Abstract
Melanoma has a propensity for lymph node metastasis. However, the incidence of lymphatic invasion detected by histology alone in primary melanoma is disproportionately low in comparison to the incidence of positive sentinel lymph nodes (SLN). With the discovery of lymphatic endothelial cell markers, such as podoplanin and LYVE-1, lymphatic vessels can be reliably detected in formalin-fixed paraffin-embedded (FFPE) tissues. There is a now consensus that lymphatic invasion detected by immunohistochemical stains in primary melanoma is much more common than previously reported by histological examination alone. Immunohistochemical stains show that lymphangiogenesis and lymphatic invasion in primary melanoma may occur intratumorally or peritumorally, and lymphatic invasion is common across the range of tumor thicknesses in primary vertical growth phase (VGP) melanomas. A number of studies have shown that lymphatic invasion in primary melanoma is associated with a positive sentinel lymph node biopsy and a worse clinical outcome. Although not currently a part of the standard of care for staging of melanoma, the detection of lymphatic invasion in primary melanoma using immunohistochemical markers may be helpful in planning of therapy in some cases and may find a routine role in primary melanoma microscopic attributes in future.
Keywords: Lymphangiogenesis, Lymphatic invasion, Melanoma, Podoplanin, D2–40, LYVE-1, Prognosis
1 Introduction
Clinically apparent distant metastasis is the cause of most deaths in patients with primary cutaneous melanoma. Tumor dissemination may occur through a number of pathways: (a) local tissue invasion, (b) direct seeding of body cavities or surfaces, (c) hematogenous spread, and (d) lymphatic spread. Melanoma has a propensity for lymph node metastasis and clinical and pathological observations suggest transport of melanoma cells via lymphatics is the most common pathway of initial dissemination, with patterns of spread via afferent lymphatics following routes of natural drainage [1, 2].
The growth of new lymphatic vessels, called lymphangiogenesis, is largely absent in normal adult tissues, but can be induced in pathological processes, such as inflammation, wound healing, and cancer [3]. Lymphangiogenesis and lymphatic invasion (LI, defined as melanoma cells in lymphatic vessels) have been under increasing investigation in the lesions of primary melanoma because of the recent availability of antibodies specific for lymphatic endothelial cells, such as antibodies to podoplanin and Lymphatic Vessel Endothelial Receptor 1 (LYVE-1) [4, 5]. Podoplanin, also known as GP36, T1 alpha, and Aggrus, is a mucin-type transmembrane glycoprotein with extensive O-glycosylation. It is specifically expressed by lymphatic endothelial cells but not blood vascular endothelial cells. In addition, a range of non-endothelial cells in numerous normal tissues also express this protein [6]. LYVE-1 is a CD44 homolog found primarily on lymphatic endothelial cells. While LYVE-1 functions continue to be defined, potential roles have been suggested in hyaluronan (HA) transport and turnover, or in promoting HA localization to the surfaces of lymphatic endothelium [7]. Unlike the pan-endothelial cell marker CD31, antibodies against podoplanin (D2–40) and LYVE-1 specifically detect lymphatic endothelial cells, but not blood vascular endothelial cells, making these markers particularly useful to study lymphangiogenesis and LI in tumors.
