were sample weighted but not adjusted for the complex sample design of NHANES as this capability was not available.
While not the primary focus of this research, we did examine the impact of age, gender, socioeconomic status (measured as poverty income ratio) and presence of hypertension (based on the answer to a question about whether a subject had previously been told by a doctor they had hypertension or elevated blood pressure) on liver markers. We also examined the interaction of alcohol intake (as measured by all three methods of assessment) with age, gender, race/ethnicity, socioeconomic status, and presence of hypertension. These regression models also contained additional covariates: physical activity (categorized as sedentary, moderate, or vigorous based on response to questions on activity), current smoking (yes/no), energy intake, and BMI. Significance for all statistical comparisons was set at P < 0.01.
Results
Approximately 58.7 % of NHANES 2001–2010 adult participants (n = 13,104) regularly consumed alcohol (questionnaire method) and 25 % of adult participants consumed alcohol (n = 6176) at least once over the 24-h survey period (24-h recall method). Regardless of the method used to identify alcohol consumers, they were more likely to be male, younger, non-Hispanic whites, smokers, have higher income, and have more physically-active lifestyles compared to non-consumers.
The mean intake of alcohol as estimated by the 24-h recall method representing the alcohol intake on the day of recall was about 3–5 times higher than the usual intake estimated by NCI method or by alcohol intake questionnaire (Table 1). Usual intake, as assessed by the NCI method using two 24-h recalls, and intake by questionnaire representing long term intake, provided very similar estimates of alcohol consumption.
Table 1.
Average intake of alcohol by three different methods of estimationa, NHANES 2001–2010
| Mean alcohol intake g/day | Gender combined | Male | Female | |
|---|---|---|---|---|
| 24-h Recall | N | 24,807 | 12,561 | 12,246 |
| Mean ± SE | 41.0 ± 0.8 | 48.2 ± 1.0 | 29.4 ± 0.8 | |
| Usual intake by NCI method | N | 24,807 | 12,561 | 12,246 |
| Mean ± SE | 10.9 ± 0.2 | 16.1 ± 0.3 | 5.9 ± 0.1 | |
| Alcohol intake questionnaire | N | 22,307 | 11,376 | 10,931 |
| Mean ± SE | 11.0 ± 0.2 | 14.6 ± 0.3 | 6.4 ± 0.2 | |
(N Sample Size; SE Standard Error; aAlcohol intake (g/day) was assessed three ways: 1) via a single 24-h recall; 2) by determining usual alcohol intakes with the NCI method, an estimate of long-term intake that employed data from two days of 24-h recalls; and 3) via an alcohol intake questionnaire quantifying annual consumption of alcohol)
The activities of liver enzymes of alcohol consumers and non-consumers were compared and alcohol consumers (gender combined) had significantly lower ALP (5–6 %, P < 0.01) and higher AST (4–7 %, P < 0.01), GGT (15–25 %, P < 0.01) and bilirubin (3 %, P < 0.01) compared to non-consumers by both methods. However, the difference in ALT activities of alcohol consumers and non-consumers, were less than 3 % and were not statistically significant (P > 0.01). To determine whether there were dose-related differences in liver enzymes levels in alcohol users, we examined the data by deciles of alcohol intake (Fig. 1) as well as alcohol intake by g/day (Fig. 2), estimated by three different methods. Table 2 provides the percentile of amount of alcohol intake by consumers using different methods of alcohol intake estimation and can be used to assess the percentage of the population consuming various levels of alcohol. Regardless of method used, a significant increasing linear trend in activities of ALT, AST, and GGT, and a decreasing linear trend in activity of ALP, was observed as alcohol intake increased (Figs. 1 and 2, Table 3). A significant curvilinear trend was noted for activity of ALP, AST and GGT with some methods but not with others (Figs. 1 and 2, Table 3) indicating modification of the direction and/or magnitude of the change as alcohol intake increases. While the relationship between intake of alcohol and activities of AST and GGT was similar in males and females, the relationship between alcohol intake and activities of ALP and ALT were largely driven by males (Figs. 1 and 2, Tables 4 and 5). The increase in activity of GGT with increase in alcohol intake was higher than the increase in activities of ALT and AST, regardless of the method used to measure alcohol intake (Figs. 1 and 2, Tables 3, 4 and 5). However, no significant trends were noted for bilirubin levels (Tables 3, 4 and 5). Dunnett post-hoc test indicated that, based on the alcohol questionnaire data, we could detect a significant difference in GGT with consumption of as little as 7–14 g alcohol per day (P < 0.01) while differences were detected for AST and ALT at 14–21 and 21–28 g alcohol per day, respectively (P < 0.01).
Fig. 1.
