Abstract
Introduction
Intra-articular hyaluronic acid (IA-HA) injections are a treatment for knee osteoarthritis (OA), although current literature provides mixed results with regard to their efficacy. We will review a randomized controlled trial (RCT) and subsequent extension trial in order to identify factors that are associated with outcomes in patients with knee OA who received IA-HA.
Methods
We used data recorded by the FLEXX trial and extension trial for secondary analysis of potential prognostic factors. Linear regression was used to examine the predictors of outcomes at 6- and 12-month follow-up visits.
Results
Sixty percent of all patients presented with a Kellgren Lawrence (K-L) grade 3. Patients with high baseline outcome scores and a K-L grade 3 demonstrated less response than individuals within an earlier stage of knee OA, although results for both K-L grade 2 and K-L grade 3 patients still showed benefit. Those with more severe radiographic change K-L grade 3 often had a better response with the second series of IA-HA injections. Significantly greater positive response in all outcomes was demonstrated for the patient subgroup classified as K-L grade 2, when compared with K-L grade 3 patients.
Conclusions
The results demonstrate that IA-HA for knee OA was of greater benefit in those with less severe radiographic changes. However, those with more severe radiographic change often had a better response with the second course of IA-HA. Similar analyses are required in order to determine if these results are unique to Euflexxa, or if these results are consistent with other available IA-HA agents.
Keywords: knee, joint involved, osteoarthritis, diagnosis, intraarticular delivery, therapeutic delivery
Introduction
Knee osteoarthritis (OA) is the most common form of OA and it is increasingly prevalent and symptomatic in the elderly. Progressive intra-articular (IA) changes associated with the disease may result in significant pain and disability.1 Socioeconomic implications such as cost of treatment, inability to work, and early retirement have a significant impact those affected by knee OA, and these direct and indirect costs can pose a large burden on society.2 IA hyaluronic acid (IA-HA) injections are a treatment option for knee OA that serves to replenish the decreasing viscoelastic and biochemical properties of the synovial fluid.3 Previous trials evaluating IA-HA have provided mixed results regarding efficacy; however, some evidence has shown greater therapeutic benefit for higher molecular weight IA-HA.4 Variable results in current publications have resulted in inconsistent recommendations among clinical practice guidelines regarding the use of IA-HA for the treatment for OA of the knee.5-7
Further insight into the efficacy and safety of IA-HA is needed in order to determine the appropriate role of IA-HA for each individual with knee OA. In a previous study on prognostic factors, radiographic guidance of the IA injection predicted a more positive outcome for IA-HA therapy.8 Further pre–IA injection prognostic studies need to be explored in order to identify additional prognostic factors associated with positive outcomes in patients treated with IA-HA. Conversely, identifying factors associated with a less than ideal outcome will help identify specific populations who respond less than ideally, to IA-HA. The objective of this study is to help clinicians determine the most appropriate patients for IA-HA therapy for OA of the knee.8
To help identify potential predictors of response to IA-HA, a detailed review will be performed on a previous randomized controlled trial (RCT).9 and subsequent extension trial10 of a biologically produced, high-molecular-weight HA treatment (Euflexxa, Ferring Pharmaceuticals Inc.). The initial study (FLEXX trial) randomized 588 patients with knee OA to 3 weekly IA injections of biologically derived HA (Bio-HA), or 3 weekly injections of IA saline. In the initial 26 week study, the Bio-HA group showed significantly greater improvement in the measured outcomes with a low incidence of adverse events.9 The 26-week extension trial demonstrated the safety and supported the efficacy of reinjection of Bio-HA after an additional 26 weeks.10 Both trials will be examined in an attempt to identify factors that may predict either a successful or less successful outcome in patients who received IA-HA.
