Abstract
Purpose of review
Added sugar (AS) consumption is associated with adverse health outcomes including weight gain and cardio-metabolic disease, yet the reliance on self-reported methods to determine AS intake continues to be a significant research limitation. The purpose of this review is to summarize recent advances in the development of two potential predictive biomarkers of added sugar intake: δ13C and urinary sugar excretion.
Recent findings
The results of numerous cross-sectional investigations have indicated modest associations of the δ13C sugar biomarker measured in a variety of sample types (e.g., fingerstick blood, serum, red blood cells, hair) with self-reported AS and sugar-sweetened beverage (SSB) intake, and δ13C values have been reported to change over time with changes in reported SSB intake. Results from large-scale trials have suggested modest associations of urinary sugar excretion with reported sugar intake, and a dose-response relation has been demonstrated between urinary sugar excretion and actual sugar intake.
Summary
Valid markers of sugar intake are urgently needed to more definitively determine the health consequences of AS intake. Adequately-powered controlled feeding studies are needed to validate and compare these two biomarkers of sugar intake, and to determine what individual characteristics and conditions impact biomarker results.
Keywords: Sugar, biomarker, dietary assessment, isotope, sucrose, fructose
Introduction
A large body of evidence has associated added sugars (AS) consumption with adverse health outcomes, including metabolic and cardiovascular diseases [1–3], weight gain [4, 5], and poor diet quality [6]. The World Health Organization recommends that “free” sugars (i.e., AS and sugars naturally present in honey, syrups, and fruit juices) be limited to less than 10% of total energy intake, and ideally to less than 5% [7], which is similar to the 10% or less AS intake recommendation in the United States[8]. Yet, current AS consumption patterns typically exceed these levels. For example, mean AS intake in the United States is 13% of total energy intake [9], and ranges from 7 to 17% in European populations [7]. Intake levels among children tend to be even higher – representing 16% of total energy among children in the United States [10], and 12 to 25% in Europe [7].
Aside from intake recommendations, significant controversy exists surrounding the health effects of AS [2, 11, 12], due in part to the methodological limitation of utilizing self-reported dietary intake assessment techniques [12, 13]. Under-reporting is a particular problem for total energy intake and for socially undesirable foods, such as those containing AS (e.g., sweets, sugar-sweetened beverages [SSB]) [13, 14]. The availability of an objective marker of dietary AS intake could overcome this research limitation [13, 14], strengthen the evidence-base for intake recommendations, and significantly advance research addressing the health consequences of AS consumption.
This review will describe recent advances in the development of two biomarkers of AS intake: δ13C in human tissues, which has been proposed as a predictive biomarker of corn and cane sugar intake, and urinary sugar excretion, which is a predictive biomarker of dietary sucrose and fructose intake.
Carbon Isotope Added Sugar Intake Biomarker: δ13C
Corn and cane plants utilize the PEP-carboxylase enzyme to pre-concentrate carbon dioxide prior to carbon fixation, a process known as “C4 photosynthesis”, and thus manufacture sugars naturally enriched in the heavy stable isotope of carbon (13C) [15 **]. As digested food is absorbed from the intestines into the bloodstream, the carbon within food becomes carbon within tissues. Thus, a high ratio of 13C:12C (measured as “δ13C”) in human blood may reflect a high ratio of 13C:12C in the diet, with corn and cane sugars as important contributors (Figure 1) [15**]. Because more than half of AS is consumed in the form of SSB, and the majority of the AS consumed in many countries is ultimately derived from corn and cane plants, the δ13C value of human blood may be affected by SSB intake [15**,18*]. Analysis of δ15N is sometimes concurrently performed with δ13C analysis; the resulting dual isotope model may be used to correct for the potential confounding effect of meat consumption, as much livestock is fed upon corn, a C4 plant [19,20].