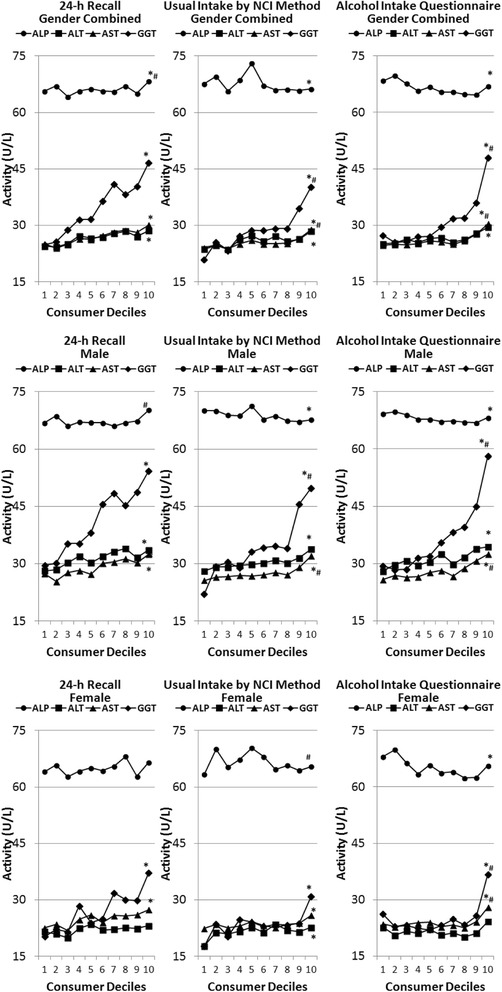
Association of alcohol consumption by consumer deciles of intake with liver enzyme function† NHANES 2001–2010. † ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase and GGT gamma glutamyl transferase; * Significant Linear Trend at P < 0.01; # Significant Curvilinear Trend at P < 0.01
Fig. 2.
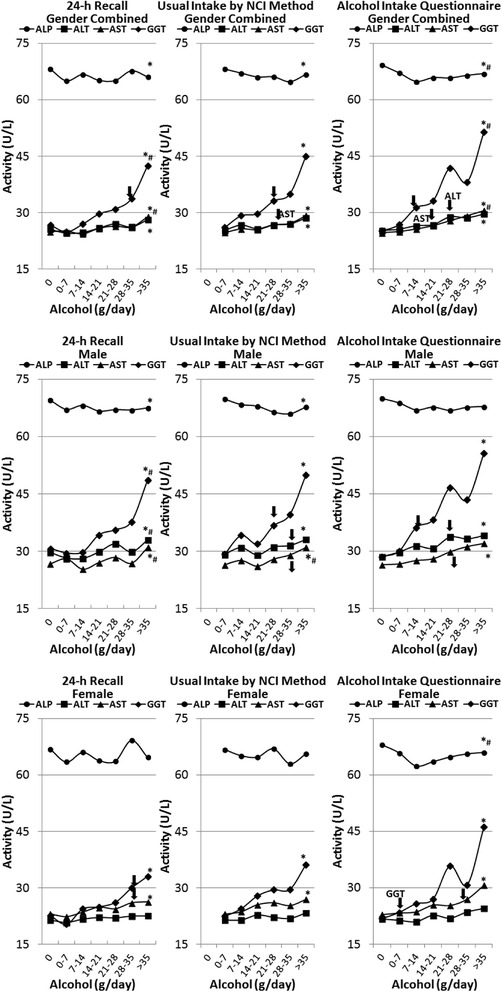
Association of alcohol consumption by g of alcohol per day with liver enzyme function† NHANES 2001–2010. † ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase and GGT gamma glutamyl transferase; * Significant Linear Trend at P < 0.01; #Significant Curvilinear Trend at P < 0.01; ↓Significant Dunnett at P < 0.01 continuous start point
Table 2.
Percentiles of amount of alcohol intake by consumers estimated by three different methods of alcohol intake estimation among adults. NHANES 2001–2010
| Alcohol intake (g/day ± SE) percentiles | |||||
|---|---|---|---|---|---|
| 10th | 25th | 50th | 75th | 90th | |
| 24-h recall | |||||
| All | 4.52 ± 1.01 | 13.96 ± 0.02 | 28.05 ± 0.03 | 55.60 ± 1.56 | 88.50 ± 2.16 |
| Male | 10.93 ± 0.56 | 15.59 ± 0.85 | 33.48 ± 0.97 | 63.07 ± 2.30 | 102.9 ± 4.02 |
| Female | 0.74 ± 0.16 | 11.04 ± 0.40 | 21.21 ± 0.53 | 36.95 ± 1.21 | 64.71 ± 2.56 |
| Usual intake by NCI method | |||||
| All | 1.84 ± 0.003 | 2.60 ± 0.003 | 5.30 ± 0.08 | 7.15 ± 0.49 | 30.20 ± 0.65 |
| Male | 3.32 ± 0.17 | 5.34 ± 0.01 | 6.70 ± 0.11 | 20.38 ± 0.74 | 42.66 ± 1.00 |
| Female | 1.38 ± 0.01 | 1.99 ± 0.05 | 2.61 ± 0.002 | 3.47 ± 0.002 | 15.94 ± 0.70 |
| Alcohol intake questionnaire | |||||
| All | 0.23 ± 0.02 | 0.92 ± 0.002 | 4.00 ± 0.001 | 13.96 ± 0.50 | 27.89 ± 0.05 |
| Male | 0.44 ± 0.02 | 1.85 ± 0.06 | 7.98 ± 0.25 | 19.96 ± 0.28 | 39.42 ± 0.60 |
| Female | 0.16 ± 0.02 | 0.46 ± 0.001 | 2.00 ± 0.20 | 7.84 ± 0.07 | 15.95 ± 1.12 |
Table 3.