Methods
The FLEXX Trial and Extension Trial
The FLEXX trial was conducted at 36 sites in the United States in accordance with the Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. The study took place from October 2006 to May 2008. Patients were included based on the following criteria: OA of the knee by American College of Rheumatology criteria; moderate to severe pain score of 41 to 90 mm recorded on 100 mm visual analog scale (VAS) immediately following a 50-foot walk; bilateral standing anterior-posterior radiograph demonstrating Kellgren and Lawrence (K-L) grade 2 or 3 OA (Grade 2: definite presence of osteophytes and definite joint space narrowing; Grade 3: multiple osteophytes present, definite joint space narrowing, some sclerosis, and possible bone deformity) of the target knee; ability and willingness to use only acetaminophen as the analgesic (rescue) study medication; unassisted walking 50 feet on a flat surface and going up and down stairs; and willingness and ability to complete efficacy and safety questionnaires.9 All individuals within the FLEXX trial were eligible for inclusion within the extension trial.10
Prognostic Factors
We used data recorded by the FLEXX trial and extension trial for secondary analysis of potential prognostic factors. Baseline variables included in the analyses were treatment received, age, body mass index (BMI), sex, alcohol use, smoking status, severity of osteoarthritis (K-L grading scale), OA in contralateral knee, history of physical therapy, history of steroid injection, history of other injection, history of arthrocentesis, history of nonsteroidal anti-inflammatory drug (NSAID) treatment, baseline VAS pain, and Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain, stiffness, and disability scores. The outcome measures analyzed were VAS pain score recorded after a 50-foot walk, WOMAC pain, stiffness and disability subscale scores and global VAS pain score. Higher scores indicate worse outcomes on all of the outcome measures.
Data Analysis
Descriptive statistics were performed for the recorded baseline variables. Continuous variables were reported as a mean with standard deviation and compared using independent-samples t test. Categorical variables were reported as frequencies and relative frequencies and compared using a chi-squared test. Multivariable linear regression was used to examine the predictors of pain outcomes at 6- and 12-month follow-up visits. Coefficients with 95% confidence intervals (CIs) are reported. Distribution of the residuals and scatterplots of residuals against predicted values were examined for goodness-of-fit of the model. Multivariable logistic regression was performed for OMERACT OARSI response, and odds ratios with 95% CIs for the predictors and P value for the Hosmer-Lemeshow goodness of fit were reported. Means with 95% CIs were plotted for visual interpretation of the pain outcomes over time. Boxplots were plotted for the visual interpretation of the change in pain outcomes by Kellgren-Lawrence (K-L) grade. A P value of 0.05 was considered for statistical significance. SPSS software (www.IBM.com) was used for analysis.
Results
Demographics and Outcome Scores
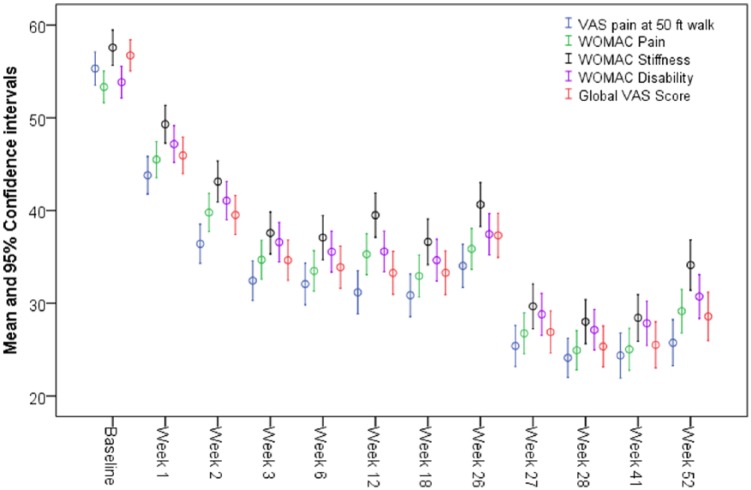
The mean age of the participants was 61.5 years. On all, 63% of patients included in the study were women and 60% of all patients presented with a K-L grade 3 ( Table 1 ). The mean primary outcome measure of pain after the 50-foot walk test for all patients was 55.1 on a 100-mm VAS scale at the time of randomization. This mean score decreased to 34 at 6 months, and to 25.6 at 12 months. The mean scores of secondary outcomes showed a similar decrease over the follow-up period. The scores from all of the outcome measures decreased within the first 3 weeks posttreatment, and plateaued until the 26-week follow-up. After receiving further treatment at week 26, a further decrease in scores was seen from weeks 26 to 40. After week 41, the outcome scores began to increase until the 52-week follow-up ( Fig. 1 ).
Table 1.