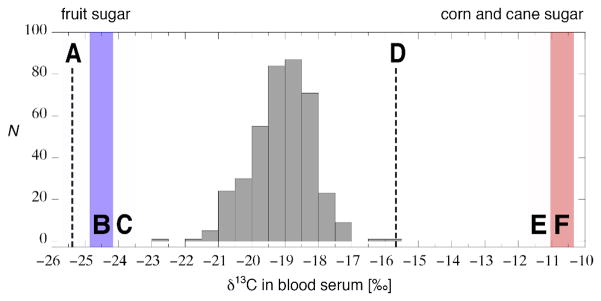
Figure 1.
Histogram of the carbon stable isotope composition of blood serum collected from 406 anonymous donors with unknown dietary history compared to values for low-added sugar menu items (A), fruit juice (B) and fruit sugar (C), as well as for high-added sugarmenu items (D), corn and cane sugars (E) and sugared sodas (F). [Data from Bostic and Jahren (unpublished results), 16, 17]
δ13C values can be determined using a variety of biological specimens, including blood (i.e., whole blood, serum, red blood cells [RBC], blood glucose, RBC alanine), bone, hair, nails, saliva, and urine. Specimen types reflect different time periods of AS intake (e.g., blood glucose reflects acute AS consumption, whole blood reflects consumption over 2–3 weeks; hair reflects consumption over 2–4 months) [15**]. Prior to isotopic analysis, samples are quantitatively combusted to CO2 (e.g., blood, nail and hair tissues) or separated from mixture using gas and/or liquid chromatography (e.g., blood lipids, blood glucose). Samples are then assessed for 13/12C16O2 and 15/14N2 using natural abundance stable isotope mass spectrometry. This technique requires minimal staff training for sample collection and storage for most sample types, no special storage or shipping conditions after collection, and can be performed on minimally invasive specimens (e.g., fingerstick blood, hair, nails). The equipment currently used for isotopic analysis is costly. However, with the advent of cavity ring-down spectroscopy technology, the future cost of high-precision δ13C analysis could decline significantly within the next decade, as combustion modules are integrated into easy-use continuous flow systems. Thus, the ability to measure δ13C value could become more widely available in the near future.
Recent Human Studies
Two recent reviews describe research investigating the δ13C method as a potential AS intake biomarker in substantial detail [15**,21**]. Studies of this biomarker published within the past 1–2 years are presented in Table 1. These studies included individuals with a range of AS and SSB intake (e.g., both low and high consumers) and weight status. Study populations have been primarily either white or Yup’ik Alaskan natives, and studies of adult populations have included a wide age range. Only two investigations have focused on children [22,23], who generally have a higher AS intake than adults. Most two studies were cross-sectional. To date, controlled feeding studies longer than seven days [28] have not been performed in adults or children to validate this AS biomarker; most existing validation studies have utilized self-reported dietary intake assessment methods to evaluate comparative validity.
Table 1.
