Abstract
Background
C5–C8 medium-chain carboxylic acids are valuable chemicals as the precursors of various chemicals and transport fuels. However, only a few strict anaerobes have been discovered to produce them and their production is limited to low concentrations because of product toxicity. Therefore, a bacterial strain capable of producing high-titer C5–C8 carboxylic acids was strategically isolated and characterized for production of medium chain length carboxylic acids.
Results
Hexanoic acid-producing anaerobes were isolated from the inner surface of a cattle rumen sample. One of the isolates, displaying the highest hexanoic acid production, was identified as Megasphaera sp. MH according to 16S rRNA gene sequence analysis. Megasphaera sp. MH metabolizes fructose and produces various medium-chain carboxylic acids, including hexanoic acid, in low concentrations. The addition of acetate to the fructose medium as an electron acceptor increased hexanoic acid production as well as cell growth. Supplementation of propionate and butyrate into the medium also enhanced the production of C5–C8 medium-chain carboxylic acids. Megasphaera sp. MH produced 5.7 g L−1 of pentanoic acid (C5), 9.7 g L−1 of hexanoic acid (C6), 3.2 g L−1 of heptanoic acid (C7) and 1.2 g L−1 of octanoic acid (C8) in medium supplemented with C2–C6 carboxylic acids as the electron acceptors. This is the first report on the production of high-titer heptanoic acid and octanoic acid using a pure anaerobic culture.
Conclusion
Megasphaera sp. MH metabolized fructose for the production of C2–C8 carbon-chain carboxylic acids using various electron acceptors and achieved a high-titer of 9.7 g L−1 and fast productivity of 0.41 g L−1 h−1 for hexanoic acid. However, further metabolic activities of Megaspahera sp. MH for C5–C8 carboxylic acids production must be deciphered and improved for industrially relevant production levels.
Electronic supplementary material
The online version of this article (doi:10.1186/s13068-016-0549-3) contains supplementary material, which is available to authorized users.
Keywords: Megasphaera sp. MH, Pentanoic acid, Hexanoic acid, Heptanoic acid, Octanoic acid, Fermentation
Background
Medium-chain carboxylic acids have 5–8 carbon chains, such as pentanoic acid (valeric acid), hexanoic acid (caproic acid), heptanoic acid (enanthic acid), and octanoic acid (caprylic acid), which can be used as platform chemicals for a broad range of organic building blocks [1]. However, production of these carboxylic acids has been rarely reported and only at low-titers because of product inhibition [2, 3].
Biological production of hexanoic acid has been reported for a few strict anaerobic bacteria. Clostridium kluyveri produced hexanoic acid from ethanol [4], a mixture of cellulose and ethanol [5] and from ethanol and acetate [6]. Strain BS-1, classified as a Clostridium cluster IV, produced hexanoic acid when cultured on galactitol [7]. Megasphaera elsdenii produced a diverse mixture of carboxylic acids such as formic acid, acetic acid, propionic acid, butyric acid, pentanoic acid, and hexanoic acid from glucose and lactate [8] and sucrose and butyrate [9]. It is postulated that hexanoic acid is produced by two consecutive condensation reactions: the first is the formation of butyric acid from two acetyl-CoAs, and the second is the formation of hexanoic from one butyryl-CoA and one acetyl-CoA [10]. The condensation reaction of two acetyl-CoAs to butyric acid has been well reported in Clostridium spp. such as Clostridium pasteurianum, C. acetobutylicum, and C. kluyveri [11–13] (Fig. 1a). The second condensation reaction was demonstrated in a metabolically engineered Escherichia coli, expressing a beta-ketothiolase (carbon–carbon bond formation) for production of hexanoic acid [14], but is yet to be demonstrated in anaerobic hexanoic acid-producing bacteria (Fig. 1b).
Fig. 1.
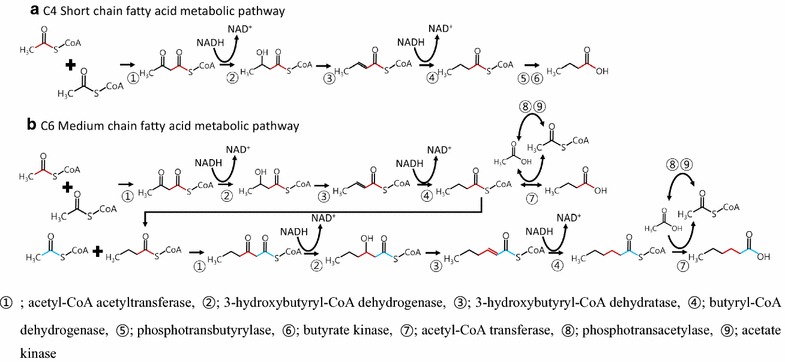
The microbial metabolic pathway for carbon-chain elongation such as a butyric acid (C4) production by the genera Clostridium and Butyrivibrio [27] and b hexanoic acid production postulated in Megasphaera elsdenii and Clostridium kluyveri [10]
Clostridium kluyveri produced hexanoic acid using either acetate or succinate as electron acceptors [6], and M. elsdenii produced butyric acid with the addition of acetate [15–18].