Patient Characteristics.
| Saline (n = 295) | Euflexxa (n = 293) | Total (n = 588) | |
|---|---|---|---|
| Age, years, mean (SD) | 60.75 (10.3) | 62.5 (10.6) | 61.5 (10.5) |
| BMI, kg/m2, mean (SD) | 33.0 (7.4) | 32.3 (7.4) | 32.6 (7.4) |
| Current smoker, n (%) | 40 (13.5) | 34 (11.5) | 74 (12.5) |
| Previously smoked, n (%) | 84 (28.5) | 101 (34.5) | 185 (31.5) |
| Never smoked, n (%) | 171 (58.0) | 158 (54.0) | 329 (56.0) |
| Sex, n (%) | |||
| Male | 109 (37.0) | 108 (37.0) | 217 (37.0) |
| Female | 186 (63.0) | 185 (63.0) | 371 (63.0) |
| Target knee, n (%) | |||
| Left | 158 (53.6) | 147 (50.2) | 305 (51.9) |
| Right | 137 (46.4) | 146 (49.8) | 283 (48.1) |
| Kellgren-Lawrence grade, n (%) | |||
| 2 | 115 (39.0) | 120 (41.0) | 235 (40.0%) |
| 3 | 180 (61.0) | 173 (59.0) | 353 (60.0%) |
| Alcohol consumption, n (%) | 133 (45.0) | 124 (42.3) | 257 (43.7%) |
| Baseline VAS score (mm) at 50 ft walk, mean (SD) | 54.6 (21.8) | 55.6 (22.0) | 55.1 (21.9) |
| Baseline WOMAC score, mean (SD) | |||
| Pain | 54.0 (20.3) | 52.3 (21.8) | 53.2 (21.0) |
| Stiffness | 57.7 (21.6) | 57.4 (25.0) | 57.6 (23.3) |
| Disability | 53.5 (20.2) | 53.8 (21.6) | 53.6 (21.0) |
| Baseline global VAS score, mean (SD) | 57.2 (19.7) | 56.4 (21.6) | 56.8 (20.7) |
| Baseline SF-36 score, mean (SD) | |||
| Pain | 49.8 (22.0) | 50.2 (21.0) | 50.0 (21.5) |
| Function | 45.3 (24.0) | 42.3 (25.2) | 43.9 (24.6) |
| Health survey | 47.5 (19.7) | 46.2 (20.4) | 47.0 (20.0) |
| Mean acetaminophen use, mean (SD) | 18.6 (28.0) | 15.0 (18.7) | 16.8 (23.7) |
BMI = body mass index; SF-36 = Short Form–36; VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Figure 1.
Mean Outcome Scores over Time.
A demographic comparison between patients with a K-L grade 2 (n = 235) and a K-L grade 3 (n = 353) demonstrated a significant difference in mean age (K-L grade 2, 59.28 ± 10.05 years vs. K-L grade 3, 63.20 ± 10.51 years, P < 0.001). There was also significant difference in mean BMI between K-L grade2 and K-L grade 3 (31.76 ± 7.31 vs 33.31 ± 7.48 kg/m2, respectively; P = 0.014). All other demographic data were similar between patients with a K-L grade 2 and those with a K-L grade 3, including baseline outcome scores for target knee VAS pain at the 50-feet walk, WOMAC pain, stiffness, and disability, and global VAS assessment ( Table 2 ).
Table 2.
Baseline Characteristics by Kellgren-Lawrence (K-L) Grades.
| K-L Grade 2 (n = 235) | K-L Grade 3 (n = 353) | P | |
|---|---|---|---|
| Age, years, mean (SD) | 59.28 (10.05) | 63.20 (10.51) | <0.001 |
| BMI, kg/m2, mean (SD) | 31.76 (7.31) | 33.31 (7.48) | 0.014 |
| Ever smoked, n (%) | 101 (43.00) | 158 (44.80) | 0.671 |
| Current smoker, n (%) | 32 (13.60) | 42 (11.90) | 0.539 |
| Men, n (%) | 154 (65.50) | 217 (61.50) | 0.316 |
| Alcohol consumption, n (%) | 109 (46.40) | 148 (41.90) | 0.287 |
| Target knee VAS pain at 50-ft walk, mean (SD) | 56.17 (21.26) | 54.43 (22.40) | 0.349 |
| WOMAC pain, mean (SD) | 54.90 (21.76) | 52.08 (20.56) | 0.114 |
| WOMAC stiffness, mean (SD) | 57.80 (25.00) | 57.47 (922.27) | 0.866 |
| WOMAC disability, mean (SD) | 54.15 (21.87) | 53.37 (20.33) | 0.660 |
| Global VAS, mean (SD) | 57.47 (20.21) | 56.39 (21.03) | 0.538 |
BMI = body mass index; VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Demographic and Disease Severity Prognostic Factors
Patients who received a saline injection had significantly higher (P = 0.029) VAS pain score after the 50-foot walk at 6-month follow-up than patients who received Euflexxa. Patients who had a higher baseline VAS pain score or a K-L grade 3 had a higher VAS pain score after the 50-foot walk at 6- and 12-month follow-up than patients who had a low baseline VAS score or a K-L grade 2. Older patients had a slightly more successful response to treatment with regard to VAS pain score after a 50-foot walk at 6- and 12-month follow-up than younger patients (coefficient = −0.3, P = 0.029). Women had a lower response than men at 6-month follow-up (coefficient = 7.1, P = 0.021), yet still significant. Patients with OA in the contralateral knee demonstrated significantly better results at 12-month follow-up with regard to VAS pain score after the 50-foot walk in the treated knee ( Table 3 ).