1.1 Lymphangiogensis and Melanoma Prognosis
Similar to the blood vasculature, lymphatic vessels in most adult tissues and organs are quiescent under physiological conditions. Tumor cells or tumor-infiltrating inflammatory cells secrete growth factors that likely promote lymphangiogenesis, which in turn may promote lymphatic metastasis. Among known lymphangiogenic factors, the VEGFC/VEGFD–VEGFR3 pathway is the best-characterized signalling system. It has a vital role in the budding of initial lymphatics from vein endothelium that expresses PROX1. Recently, several other lymphangiogenic factors have been reported. In addition to members of the VEGF family, these factors include members of the FGF, PDGF, and angiopoietin families, and they seem to have interdependent or collaborative roles with each other or with the members of the VEGF family in the establishment of functional lymphatics [8]. Melanoma cells and activated leukocytes in inflammatory sites produce a broad spectrum of growth factors, including members of the FGF, VEGF, and PDGF families, as well as proinflammatory cytokines and chemokines that might stimulate proliferation, migration, and survival of isolated lymphatic endothelial cells [9, 10]. Macrophages have been shown to be a rich source of lymphangiogenic factors such as VEGFC and VEGFD [11]. Indeed, we recently showed that in melanomas with radial growth phase (RGP) regression, which is characterized by a dense lymphocytic and melanophage infiltrate and an absence of melanoma cells, lymphatic vessel density was significantly higher in the areas with complete regression (mean ± SD, 23.7 ± 12.3/mm2) compared with adjacent normal dermis (7.3 ± 3.5/mm2) and distant normal dermis (5.5 ± 2.6/mm2). This observation supports the hypothesis that tumor stromal-infiltrating inflammatory cells contribute to lymphangiogenesis [12].
Dilated lymphatic vessels are commonly observed within or at the periphery of malignant tumors, including melanoma [13]. Valencak et al. showed that those patients whose primary lesions had high lymphatic density had shorter overall and disease-free survival [14]. Dadres et al. noted that intratumoral lymphatics detected by immunohistochemical (IHC) staining of LYVE-1 were more frequent in primary melanomas excised from patients whose sentinel lymph nodes (SLN) had metastases than in those taken from SLN-negative patients [15]. We have previously demonstrated in univariate analyses that higher intratumoral lymphatic density was significantly associated with metastasis and melanomarelated death, whereas peritumoral lymphatic density was associated only with melanoma-specific death [13]. These studies provide evidence that lymphangiogenesis is a potential prognostic marker for sentinel lymph node metastasis and melanoma-related death. Nevertheless, the cohorts used in these studies were relatively small and additional studies are needed to clarify the prognostic role of lymphangiogenesis in melanoma while controlling for other potential explanatory factors.
1.2 Frequency of Lymphatic Invasion in Melanoma
The detection of lymphatic invasion by routine histology alone in primary melanoma is disproportionately low at 0–7.8 % [16, 17], relative to the incidence of sentinel lymph node (SLN) positivity (16–20 %) [18, 19]. Although blood vascular invasion is even more uncommon than lymphatic invasion, ranging from 1 to 3 % [14, 20], it potentially confounds the detection of LI. Thus, routine histology detects lymphovascular invasion (LVI). With the use of markers specific for lymphatic endothelial cells, however, the rate of LI detected by IHC stains increases dramatically. When only a lymphatic endothelial cell marker was used for IHC, the rate of LI in primary invasive melanoma ranged from 16 to 37% [16, 21–27]; whereas when double staining of lymphatic endothelial cells and melanoma cells was used, the rate of LI in primary invasive melanoma ranged from 33 to 43% [13, 28](Table 1). In thin melanomas (Breslow thickness ≤1 mm) with vertical growth phase, we found that the LI rate is 9.6 % (12/125) [28]. The lymphatic invasion rate in thin to intermediate thickness (Breslow thickness ≤2 mm) melanoma was 21.9 % [27]. These results indicate that LI is frequent in primary melanoma and suggest that it occurs early during melanoma progression.
Table 1.