Association of liver enzyme function with alcohol consumption level (Deciles and g/day) by three different methods of alcohol intake estimation among all adults. NHANES 2001–2010a
| 24-h recall | Usual intake by NCI method | Alcohol intake questionnaire | ||
|---|---|---|---|---|
| Regression coefficientb | ||||
| Alkaline phosphatase (ALP), U/L | ||||
| N | 21,245 | 21,245 | 19,523 | |
| By Decile | Linear | 47.82/−0.24* | 50.70/−0.38* | 48.37/−0.45* |
| Curvilinear | 48.14/−1.10/0.11# | ns | ns | |
| By g/day | Linear | 48.21/−0.37* | 48.70/−0.45* | 47.58/−0.53* |
| Curvilinear | ns | ns | 51.08/−3.14/0.36# | |
| Alanine aminotransferase (ALT), U/L | ||||
| N | 21,179 | 21,179 | 19,460 | |
| By Decile | Linear | 9.95/0.26* | 7.17/0.37* | 9.22/0.27* |
| Curvilinear | ns | ns | ns | |
| By g/day | Linear | 9.59/0.34* | 8.84/0.57* | 8.91/0.74* |
| Curvilinear | ns | ns | ns | |
| Aspartate aminotransferase (AST), U/L | ||||
| N | 21,177 | 21,177 | 19,458 | |
| By Decile | Linear | 18.57/0.42* | 15.58/0.40* | 17.06/0.33* |
| Curvilinear | ns | 17.82/−0.36/0.06# | 17.75/−0.35/0.08# | |
| By g/day | Linear | 18.00/0.54* | 17.01/0.79* | 16.64/0.93* |
| Curvilinear | 19.28/−0.70/0.17# | ns | 17.86/0.02/0.12# | |
| Gamma glutamyl transferase (GGT), U/L | ||||
| N | 21,245 | 21,245 | 19,523 | |
| By Decile | Linear | −0.64/1.65* | −13.55/1.72* | −5.78/1.43* |
| Curvilinear | ns | −8.30/−0.05/0.15# | −3.01/−1.30/0.31# | |
| By g/day | Linear | −2.94/2.21* | −7.09/3.29* | −7.36/3.88* |
| Curvilinear | 2.31/−2.95/0.69# | ns | −3.21/0.80/0.42# | |
| Bilirubin (mg/dl) | ||||
| N | 21,236 | 21,236 | 19,514 | |
| By Decile | Linear | 0.87/0.003* | 0.86/0.001 | 0.84/0.005* |
| Curvilinear | ns | ns | ns | |
| By g/day | Linear | 0.87/0.004* | 0.86/0.004 | 0.85/0.01* |
| Curvilinear | ns | ns | ns | |
aRegression analyses examining linear and/or quadratic effect of alcohol intake conducted with the following covariates: age, gender, race/ethnicity, current smoking status (yes/no), poverty income ratio, physical activity level (sedentary, moderate, or vigorous based on feedback on questions on activity), energy intake, and body mass index. bβ0/β1 for Linear Trend and β0/β1/β2 for Curvilinear Trend (β0: intercept, β1: linear regression coefficient, and β2: curvilinear regression coefficient); * Significant Linear Trend at P < 0.01; # Significant Curvilinear Trend at P < 0.01, N Sample Size; ns Non-significant Curvilinear Trend
Table 4.