Table 3.
Factors Associated with a High VAS Pain Score After a 50-Foot Walk at 6- and 12-Month Follow-up.a
| Coefficient (95% CI) | P | |
|---|---|---|
| At 6 months | ||
| Saline injection | 6.2 (0.6, 12.0) | 0.029 |
| Baseline VAS pain score (mm) at 50 ft walk | 0.2 (0.1, 0.4) | 0.001 |
| Kellgren-Lawrence grade 3 | 7.5 (1.5, 13.5) | 0.014 |
| Females | 7.1 (1.1, 13.1) | 0.021 |
| Age | −0.3 (–0.6, –0.03) | 0.029 |
| History of steroid treatment | 6.8 (0.1, 13.4) | 0.044 |
| History of arthrocentesis | −13.3 (–25.6, –1.1) | 0.033 |
| At 12 months | ||
| Baseline VAS pain score (mm) at 50 ft walk | 0.3 (0.1, 0.4) | <0.001 |
| Kellgren-Lawrence grade 3 | 8.7 (2.5, 15.0) | 0.009 |
| Age | −0.3 (–0.6, –0.02) | 0.033 |
| OA in contralateral knee | −6.6 (–13.0, –0.2) | 0.042 |
CI = confidence interval; OA = osteoarthritis; VAS, visual analog scale.
Variables entered into the models were treatment received, sex, Kellgren-Lawrence grade, age, body mass index (BMI), smoking status, alcohol consumption, OA in contralateral knee, history of physical therapy, history of steroid injection, history of other injection, history of arthrocentesis, history of nonsteroidal anti-inflammatory drug (NSAID) treatment, and baseline VAS pain after 50-foot walk.
A higher baseline WOMAC pain score, and a K-L grade 3 were associated with a higher WOMAC pain score at 6- and 12-month follow-up ( Table 4 ). Saline injection and women were also associated with a higher WOMAC pain score at 6-month follow-up only ( Table 4 ). A higher baseline WOMAC stiffness score was associated with a higher WOMAC stiffness score at 6 months. A higher baseline WOMAC stiffness score, and a K-L grade 3 were both associated with higher WOMAC stiffness scores at 6- and 12-month follow-up ( Table 5 ). A higher baseline WOMAC disability and a K-L grade 3 were also associated with a higher WOMAC disability score at 6- and 12-month follow-up, and saline injection and women were associated with higher scores at 6-month follow-up ( Table 6 ). Saline was not provided within the extension trial, so the results could not be assessed at 12 months. A higher global VAS score and a K-L grade 3 were associated with higher global VAS scores at both 6- and 12-month follow-up. Additionally, younger patients and patients with unilateral knee OA were associated with slightly higher global VAS scores at 12-month follow-up only ( Table 7 ).
Table 4.
Factors Associated with a High WOMAC Pain Scores at 6- and 12-Month Follow-up.
| Coefficient (95% CI) | P | |
|---|---|---|
| At 6 months | ||
| Baseline WOMAC pain score | 0.4 (0.3, 0.5) | <0.001 |
| Kellgren-Lawrence grade 3 | 6.7 (1.4, 12.0) | 0.013 |
| Saline injection | 5.6 (0.5, 10.8) | 0.031 |
| Females | 5.5 (–0.1, 11.1) | 0.052 |
| At 12 months | ||
| Baseline WOMAC pain score | 0.38 (0.27, 0.50) | <0.001 |
| Kellgren-Lawrence grade 3 | 9.3 (3.6, 15.0) | 0.001 |
CI = confidence interval; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Variables entered into the models were treatment received, sex, Kellgren-Lawrence grade, age, BMI, smoking status, alcohol consumption, osteoarthritis (OA) in contralateral knee, history of physical therapy, history of steroid injection, history of other injection, history of arthrocentesis, history of nonsteroidal anti-inflammatory drug (NSAID) treatment, and baseline WOMAC pain score.
Table 5.