Summary: Recent research* investigating the δ13C sugar intake biomarker
| Study | Substrate Assessed | Population | Design | Results | Comments |
|---|---|---|---|---|---|
| Chi et al., [22] | Hair | US children aged 6–17 years, Yup’ik Alaska Natives, 50% female (n=51) | Cross-sectional | 40g/d increase in AS intake (determined by hair δ13C analysis) was associated with 6.4% absolute increase in the proportion of carious tooth surfaces (p=0.02). No associations of self-reported sugary foods/beverage intake with tooth decay. | 49% of children reported consuming SSB 2–3 times/day. Weight status was not reported. Equation used to estimate AS intake from δ13C hair analysis was developed using prior data from 14–79 year old Yup’ik. |
| Davy et al., [23] | Finger-stick blood | US children aged 6–18 years, 92% white, 46% female (n=140) | Cross-sectional | δ 13C test-retest reliability, r=0.99 (p<0.0001). δ13C value was associated (P<0.0001) with self-reported SSB kcal, r=0.35. | Mean reported SSB intake was 116+11 kcal/d. 20% of sample was overweight/obese. |
| Fakhouri et al., [24] | Blood serum | US adults aged 25–79 years, 81% black, 66% female (n=144) | 18-month trial, subset of PREMIER | A 12 fl oz/d reduction in self-reported SSB intake was associated with 0.17‰ reduction in serum δ13C value (p<0.0001) over 18 months. | Results remained significant after controlling for multiple confounders, including corn consumption and δ 15N. Mean BMI=34kg/m2 |
| Hedrick et al., [19] | Finger-stick blood | US adults aged ≥18 years, 91% white, 77% female (n=257) | Cross-sectional | δ13C value was associated (p≤0.01) with AS (r=0.32) and SSB (r=0.39). Including δ15N resulted in minimal changes to the model’s ability to predict AS/SSB intake. | 74% of sample was overweight/obese. Mean reported SSB intake was 359±347 kcal/d. Non-sweetener corn intake was not associated with δ13C value. |
| Hedrick et al., [18] | Finger-stick blood | US adults aged ≥18 years, 94% white, 83% female (n=216) | Cross-sectional | Regression models demonstrated that HEI-2010 (i.e., overall diet quality)(R2=0.16), AS (R2=0.15) and SSB (R2=0.14) were all significant predictors of fingerstick δ13C value. HEI-2010 was significantly different across δ13C tertiles. | High habitual SSB consumers according to self-report (>200 kcal/d). Mean BMI=33kg/m2 |
| Nash et al., [25] | Red blood cells | US adults aged 19–94 years, 55% female, Yup’ik Alaska Natives (n=1076) | Cross-sectional | Total sugar intake was estimated via prediction equation using RBC δ13C and δ15N values; positive associations of sugar intake noted with BP and triglyceride concentrations; inverse associations noted with total-, HDL-and LDL-cholesterol concentrations. | 68% were overweight or obese; BMI and waist circumference were not associated with estimated sugar intake. |
| Nash et al., [26] | Plasma, plasma glucose, RBC, hair | US adults aged 14–79 years, Yup’ik Alaska Natives Two sets of participants (n=52 with complete data; n=68 with partial data; ~50% female overall) | Cross-sectional | RBC and hair dual isotope (δ13C and δ15N) models predicated self-reported total sugar (R2=0.52–0.53), AS (R2=0.47–0.48) and SSB intake (R2=0.34). Plasma dual isotope models predicted SSB (R2=0.28) but not total sugars or AS. Fasting plasma glucose was not associated with any of the self-reported sugar intake variables. | 55–56% in each sample were overweight or obese; most were >21 years or older (84–90% of sample). |
| Patel et al., [27] | Blood serum | UK adults aged 40–79 years, 59% female (total n=1178; 476 T2D cases and 718 in subcohort) | Case-cohort study, longitudinal | δ13C values were lower in cases than in the subcohort (p=0.009), and inversely associated with T2D (HR per tertile 0.74, p<0.001). No associations between δ13C values and sugar intake determined by FFQ. | European population with different primary sources of sugar intake than US samples. Mean BMI=26 kg/m2 |
Published in 2014–2015.
Abbreviations used: US = United States; AS = Added Sugar; SSB = Sugar-sweetened Beverage; PA = physical activity; BMI = body mass index; HEI-2010 = Healthy Eating Index – 2010; BP = blood pressure; HDL = high-density lipoprotein; LDL-low-density lipoprotein; UK = United Kingdom; T2D = type 2 diabetes; HR = hazard ratio; FFQ = food frequency questionnaire.
Results from the literature available are promising, in that significant associations have been reported between the δ13C sugar biomarker and self-reported total sugar, AS and SSB intake. Associations have also been noted between biomarker values and overall diet quality [18*], and with objectively assessed health outcomes, including carious tooth surfaces [22], indicators of cardio-metabolic risk [25*], and diabetes [27]. Changes in δ13C values also have been shown to change in response to reported changes in AS and SSB intake [24*]. Overall, the magnitude of associations are generally modest (i.e., r=0.3–0.4; r2=0.2–0.5), possibly in part due to reliance on self-reported dietary assessment methods as the primary method of comparison which could underestimate actual AS intake.