Association of liver enzyme function with alcohol consumption level (Deciles and g/day) by three different methods of alcohol intake estimation among male adults. NHANES 2001–2010a
| 24-h recall | Usual intake by NCI method | Alcohol intake questionnaire | ||
|---|---|---|---|---|
| Regression coefficientb | ||||
| Alkaline phosphatase (ALP), U/L | ||||
| N | 10,858 | 10,858 | 10,025 | |
| By Decile | Linear | 66.64/−0.25 | 68.96/−0.37* | 65.82/−0.29* |
| Curvilinear | 67.25/−1.24/0.12# | ns | ns | |
| By g/day | Linear | 67.20/−0.40* | 68.07/−0.53* | 65.67/−0.40 |
| Curvilinear | ns | ns | ns | |
| Alanine aminotransferase (ALT), U/L | ||||
| N | 10,820 | 10,820 | 9987 | |
| By Decile | Linear | 15.08/0.36* | 12.14/0.47* | 14.24/0.52* |
| Curvilinear | ns | ns | ns | |
| By g/day | Linear | 14.46/0.45* | 13.25/0.68* | 13.94/0.93* |
| Curvilinear | 16.10/−1.14/0.21# | ns | ns | |
| Aspartate aminotransferase (AST), U/L | ||||
| N | 10,819 | 10,819 | 9986 | |
| By Decile | Linear | 22.45/0.48* | 19.35/0.50* | 21.06/0.45* |
| Curvilinear | ns | 23.03/−0.75/0.11# | 21.92/−0.35/0.09# | |
| By g/day | Linear | 21.66/0.57* | 20.30/0.80* | 20.47/0.94* |
| Curvilinear | 23.50/−1.22/0.23# | 23.07/−0.75/0.18# | ns | |
| Gamma glutamyl transferase (GGT), U/L | ||||
| N | 10,858 | 10,858 | 10,025 | |
| By Decile | Linear | 7.97/2.12* | −6.88/2.38* | 0.44/2.14* |
| Curvilinear | ns | 3.35/−1.07/0.30# | 4.23/−1.37/0.39# | |
| By g/day | Linear | 4.36/2.60* | −1.90/3.66* | −1.95/4.33* |
| Curvilinear | 10.81/−3.66/0.82# | ns | ns | |
| Bilirubin (mg/dl) | ||||
| N | 10,854 | 10,854 | 10,021 | |
| By Decile | Linear | 1.11/0.002 | 1.10/0.002 | 1.07/0.004 |
| Curvilinear | ns | ns | ns | |
| By g/day | Linear | 1.11/0.003 | 1.11/0.002 | 1.07/0.01 |
| Curvilinear | ns | ns | ns | |
aRegression analyses examining linear and/or quadratic effect of alcohol intake conducted with the following covariates: age, race/ethnicity, current smoking status (yes/no), poverty income ratio, physical activity level (sedentary, moderate, or vigorous based on feedback on questions on activity), energy intake, and body mass index. bβ0/β1 for Linear Trend and β0/β1/β2 for Curvilinear Trend (β0: intercept, β1: linear regression coefficient, and β2: curvilinear regression coefficient);* Significant Linear Trend at P < 0.01; # Significant Curvilinear Trend at P < 0.01; N Sample Size; ns Non-significant Curvilinear Trend
Table 5.
Association of liver enzyme function with alcohol consumption level (Deciles and g/day) by three different methods of alcohol intake estimation among female adults. NHANES 2001–2010a
| 24-h recall | Usual intake by NCI method | Alcohol intake questionnaire | ||
|---|---|---|---|---|
| Regression coefficientb | ||||
| Alkaline phosphatase (ALP), U/L | ||||
| N | 10,387 | 10,387 | 9498 | |
| By Decile | Linear | 36.96/−0.21 | 38.96/−0.25 | 38.16/−0.54* |
| Curvilinear | ns | 32.91/1.73/−0.17# | ns | |
| By g/day | Linear | 37.31/−0.33 | 37.79/−0.42 | 37.73/−0.94* |
| Curvilinear | ns | ns | 41.56/−3.92/0.46# | |
| Alanine aminotransferase (ALT), U/L | ||||
| N | 10,359 | 10,359 | 9473 | |
| By Decile | Linear | 10.77/0.13 | 8.58/0.28* | 10.55/0.004 |
| Curvilinear | ns | ns | ns | |
| By g/day | Linear | 10.57/0.19 | 10.09/0.35 | 10.05/0.27 |
| Curvilinear | ns | ns | ns | |
| Aspartate aminotransferase (AST), U/L | ||||
| N | 10,358 | 10,358 | 9472 | |
| By Decile | Linear | 18.10/0.34* | 15.88/0.28* | 16.62/0.21* |
| Curvilinear | ns | ns | 17.15/−0.29/0.06# | |
| By g/day | Linear | 17.58/0.50* | 16.49/0.83* | 15.78/0.88* |
| Curvilinear | ns | ns | ns | |
| Gamma glutamyl transferase (GGT), U/L | ||||
| N | 10,387 | 10,387 | 9498 | |
| By Decile | Linear | 0.04/0.99* | −7.01/0.89* | −2.16/0.67* |
| Curvilinear | ns | ns | −0.44/−0.96/0.19# | |
| By g/day | Linear | −1.49/1.47* | −4.76/2.47* | −4.73/2.77* |
| Curvilinear | ns | ns | ns | |
| Bilirubin (mg/dl) | ||||
| N | 10,382 | 10,382 | 9493 | |
| By Decile | Linear | 0.83/0.005* | 0.81/0.002 | 0.81/0.01* |
| Curvilinear | ns | 0.86/−0.02/0.001# | ns | |
| By g/day | Linear | 0.82/0.01* | 0.81/0.01 | 0.81/0.01* |
| Curvilinear | ns | 0.73/0.06/−0.01# | 0.78/0.04/−0.004# | |
aRegression analyses examining linear and/or quadratic effect of alcohol intake conducted with the following covariates: age, race/ethnicity, current smoking status (yes/no), poverty income ratio, physical activity level (sedentary, moderate, or vigorous based on feedback on questions on activity), energy intake, and body mass index. bβ0/β1 for Linear Trend and β0/β1/β2 for Curvilinear Trend (β0: intercept, β1: linear regression coefficient, and β2: curvilinear regression coefficient); * Significant Linear Trend at P < 0.01; # Significant Curvilinear Trend at P < 0.01; N Sample Size; ns Non-significant Curvilinear Trend
There were significant effects of age (as age increased ALP decreased and AST, ALP, and GGT increased), gender (males were higher for all markers), and ethnicity (the effects varied depending on the specific marker in question) on liver markers; ALP and GGT decreased as poverty income ratio increased and there was no effect of hypertension status on liver markers (data not shown). All these effects were similar regardless of method used to assess alcohol intake. There were no significant interactions or only a few sporadic significant interactions (which may be due to chance given the large number of interactions evaluated) of alcohol intake with gender, race/ethnicity, socioeconomic status, and presence of hypertension (data not shown). However there were significant interactions of alcohol intake for all three measures of intake assessment with age for ALP and GGT. For ALP there was a positive relationship of alcohol intake up until about age 35–40 years and thereafter higher intake was associated with slightly lower ALP. Also, GGT increased as age increased (data not shown).