Factors Associated with a High WOMAC Stiffness Scores at 6- and 12-Month Follow-up.a
| Coefficient (95% CI) | P | |
|---|---|---|
| At 6 months | ||
| Baseline WOMAC stiffness score | 0.4 (0.2, 0.5) | <0.001 |
| Kellgren-Lawrence grade 3 | 7.0 (1.2, 12.7) | 0.018 |
| History of steroid injection | 6.8 (0.2, 13.5) | 0.044 |
| History of arthrocentesis | −16.9 (–29.0, –4.7) | 0.007 |
| At 12 months | ||
| Baseline WOMAC stiffness score | 0.4 (0.2, 0.5) | <0.001 |
| Kellgren-Lawrence grade 3 | 10.6 (4.2, 17.0) | 0.001 |
CI = confidence interval; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Variables entered into the models were treatment received, sex, Kellgren-Lawrence grade, age, body mass index (BMI), smoking status, alcohol consumption, osteoarthritis (OA) in contralateral knee, history of physical therapy, history of steroid injection, history of other injection, history of arthrocentesis, history of nonsteroidal anti-inflammatory drug (NSAID) treatment, and baseline WOMAC stiffness score.
Table 6.
Factors Associated with a High WOMAC Disability Scores at 6- and 12-Month Follow-up.a
| Coefficient (95% CI) | P | |
|---|---|---|
| At 6 months | ||
| Baseline WOMAC disability score | 0.5 (0.4, 0.6) | <0.001 |
| Kellgren-Lawrence grade 3 | 6.0 (1.0, 11.0) | 0.021 |
| Saline injection | 5.5 (0.6, 10.4) | 0.027 |
| Females | 5.6 (0.3, 11.0) | 0.038 |
| At 12 months | ||
| Baseline WOMAC disability score | 0.45 (0.33, 0.5) | <0.001 |
| Kellgren-Lawrence grade 3 | 8.1 (2.7, 13.6) | 0.004 |
CI = confidence interval; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Variables entered into the models were treatment received, sex, Kellgren-Lawrence grade, age, body mass index (BMI), smoking status, alcohol consumption, osteoarthritis (OA) in contralateral knee, history of physical therapy, history of steroid injection, history of other injection, history of arthrocentesis, history of nonsteroidal anti-inflammatory drug (NSAID) treatment, and baseline WOMAC disability score.
Table 7.
Factors Associated with a High Global VAS Assessment Scores at 6- and 12-Month Follow-up.a
| Coefficient (95% CI) | P | |
|---|---|---|
| At 6 months | ||
| Baseline global VAS pain score (mm) | 0.5 (0.3, 0.6) | <0.001 |
| Kellgren-Lawrence grade 3 | 7.5 (1.8, 13.0) | 0.010 |
| Arthrocentesis | −14.7 (–26.2, –3.2) | 0.012 |
| At 12 months | ||
| Baseline global VAS pain score (mm) | 0.4 (0.3, 0.6) | <0.001 |
| Kellgren-Lawrence grade 3 | 10.0 (3.7, 16.6) | 0.002 |
| Age | −0.3 (–0.6, –0.01) | 0.026 |
| OA in contralateral knee | −7.5 (–14.1, –0.9) | 0.026 |
CI = confidence interval; OA = osteoarthritis; VAS = visual analog scale.
Variables entered into the models were treatment received, sex, Kellgren-Lawrence grade, age, body mass index (BMI), smoking status, alcohol consumption, OA in contralateral knee, history of physical therapy, history of steroid injection, history of other injection, history of arthrocentesis, history of nonsteroidal anti-inflammatory drug (NSAID) treatment, and baseline global VAS assessment score.
Treatment History Prognostic Factors
Patients who had previously received steroid injection treatment prior to IA-HA administration demonstrated significantly higher (P = 0.044) VAS scores after a 50-foot walk test at 6-month follow-up, while patients who had previously received arthrocentesis demonstrated significantly lower (P = 0.033) VAS scores after a 50-foot walk test at 6-month follow-up ( Table 3 ). Similarly, patients who had previously received steroid treatment (n = 107) prior to IA-HA administration demonstrated significantly higher WOMAC stiffness scores at 6-month follow-up, and patients who had previously received arthrocentesis (n = 24) demonstrated significantly lower WOMAC stiffness scores ( Table 5 ) and global VAS assessment scores ( Table 7 ) at 6-month follow-up. A significantly greater proportion of patients who were previously treated with steroid injection had a K-L grade 3 than patients who had not received previous steroid injection (71.96% vs. 56.5%, P = 0.004), which is a factor affecting the aforementioned results regarding the results of the steroid injection group.
