Abstract
Aims
To examine among Hispanics in the U.S., a population with increased reliance on informal healthcare support structures, 1) the association between cognitive function and control of diabetes; and 2) whether this association is modified by family support.
Methods
The Digit Symbol Substitution Test (DSST), Word Fluency, and learning and delayed recall components of the Spanish English Verbal Learning Test were administered to 1,794 Hispanic adults aged 45–76 years with diagnosed diabetes. An executive function index and global cognitive function index (GCFI) were derived. Uncontrolled diabetes (HbA1c≥7% [53 mmol/mol]) was compared across quartiles of cognitive function using multivariable logit models with interaction terms for cognitive function and family support.
Results
After adjustment, lower DSST scores were associated with uncontrolled diabetes (P=0.03). Family support modified the relationship between other measures of cognition and diabetes control (Pinteraction: 0.002 to 0.09). Among individuals with low family support, as cognitive function declined, the odds of uncontrolled diabetes increased (P-trend across quartiles of the GCFI, 0.015). Among those with low family support, persons in the lowest quartile of global cognitive function were more than twice as likely to have uncontrolled diabetes as those in the highest performing quartile (OR=2.31; 95% CI: 1.17, 4.55). There was no similar effect among those with high family support.
Conclusions
Family support may buffer the negative association between low cognitive functioning and diabetes control in US Hispanics/Latinos. Educational programs targeted at family members of middle-age and older persons with diabetes regardless of neurocognitive status may help improve population-level glycemic control.
Keywords: Hispanic, cognitive function, diabetes, glycemic control, social support
1. Introduction
Both type 2 diabetes and cognitive decline are common problems in older adults [1,2] and both may disproportionally affect Hispanic individuals.[2–4] Type 2 diabetes is a risk factor for cognitive decline and dementia.[5–9] Cognitive problems, in turn, may make optimal glycemic self-control more difficult, potentially shifting the burden of care to others.[10,11]
Cognitive decline in older adults ranges from subtle executive dysfunction and memory difficulties to dementia.[1] Cross-sectional and longitudinal results from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial revealed inverse associations between cognitive function and glycemic control.[12] In a separate urban clinic-based study of older adults with diabetes, executive dysfunction and memory impairment were independent predictors of inadequate glycemic control.[13] Further cross-sectional studies have also reported a link between cognitive function and control of diabetes.[14,15]
Taken together, prior studies suggest that cognitive dysfunction may interfere with diabetes self-management and that poor diabetes control may contribute to cognitive decline. Diabetes self-management tasks, including close attention to diet, exercise, medication administration, and glucose monitoring, are cognitively demanding.[11] Evidence also suggests that family support improves diabetes control in older adults with impaired cognition. In the Health and Retirement Study (HRS) of over 1000 persons with self-reported diabetes ages 50 years and older, respondents in the lowest quartile of cognitive scores with low levels of social support had significantly higher hemoglobin A1c (HbA1c) levels than those in the highest quartile of cognition or those with high social support.[15] Importantly, higher levels of family support ameliorated this association between cognitive impairment and glycemic control. A more recent study of mild cognitive dysfunction in a largely Hispanic urban population found no association with glycemic control, though it was noted by the authors that assistance from informal care givers could not be ruled out.[16] Indeed, family support may be especially relevant to health outcomes among Hispanic individuals,[17–19] and particularly important for the cognitively-demanding activity of diabetes self-care.
In this study we hypothesized that lower levels of memory, executive functioning, and overall cognitive function would be associated with poor glycemic control in community-dwelling Hispanic/Latino adults with diabetes age 45 years and older. We also sought to examine whether these relationships were modified by family support.
2. Subjects, Materials and Methods
2.1 Study population and data collection
The Hispanic Community Health Study / Study of Latinos (HCHS/SOL) is a population-based study of 16,415 Hispanic adults age 18–74 years at recruitment living in 4 U.S. urban centers (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA). Participants were recruited using a 2-stage area probability sample design, as detailed previously.[20,21] The household-level response rate was 33.5% and 41.7% of screened individuals were enrolled, representing 16,415 persons from 9,872 households. Of 2,007 adults age 45–74 years at enrollment who reported a previous diagnosis of diabetes and/or taking antidiabetic medications within four weeks prior to the baseline interview, the current study was limited to those for whom complete data were available with regard to glycemic control, cognitive function, family support, and educational attainment (n=1,794 or 89%).