Strengths and Limitations
Advantages of this approach include the ability to use a wide variety of sample types – some which are minimally invasive – and the ease of sample collection, processing and storage. Disadvantages include the potential confounding effects of non-sweetener corn and meat consumption which may or may not be an issue depending upon the study population [19,20], an inability to reflect all dietary sources of AS (e.g., beet sugar), the potential impact of additives or preservatives to samples such as blood or urine [15**,21**], and analytical equipment requirements. However, analytical challenges may be more feasible in the future with the availability of an easily operable instrument that will be within the financial reach of most hospitals. Approaches which may be less sensitive but more feasibly implemented in large-scale, community-based trials (e.g., fingerstick blood) may be more better suited to studies in which categorizing AS intake levels is of interest. Therefore, the δ13C biomarker approach has the potential for widespread application – ranging from clinical- and field-based nutrition research to clinical practice.
Future Research Needs
The most pressing research need for this potential marker of sugar intake is a large-scale validation study, greater than 2–3 weeks in duration, using a controlled feeding design. A study with this design is also needed to evaluate the biomarker’s sensitivity to detect changes in AS intake over time. Study samples should include more diverse populations in terms of geography, race/ethnicity, and age, as variations in non-sweetener corn/meat consumption patterns, regional agricultural practices, and tissue turnover times may differ according to stage of growth [15**,21**]. More investigation is needed to examine a variety of specimen types [26*], in order to more definitively determine the time frame of AS consumption reflected by marker values [15**,21**]. Although challenging from an analytical standpoint, research is also needed to investigate HbA1C as a potential substrate, which may reflect habitual AS intake over longer periods of time.
Urinary Sugar Excretion: Total Sugar Intake Biomarker
When the disaccharide sucrose is consumed, most is broken down to glucose and fructose and absorbed in the small intestine. However, small amounts (i.e., mg quantities, or ~0.05% of intake) of intact sucrose are also absorbed in the small intestine and, along with fructose, excreted in the urine [29**]. This observation suggested that urinary sucrose and fructose excretion could represent a biomarker of recent sugar intake, although both AS and naturally-occurring sources of sugar (i.e., fructose from fruit/fruit juices) are reflected by the urinary sugar excretion measurement.
To evaluate urinary sugar excretion, two or more 24-hour urine samples are collected per participant. A preservative (e.g., thymol, boric acid) should be added to the collection container to prevent degradation of the excreted urinary sugars. Sample processing is minimal. Several analytical approaches can be used, including a commercially available assay kit and a spectrophotometer. To verify the completeness of 24-hour urine collections, para-aminobenzoic acid (PABA) may be administered to participants prior to sample collection in tablet form or added into study foods. PABA excretion is assessed in urine samples using a colorimetric analysis [30], and urine samples with ≥85% PABA recovery are generally considered complete and acceptable for inclusion in biomarker analyses (30). Spot urine samples have also been evaluated for urinary sugar excretion [29**,31*].
Recent Human Studies
A recent review describes prior investigations of the urinary sugar excretion method as a potential sugar intake biomarker in detail [29**]. Studies published within the past 1–2 years are presented in Table 2. Both studies included large sample sizes of adults, and reported associations of biomarker values with self-reported sugar intake [31*,32]. Tasevska and colleagues [32] utilized a biomarker-based calibration equation to predict total sugar intake, and compared this to self-reported intake in a US sample. Kuhnle and colleagues [31*] collected spot urine samples from a UK sample. In general, associations appear low to modest (i.e., r=0.2).
Table 2.