Summary of studies using lymphatic endothelial cell markers to detect LI in primary melanoma
| Series | Markers | No. cases |
LVI by histology (%) |
LI by IHC (%) |
LI and microstage attributes |
LI and SLN status |
LI and DFS/OS |
|---|---|---|---|---|---|---|---|
| Xu et al. | D2–40/ S100 |
106 | 5 | 33 | Thickness | N/A | DFS, univariate |
| Xu et al. | D2–40/ S100 |
251 | 4.6 | 43 | Thickness, mitotic rate, ulceration, gender |
N/A | OS, multivariate |
| Sahni et al. |
LYVE-1 | 36 | 0 | 16 | No associate ions noted |
N/A | N/A |
| Niakosari et al. |
D2–40 | 96 | N/A | 33 | Clark level, thickness | Significant, multivariate |
N/A |
| Doeden et al. |
D2–40, LYVE-1, CD31 |
94 | 6 | 16 | Stage, histologic subtype |
Significant, univariate |
No associations noted |
| Petersson et al. |
D2–40 | 74 | 0 | 23 | N/A | Significant, univariate |
DFS and OS, multivariate |
| Petitt et al. |
D2–40 | 27 | 4 | 37 | No associations noted |
Not significant | N/A |
| Rose et al. |
D2–40, CD34 |
246 | 3 | 18 | Thickness, ulceration, mitotic rate, histologic subtype |
Not significant | DFS and OS, multivariate |
| Storr et al. |
D2–40, CD34 |
202 | 8 | 30 | Thickness, ulceration, mitotic rate, histologic subtype, microsatellite |
Not significant | No assciateions noted |
| Fohn et al. |
D2–40 | 64 | 3.1 | 22 | Thickness, Mitotic rate |
Significant, multivariate |
N/A |
Based on our studies, we have found that double staining of tissue sections with D2–40 and a melanocytic marker is the most sensitive method to detect melanoma LI [12, 13, 28]. On sections stained with D2–40 alone, we occasionally observe single cells in the lymphatic vessels and it is difficult without doing S-100 staining to decide whether some of the single cells are melanoma cells or hematopoietic cells (Fig. 1a, b). A double stain more reliably and accurately detects single or cluster of melanoma cells in the lymphatic space (Fig. 1b, c).
Fig. 1.
Lymphatic invasion detected by double S-100 and D2–40 staining using light microscopy. Sections of melanocytic lesions were sequentially stained with D2–40 and S-100. (a) A single DAB-positive lymphatic endothelial cell is inside a lymphatic vessel highlighted by staining of endothelial cells with DAB. (b) A single Fast Red-positive melanoma cell is inside a lymphatic vessel highlighted by DAB-stained lymphatic endothelial cells. The hematopoietic cells in the lymphatic vessel are negative for Fast Red or DAB staining. (c) A cluster of Fast Red positive melanoma cells is inside a lymphatic vessel highlighted by DAB-stained lymphatic endothelial cells. Arrow heads point to D2–40 (DAB) positive endothelial cells aligning lymphatic vessel; arrows point to S-100 (Fast Red)- positive melanoma cells
1.3 Lymphatic Invasion, Lymphovascular Invasion, and Sentinel Lymph Status
The most powerful predictor of survival for patients with early-stage melanoma is the presence of regional lymph node metastases, currently assessed by sentinel lymph node (SLN) biopsy. Given that melanoma metastasizes through lymphatic channels to the regional lymph nodes, it is logical to consider invasion into lymphatic vessels by the primary tumor a sign of aggressive disease. Investigators have studied whether lymphovascular invasion and LI can predict SLN metastasis, thus identifying higher or lower risk patients to refine our patient selection for sentinel node biopsy.