The HCHS/SOL baseline examination was conducted between 2008 and 2011 by bilingual interviewers in either English or Spanish, including blood collection, questionnaires covering a comprehensive assortment of sociodemographic, medical, environmental and lifestyle components, and a cognitive battery administered to those age 45 years and older. This study was approved by the Institutional Review Boards at each participating institution and all subjects gave informed consent.
2.2 Cognitive variables
The cognitive tests administered to HCHS/SOL participants have been previously described:[22] 1) Spanish English Verbal Learning Test (SEVLT), 2) Word Fluency (WF) Test, and 3) Digit Symbol Substitution Test (DSST). All examiners were trained to a common proficiency level in the scoring and administration of cognitive measures; certification was required prior to test administration and audio-taped exams from each examiner, with associated paper responses, were reviewed periodically by the Neurocognitive Reading Center to maintain certification throughout the examination period.[23]
The SEVLT is a measure of episodic verbal learning and memory constructed to be psychometrically equivalent in English and Spanish versions.[24] It consists of three 15-word learning trials, a fourth interference trial; and a fifth recall trial. We analyzed two measures from the SEVLT: 1) the sum of the first 3 trials to represent “learning,” with a maximum of 45; and 2) the fifth trial, termed “delayed recall,” which represents retention of the previously learned material. Both learning and delayed recall components of the SEVLT have been previously judged as similarly discriminant of cognitive decline regardless of language of administration.[25] In the WF test, participants were asked to produce as many words as possible beginning with the letter F, then the letter A, in consecutive 1-minute trials.[26] Unique English and Spanish words were accepted. In the DSST, the participant has 90 seconds to transcribe numbers into symbols using a key that pairs each of the digits 1–9 with a unique symbol.[27] The WF test and DSST were translated from English to Spanish and backtranslated from Spanish to English.[22]
A variety of methods have been used to assess executive function, tapping into the constructs of planning, attention control, cognitive flexibility, and verbal/nonverbal fluency.[28,29] Due to the complex, multi-construct nature of executive function, and based on prior research [29,30] supporting a latent variable approach to its measurement, we created an “executive function index” that was specified a priori by summing standardized scores ([individual value – mean value]/SD) from the DSST and WF. Though the DSST had traditionally been considered an index of processing speed, recent studies [31,32] have shown that DSST performance among older adults clearly involves inhibition, shifting and to a lesser extent updating, the three main components of executive function.[30] Preliminary internal consistency analyses of this executive function index yielded a satisfactory Cronbach’s alpha of 0.64 among HCHS/SOL participants age 45–76 years.
In addition, we created a global cognitive function index by summing standardized scores of DSST, WF, SEVLT learning, and SEVLT recall (alpha=0.77). The use of composite scores increases measurement precision and limits the impact of measurement error and confounding biases relating to any one test.[33] Composite scores may also reveal subtle cognitive deficits not apparent in a single test score [34] and have been shown to better track with disease-related cognitive decline than individual scores.[35,36] Quartile categorizations were created for each cognitive measure with the first quartile representing those with the lowest cognitive functioning.
2.3 Diabetes-related variables
Participants were asked to bring in all prescribed and over-the-counter medications taken within the past 4 weeks with them to the clinic examination. Medications were then scanned or transcribed and compared with a master drug list drawn from multiple commercial databases to assign therapeutic classification codes according to the convention of Medi-Span’s Master Drug Data Base (MDDB®, version 2, 2003). Those who reported not bringing in all current medications were followed up over the phone, with complete medications recorded for over 97% of participants. Therefore, participants taking insulin or oral hypoglycemic agents (OHGAs), were included in this analysis. In addition, those who self-reported taking “high blood sugar or diabetes” medications in the last 4 weeks, and those reporting a previous doctor diagnosis of diabetes that was not restricted to pregnancy, were also included. Participants taking insulin were identified either through review of medications or by an affirmative response to the question, “Are you being treated with insulin?” Since those taking insulin alone or in combination with OHGA had a similar prevalence of uncontrolled diabetes, and because relatively few took insulin, we created a single insulin treatment group.