Summary: Recent research* investigating the urinary sugar excretion biomarker
| Study | Sample type | Population | Design | Results | Comments |
|---|---|---|---|---|---|
| Kuhnle et al., [31] | Spot urine sample | UK adults aged 39–77 years, 54% female (n=1734) | Longitudinal; 3-year follow up period | Baseline urinary sucrose concentration was associated with an increased risk of overweight/obesity and BMI at year 3, and with baseline self-reported sugar intake determined by 3 methods: 7 day diaries, 24-hr recall, and FFQ. | Self-reported sucrose intake was inversely associated with BMI. Mean baseline BMI =26kg/m2 |
| Tasevska et al., [32] | 24-hour urine sample | US adults aged 60–91 years, 100% female, postmenopausal 64% white (n=450) | Cross-sectional | Associations between (log) biomarker-based total sugars intake and self-reported intake using three methods (FFQ, 4d diary, three 24-hr recalls) ranged r=0.13 – 0.16. | Using biomarker values, calibration equations were used to predict total sugar intake. 66% of sample was overweight/obese. A second follow-up sample (n=88) was included for reliability assessment at 6 mo. |
Published in 2014–2015.
Abbreviations used: UK = United Kingdom; BMI = body mass index; FFQ = food frequency questionnaire; US = United States.
Strengths and Limitations
Advantages of this technique include the use of a minimally invasive sample type, minimal sample processing requirements, the availability of a commercially available assay kit for urinary sugar analysis, the possibility of performing assessments in spot urine samples, and the ability to be used in populations which consume a greater variety of sugar sources (e.g., beet sugar). However, limitations include the short time period reflected by this sugar biomarker (e.g., several hours to one day), which is not ideal for determining diet/disease associations as intake can vary over time, and the assessment of total sugar intake rather than AS, the latter of which is primarily targeted by dietary intake recommendations. Two or more 24-hour urine collections per participant are needed at each assessment point, which is burdensome for participants. Therefore compliance can be problematic, and should be assessed to insure that samples are complete, although possibly selectively [33]. Dose-response associations between sugar intake and biomarker values have been reported, yet there is substantial within-subject variability in sugar excretion at the same level of sugar intake [29**]. Results can be impacted by numerous factor such as differences in intestinal permeability, which can vary with gastrointestinal disorders, infections, non-steroidal anti-inflammatory drugs, and alcohol usage [29**].
Future Research Needs
Literature dating back several decades is available on the urinary sugar excretion marker of sugar intake, much more than on the carbon isotope sugar biomarker method in modern populations. Data are also available from several large-scale epidemiological trials, and at least two controlled feeding studies [30,34]. Several studies had more of an emphasis on developing and utilizing calibration equations from biomarker values to predict sugar intake. Controlled feeding studies are needed to better understand what individual characteristics and conditions impact intestinal absorption and sugar excretion, and thus biomarker values. To date, almost all studies have focused on adults [29**].
Conclusions
An ideal dietary biomarker is minimally invasive with a low participant burden, feasibly implemented in clinical and field research settings, inexpensive, valid, reliable, and sensitive to reflect changes in dietary intake. Both markers of sugar intake discussed in this review have shown promise, although both techniques have limitations including expensive instrumentation. A panel of urinary metabolites has also recently been suggested as a potential biomarker of acute SSB intake [35*].To advance this area of research, adequately-powered validation studies using a controlled feeding design, which would ideally compare the markers of sugar intake, are needed. Valid sugar intake biomarkers are urgently needed to more definitively determine the health impacts of AS intake on body weight and cardio-metabolic outcomes [2].
Key Points.
Controversy exists surrounding the health effects of added sugar intake, due in part to the well-recognized limitations of self-reported dietary assessment methods.
In recent years, two approaches to objectively assess added sugar intake have received the most attention – the carbon isotope biomarker δ13C, and the urinary sugars excretion biomarker.
Most studies evaluating the δ13C biomarker have been cross-sectional; only one short-term controlled feeding study has been conducted to date.