Discussion
Approximately 25 % of adults consume alcohol on a given day as assessed by the 24-h recall method and ~60 % adults regularly consume alcohol as assessed by the alcohol questionnaire method. Previous studies have shown that approximately 50 % of US adults are regular drinkers [2]. An analysis of data from NHANES 2003–2006 using 24-h recall also found that about 33 % of men and 17 % of women consumed some amount of an alcoholic beverage on a given day [25]. The average regular intake of alcohol observed in this study, as assessed by either by questionnaire or the usual intake NCI method, was about 11 g/d (~16 g/d for men and 6 g/d for women) and is considered moderate intake by the Dietary Guidelines for Americans, 2010 definition [2].
Effects of alcohol on liver function
To the best of our knowledge, this study provides the most current, detailed data on the association of liver enzymes with graded levels of alcohol intake in a very large (most likely the largest) representative sample of the U.S. population. By combining data collected by NHANES over the last 10 years on over 20,000 adults, quantitative estimates of the relationship between alcohol intake and multiple liver enzymes could be calculated with a very high level of sensitivity to graded changes in alcohol intake of the US population. This study demonstrates that even very modest levels of alcohol intake can significantly affect liver enzymes and the most sensitive measure of alcohol intake is the enzyme GGT which is potentiated by alcohol intake as low as 7–14 g/day.
Our data present both linear and curvilinear (quadratic) equations (when the latter are significant) that can be used to assess impact of alcohol on liver enzymes. The linear component of both sets of equations have the same direction, but the curvilinear equations have the quadratic element which modifies the direction and/or magnitude of change as alcohol intake increases. For example, both the linear elements of ALP (in both linear and curvilinear equations) are all negative, but the curvilinear component is positive which indicates as alcohol intake increases further the magnitude of the drop in ALP decreases. While the linear equations are useful, when curvilinear equations were significant these will provide a better fit of the relationship of alcohol intake with liver enzymes.
Excessive alcohol consumption can cause liver diseases including fatty liver, hepatitis and cirrhosis [26–28]. Since alcohol is mainly metabolized by the liver, it is a primary site of alcohol-induced adverse health effects. Alcohol consumers had significantly higher AST and GGT activities compared to non-consumers confirming previous findings demonstrating that alcohol intake is associated with increased hepatic enzyme activities [12, 13]. Changes in liver enzymes activities are biomarkers of liver damage and are routinely assessed for diagnostic purposes and as part of physical examinations [22–24]. Abnormal activities of liver enzymes are also strong predictors of mortality associated with liver disease, cardiovascular disease, diabetes and cancer [29–33]. However, the activities of liver enzymes AST and GGT in alcohol consumers in our study did not approach levels that would be considered clinically abnormal [34]. It may be possible that the changes in the activities of liver enzyme within the normal range are not benign and additional research is required to determine if they are associated with subsequent development of liver disease.
The association between alcohol consumption and GGT has previously been demonstrated and is a widely used index of excessive alcohol intake [12, 13, 35, 36]. Consistent with the literature, in our study serum GGT appears to be the most sensitive measure of alcohol consumption as assessed by 24-h recall, as well as alcohol questionnaire, with respect to the difference between alcohol consumers and non-consumers. Our results support previous findings that GGT is a more sensitive indicator of moderate levels of alcohol consumption than AST and ALT [12, 13, 16]. Elevated serum GGT has also been shown to be associated with metabolic syndrome [37, 38] and is considered to be the most sensitive indicator of liver disease [39]. Previous studies have suggested the presence of a graded dose–response relationship between alcohol intake and risk of liver disease [40–43] and that GGT induction can be initiated at low doses of alcohol intake [12, 13]. In the present study we noted a gradual increase in liver enzyme activities with increasing alcohol dose with the largest dose-dependent increase noted for GGT activity. This is in agreement with studies reporting a gradual effect of increasing dose of alcohol on liver enzyme induction [12, 13, 16].