OMERACT-OARSI Responders Analysis
The calculated odds ratios demonstrated a positive association between receiving Euflexxa injection and OMERACT-OARSI responders for VAS pain after the 50-foot walk and WOMAC pain subscale score at 6-month follow-up. It was shown that patients who received Euflexxa had 1.5 times better OMERACT-OARSI responders than patients who received saline for both VAS pain after 50-foot walk and WOMAC pain score. At 12-month follow-up, the only predictor of OMERACT-OARSI responders was high baseline VAS pain score after the 50-foot walk, as patients with a higher baseline score were more likely to categorize as an OMERACT-OARSI responder at the end of the extension trial ( Table 8 ).
Table 8.
OMERACT-OARSI Responders.a
| Odds Ratio (95% CI) | P | |
|---|---|---|
| 50-ft walk VAS pain at 6 months | ||
| Euflexxa injection | 1.6 (1.0, 2.5) | 0.033 |
| Hosmer-Lemeshow goodness-of-fit P = 1.00 | ||
| 50 ft walk VAS pain at 12 months | ||
| Baseline VAS pain score | 1.01 (1.0, 1.03) | 0.024 |
| Hosmer-Lemeshow goodness-of-fit P = 0.903 | ||
| WOMAC pain at 6 months | ||
| Euflexxa injection | 1.5 (1.0, 2.3) | 0.046 |
| Hosmer-Lemeshow goodness-of-fit P = 1.00 |
CI = confidence interval; VAS, visual analog scale; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Variables entered into the models were treatment received, sex, Kellgren-Lawrence grade, age, body mass index (BMI), smoking status, alcohol consumption, osteoarthritis (OA) in contralateral knee, history of physical therapy, history of steroid injection, history of other injection, history of arthrocentesis, history of nonsteroidal anti-inflammatory drug (NSAID) treatment, and baseline VAS, WOMAC pain, WOMAC stiffness, WOMAC disability, or global VAS assessment score.
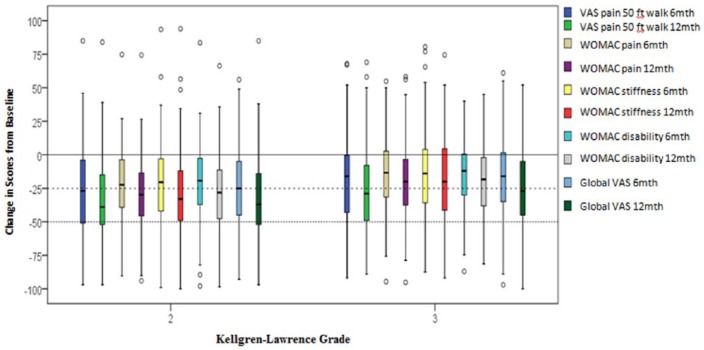
Mean Change in Outcome Scores by K-L Grades
Significantly greater reduction in all outcome assessments was demonstrated for the patient subgroup classified as K-L grade 2, when compared to patients with a K-L grade 3. These significantly greater differences in mean score reduction were demonstrated at both 6 and 12 months for VAS pain at 50-foot walk, WOMAC pain, stiffness, and disability subscales, and global VAS assessment ( Table 9 , Fig. 2 ). Figure 2 demonstrates that the mean score for all pain and functional assessments for patients with K-L grade 2 or K-L grade 3 was reduced at 6- and 12-month follow-up times.
Table 9.
Mean Change in Outcome Scores for Kellgren-Lawrence (K-L) Grades at 6 and 12 Months.
| K-L Grade 2 (n = 235), Mean (95% CI) | K-L Grade 3 (n = 353), Mean (95% CI) | P | |
|---|---|---|---|
| Mean change in VAS pain target knee | |||
| 6 months | −24.87 (−28.81, −20.93) | −17.19 (−20.55, −13.82) | 0.004 |
| 12 months | −34.24 (−38.49, −29.98) | −27.22 (−30.94, −23.51) | 0.016 |
| Mean change in WOMAC pain | |||
| 6 months | −21.08 (−24.73, −17.43) | −13.22(−16.01, −10.43) | 0.001 |
| 12 months | −29.44 (−33.26, −25.62) | −19.62 (−22.94, −16.31) | <0.001 |
| Mean change in WOMAC stiffness | |||
| 6 months | −20.02 (−23.90, −16.15) | −14.09 (−17.36, −10.82) | 0.023 |
| 12 months | −30.07 (–34.40, −25.75) | −19.46 (−23.28, −15.63) | <0.001 |
| Mean change in WOMAC disability | |||
| 6 months | −19.39 (−22.88, −15.89) | −13.38 (−15.98, −10.78) | 0.006 |
| 12 months | −28.55 (−32.42, −24.68) | −19.86 (−22.96, −16.76) | 0.001 |
| Mean change in global VAS assessment | |||
| 6 months | −23.21 (−27.08, −19.34) | −15.73 (−18.84, −12.61) | 0.003 |
| 12 months | −33.29 (−37.58, −29.01) | −24.94 (−28.52, −21.36) | 0.003 |
CI = confidence interval; VAS, visual analog scale; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Figure 2.