Dose-response associations between actual sugar intake and urinary sugar excretion have been reported, although individual variability is substantial at the same level of sugar intake.
Controlled feeding studies are needed to understand what individual characteristics and conditions impact biomarker values (e.g., age, disease states); the validity, reliability, and sensitivity of biomarker values using a variety of sample types and analytical approaches.
Acknowledgments
Financial support
This work was supported in part by the National Institutes of Health (R01CA154364 to J. Zoellner and R21HD078636 to B. Davy)
Abbreviations
- AS
added sugar
- BMI
body mass index
- BP
blood pressure
- C
carbon
- FFQ
food frequency questionnaire
- HDL
high-density lipoprotein
- HEI-2010
Healthy Eating Index – 2010
- HR
hazard ratio
- LDL
low-density lipoprotein
- PA
physical activity
- PEP
phosphoenolpyruvate
- SSB
sugar-sweetened beverage
- T2D
type 2 diabetes
- UK
United Kingdom
- US
United States
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Kell KP, Cardel MI, Bohan Brown MM, Fernandez JR. Added sugars in the diet are positively associated with diastolic blood pressure and triglycerides in children. Am J Clin Nutr. 2014;100(1):46–52. doi: 10.3945/ajcn.113.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanhope KL. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci. 2016;53(1):52–67. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, Zhang Z, Gregg EW, et al. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174(4):516–24. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong D, Bilger M, van Dam RM, Finkelstein EA. Consumption Of Specific Foods And Beverages And Excess Weight Gain Among Children And Adolescents. Health Aff (Millwood) 2015;34(11):1940–8. doi: 10.1377/hlthaff.2015.0434. [DOI] [PubMed] [Google Scholar]
- 5.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 6.Louie JC, Tapsell LC. Association between intake of total vs added sugar on diet quality: a systematic review. Nutr Rev. 2015;73(12):837–57. doi: 10.1093/nutrit/nuv044. [DOI] [PubMed] [Google Scholar]
- 7.Sugars intake for adults and children. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 8.FDA revises proposed Nutrition Facts label rule to include a daily value for added sugars [press release] 2015 Jul 24; [Google Scholar]
- 9.Ervin RB, Ogden CL. Consumption of added sugars among U.S. adults, 2005–2010. NCHS Data Brief. 2013;(122):1–8. [PubMed] [Google Scholar]
- 10.Ervin RB, Kit BK, Carroll MD, Ogden CL. Consumption of added sugar among U.S. children and adolescents, 2005–2008. NCHS Data Brief. 2012;(87):1–8. [PubMed] [Google Scholar]
- 11.Bray GA, Popkin BM. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! Pour on the sugar. Diabetes Care. 2014;37(4):950–6. doi: 10.2337/dc13-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn R, Sievenpiper JL. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: we have, but the pox on sugar is overwrought and overworked. Diabetes Care. 2014;37(4):957–62. doi: 10.2337/dc13-2506. [DOI] [PubMed] [Google Scholar]
- 13.Subar AF, Freedman LS, Tooze JA, et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J Nutr. 2015;145(12):2639–45. doi: 10.3945/jn.115.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhnle GG. Nutritional biomarkers for objective dietary assessment. J Sci Food Agric. 2012;92(6):1145–9. doi: 10.1002/jsfa.5631. [DOI] [PubMed] [Google Scholar]
- *15.Jahren AH, Bostic JN, Davy BM. The potential for a carbon stable isotope biomarker of dietary sugar intake. J Anal Atom Spectrom. 2014;29(5):795–816. This comprehensive tutorial review presents background information on the development of the carbon isotope biomarker of added sugar intake, including carbon isotope values in foods and in sources of added sugar. Investigations which included the carbon isotope biomarker assessed using a variety of specimen types and analytical approaches are reviewed. [Google Scholar]