Duration of diabetes (in years) was calculated by subtracting the reported age of diagnosis from the age at clinic visit. To assess diabetes control, blood specimens were collected and processed according to a standardized protocol.[21] HbA1c was measured in EDTA whole blood using a Tosoh G7 HPLC Analyzer (Tosoh Bioscience, Inc., South San Francisco, CA). In a repeatability study of HCHS/SOL participants of all ages (n=57), there was high test-retest reliability of HbA1c laboratory measures (r=0.96). Uncontrolled diabetes was defined as HbA1c ≥ 7% (53 mmol/mol).[37]
2.4 Other covariates
The Social Network Index (SNI), first described by Cohen and colleagues,[38] is a 13-item questionnaire of the diversity and size of participants’ social networks, and is commonly used to assess the number of people with whom the respondent has regular contact (at least once every two weeks).[39] HCHS/SOL participants completed an abbreviated 7-item version of the SNI consisting exclusively of questions regarding ties within the family. In accordance with recommended scoring of the full version of the SNI (http://www.psy.cmu.edu/~scohen/SNI.html), we summed item 2, “How many children do you see or talk to on the phone at least once every 2 weeks?” and item 7, “How many other relatives (other than your spouse, parents & children) do you feel close to?” and added 1 point if married or living with a partner, to approximate the number of non-parent relatives with close ties that could have provided support with diabetes management. Parents and parents-in-law were presumed to not play a supportive role in diabetes management in this middle-aged and older adult population. In bivariate and multivariable analyses, tertile cut-points were used to categorically classify levels of family support; a median split dichotomous variable was employed in interaction analyses to increase power.
Additional covariates considered were sex, age, educational attainment, health insurance status, field center, self-identified Hispanic/Latino background, depressive symptomology assessed with a 10-item version of the Center for Epidemiologic Studies Depression Scale (CESD-10),[40] language of cognitive test administration, and healthcare utilization as defined by the number of physician visits in the past 12 months. We also adjusted for body mass index (BMI; kg·m−2), as well as a history of cardiovascular disease (CVD), defined as self-report of a doctor-diagnosed previous stroke or heart attack, self-report of prior balloon angioplasty, stent or bypass surgery, or electrocardiographic indicative of previous myocardial infarction (MI). Lastly, an aggregate measure of physical disability from the participant’s perspective was derived from the Physical Component Score (PCS) of the SF-12 Health Survey Version 2.0 (QualityMetric Inc., Lincoln, RI), with the lowest quartile defined as “poor physical functioning.”
2.5 Statistical Analysis
In accordance with procedures standard to large population-based studies,[41] all reported values were non-response adjusted, trimmed, and calibrated by age, sex, and Hispanic/Latino national background to the characteristics of each field center’s target population from the 2010 U.S. Census. All analyses also account for cluster sampling and the stratification in the sample selection. P-values corresponding to Wald F-statistics and 95% confidence intervals (CI) were computed based on variance estimates derived from Taylor series linearization to account for the complex sampling scheme. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) and SUDAAN release 11.0.1 (RTI International, Research Triangle Park, NC), and all statistical tests are two-sided with a level of significance of 5%.
The prevalence of uncontrolled diabetes was examined among subgroups defined by covariates of interest. Logistic regression models were constructed with uncontrolled diabetes (HbA1c ≥ 7% [53 mmol/mol]) as the outcome of interest and quartiles of cognitive function as independent variables. Analyses were adjusted for potential confounders defined a priori. Participants with missing values for any of the covariates were excluded from all models (7% excluded, yielding n=1,661). Odds ratios and 95% CIs are presented with the highest performing group (group 4) serving as the reference. We also present p-values for a trend over quartiles of cognitive function, derived from fully adjusted models in which cognitive scores grouped in quartiles were entered into models as continuous variables. Quartiles analyses are presented as primary to better capture the nature of associations with the outcome. However, models using continuous cognitive measures were run in parallel for increased power.
Interaction models were fully-adjusted and contained interaction terms between quartiles of cognitive function measures and family support. Odds ratios and 95% CIs were computed using a common referent group within each model (high cognitive function and low family support). P-values for the interaction between family support and cognitive variables were computed with cognitive function quartiles entered into the model as continuous variables. Additional interaction models were constructed to test for heterogeneity across subgroups of age (60 years or older versus 45–60 years), gender, and antidiabetic medication use (none versus OHGA only versus insulin). Lastly, two sensitivity analyses were carried out. First, analyses were repeated while excluding individuals not taking antidiabetic medications and therefore presumably managing their diabetes through lifestyle modification. Second, because language of test administration was previously related to cognitive test performance in the HCHS/SOL population[22] and to exclude any artifactual influence of test language on the results, we conducted parallel analyses in which the minority of participants who were given the tests in English (n=237; 13% of the sample) were excluded.