In this study, we isolated a hexanoic acid producing rumen bacterium using a medium supplemented with hexanoic acid. After the isolation of the hexanoic acid producer, its taxonomy was identified using 16S rRNA gene sequence analysis, and productions of C5, C6, C7, and/or C8 medium-chain carboxylic acids by the isolate were studied in media with fructose supplemented with C2, C3, C4, C5, and/or C6 medium-chain carboxylic acids as electron acceptors. This is the first report on the production of heptanoic acid and octanoic acid and high-titer production of medium-chain (C5–C8) carboxylic acids using an anaerobic pure culture.
Results and discussion
Isolation of hexanoic acid-producing bacteria
For the isolation of hexanoic acid-producing bacteria, RCM supplemented with hexanoic acid was used as a selection medium. Hexanoic acid has been shown to be toxic for microbial growth [19, 20]; therefore, the suppression of bacteria that do not produce hexanoic acid was expected by supplementing hexanoic acid (5 g/L) to RCM. The metabolites in the culture broth for isolation were analyzed by GC-FID after 7 days cultivation, and then the culture broth containing over 5 g L−1 of hexanoic acid production was transferred to a fresh selection medium and subcultured for 3 days. The final sub-culture on selection medium was transferred to RCM not containing hexanoic acid, and the bacterial consortium was observed to produce over 4.5 g L−1 of hexanoic acid. The culture broth was spread on RCM agar and were observed to form colonies with two-types of morphologies. One of these colony types was isolated and designated strain MH.
The strain MH was cultivated in the RCM broth without hexanoic acid supplement for 3 days, and the amount of hexanoic measured by GC/FID and its identity confirmed by GC/MS. From the RCM containing 20 g L−1 of glucose, the strain MH produced approximately 0.5 g L−1 hexanoic acid and approximately 0.1 g L−1 pentanoic acid on the RCM medium.
Identification and phylogenetic analysis
The isolate is closely related to the type strain for Megasphaera and was identified as Megasphaera sp. MH. The 16S rRNA sequence similarity between the strain MH and the type strain of Megasphaera species was 93.1–93.9 %. The closest type strain to the strain MH was Megasphaera paucivorans VTT E-032341T, with 93.9 % of 16S rRNA gene sequence similarity, and the next similarity domain present was Megasphaera micronuciformis AIP 412.00T (93.8 %). In the neighbor-joining phylogenetic tree, the strain MH clustered with Megasphaeraelsdenii and Megasphaera paucivorans (Fig. 2). The GenBank number for the strain is KX021300. Megasphaera sp. MH was deposited in the Korean Culture Center of Microorganism as KFCC11466P.
Fig. 2.
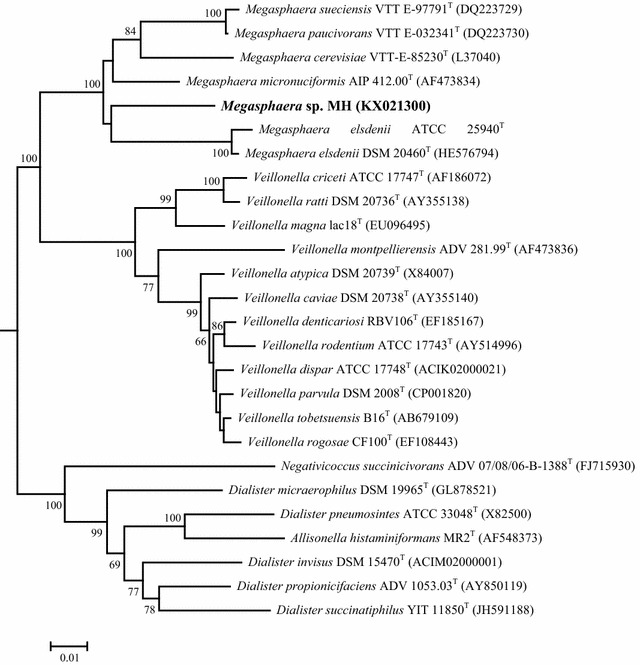
Phylogenetic tree of Megasphaera sp. MH
The production of hexanoic acid by Megasphaera sp. MH
Using an API 50 CH test strip, the utilization of other carbohydrates by Megasphaera sp. MH was investigated. Megasphaera sp. MH fermented d-arabinose, d-fructose, d-arabitol, inositol, potassium gluconate, and 5-keto-gluconate after 2 days of cultivation at 37 °C, but did not ferment 43 other carbohydrates including glucose. The reason of the preference of fatty acids by rumen bacteria seems to be enough and various organic acids present in rumen environment. Therefore, fructose was selected as the carbon source for the strain MH culture and was added into the PYG medium (denoted as mPYF). Megasphaera sp. MH produced 0.88 g L−1 hexanoic acid in mPYF medium (Fig. 3a). Other carboxylic acids were also detected in the culture broth as final products, such as 0.04 g L−1 of pentanoic acid (C5), 0.12 g L−1 of heptanoic acid (C7), and 0.6 g L−1 of octanoic acid (C8). Interestingly, the medium chain fatty acids such as hexanoic acid and octanoic acid were produced more than butyrate, a typical product of fermentation. However, the maximum O.D. for the microbial growth was just 2.9 ± 0.21, and only 5.1 ± 1.5 g/L of the initial 20 g/L was consumed.