In the Sunbelt Melanoma trial, 171/2183 (7.8 %) patients had lymphovascular invasion (LVI) identified by routine histology alone, and therefore did not specifically distinguish between LI and LVI [17]. Median follow-up was 68 months. Factors significantly associated with the presence of LVI included tumor thickness, ulceration, and histologic subtype (P < 0.05). LVI was associated with a greater risk of SLN metastasis (P < 0.05). In more recent studies, the presence of LI detected by IHC in primary melanoma has been shown to be independently predictive of the presence of SLN metastasis. Fohn et al. estimated the sensitivity, specificity, and predictive value of LI detected by D2–40 staining in patients with thin to intermediate thickness (Breslow thickness: ≤2.0 mm) melanomas [27]. Among the 64 patients in this study, 12 of 14 patients with D2–40 LI were SLN positive (positive predictive value, 85.7 %). D2–40 LI was detected in the primary biopsy specimen of 12 of 18 patients with a positive SLN (sensitivity 66.7 %). Of 50 patients without D2–40 LI, 44 were SLN negative (negative predictive value, 88.0 %), and among the 46 SLN-negative patients, 44 did not have D2–40 LI (specificity, 95.7 %). In their multivariate analysis, D2–40-detected LI was the only significant predictor of SLN status. Niakosari et al. demonstrated that LI identified by D2–40 was present in 15 of 23 SLN-positive cases (sensitivity, 65 %), whereas no lymphatic invasion was present in 56 of 73 SLN-negative cases (specificity 77 %) [21]. In addition, the assessment of LI detected by D2–40 had a positive predictive value of 46.9 % and a negative predictive value of 87.5 %. In their multivariate analysis, LI detected by D2–40 was significantly associated with SLN positivity (P = 0.01; odds ratio, 6.7; 95 % confidence interval, 1.64–27.5). However, in three other studies LI detected by immunohistochemical staining was found to be not associated with SLN status [23, 25, 26]. Therefore, the impact of LI (or LVI) on SLN metastases remains an area of controversy within the literature.
1.4 Lymphatic Invasion, Lymphovascular Invasion, and DFS/OS in Melanoma
In the initial report of the Sunbelt Melanoma trial where the median follow-up was 68 months and LVI was identified by histology, LVI was reported to be significantly associated with worse OS (P = 0.0009) by comparing the KM curves [17]. LVI was not an independent predictor of OS in the multivariate analysis. However in a subset analysis of patients with radial growth phase regression, the 5-year OS rate was 49.4 % for patients with LVI compared to 81.1 % those who did not have LVI (P < 0.0001).
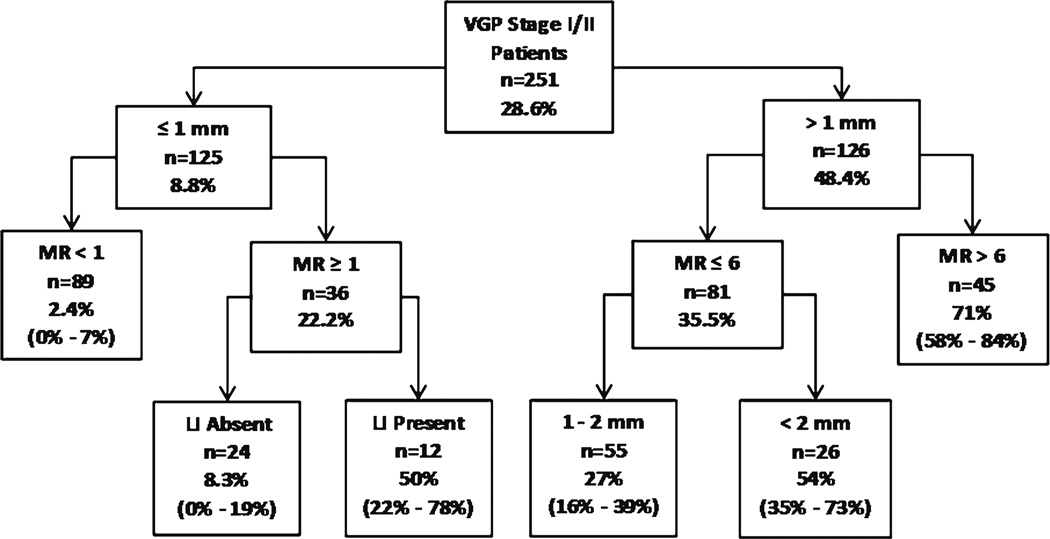
In another study of 251 melanoma patients with at least 10 years of protocol-driven, prospective follow-up and who had paraffin blocks available for immunohistochemical staining, double staining of S-100, and D2–40 identified 43 % of the primary melanomas had LI [28]. Four independent prognostic factors were identified in a multivariate model for 10-year metastasis: tumor thickness, mitotic rate, LI, and anatomic site. Of those who had a first metastasis within 10 years of treatment (n = 72), 65.2 % had LI (95 % CI = 54.3–76.3); of those without a 10-year metastasis (n = 179), only 34.0 % had LI (95 % CI = 27.2–41.0). LI was a significant independent prognostic factor (adjusted OR = 2.2). For the group of patients with thin melanomas, LI was also independently associated with 10-year metastasis with an unadjusted OR of 4.3. Interesting, in patients with thin melanoma with dermal mitotic activity (stage IB), LI can further distinguish two groups with different metastasis rates; the 10-year metastasis rate for those with LI was 50 % (95 % CI = 22–78) and it was six-fold higher than the rate of 8.3 % (95 % CI = 0–19) for those without LI (Fig. 2).