The health effects of alcohol also vary across population groups. A negative dose response relationship between consumption of alcohol and prevalence of suboptimal health was reported in a cross sectional survey from Spain [44] while a curvilinear relationship (inverse J shaped) was observed between alcohol intake and health related quality of life in Dutch population [45]. Intoxication and liquor consumption were associated with poor mental and physical health while moderate intake were associated with better health in another cross sectional study conducted in New York State [46]. Future studies, including epidemiological investigations and clinical trials, should be conducted to investigate the relationship between intake of various levels of alcohol, multiple health outcomes and all-cause mortality.
The factors responsible for the beneficial effects of moderate intake of alcohol are uncertain, although the adverse effects of higher doses of alcohol on various organ systems have been well documented. Increased HDL, apolipoprtotein A-1 and adiponectin levels, and reduction of LDL concentration, blood pressure, coronary blood flow, platelet aggregation, fibrinogen levels and inflammation resulting from moderate alcohol intake have been suggested as mechanisms that could explain the beneficial effect of moderate alcohol intake [47, 48]. One additional mechanism that may explain some of the beneficial effects of lower doses of alcohol could be its effects on the liver. The liver is the organ primarily responsible for detoxifying a wide variety of metabolic and environmental toxins so consumption of low doses of alcohol could, by potentiation of key liver enzyme systems such as cytochrome P450, enhance its ability to remove toxic compounds from the body. It should be noted that levels of GGT increase with obesity but the effects of BMI, as well as other covariates, were adjusted for in our analyses [49].
Methods for assessing alcohol intake
In this study we used data from 3 methods of assessing alcohol intake, and evaluated the association of these intake estimates with liver enzyme functions. It is interesting to note that, regardless of the method used to assess alcohol intake, each with their own measurement error issues, alcohol consumption significantly altered AST, ALT & GGT. In large surveillance studies of the U.S. population, 24-h recall is the preferred method of assessing of dietary intake and determining changes in consumption patterns, and these data are used in developing regulations, policies, and dietary standards. The 24-h recall method provides as estimate of an individual’s alcohol consumption on the day of recall (acute intake) but does not capture day-to-day variation in intake and appears to substantially overestimate annual alcohol consumption. The alcohol intake questionnaire used in NHANES is designed to assess long term consumption of alcohol (during the past 12 months as well as over the lifetime) but might not accurately represent actual alcohol intake since it relies on memory and estimation of the actual amount consumed by the consumer and may be difficult for subjects to report accurately. The NCI method can estimate usual intake distributions for episodically-consumed dietary components like alcohol even when there are large proportions of zero intakes on any given day. In this study we found that the NCI method and the alcohol intake questionnaire provided very similar estimates of intake with the population means varying by only 0.1 g/d and were predictive of multiple markers of liver function, the biological measures of alcohol intake we examined. Therefore, they both appear to be useful methods to estimate alcohol intake. While various methods of alcohol assessment appear to be useful in assessing the relationship with the specific physiological measures evaluated in this study, namely liver enzymes, researchers should carefully consider specific objectives of their research and the impact of measurement error of the method used and study participant burden to provide intake information before selecting an alcohol assessment approach.
Study limitations and strengths
The limitations of our study include the inability to determine cause-effect relationship due to cross-sectional design of NHANES. Another limitation is the potential for bias in self-reported intakes since alcohol intake is often underestimated [50] and the potential for measurement error of intake instruments to influence our results. However, it should be noted that alcohol intake as estimated by the questionnaire and NCI method were quite similar and while we would expect measurement error to attenuate our ability to detect relationships, various significant relationship were still found. Other factors which may impact markers of liver function (e.g., health condition, concomitant medication use. etc.) were not considered in these analyses and therefore residual confounding may have influenced our results. A major strength of our study is the use of a large nationally representative population-based sample of adults.
Conclusions
The data presented in this study show that moderate alcohol consumption affects liver function in a dose-dependent manner. Use of data from the NHANES survey, especially when combined over multiple years, provides an opportunity to study potential adverse effects of dietary constituents, especially those thought to be associated with changes in biomarkers routinely assessed as part of the NHANES survey. In addition, this study indicated usual intakes determined using the NCI method and an alcohol intake questionnaire yield similar estimates of alcohol intakes and relationships with liver enzymes.