Fig. 3.
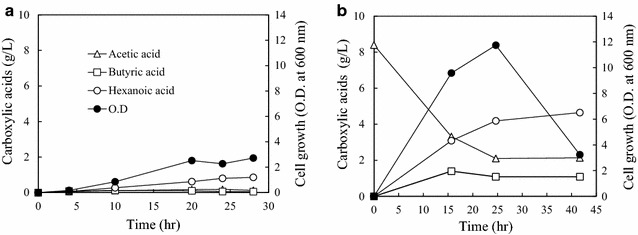
The hexanoic acid production by Megasphaera sp. MH using fructose a without supplemented electron acceptors and b with acetate as an electron acceptor
Sodium acetate (100 mM) into mPYF medium led to an increase in the production of butyric acid and hexanoic acid, which were 0.88 g L−1 (9.9 mM) and 4.37 g L−1 (37.7 mM), respectively (Fig. 3b; Table 1). Microbial growth also increased up to OD600 = 5.45 (Fig. 3b; Table 1). The OD decreased during stationary phase, which may have been due to the toxicity of the products or pH inhibition [7], and fructose was consumed up to 11.1 ± 1.4 g/L. It seems that the produced butyrate by Megasphaera sp. MH could be reused by itself and converted to hexanoate with acetate (Table 1). Therefore, butyrate and hexanoate more increased in acetate-mPYF medium than in mPYF medium only. Previous papers also showed that the production of hexanoic acid by Clostridium sp. BS-1 [7] was increased by adding acetate. When the electron flows inside the cell were changed by the inhibition of hydrogenase activity and adding acetic acid into the medium, the production of hexanoic acid by M. elsdenii NIAH-1102 observed to increase [15].
Table 1.
The fermentation products according to various electron acceptors by Megasphaera sp. MH using fructose
| Electron acceptor (EA, 100 mM) |
Fermentation products (g/L)a | |||||
|---|---|---|---|---|---|---|
| Butyrate (C4) | Pentanoate (C5) | Hexanoate (C6) | Heptanoate (C7) | Octanoate (C8) | O.D.max | |
| Without supplementary EA | 0.3 ± 0.0 | 0.0 ± 0.0 | 0.9 ± 0.1 | 0.1 ± 0.0 | 0.6 ± 0.0 | 2.9 ± 0.2 |
| Acetate (C2) | 0.9 ± 0.1 | 0.2 ± 0.2 | 4.4 ± 0.0 | 0.1 ± 0.0 | 0.6 ± 0.0 | 5.5 ± 0.6 |
| Propionate (C3) | 0.2 ± 0.0 | 4.1 ± 0.3 | 0.2 ± 0.0 | 2.0 ± 0.1 | 0.1 ± 0.0 | 5.4 ± 0.3 |
| Butyrate (C4) | 1.6 ± 0.1b | 0.2 ± 0.0 | 6.3 ± 0.0 | ND | 0.6 ± 0.0 | 3.9 ± 0.0 |
| Acetate based (dual electron acceptors)c | ||||||
| Acetate(C2) +propionate(C3) | 1.0 ± 0.0 | 5.7 ± 0.1 | 1.5 ± 0.1 | 2.7 ± 0.1 | 0.2 ± 0.0 | 6.4 ± 0.4 |
| Acetate(C2) + butyrate (C4) | 2.5 ± 0.1b | 0.3 ± 0.0 | 9.7 ± 0.2 | ND | 0.6 ± 0.0 | 6.2 ± 0.5 |
| Acetate(C2) + pentanoate (C5) | 0.2 ± 0.0 | 5.8 ± 0.1b | ND | 3.6 ± 0.1 | 0.2 ± 0.0 | 2.2 ± 0.0 |
| Acetate(C2) + hexanoate (C6) | 1.6 ± 0.0 | 0.2 ± 0.0 | 8.7 ± 0.0b | ND | 1.2 ± 0.0 | 2.0 ± 0.0 |
The italic numbers indicate higher production than in mPYF without supplementary electron acceptors
ND not detected
aThe value is average of duplicates
bThe value is undefined as products or non-used electron acceptor
cEach concentration was 100 mM except hexanoate (50 mM) and total concentration of extracellular electron acceptors was 200 mM (for acetate + hexanoate, 150 mM)
The production of longer carbon-chain carboxylic acids by Megasphaera sp. MH
Other carboxylic acids, such as propionate (C3), butyrate (C4), pentanoate (C5), and hexanoate (C6), were investigated as electron acceptors. Interestingly, when the C3–C6 carboxylic acids were added into the medium, longer carbon-chain carboxylic acids, such as pentanoic acid, heptanoic acid, and octanoic acid, were detected (Table 1).