Fig. 2.
Prognostic tree developed using recursive partitioning with 10-year metastasis rates and 95 % CIs for each risk group and prognostic value of LI in thin melanoma (≤1 mm)
In another cohort of 246 melanoma patients with median follow-up time of 6.0 years, Rose et al. found that LI detected using IHC is a significant predictor of reduced DFS and OS in a multivariate model controlling for clinical stage [25]. In the multivariate model controlling for clinical stage at diagnosis (I/II vs. III/IV), LI detected using IHC markers remained a significant predictor of reduced DFS [hazard ratio (HR) 2.01; 95 % CI: 1.27–3.18] and OS (HR 2.08; 95 % CI: 1.25–3.46). Similar association of LI detected by D2–40 with DFS/OS was observed by Petersson et al. [23]. However, two other studies failed to show definitive associations of LI with DFS and OS in multivariate models [22, 26]. Nevertheless, one of the two studies did show a significant association of LI detected using IHC markers with the presence of microsatellites and with disease stage [26].
In conclusion, IHC-based lymphatic markers can reliably detect lymphatic vessels in formalin-fixed paraffin-embedded (FFPE) tissues. LI is underappreciated using H&E staining alone. LI detected by IHC has been demonstrated to be associated with significantly worse clinical outcome (SLN metastasis and/or DSF/OS) in 7/9 studies (Table 1), and it is likely predictive of regional nodal metastasis and prognostic of poorer measures of survival. In addition to prognostic value, LI detected by IHC may also have diagnostic value in further classifying atypical and ambiguous melanocytic lesions (i.e. melanocytic tumor of uncertain malignant potential). We are currently investigating the predictive value of LI detected by IHC in atypical and ambiguous melanocytic lesions. Some authors have proposed that detection of LI in primary melanoma using IHC markers should be incorporated in routine melanoma biopsy evaluation. Nevertheless, larger studies are needed to confirm the association of LI detected by IHC with SLN status and DFS/OS and to evaluate more precisely the potential contributions of this marker to multivariate predictive and prognostic modeling and to staging.
2 Materials
2.1 Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Sections
Immunohistochemistry (IHC) assays are performed on 5-µm-thick, formalin-fixed, paraffin-embedded sections.
2.2 Reagents for IHC Staining of FFPE Sections with D2–40 Antibody
Primary D2–40 antibody specific for podoplanin (mouse monoclonal antibody, to stain lymphatic vessels (Signet Laboratories, Dedham, MA).
Secondary antibody for use with mouse primary antibody: HRP-labeled polymer Dako Envision™ + System (DakoCytomation).
3,3′-diaminobenzidine (DAB) peroxidase substrate system produces brown reaction product for staining tissue sections. DAB is diluted using Dilution Buffer included in the Dako EnVision™ + System (DakoCytomation).
EDTA buffer: 1 mM EDTA, 0.05 % Tween 20, pH 8.0, for antigen retrieval.
Washing Buffer: 50 mM Tris–HCl, 150 mM NaCl, 0.05 % Tween 20, pH 7.6.
Diluted hydrogen peroxide solution for blocking of endogenous peroxidase. Prepare solution by diluting 5 ml of 30 % hydrogen peroxidase in 145 ml of deionized water.