- 16.Jahren AH, Saudek C, Yeung EH, et al. An isotopic method for quantifying sweeteners derived from corn and sugar cane. American Journal of Clinical Nutrition. 2006;84(6):1380–4. doi: 10.1093/ajcn/84.6.1380. [DOI] [PubMed] [Google Scholar]
- 17.Kraft RA, Jahren AH, Saudek CD. Clinical-scale investigation of stable isotopes in human blood: delta(13)C and delta(15)N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun Mass Sp. 2008;22(22):3683–92. doi: 10.1002/rcm.3780. [DOI] [PubMed] [Google Scholar]
- *18.Hedrick VE, Davy BM, Wilburn GA, et al. Evaluation of a novel biomarker of added sugar intake (delta 13C) compared with self-reported added sugar intake and the Healthy Eating Index-2010 in a community-based, rural US sample. Public Health Nutr. 2015:1–8. doi: 10.1017/S136898001500107X. This cross-sectional investigation examined associations between the fingerstick blood δ13C value and dietary outcomes including AS and SSB intake, and overall dietary quality (HEI-2010). The study was conducted in a rural community setting. An increase in HEI-2010 score was associated with a reduction in δ13C value, indicating that higher diet quality was linked to a lower AS intake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedrick VE, Zoellner JM, Jahren AH, et al. A Dual-Carbon-and-Nitrogen Stable Isotope Ratio Model Is Not Superior to a Single-Carbon Stable Isotope Ratio Model for Predicting Added Sugar Intake in Southwest Virginian Adults. J Nutr. 2015;145(6):1362–9. doi: 10.3945/jn.115.211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nash SH, Kristal AR, Bersamin A, et al. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup’ik study population. J Nutr. 2013;143(2):161–5. doi: 10.3945/jn.112.169425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.O’Brien DM. Stable Isotope Ratios as Biomarkers of Diet for Health Research. Annu Rev Nutr. 2015;35:565–94. doi: 10.1146/annurev-nutr-071714-034511. This review presents an overview of stable isotope ratios as dietary intake biomarkers. In addition to the carbon isotope biomarker of added sugar intake, nitrogen and sulfur stable isotopes are discussed as potential biomarkers of animal protein and fish intake, and seafood intake, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi DL, Hopkins S, O’Brien D, et al. Association between added sugar intake and dental caries in Yup’ik children using a novel hair biomarker. BMC Oral Health. 2015;15(1):121. doi: 10.1186/s12903-015-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davy BM, Jahren AH, MacDougall C, et al. Evaluation of d13C in fingerstick blood as a biomarker of sugar-sweetened beverage intake in children and adolescents: Preliminary findings. 9th International Conference on Diet and Activity Methods; September 9, 2015; Brisbane, Australia. abstract. [Google Scholar]
- *24.Fakhouri TH, Jahren AH, Appel LJ, et al. Serum carbon isotope values change in adults in response to changes in sugar-sweetened beverage intake. J Nutr. 2014;144(6):902–5. doi: 10.3945/jn.113.186213. This 18-month longitudinal investigation of the PREMIER trial (subset) reported changes in serum δ13C values and in self-reported SSB intake. A reduction in reported SSB intake of 12 fl oz/day was associated with a 0.17‰ reduction in δ13C value, thus biomarker values may be used to detect changes in SSB intake over time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Nash SH, Kristal AR, Bersamin A, et al. Isotopic estimates of sugar intake are related to chronic disease risk factors but not obesity in an Alaska native (Yup’ik) study population. Eur J Clin Nutr. 2014;68(1):91–6. doi: 10.1038/ejcn.2013.230. This cross-sectional study investigated the association of isotopic estimates of sugar intake, based upon RBC carbon and nitrogen isotope ratios, with anthropometric and cardio-metabolic outcomes in the Yup’ik Alaskan native population. Isotopic estimates of sugar intake were not associated with anthropometric variables, but were associated with blood pressure and blood lipid/lipoprotein concentrations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Nash SH, Kristal AR, Hopkins SE, et al. Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a Yup’ik study population. J Nutr. 2014;144(1):75–80. doi: 10.3945/jn.113.182113. This cross-sectional study evaluated the ability of dual-isotope (carbon and nitrogen) models using RBC, plasma, and hair to predict self-reported AS and SSB intake in the Yup’ik Alaskan native population, and determined that models using RBC and hair were the strongest predictive models. Fasting glucose δ13C was not associated with reported sugar intake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel PS, Cooper AJ, O’Connell TC, et al. Serum carbon and nitrogen stable isotopes as potential biomarkers of dietary intake and their relation with incident type 2 diabetes: the EPIC-Norfolk study. Am J Clin Nutr. 2014;100(2):708–18. doi: 10.3945/ajcn.113.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook CM, Alvig AL, Liu YQ, Schoeller DA. The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. J Nutr. 2010;140(2):333–7. doi: 10.3945/jn.109.114777. [DOI] [PubMed] [Google Scholar]
- **29.Tasevska N. Urinary Sugars--A Biomarker of Total Sugars Intake. Nutrients. 2015;7(7):5816–33. doi: 10.3390/nu7075255. This comprehensive review is focused on the development and validation of the urinary sugars excretion biomarker of total sugar intake. Applications of this biomarker approach include the development of calibration equations to account for measurement error in self-reported dietary intake assessment methods, and its use as a predictive biomarker of dietary sugar intake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tasevska N, Runswick SA, McTaggart A, Bingham SA. Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1287–94. doi: 10.1158/1055-9965.EPI-04-0827. [DOI] [PubMed] [Google Scholar]
- *31.Kuhnle GG, Tasevska N, Lentjes MA, et al. Association between sucrose intake and risk of overweight and obesity in a prospective sub-cohort of the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) Public Health Nutr. 2015;18(15):2815–24. doi: 10.1017/S1368980015000300. This prospective cohort study examined associations between self-reported sucrose intake, sucrose excretion assessed in spot urine samples, and BMI at three years of follow-up. Associations were noted between reported sucrose intake and urinary sucrose excretion, and between sucrose excretion and BMI at follow-up. An inverse association was reported between self-reported sucrose intake and BMI, yet a positive association was reported between sucrose excretion and BMI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasevska N, Midthune D, Tinker LF, et al. Use of a urinary sugars biomarker to assess measurement error in self-reported sugars intake in the nutrition and physical activity assessment study (NPAAS) Cancer Epidemiol Biomarkers Prev. 2014;23(12):2874–83. doi: 10.1158/1055-9965.EPI-14-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subar AF, Midthune D, Tasevska N, et al. Checking for completeness of 24-h urine collection using para-amino benzoic acid not necessary in the Observing Protein and Energy Nutrition study. Eur J Clin Nutr. 2013;67(8):863–7. doi: 10.1038/ejcn.2013.62. [DOI] [PubMed] [Google Scholar]
- 34.Joosen AM, Kuhnle GG, Runswick SA, Bingham SA. Urinary sucrose and fructose as biomarkers of sugar consumption: comparison of normal weight and obese volunteers. Int J Obes (Lond) 2008;32(11):1736–40. doi: 10.1038/ijo.2008.145. [DOI] [PubMed] [Google Scholar]
- *35.Gibbons H, McNulty BA, Nugent AP, et al. A metabolomics approach to the identification of biomarkers of sugar-sweetened beverage intake. Am J Clin Nutr. 2015;101(3):471–7. doi: 10.3945/ajcn.114.095604. This article describes a cross-sectional investigation that identified four urinary metabolites reflecting SSB consumption using a metabolomics approach, and a small study (n=10) to validate this panel of SSB biomarkers in urine following acute SSB consumption. This combination of SSB metabolites is a potential biomarker of acute SSB intake. [DOI] [PubMed] [Google Scholar]