When propionate (C3) was added to the medium, pentanoic acid (C5) and heptanoic acid (C7) produced up to 39.8 and 15.6 mM, respectively. In the mPYF medium with acetate, hexanoic acid increased in addition to butyric acid presumably because some of the butyrate produced reacted with acetate. Additionally, in the mPYF culture with propionate, the increase in heptanoic acid seemed to be due to the reuse of produced pentanoate. However, hexanoate did not react in the mPYF medium with butyrate (Table 1); there was no octanoic acid production. Finally, the greatest amount of hexanoic acid was produced in the mPYF culture with added butyrate (Table 1; Fig. 4). Therefore, a targeted increase in production of specific carboxylic acid was accomplished by selecting the optimal electron acceptor.
Fig. 4.

The fermentation products according to various electron acceptors by Megasphaera sp. MH using fructose
Adding either acetate or a mixture of propionate and butyrate to the mPYF medium increased cell growth relative to the control culture (Table 1). Finally, the hexanoic acid was the highest concentration at 9.7 g/L (0.53 molar yield, see detail calculation in Additional file 1: Table S1) in the mPYF medium with acetate and butyrate. The pentanoic acid was 5.7 g/L in the mPYF medium with acetate and propionate (Table 1). The conversion efficient of electron acceptors to attend chain-elongation process could not be analyzed because products could not be distinguished as whether unused electron acceptors or the real products. It may be a future study to elucidate the origin using isotope-labeled electron acceptor for tracking.
The strain produced medium chain carboxylic acid using supplemented short chain fatty acid, different from fatty acid producing process using carbon-rich mediums such as wastewater [30]. In our study, the major product was controlled by the selection of appropriate short chain fatty acid, showing high productivity and titer as shown Table 2.
Table 2.
Performance comparison for biological hexanoic acid production
| Substrate | Inoculum | Time (Day) | Maximum hexanoic acid (g L−1) | Productivity (g L−1H) | References |
|---|---|---|---|---|---|
| Fructose, acetate, butyrate | Megasphaera sp. MH (pure culture) | 1 | 9.7 | 0.41 | This study |
| Galactitol, acetate, butyrate | Clostridium sp. BS-1 (pure culture) | 3–16a | 6.96–32.0a | 0.28–0.34 | Jeon et al. [28] |
| Glucose | Megasphaera elsdenii ATCC 25940 (pure culture) | 5–8.3a | 2.6–11.4a | 0.03–0.13 | Roddick and Britz [29] |
| Ethanol, acetate | C. kluyveri 3231B (pure culture) | 3 | 12.8 | 0.175 | Weimer and Stevenson [6] |
| Lactate | Mature pit mud, enriched Clostridium cluster IV | 5–16a | 12.93–23.93a | 0.06–0.108a | Zhu et al. [30] |
| Acetate, butyrate, ethanol | Mixed culture | 500 | 0.9 | 0.0375 | Agler et al. [31] |
a Fed batch or product removal during fermentation
The proposed synthetic process of C5–C8 carbon-chain carboxylic acids in Megasphaera sp. MH is the transformation of both metabolites and supplemented carboxylic acids to more reduced forms; i.e., longer carbon-chain carboxylic acids, for the disposal of reducing equivalents. The oxidation of fructose to acetyl-CoA will liberate reducing equivalents as NADH or FADH2. The discharge of overflowing reducing equivalents is required for cell growth, and it may be excreted as H2 gas or may be transferred to electron acceptors for the synthesis of C4–C8 carboxylic acids (2–6 NADH consumption per one mol from pyruvate, Additional file 1: Table S2). Megasphaera sp. MH has the pathway in which supplemented carboxylic acids are used as the electron acceptors and the enzymes related to carbon-chain elongation (Additional file 1: Figure S1). Electron acceptors supplemented reducing equivalent and carbon sources for chain elongation. Less production of H2 (73–78 % of that produced without electron acceptor, individual data not shown), more fructose consumption (almost two times, individual data not shown), and more cell growth (OD600 = 3.9–5.5 vs. 2.9, Table 1) were observed in the presence of an electron acceptor. This means that the growth of Megasphaera sp. MH was stimulated by the supplementation of the electron accepters and the surplus reducing equivalents were used for production of C5–C8 linear chain carboxylic acids. However, the addition of C5 and C6 reduced the microbial growth compared with C2–C4 supplemented medium probably due to its toxicity (Table 1).