Normal mouse serum (Jackson ImmunoResearch) (1:1,000 dilution) was substituted for the primary antibody in a negative control reagent.
Hematoxylin solution (Fisher Scientific).
Antibody diluent for preparing antibody solutions (DakoCytomation).
Coverslips.
Permount slide mounting medium (Fisher Scientific).
LiCO3 (30 mM, Sigma).
2.2.1 Reagents for IHC S-100 Staining
Rabbit polyclonal S-100 antibody, diluted, ready to use (DakoCytomation).
Secondary antibody conjugated to HRP-labeled polymer Dako Envision™ + System for use with rabbit primary antibody (DakoCytomation).
Fast Red chromogen system (Covance, Inc). Fast Red produces bright red reaction product for staining tissue sections. Prepare Fast Red substrate solution immediately before use by dissolving one Fast Red chromogen tablet in 5 ml of substrate buffer which is included in the system.
3 Methods
3.1 D2–40 Staining
Bake the slides in 45–50 °C oven overnight and let it cool for 30 min.
Deparaffinize sections in xylene, 3×, 5 min each time.
Hydrate with 100 % ethanol, 2×, 3 min each time and with 95 % ethanol, 1× for 1 min.
Rinse slides in deionized water 2×, 5 min each time.
Antigen Retrieval: Bring EDTA buffer to boil at 100 °C using a hot plate, place the slides in boiling buffer for 20 min. Allow them to cool for 20 min on bench top (see Note 1).
Wash sections in deionized water 3×, 5 min each time.
Incubate sections in diluted hydrogen peroxide solution for 10 min to quench endogenous peroxidase activity.
Wash sections in deionized water 3×, 5 min each time.
Wash sections in Washing Buffer 2×, 5 min each time.
Remove extra buffer from the slides, dilute primary Antibody D2–40 (1:25 dilution with Antibody Diluent) and incubate sections at room temperature for 1 h (see Note 2). Normal mouse serum (1:1,000 dilution in Antibody Diluent) is substituted for the primary antibody in negative control reagent.
Remove antibody solution by rinsing in Washing Buffer 3×, 5 min each time.
Incubate sections with secondary HRP-labeled antibody (antimouse) at room temperature for 30 min (see Notes 3 and 4).
Wash sections in Washing Buffer for 3×, 5 min each time.
Incubate sections in DAB solution at room temperature for 5–10 min (see Note 5).
Wash sections with Washing Buffer for 5 min.
Counterstain sections with Hematoxylin (not diluted) for exactly 30 s, dip in tap water, then in 30 mM LiCO3 solution for 10 s, and dip in tap water.
Dehydrate slides in ethanol (100 %) 3×, 3 min each time and then in xylene 3×, 3 min each time.
Coverslip mounting with Permount for permanent sealing. Apply 1–2 drops of Permount onto the tissue section and gently place the coverslip eliminating any air bubbles.
Examine the slides microscopically to identify tumor cells in D2–40 antibody-stained lymphatic vessels (brown) (see Note 6).
3.2 Double Staining with D2–40 and S-100 Antibodies
For the second color staining for detection of S-100 antigen please follow exactly the steps for the first color staining with D2–40 antibody using DAB peroxidase substrate described above in the Subheading 3.1 steps 1–15. After these steps are completed continue second color staining to visualize S-100 antigen using Fast Red peroxidase substrate as chromogen with the following steps (1–9) described below:
-
Remove extra buffer from the slides, apply diluted primary antibody S-100 to slides and incubate at room temperature for 30 min.
Remove antibody solution by rinsing in Washing Buffer 3×, 5 min each time.
Incubate the sections in secondary HRP-labeled antibody (anti-rabbit) in room temperature for 30 min.
Rinse sections in Washing Buffer 3×, 5 min each time.
Add Fast Red solution and let it incubate at room temperature for 10–20 min. Stop the reaction by immersing in deionized water.