3. Results
Included were 1,794 HCHS/SOL participants age 45 years or older with a previous diagnosis of diabetes, after exclusion of 11% with missing data for key variables. Subjects were predominantly female, under 65 years of age, and most were taking OHGA but not insulin (Table 1). Correlates of uncontrolled diabetes were younger age, OHGA and insulin use, more time since diabetes diagnosis, and less frequent use of healthcare services. Study participants reported a median of 6 close relatives excluding parents and parents-in-law (data not shown).
Table 1.
Diabetes Control as a Function of Demographics, Diabetes Indicators, and Neurocognitive Test Scores, among Hispanics with Diabetes Age 45 and Older Living in Four U.S. Urban Centers, 2008–2011
| n | Individuals with controlled HbA1c (<7% [53 mmol/mol]) (n=810)
|
Individuals with uncontrolled HbA1c (≥7% [53 mmol/mol]) (n=984)
|
Pa | |||
|---|---|---|---|---|---|---|
| mean/row % | 95% CI | mean/row % | 95% CI | |||
| HbA1c, mean % | 1794 | 6.2 | (6.2, 6.3) | 8.9 | (8.8, 9.1) | n/a |
| Sex | 0.92 | |||||
| Female | 1147 | 45.9 | (41.5, 50.4) | 54.1 | (49.6, 58.5) | |
| Male | 647 | 46.4 | (40.6, 52.2) | 53.6 | (47.8, 59.4) | |
| Age | 0.045 | |||||
| 45–54 | 648 | 41.3 | (36.5, 46.3) | 58.7 | (53.7, 63.5) | |
| 55–64 | 760 | 44.9 | (40.2, 49.8) | 55.1 | (50.2, 59.9) | |
| 65–76 | 386 | 52.3 | (45.9, 58.6) | 47.7 | (41.4, 54.1) | |
| Education | 0.84 | |||||
| 5th Grade or less | 418 | 47.8 | (40.2, 55.5) | 52.2 | (44.5, 59.8) | |
| Some high school | 470 | 44.0 | (37.6, 50.6) | 56.0 | (49.4, 62.4) | |
| High school graduate or equivalent | 366 | 45.0 | (38.6, 51.5) | 55.0 | (48.5, 61.4) | |
| Greater than high school | 540 | 47.5 | (41.5, 53.5) | 52.5 | (46.5, 58.5) | |
| Hispanic/Latino background | 0.21 | |||||
| Dominican | 147 | 50.6 | (40.3, 60.9) | 49.4 | (39.1, 59.7) | |
| Central American | 159 | 37.0 | (27.3, 47.8) | 63.0 | (52.3, 72.7) | |
| Cuban | 229 | 53.5 | (46.3, 60.6) | 46.5 | (39.4, 53.7) | |
| Mexican | 704 | 43.7 | (37.7, 49.8) | 56.3 | (50.2, 62.3) | |
| Puerto Rican | 444 | 43.4 | (38.6, 48.2) | 56.7 | (51.8, 61.4) | |
| South American | 80 | 47.6 | (33.9, 61.7) | 52.4 | (38.3, 66.1) | |
| Other/more than one background | 28 | 38.6 | (13.3, 72.0) | 61.5 | (28.0, 86.7) | |
| Health insurance status | 0.28 | |||||
| No | 576 | 43.1 | (37.0, 49.5) | 56.9 | (50.5, 63.1) | |
| Yes | 1203 | 47.5 | (43.5, 51.6) | 52.5 | (48.5, 56.5) | |
| Antidiabetic medications | <0.0001 | |||||
| None | 249 | 69.8 | (61.3, 77.1) | 30.3 | (22.9, 38.8) | |
| OHGA only | 1151 | 50.5 | (46.5, 54.6) | 49.5 | (45.4, 53.5) | |
| Insulin with or without OHGA | 394 | 18.5 | (13.8, 24.3) | 81.5 | (75.7, 86.2) | |
| Family support | 0.48 | |||||
| Low | 529 | 47.5 | (42.1, 53.0) | 52.5 | (47.0, 57.9) | |
| Med | 702 | 43.3 | (37.7, 49.1) | 56.7 | (50.9, 62.3) | |
| High | 563 | 48.1 | (42.1, 54.2) | 51.9 | (45.8, 57.9) | |
| Depressive symptoms | 0.41 | |||||
| CES-D < 10 | 1089 | 47.5 | (42.9, 52.2) | 52.5 | (47.8, 57.1) | |
| CES-D ≥ 10 | 691 | 43.8 | (38.0, 49.8) | 56.2 | (50.2, 62.0) | |