We have concerned the separation process for mixture of fatty acid and have informed extraction process for hexanoic acid through previous study or reports [9]. The C5–C8 fatty acid is easily separated at lower pH than each pKa [9]. Also, most of minor products were below 1 g/L. Therefore, we thought that mixture could be selectively extracted as pure products.
A recently isolated C. kluyveri 3231B produced 12.8 g L−1 of hexanoic acid from ethanol and acetate during 72 h of cultivation [6]. Although Megasphaera sp. MH produced a smaller amount of hexanoic acid than C. kluyveri 3231B, the production rates of hexanoic acid by C. kluyveri 3231B and Megasphaera sp. MH were 0.18 g L−1 h−1 and 0.41 g, respectively. The rapid production of hexanoic acid by Megasphaera sp. MH might be indicative of highly active enzymes performing key reactions in the metabolic pathway for the synthesis of hexanoic acid. Among the related enzymes, acetyl-CoA acetyl transferase and acyl-CoA hydrolase were expected to be the most important enzymes in the putative pathway for the production of hexanoic acid (Fig. 1). Therefore, the genomic and proteomic analyses of Megaspahera sp. MH are required for confirming the pathway for the production of C5–C8 carboxylic acids, including acetyl-CoA acetyl transferase and acyl-CoA hydrolase. In addition, the supplementation of the C13-labeled acetic acid, propionic acid, and/or butyric acid to culture medium for the chase of C13-labeled products will be investigated in future studies.
Conclusions
An anaerobe strain designated MH isolated from the cattle rumen was identified as Megasphaera sp. MH by phylogenic analysis of the 16S rRNA gene sequence. Megasphaera sp. MH metabolized fructose for the production of C2–C8 carbon-chain carboxylic acids using various electron acceptors. The addition of C2–C6 carbon-chain carboxylic acids into the medium increased the growth of Megasphaera sp. MH and followed the production of pentanoic acid, hexanoic acid, heptanoic acid, and octanoic acid. Megasphaera sp. MH produced 5.7 g L−1 of pentanoic acid and 9.7 g L−1 of hexanoic acid using fructose and the supplemented C2–C4 carboxylic acids within 24 h. Megasphaera sp. MH demonstrated the fastest productivity of hexanoic acid (0.41 g L−1 h−1) in batch culture reported yet.
Methods
Media and culture conditions
All bacterial cultures were performed in an anaerobic environment. Cells on an agar plate were incubated in an anaerobic flexible vinyl chamber (Coy Products, Grass Lake, MI, USA) maintaining an anaerobic atmosphere with a mixed gas (N2:CO2:H2 = 8:1:1, v/v). Liquid broth was prepared as 20 mL of media in a 50 mL serum bottle under argon purging. Reinforced Clostridia Medium (RCM, BD, USA) containing 20 g L−1 of glucose and 5 g L−1 hexanoic acid (pH 7) was used as a selection medium for the isolation of hexanoic acid-producing bacteria. For cultivation of an isolated Megasphaera sp. strain, mPYG and mPYF media were used. The medium of mPYG is suggested for Megasphaera growth by the German Collection of Microorganisms and Cell Cultures (https://www.dsmz.de). The mPYG medium contained the following components dissolved in distilled water to a final volume of 1 L: yeast extract, 10 g; peptone, 5 g; tryptone, 5 g; beef extract, 5 g; fructose, 20 g; K2HPO4, 2 g; Tween 80, 1 mL; cysteine HCl·H2O, 0.5 g; hemin solution, 10 mL; salt solution, 40 mL; and vitamin K1 solution, 0.2 mL. Salt solution was prepared in distilled water to a final volume of 1 L: CaCl2·2H2O, 0.25 g; MgSO4·7H2O, 0.5 g; K2HPO4, 1 g; KH2PO4, 1 g; NaHCO3, 10 g; and NaCl, 2 g. For hemin solution, 50 mg of hemin (Sigma Aldrich) was dissolved in 1 mL of 1 N NaOH and then diluted into distilled water to a final volume of 100 mL. Vitamin K1 solution was made by diluting 0.1 mL of vitamin K1 stock (Sigma Aldrich) in 20 mL of 95 % ethanol. The pH of the medium was adjusted to 7.2 using 8 N NaOH. The mPYG, except for vitamin K1 and hemin solution, was autoclaved and cooled, and then vitamin K1 and hemin solutions were added separately after sterilization by filtration and argon purging. The mPYF medium, containing fructose instead of glucose in the mPYG medium, was used for maintenance of the isolated strain and for the production of C5–C8 saturated linear chain carboxylic acid. Bacteria were cultured in a shaking incubator with rotation at 150 rpm at 37 °C. For the production of C2–C8 carboxylic acids by the isolate, 3 % (v/v) of seed culture in mPYF supplemented with 0.1 M of sodium acetate and 0.1 M of sodium butyrate was inoculated to fresh mPYF medium. The effects of C2–C6 carboxylic acids as the electron acceptors on the production C5–C8 carboxylic acids by the isolated strain were observed in mPYF medium supplemented with sodium acetate (C2), sodium propionate (C3), sodium butyrate (C4), sodium pentanoate (C5) or sodium hexanoate (C6). All experiments were performed in duplicate, and the results are shown as an average of duplicate experiments.