Counterstain sections with Hematoxylin (no dilution) for exactly 30 s, dip in tap water, then in 30 mM LiCO3 solution for 10 s, and dip in tap water.
Dehydrate slides in ethanol (100 %) 3×, 3 min each time and then in xylene 3×, 3 min each time.
Coverslip with Permount as described above.
Examine the slides microscopically to identify melanoma cells (red) in lymphatic vessels (brown) (see Notes 6 and 7).
Acknowledgments
We would like to thank Jie Dai for technical assistance. This work was supported by NIH grant CA-093372.
Footnotes
Antigenic determinants masked by formalin fixation and paraffin embedding often may be exposed by heat-induced epitope retrieval, epitope unmasking using enzymatic digestion (e.g., trypsin) or saponin, etc. Different antigens may require different antigen unmasking methods. Do not use EDTA buffer boiling as antigen unmasking method with frozen sections or cultured cells that are not paraffin-embedded.
It is critical to keep the sections wet during the staining process. Drying at any stage will lead to nonspecific binding and ultimately nonspecific background staining. If staining is performed manually, placing wet tissue paper in the bottom of slide tray helps to prevent slides from drying.
Reducing of nonspecific binding by incubating sections with serum or immunoglobulin from the same species may be helpful to decrease background staining. However, we found that this step is unnecessary in the protocol that we use.
Incubation time with DAB, Fast Red, or other chromogens varies depending on the amount of tissue antigen expression and the antibody used. The optimal developing time should be determined empirically for each chromogen, each antigen and antibody, and the type of tissue immunohistochemically stained. In general DAB usually requires less time to develop than other chromogens such as Nova Red or Fast Red. Because melanomas often contain abundant melanin pigment which may be difficult to separate from DAB staining histologically, Nova red, Fast Red, or AEC (3-amino-9-ethylcarbazole) may be used as a chromogen when only one antibody is needed. The advantage of using DAB is that it is very stable and does not dissolved in alcohols or other organic liquids.
D2–40 (DAB) staining allows for rapid identification of endothelial cells and S-100 (Fast Red) allows for rapid identification of melanoma cells. With double staining, it is easy to identify a cluster of melanoma cells in the lymphatic vessels (Fig. 1c). However, single cell metastases are difficult to observe even with double staining (Fig. 1b). Therefore, it is critical to examine the slides carefully and thoroughly. Based on our experience we recommend that at least two well-trained observers assess slides to reach consensus regarding for lymphatic invasion.
Currently, LI is reported by pathologists as a binary variable (present or absent) based on light microscopy observation. Ability to assess the level of LI quantitatively (number of vessels containing melanoma cells and number of melanoma cells in the lymphatic vessels) might increase the prognostic value of LI in primary melanomas and future studies should consider reading LI quantitatively.
Supported provided by: Specialized Program of Research Excellence (SPORE) on Skin Cancer (P50-CA-093372).