Mean change in pain and functional outcome assessments for Kellgren-Lawrence (K-L) grades at 6 and 12 months.
Discussion
It is apparent that the pain and functional scores decreased continually until the first 6 weeks and then somewhat increased by the end of the FLEXX trial, with mean reductions of 25.7 and 19.5 mm from baseline, respectively.9 Similar trends of changes in the pain and function scores was shown for the extension trial, with additional mean reductions of 5.6 and 3.5 mm, respectively, although patients who participated in the extension trial started with lower mean pain and functional scores than the final visit of the FLEXX trial.10
In these studies of IA Bio-HA for OA of the knee, patients with K-L grade 2 and lower baseline demographics and outcome measures did better than those with K-L grade 3 and high baseline data, although both groups showed benefit. This suggests that those with less severe OA, and perhaps earlier disease, of the knee tend to have better responses to Bio-HA.
Previous publications have suggested a reduction of >19.9 mm (100 mm VAS) to be clinically meaningful.11,12 This minimum clinically important difference was demonstrated by the 6-month follow-up in those with K-L grade 2, while reinjection at the 12-month follow-up was needed for those with K-L grade 3 to reach the minimum clinically important difference. For K-L grade 2 patients, a mean reduction of 25 scores was consistent for all outcome assessments, while this was not apparent for K-L grade 3 patients. These results are consistent with, and may partially explain, the results of the AMELIA trial, where some patients required more than one series of IA-HA injections to achieve benefit.13
Previous research has found that IA-HA treatment provides greater therapeutic benefit in the treatment of less severe OA in comparison with the treatment of more advanced or “late-stage” OA.14,15 However, in other studies, disease severity was not specifically correlated to the efficacy of IA-HA treatment.8 Our analyses agree with the former, as patients with a K-L grade 3 demonstrated higher values in specified outcome scores than patients with a K-L grade 2. Patients with a K-L grade 2 and K-L grade 3 had similar baseline outcome scores; however, a larger change from baseline was demonstrated across all outcome scores for patients with a K-L grade 2, when compared with those with a K-L grade 3.
Analyses presented within this report demonstrate that gender and age may also play a role in the outcome of IA-HA treatment. The analyses found that females had specific higher assessment scores at 6- and 12-month follow-up. The weak correlation between a younger age and a slightly worse response is not entirely clear, as our findings consistently suggested that treatment was significantly more successful for patients with earlier stages of OA (K-L grade 2 vs. K-L grade 3). The association to a greater response for all outcome variables is more closely correlated to K-L grade than to age; demonstrating that K-L grade is likely to be the prominent prognostic factor with respect to these two variables. The correlation between age and gender is not apparent within the current literature, making these findings of interest for future research to provide additional reporting of the potential correlation between age, gender, and IA-HA treatment outcomes. Patients with OA in the contralateral knee also demonstrated some significantly lower assessment scores at 12-month follow-up. This may suggest that the benefit of IA-HA was more obvious to the patient when the patient was able to compare the treated to the untreated knee with OA. These results differ from results of Orthovisc trials for FDA approval,16 in which patients with contralateral knee pain demonstrated significantly worse results in a post hoc subgroup analysis, and were subsequently excluded from further analyses.17 This comparison further illustrates the variability in results based on products and their intrinsic characteristics, alluding to the notion that all HA products do not perform similarly.
This study is strengthened by its in-depth and thorough analysis of prognostic factors for the treatment of knee OA with IA-HA, specifically Euflexxa. This exploration of the FLEXX trail and extension database9,10 for prognostic factors provides insight into the situations into where IA-HA treatment may be of greater benefit to patients, and should be considered in future IA-HA trial analyses to reinforce the findings from this report. This study is limited by its use of a single trial database. Because of the nature of the study design, we were only able to analyze the data that were originally collected. This prevented us from exploring radiographic factors that may provide prognostic value. Similar analyses are required for a variety of IA-HA products in order to determine the appropriateness of these results, as they may be specific to this study and not to IA-HA treatments in general.