Isolation of hexanoic acid-producing bacteria
All isolation procedures were performed under an anaerobic environment. A cattle rumen sample was used as a bacterial source. The inner surface of the cattle rumen was sliced and chopped, and the bacteria on the rumen samples were extracted into the sterilized 10 % (v/v) glycerol solution by vigorous vortex mixing. The extracted bacterial samples were inoculated in the selection media for the isolation of hexanoic acid producer and cultured at 37 °C in a standing culture. After 7 days of cultivation, the enriched broths were transferred to the fresh selection media, and this procedure was repeated successively ten times. Then, the last enriched broths were inoculated in the fresh selection media not containing hexanoic acid and were cultured for 3 days. After confirming the presence of hexanoic acid at the final culture broth, the culture broth was serially diluted with sterilized saline solutions and spread on RCM solid plates. The plates were incubated for 7 days in an anaerobic chamber. Bacterial colonies grown on the RCM plates were serially sub-cultured to fresh plates to acquire a pure bacterial strain. Carbohydrate usage of the isolate was evaluated using API 50 CH strips (bioMérieux, France) according to the manufacturer’s instructions.
16S rRNA gene sequence and phylogenic analysis
The genomic DNA of the isolate was extracted using a DNA isolation kit (iNtRON Biotechnology, Korea). The 16S rRNA gene of the isolate was amplified by PCR using universal primers 27F and 1492R (Lane, 1991) and analyzed as described by Kim et al. [9]. The closely related type strains of the isolate were determined by a database search, and the 16S rRNA gene sequences of relative strains were retrieved from GenBank using the BLAST program (http://www.ncbi.nlm.nih.gov/blast/) and from the EzTaxon-e (http://eztaxone.ezbiocloud.net/) server [21]. Multiple alignments of the 16S rRNA gene sequences were performed using Clustal_X [22].
The phylogenetic trees of 16S rRNA gene sequences of the isolate with their closely related strains were constructed by the neighbor-joining method [23] using MEGA5 software [24] based on an alignment with a length of 1308 nucleotides. Phylogenetic distances were calculated using Kimura’s two-parameter method [25]. The confidence limit for a phylogenetic tree was estimated from bootstrap analysis [26] using 1000 replicates.
Analytical methods
Cell growth in broth medium was measured by OD600 using a spectrophotometer (Simazu-1240). Metabolites produced by isolates were analyzed with a gas chromatograph (GC) equipped with a flame ionized detector (FID) and with a thermal conductivity detector (TCD) for the presence of C2–C8 carboxylic acids in the liquid phase and H2 and CO2 in the gas phase, respectively, according to methods described previously [32]. Culture broth was taken using a syringe and stored at −20 °C before analysis of metabolites in the liquid phase. Cell mass was removed by filtration, and the pH of the filtrate was dropped below pH 4 using 10 % (v/v) phosphoric acid before gas chromatograph (GC) analysis for the protonation of acids.
A GC (Agilent 6890) equipped with a time-of-flight (TOF) mass spectrometer (MS, Leco) equipped with a HP-Innowax column (30 m × 0.25 mm i.d., 0.25 µm film thickness; Agilent Technologies) was used for confirmation of pentanoic acid, hexanoic acid, heptanoic acid, and octanoic acid. To perform GC/TOF/MS analysis, the filtrate of the culture broth was adjusted to pH 4 with 10 % (v/v) phosphoric acid, and carboxylic acid in the filtrate was extracted two times with an equal volume of diethyl ether. Then, 2 µL of the resulting solution was injected into the GC/TOF/MS. The samples were introduced by split mode at a split ratio of 20:1. The injector temperature was set at 120 °C. The column temperature was 130 °C initially and then was ramped up to 180 °C at 6 °C min−1. Helium (99.9999 %) was used as the carrier gas at 1.0 mL min−1. The ion source temperature was 230 °C. The mass selective detector was operated at 70 eV in the electron impact mode with full scan mode over a mass range of 10–300 m/z. Compounds were identified using the National Institute of Standards and Technology (NIST)-library spectra and the published MS data.
Authors’ contributions
BJ performed experiments and contributed to design, acquisition and analysis of data. OC and YU contributed to design of the study and analysis of data. BS developed the concept of the study, contributed to data analysis and preparation of the manuscript. All the authors were involved in the drafting and editing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the research fund of New & Renewable Energy of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) Grant funded by the Korea Government Ministry of Trade, Industry and Energy (No. 20133030000300) and was also supported by the National Research Council of Science & Technology (NST) grant by the Korea government (MSIP) (No. CAP-11-04-KIST)/Korea Institute of Science and Technology (KIST).