References
- 1.Balch CM, Cascinelli N. Sentinel-node biopsy in melanoma. N Engl J Med. 2006;355:1370–1371. doi: 10.1056/NEJMe068147. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 3.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 4.Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giorgadze TA, Zhang PJ, Pasha T, et al. Lymphatic vessel density is significantly increased in melanoma. J Cutan Pathol. 2004;31:672–677. doi: 10.1111/j.0303-6987.2004.00249.x. [DOI] [PubMed] [Google Scholar]
- 6.Al-Rawi MA, Mansel RE, Jiang WG. Molecular and cellular mechanisms of lymphangiogenesis. Eur J Surg Oncol. 2005;31:117–121. doi: 10.1016/j.ejso.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 9.Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216:347–354. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- 10.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2011 doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 11.Gordon EJ, Rao S, Pollard JW, et al. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137:3899–3910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun SJ, Gimotty PA, Hwang WT, et al. High lymphatic vessel density and lymphatic invasion underlie the adverse prognostic effect of radial growth phase regression in melanoma. Am J Surg Pathol. 2011;35:235. doi: 10.1097/PAS.0b013e3182036ccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Gimotty PA, Guerry DP, et al. Lymphatic invasion revealed by multispectral imaging is common in primary melanomas and associates with prognosis. Hum Pathol. 2008;39:901–909. doi: 10.1016/j.humpath.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valencak J, Heere-Ress E, Kopp T, et al. Selective immunohistochemical staining shows significant prognostic influence of lymphatic and blood vessels in patients with malignant melanoma. Eur J Cancer. 2004;40:358–364. doi: 10.1016/j.ejca.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Dadras SS, Lange-Asschenfeldt B, Muzikansky A, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. J Cutan Pathol. 2005;32:84–84. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 16.Sahni D, Robson A, Orchard G, et al. The use of LYVE-1 antibody for detecting lymphatic involvement in patients with malignant melanoma of known sentinel node status. J Clin Pathol. 2005;58:715–721. doi: 10.1136/jcp.2004.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger ME, Gilbert JE, Burton AL, et al. Lymphovascular invasion as a prognostic factor in melanoma. Am Surg. 2011;77:992–997. [PubMed] [Google Scholar]
- 18.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JF, Shaw HM. Sentinel node mapping for melanoma: results of trials and current applications. Surg Oncol Clin N Am. 2007;16:35–54. doi: 10.1016/j.soc.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Schuchter L, Schultz DJ, Synnestvedt M, et al. A prognostic model for predicting 10-year survival in patients with primary melanoma. Ann Intern Med. 1996;125:369–375. doi: 10.7326/0003-4819-125-5-199609010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Niakosari F, Kahn HJ, McCready D, et al. Lymphatic invasion identified by monoclonal antibody D2–40, younger age, and ulceration: predictors of sentinel lymph node involvement in primary cutaneous melanoma. Arch Dermatol. 2008;144:462. doi: 10.1001/archderm.144.4.462. [DOI] [PubMed] [Google Scholar]
- 22.Doeden K, Ma Z, Narasimhan B, et al. Lymphatic invasion in cutaneous melanoma is associated with sentinel lymph node metastasis. J Cutan Pathol. 2009;36:772–780. doi: 10.1111/j.1600-0560.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 23.Petersson F, Diwan AH, Ivan D, et al. Immunohistochemical detection of lymphovascular invasion with D2–40 in melanoma correlates with sentinel lymph node status, metastasis and survival. J Cutan Pathol. 2009;36:1157–1163. doi: 10.1111/j.1600-0560.2008.01242.x. [DOI] [PubMed] [Google Scholar]
- 24.Petitt M, Allison A, Shimoni T, et al. Lymphatic invasion detected by D2–40/S-100 dual immunohistochemistry does not predict sentinel lymph node status in melanoma. J Am Acad Dermatol. 2009;61:819–828. doi: 10.1016/j.jaad.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Rose AE, Christos PJ, Lackaye D, et al. Clinical relevance of detection of lymphovascular invasion in primary melanoma using endothelial markers D2–40 and CD34. Am J Surg Pathol. 2011;35:1441. doi: 10.1097/PAS.0b013e31822573f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storr SJ, Safuan S, Mitra A, et al. Objective assessment of blood and lymphatic vessel invasion and association with macrophage infiltration in cutaneous melanoma. Mod Pathol. 2012;25:493–504. doi: 10.1038/modpathol.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fohn LE, Rodriguez A, Kelley MC, et al. D2–40 lymphatic marker for detecting lymphatic invasion in thin to intermediate thickness melanomas: association with sentinel lymph node status and prognostic value – a retrospective case study. J Am Acad Dermatol. 2011;64:336–345. doi: 10.1016/j.jaad.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Chen L, Guerry DP, et al. Lymphatic invasion is independently prognostic of metastasis in primary cutaneous melanoma. Clin Cancer Res. 2012;18:229–237. doi: 10.1158/1078-0432.CCR-11-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]