Abbreviations
NHANES, National Health and Nutrition Examination Survey; NCI, National Cancer Institute; NCHS, National Center for Health Statistics; CDC, Center for Disease Control and Prevention; ALP, alkaline phosphatase; ALT alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase; LS, least square; SE, standard errors
Acknowledgements
The views, opinions, and findings in this report are those of the authors and should not be construed as an official Department of Defense or Army position, policy, or decision, unless so designated by other official documentation. Citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations. The investigators have adhered to the policies for protection of human subjects as prescribed in DOD Instruction 3216.02 and the research was conducted in adherence with the provisions of 32 CFR Part 219. We thank U.S. Army Medical Research and Material Command and Department of Defense Center Alliance for Dietary Supplement Research for funding the research. This research was also supported in part by an appointment to the Research Participation Program at the U.S. Army Medical Research Institute of Environmental Medicine administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USAMRMC.
Footnotes
Competing interest
Sanjiv Agarwal and Victor L. Fulgoni, III are nutrition consultants and provide services to industry; Harris R. Lieberman – None.
Authors’ contributions
HRL – participated in formulating the research question, design, interpretation of the data, drafting the manuscript, revising the manuscript and the approval of final version; VLF, III - participated in the design, NHANES dietary data analysis, interpretation of the data, drafting the manuscript, revising the manuscript and the approval of final version; and SA – participated in interpretation of the data, drafting the manuscript, revising the manuscript and the approval of final version.
References
- 1.Breslow RA, Chen CM, Graubard BI, Jacobovits T, Kant AK. Diets of drinkers on drinking and nondrinking days: NHANES 2003–2008. Am J Clin Nutr. 2013;97:1068–75. doi: 10.3945/ajcn.112.050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. USDA . Dietary Guidelines for Americans, 2010. 7. Washington, DC: U.S. Government Printing Office; 2011. [Google Scholar]
- 3.Lichtenstein AH, Appel LJ, Brands B, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 4.Kanny D, Liu Y, Brewer RD, Lu H. Binge drinking-United States, 2011. MMWR Surveill Summ. 2013;62:s77–80. [PubMed] [Google Scholar]
- 5.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:130293. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the United States, 2006. Am J Prev Med. 2011;41:516–24. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 8.USDA Center for Nutrition Policy and Promotion . The report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010 [internet] Alexandria (VA): USDA Center for Nutrition Policy and Promotion; 2010. [Google Scholar]
- 9.Sharpe PC. Biochemical detection and monitoring of alcohol abuse and abstinence. Ann Clin Biochem. 2001;38:652–64. doi: 10.1258/0004563011901064. [DOI] [PubMed] [Google Scholar]
- 10.Niemela O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39–49. doi: 10.1016/j.cca.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Bilban M, Vrhovec S, Karlovsek MZ. Blood biomarkers of alcohol abuse. Arh Hig Rada Toksikol. 2003;54:253–9. [PubMed] [Google Scholar]
- 12.Alatalo P, Koivisto H, Puukka K, Hietala J, Anttila P, Bloigu R, et al. Biomarkers of liver status in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol. 2009;44:199–203. doi: 10.1093/alcalc/agn099. [DOI] [PubMed] [Google Scholar]
- 13.Hietala J, Puukka K, Koivisto H, Anttila P, Niemela O. Serum gamma-glutamyl transferase in alcoholics, moderate drinkers and abstainers: effect on GT reference intervals at population level. Alcohol Alcohol. 2005;40:511–4. doi: 10.1093/alcalc/agh201. [DOI] [PubMed] [Google Scholar]
- 14.Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98:31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsai J, Ford ES, Li C, Zhao G. Past and current alcohol consumption patterns and elevations in serum hepatic enzymes among U.S. adults. Addict Behav. 2012;37:78–84. doi: 10.1016/j.addbeh.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Stranges S, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH, et al. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcohol Clin Exp Res. 2004;28:949–56. doi: 10.1097/01.ALC.0000128229.23396.42. [DOI] [PubMed] [Google Scholar]
- 17.Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39:336–9. doi: 10.1093/alcalc/agh074. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: National Center for Health Statistics. Available from: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 29 Nov 2013.
- 19.Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. National Center for Health Statistics. Vital Health Stat. 2013;2(161):1–24. [PubMed] [Google Scholar]
- 20.Kipnis V, Midthune D, Buckman DW, Dodd KW, Guenther PM, Krebs-Smith SM, et al. Modeling data with excess zeros and measurement error: application to evaluating relationships between episodically consumed foods and health outcomes. Biometrics. 2009;65:1003–10. doi: 10.1111/j.1541-0420.2009.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowman SA, Clemens JC, Thoerig RC, Friday JE, Shimizu M, Moshfegh AJ. Food Patterns Equivalents Database 2009–10: Methodology and User Guide [Online]. Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture, Beltsville, Maryland. 2013. Available at: http://www.ars.usda.gov/ba/bhnrc/fsrg. Accessed 15 Jan 2015.
- 22.Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J. 2003;79:307–12. doi: 10.1136/pmj.79.932.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172:367–79. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woreta TA, Alqahtani SA. Evaluation of abnormal liver tests. Med Clin N Am. 2014;98:1–16. doi: 10.1016/j.mcna.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Guenther PM, Bowman SA, Goldman JD. Alcoholic beverage consumption by adults 21 years and over in the United States: results from the National Health and Nutrition Examination Survey, 2003–2006: Technical Report. Center for Nutrition Policy and Promotion, and Agricultural Research Service, U.S. Department of Agriculture. 2010.