Competing interests
The authors declare that they have no competing interests.
Availability of supporting data
Not applicable.
Consent for publication
Not applicable.
Ethical approval and consent to participate
Not applicable.
Funding
This work was financially supported by the National Research Council of Science & Technology (NST) grant by the Korea government (MSIP) (No. CAP-11-04-KIST)/Korea Institute of Science and Technology (KIST) to Byoung Seung Jeon.
This work was financially supported by the research fund of New & Renewable Energy of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) Grant funded by the Korea Government Ministry of Trade, Industry and Energy (No. 20133030000300) to Okkyoung Choi.
Abbreviations
- mPYF/G
modified peptone yeast extract fructose/glucose medium
- RCM
reinforced clostridia medium
- GC/FID
gas chromatography equipped with flame ionized detector
- GC/TOF MS
gas chromatography equipped with time-of-flight mass spectrometer
Additional file
10.1186/s13068-016-0549-3 Table S1. Theoretical and experimental molar yield of hexanoic acid; Table S2. The electron equivalent of C2-C8 carboxylic acids NADH consumption for the production; Figure S1. Proposed synthesis pathways of carbon C2–C8 linear chain carboxylic acids and the electron flows in Megasphaera sp. MH.
Contributor Information
Byoung Seung Jeon, Email: jbstrust@gmail.com.
Okkyoung Choi, Email: okgii77@hanmail.net.
Youngsoon Um, Email: yum@kist.re.kr.
Byoung-In Sang, Phone: +82-2-2220-2328, Email: biosang@hanyang.ac.kr.
References
- 1.Gavrilescu M, Chisti Y. Biotechnology—a sustainable alternative for chemical industry. Biotechnol Adv. 2005;23(7–8):471–499. doi: 10.1016/j.biotechadv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SJ, Candry P, Basadre T, Khor WC, Roume H, Hernandez-Sanabria E, et al. Electrolytic extraction drives volatile fatty acid chain elongation through lactic acid and replaces chemical pH control in thin stillage fermentation. Biotechnol Biofuels. 2015;8(1):1–14. doi: 10.1186/s13068-015-0396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Guzman JJL, Andersen SJ, Rabaey K, Angenent LT. In-line and selective phase separation of medium-chain carboxylic acids using membrane electrolysis. Chem Commun. 2015;51(31):6847–6850. doi: 10.1039/C5CC01897H. [DOI] [PubMed] [Google Scholar]
- 4.Barker HA, Taha SM. Clostridium kluyveri, an organism concerned in the formation of caproic acid from ethyl alcohol. J Bacteriol. 1942;43(3):347–363. doi: 10.1128/jb.43.3.347-363.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenealy WR, Cao Y, Weimer PJ. Production of caproic acid by cocultures of ruminal cellulolytic bacteria and Clostridium kluyveri grown on cellulose and ethanol. Appl Microbiol Biotechnol. 1995;44(3–4):507–513. doi: 10.1007/BF00169952. [DOI] [PubMed] [Google Scholar]
- 6.Weimer PJ, Stevenson DM. Isolation, characterization, and quantification of Clostridium kluyveri from the bovine rumen. Appl Microbiol Biotechnol. 2012;94(2):461–466. doi: 10.1007/s00253-011-3751-z. [DOI] [PubMed] [Google Scholar]
- 7.Jeon BS, Kim BC, Um Y, Sang BI. Production of hexanoic acid from d-galactitol by a newly isolated Clostridium sp. BS-1. Appl Microbiol Biotechnol. 2010;88(5):1161–1167. doi: 10.1007/s00253-010-2827-5. [DOI] [PubMed] [Google Scholar]
- 8.Marounek M, Fliegrova K, Bartos S. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl Environ Microbiol. 1989;55(6):1570–1573. doi: 10.1128/aem.55.6.1570-1573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi K, Jeon B, Kim BC, Oh MK, Um Y, Sang BI. In situ biphasic extractive fermentation for hexanoic acid production from sucrose by Megasphaera elsdenii NCIMB 702410. Appl Biochem Biotechnol. 2013;171(5):1094–1107. doi: 10.1007/s12010-013-0310-3. [DOI] [PubMed] [Google Scholar]
- 10.Khan MA. Regulation of volatile fatty acid synthesis in Megasphaera elsdenii and hexanoic acid utilisation by Pseudomonas putida. Melbourne: Victoria University; 2006. [Google Scholar]
- 11.Berndt H, Schlegel HG. Kinetics and properties of beta-ketothiolase from Clostridium pasteurianum. Arch Microbiol. 1975;103(1):21–30. doi: 10.1007/BF00436325. [DOI] [PubMed] [Google Scholar]