| Doctor visits in past year | 0.005 | |||||
| 0 | 160 | 31.5 | (24.1, 40.0) | 68.5 | (60.0, 75.9) | |
| 1 | 111 | 51.7 | (36.2, 66.9) | 48.3 | (33.1, 63.9) | |
| >1 | 1496 | 47.1 | (43.4, 50.8) | 52.9 | (49.2, 56.6) | |
| Duration of diabetes | <0.0001 | |||||
| 0–2 years | 395 | 62.7 | (53.9, 70.6) | 37.4 | (29.4, 46.1) | |
| 3–10 years | 731 | 46.8 | (41.2, 52.4) | 53.2 | (47.6, 58.8) | |
| > 10 years | 590 | 30.5 | (25.3, 36.3) | 69.5 | (63.7, 74.7) | |
| History of CHD or stroke | 0.49 | |||||
| No | 1502 | 46.8 | (43.3, 50.3) | 53.2 | (49.7, 56.7) | |
| Yes | 291 | 43.5 | (35.5, 51.7) | 56.6 | (48.3, 64.5) | |
| Self-report global physical functioning | ||||||
| Poor (bottom quartile) | 444 | 48.5 | (41.9, 55.1) | 51.5 | (44.9, 58.1) | |
| Moderate - high | 1333 | 45.3 | (41.8, 48.9) | 54.7 | (51.1, 58.2) | |
P-values correspond to Wald F-statistics derived from Taylor series linearization methods to account for the complex survey design
Abbreviations: BMI, body mass index; CHD, coronary heart disease; HbA1c, glycosylated hemoglobin; OHGA, oral hypoglycemic agents; NS, not significant; SEVLT, Spanish English Verbal Learning Test
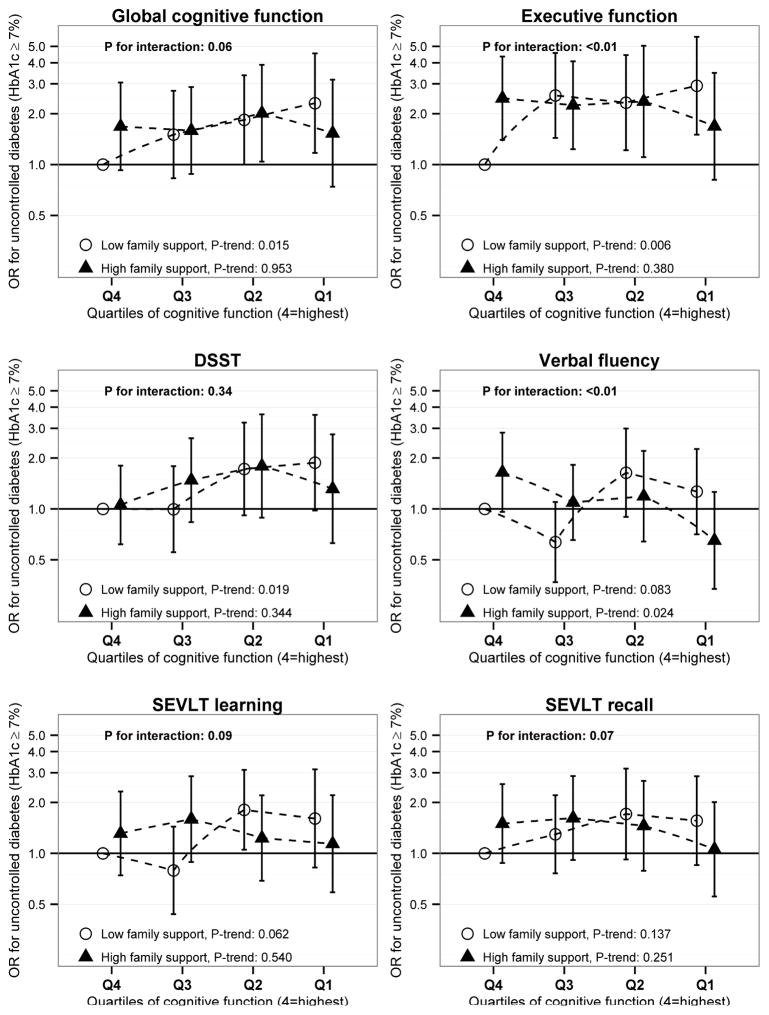
Table 2 shows the relative odds for uncontrolled diabetes (HbA1c ≥ 7% [53 mmol/mol]) among those with varying levels of cognitive function. In fully adjusted models we observed higher odds of uncontrolled diabetes with lower scores on the DSST (P-for-trend=0.03). We did not observe an overall association with respect to the other cognitive tests. However, interaction analyses (Figure 1) suggested that family support modifies the association of cognitive test performance with glycemic control. Specifically, associations between poor cognitive function and glycemic control were present only among the low family support group. Among individuals with low family support (0–6 close family members), those with lower quartiles of cognition had higher odds of uncontrolled diabetes compared to those in the higher quartiles for each test (Ptrend between 0.006 and 0.14 for each cognitive score). Among those with low family support, we observed a monotonic increase in the odds of uncontrolled diabetes over decreasing quartiles of global cognitive function, with those in the lowest quartile having 2.31 times the odds of uncontrolled diabetes than those in the highest quartile (95% CI: 1.17, 4.55). Individuals with low family support and membership in group 1 (low performance) for executive function index, the measure with the largest observed effect magnitude, was associated with a nearly three-fold increased odds for uncontrolled diabetes (OR=2.92, 95% CI: 2.04, 8.53), when compared to those with low family support and high executive function (group 4). These associations were not observed in those with high family support. Interactions were statistically significant between family support and both word fluency and the executive function index (P<0.01), with trends for interaction with family support observed for the global cognitive function index (P=0.06), as well as SEVLT learning (P=0.09) and delayed recall (P=0.07).