Additionally, patients who had previously received steroid injections responded less to Bio-HA treatment, while patients who had previously received arthrocentesis demonstrated greater response to Bio-HA treatment. However, these findings are also not entirely clear, as we do not have data regarding the indication or frequency of use with respect to patients previously treated with steroid injection, such as the higher number of K-L grade 3 grade patients in this group, as well as other potential demographic differences between patients who had and had not previously received steroid injection or arthrocentesis. These results should be further investigated in future studies, as it would be of importance for health care professionals to understand how steroid injections or arthrocentesis affect the outcomes of future treatment using Bio-HA. It is important to understand potential prognostic factors when considering IA-HA as a treatment option in order to provide patients with an advantageous and informed treatment decision.
Conclusions
The results demonstrate that IA-HA for OA of the knee was of greater benefit in those with less severe radiographic changes. However, those with more severe radiographic change often had a better response with the second course of IA-HA. Similar analyses are required in order to determine if these results are unique to Euflexxa, or if these results are consistent with other available IA-HA agents.
Footnotes
Acknowledgments and Funding: This project was funded by Ferring Pharmaceuticals Inc.
Trial Registration: Not applicable.
Declaration of Conflicting Interests: Roy Altman: Consultant for: Cytori, Dupuy, Ferring, Flexion, Iroko, McNeil, Novartis, Oletec, Pfizer, Q-Med, Rottapharm, Strategic Science & Technologies, Eva. Forough Farrokhyar: No conflicts of interest to declare Anke Fierlinger: Paid employee of Ferring Inc. Faizan Niazi: Paid employee of Ferring Inc. Jeffrey Rosen: Part of Ferring’s speaker bureau and scientific advisory board.
Ethical Approval: Ethical approval was not sought for the present study because all data was obtained from a previously conducted trial. No patients were directly enrolled within this investigation.
Informed Consent: Informed consent was not sought for the present study because all data was obtained from a previously conducted trial. No patients were directly enrolled within this investigation.
References
- 1. Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs. 2012;26(4):257-68. [DOI] [PubMed] [Google Scholar]
- 2. Trigkilidas D, Anand A. The effectiveness of hyaluronic acid intra-articular injections in managing osteoarthritic knee pain. Ann R Coll Surg Engl. 2013;95(8):545-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(2):154-62. [DOI] [PubMed] [Google Scholar]
- 4. Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180-91. [DOI] [PubMed] [Google Scholar]
- 5. Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: Treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am. 2013;95(20):1885-1886. [DOI] [PubMed] [Google Scholar]
- 6. NICE. Osteoarthritis: care and management in adults. Clinical guideline CG177 methods, evidence and recommendations. 2014. [Google Scholar]
- 7. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-88. [DOI] [PubMed] [Google Scholar]
- 8. Conrozier T, Mathieu P, Schott A-M, Laurent I, Hajri T, Crozes P, et al. Factors predicting long-term efficacy of Hylan GF-20 viscosupplementation in knee osteoarthritis. Joint Bone Spine. 2003;70(2):128-33. [DOI] [PubMed] [Google Scholar]
- 9. Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA® for treatment of painful osteoarthritis of the knee, with an open-label safety extension (The FLEXX Trial). Semin Arthritis Rheum. 2009;39(1):1-9. [DOI] [PubMed] [Google Scholar]
- 10. Altman RD, Rosen JE, Bloch DA, Hatoum HT. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: results of the open-label Extension Study of the FLEXX Trial. Osteoarthritis Cartilage. 2011;19(10):1169-75. [DOI] [PubMed] [Google Scholar]
- 11. Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Navarro-Sarabia F, Coronel P, Collantes E, Navarro FJ, de la Serna AR, Naranjo A, et al. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70(11):1957-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012;13(6):740-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jubb R, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomized, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract. 2003;57(6):467-74. [PubMed] [Google Scholar]
- 16. Anika Therapeutics. Summary of Safety and Effectiveness Data: US FDA; 2004: http://www.accessdata.fda.gov/cdrh_docs/pdf3/P030019b.pdf.
- 17. Kuo JW. Practical aspects of hyaluronan based medical products. Boca Raton, FL: CRC Press; 2005. [Google Scholar]