- 26.Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–9. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 27.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14–32. doi: 10.1038/ajg.2009.593. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis. 2012;16:659–66. doi: 10.1016/j.cld.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. Brit Med J. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TH, Kim WR, Benson JT, Therneau TM, Melton LJ., III Serum aminotransferase activity and mortality risk in a United States community. Hepatology. 2008;47:880–7. doi: 10.1002/hep.22090. [DOI] [PubMed] [Google Scholar]
- 31.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–85. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 32.Targher G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer-a narrative review. Clin Chem Lab Med. 2010;48:147–57. doi: 10.1515/CCLM.2010.031. [DOI] [PubMed] [Google Scholar]
- 33.Kunutsor SK, Apekey TA, Seddoh D, Walley J. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:187–201. doi: 10.1093/ije/dyt192. [DOI] [PubMed] [Google Scholar]
- 34.Mayo Clinic, Liver Function Tests, updated Sept 26, 2012. http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/results/PRC-20012602. Accessed 15 Jan 2015.
- 35.Puukka K, Hietala J, Koivisto H, Anttila P, Bloigu R, Niemela O. Age-related changes on serum GGT activity and the assessment of ethanol intake. Alcohol Alcohol. 2006;41:522–7. doi: 10.1093/alcalc/agl052. [DOI] [PubMed] [Google Scholar]
- 36.Anton RF, Lieber C, Tabakoff B. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcoholism Clin Exp Res. 2002;26:1215–22. doi: 10.1097/01.ALC.0000023986.42254.F5. [DOI] [PubMed] [Google Scholar]
- 37.Rantala AO, Lilja M, Kauma H, Savolainen MJ, Reunanen A, Kesaniemi YA. Gamma-glutamyl transpeptidase and the metabolic syndrome. J Int Med. 2000;248:230–8. doi: 10.1046/j.1365-2796.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto Y, Futamura A, Nakarai H, Nakahara K. Relationship between response of γ-glutamyl transpeptidase to alcohol drinking and risk factors for coronary heart disease. Atherosclerosis. 2001;158:465–70. doi: 10.1016/S0021-9150(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 39.MAYO CLINIC. http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8677. Accessed 25 Oct 2014.
- 40.Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol. 2001;35:531–7. doi: 10.1016/S0168-8278(01)00151-9. [DOI] [PubMed] [Google Scholar]
- 41.Sherlock S. Alcoholic liver disease. Lancet. 1995;345:227–9. doi: 10.1016/S0140-6736(95)90226-0. [DOI] [PubMed] [Google Scholar]
- 42.Day CP. Alcoholic liver disease: dose and threshold-new thoughts on an old topic. Gut. 1997;41:857–8. doi: 10.1136/gut.41.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorensen TIA. Alcohol and liver injury: dose-related or permissive effect. Liver. 1989;9:189–97. doi: 10.1111/j.1600-0676.1989.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 44.Guallar-Castillon P, Rodriguez-Artalejo F, Diez Ganan LD, Banegas Banegas JR, Lafuente Urdinguio PL, Herruzo Cabrera RH. Consumption of alcoholic beverages and subjective health in Spain. J Epidemiol Community Health. 2001;55:648–52. doi: 10.1136/jech.55.9.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Dijk AP, Toet J, Verdurmen JE. The relationship between health-related quality of life and two measures of alcohol consumption. J Stud Alcohol. 2004;65:241–9. doi: 10.15288/jsa.2004.65.241. [DOI] [PubMed] [Google Scholar]
- 46.Stranges S, Notaro J, Freudenheim JL, Calogero RM, Muti P, Farinaro E, et al. Alcohol drinking pattern and subjective health in a population-based study. Addiction. 2006;101:1265–76. doi: 10.1111/j.1360-0443.2006.01517.x. [DOI] [PubMed] [Google Scholar]
- 47.Nova E, Baccan GC, Veses A, Zapatera B, Marcos A. Potential health benefits of moderate alcohol consumption: current perspectives in research. Proc Nutr Soc. 2012;71:307–15. doi: 10.1017/S0029665112000171. [DOI] [PubMed] [Google Scholar]
- 48.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. Brit Med J. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang A-C, Lin Y-C, Liu P-T, Kao Y-M, Chen J-D. Synergistic effect of gamma glutamyltransferase and obesity on metabolic syndrome, independent of hepatic steatosis. Ann Epidemiol. 2012;22:876–80. doi: 10.1016/j.annepidem.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Stockwell T, Zhao J, Macdonald S. Who under-reports their alcohol consumption in telephone surveys and by how much? An application of the ‘yesterday method’ in a national Canadian substance use survey. Addiction. 2014;109:1657–66. doi: 10.1111/add.12609. [DOI] [PubMed] [Google Scholar]