- 12.Sliwkowski MX, Hartmanis MGN. Simultaneous single-step purification of thiolase and nadp-dependent 3-hydroxybutyryl-CoA dehydrogenase from Clostridium kluyveri. Anal Biochem. 1984;141(2):344–347. doi: 10.1016/0003-2697(84)90052-6. [DOI] [PubMed] [Google Scholar]
- 13.Wiesenborn DP, Rudolph FB, Papoutsakis ET. Thiolase from Clostridium acetobutylicum atcc 824 and its role in the synthesis of acids and solvents. Appl Environ Microbiol. 1988;54(11):2717–2722. doi: 10.1128/aem.54.11.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekishima Y, Lan EI, Shen CR, Cho KM, Liao JC. Extending carbon chain length of 1-butanol pathway for 1-hexanol synthesis from glucose by engineered Escherichia coli. J Am Chem Soc. 2011;133(30):11399–11401. doi: 10.1021/ja203814d. [DOI] [PubMed] [Google Scholar]
- 15.Hino T, Miyazaki K, Kuroda S. Role of extracellular acetate in the fermentation of glucose by a ruminal bacterium, Megasphaera elsdenii. J Gen Appl Microbiol. 1991;37(1):121–129. doi: 10.2323/jgam.37.121. [DOI] [Google Scholar]
- 16.Ahmed I, Ross RA, Mathur VK, Chesbro WR. Growth-rate dependence of solventogenesis and solvents produced by Clostridium beijerinckii. Appl Microbiol Biotechnol. 1988;28(2):182–187. doi: 10.1007/BF00694309. [DOI] [Google Scholar]
- 17.George HA, Chen JS. Acidic conditions are not obligatory for onset of butanol formation by Clostridium beijerinckii (Synonym, Clostridium butylicum) Appl Environ Microbiol. 1983;46(2):321–327. doi: 10.1128/aem.46.2.321-327.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SM, Cho MO, Park CH, Chung YC, Kim JH, Sang BI. Continuous butanol production using suspended and immobilized Clostridium beijerinckii NCIMB 8052 with supplementary butyrate. Energ Fuel. 2008;22(5):3459–3464. doi: 10.1021/ef800076j. [DOI] [Google Scholar]
- 19.Fay JP, Farias RN. The inhibitory action of fatty acids on the growth of Escherichia coli. J Gen Microbiol. 1975;91(2):233–240. doi: 10.1099/00221287-91-2-233. [DOI] [PubMed] [Google Scholar]
- 20.Miller RD, Brown KE, Morse SA. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect Immun. 1977;17(2):303–312. doi: 10.1128/iai.17.2.303-312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Micr. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitou N, Nei M. The neighbor-joining method—a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 24.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide-sequences. J Mol Evol. 1980;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 26.Felsenstein J. Confidence-limits on phylogenies—an approach using the bootstrap. Evolution. 1985;39(4):783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 27.Kim BH, Bellows P, Datta R, Zeikus JG. Control of carbon and electron flow in Clostridium acetobutylicum fermentations: utilization of carbon monoxide to inhibit hydrogen production and to enhance butanol yields. Appl Environ Microbiol. 1984;48(4):764–770. doi: 10.1128/aem.48.4.764-770.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon BS, Moon C, Kim BC, Kim H, Um Y, Sang BI. In situ extractive fermentation for the production of hexanoic acid from galactitol by Clostridium sp BS-1. Enzyme Microb Tech. 2013;53(3):143–151. doi: 10.1016/j.enzmictec.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Roddick FA, Britz ML. Production of hexanoic acid by free and immobilised cells of Megasphaera elsdenii: influence of in situ product removal using ion exchange resin. J Chem Technol Biotechnol. 1997;69(3):383–391. doi: 10.1002/(SICI)1097-4660(199707)69:3<383::AID-JCTB723>3.0.CO;2-H. [DOI] [Google Scholar]
- 30.Zhu XY, Tao Y, Liang C, Li XZ, Wei N, Zhang WJ, et al. The synthesis of n-caproate from lactate: a new efficient process for medium-chain carboxylates production. Sci Rep-Uk. 2015 doi: 10.1038/srep14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agler MT, Spirito CM, Usack JG, Werner JJ, Angenent LT. Development of a highly specific and productive process for n-caproic acid production: applying lessons from methanogenic microbiomes. Water Sci Technol. 2014;69(1):62–68. doi: 10.2166/wst.2013.549. [DOI] [PubMed] [Google Scholar]
- 32.Jeon BS, Um YS, Lee SM, Lee SY, Kim HJ, Kim YH, et al. Performance analysis of a proton exchange membrane fuel cell (PEMFC) integrated with a trickling bed bioreactor for biological high-rate hydrogen production. Energ Fuel. 2008;22(1):83–6. doi: 10.1021/ef700270y. [DOI] [Google Scholar]