Table 2.
Level of neurocognitive function and relative odds of uncontrolled diabetes: Results from multivariable logistic regression a
| Neurocognitive function quartile, range b | n | OR for having HbA1c≥7% [53 mmol/mol] (95% CI) | P | P-trend |
|---|---|---|---|---|
| Global cognitive function index | ||||
| Quartile 4, [3.03, 11.3] (best function) | 413 | 1 (ref) | 0.08 | |
| Quartile 3, [0.26, 3.03) | 418 | 1.19 (0.78, 1.81) | 0.43 | |
| Quartile 2, [−2.35, 0.26) | 421 | 1.51 (0.94, 2.42) | 0.09 | |
| Quartile 1, [−11.2, −2.35) | 409 | 1.54 (0.90, 2.64) | 0.12 | |
| Executive function index | ||||
| Quartile 4, (1.51, 6.92] | 411 | 1 (ref) | 0.24 | |
| Quartile 3, [−0.03, 1.51] | 427 | 1.49 (0.98, 2.26) | 0.06 | |
| Quartile 2, [−1.56, −0.04] | 420 | 1.45 (0.85, 2.47) | 0.18 | |
| Quartile 1, [−5.9, −1.56) | 403 | 1.43 (0.82, 2.53) | 0.21 | |
| Digit Symbol Substitution Test | ||||
| Quartile 4, [39,81] | 453 | 1 (ref) | 0.03 | |
| Quartile 3, [30,38] | 385 | 1.19 (0.81, 1.76) | 0.37 | |
| Quartile 2, [21,29] | 444 | 1.74 (1.06, 2.88) | 0.03 | |
| Quartile 1, [0,20] | 379 | 1.62 (0.93, 2.81) | 0.09 | |
| Word fluency | ||||
| Quartile 4, [22,40] | 437 | 1 (ref) | 0.51 | |
| Quartile 3, [17,21] | 414 | 0.64 (0.42, 0.97) | 0.04 | |
| Quartile 2, [12,16] | 439 | 1.08 (0.68, 1.71) | 0.74 | |
| Quartile 1, [0,11] | 371 | 0.72 (0.42, 1.21) | 0.21 | |
| SEVLT learning | ||||
| Quartile 4, [26, 37] | 432 | 1 (ref) | 0.33 | |
| Quartile 3, [22, 25] | 444 | 0.99 (0.64, 1.53) | 0.97 | |
| Quartile 2, [18, 21] | 416 | 1.33 (0.89, 1.99) | 0.17 | |
| Quartile 1, [3, 17] | 369 | 1.21 (0.76, 1.92) | 0.43 | |
| SEVLT delayed recall | ||||
| Quartile 4, [10, 15] | 505 | 1 (ref) | 0.69 | |
| Quartile 3, [8, 9] | 466 | 1.18 (0.79, 1.78) | 0.41 | |
| Quartile 2, [6, 7] | 371 | 1.30 (0.84, 2.01) | 0.24 | |
| Quartile 1, [0, 5] | 319 | 1.09 (0.70, 1.71) | 0.69 | |
Uncontrolled diabetes defined as HbA1c ≥ 7% (53 mmol/mol); those with missing covariates excluded from all analyses; models adjusted for Hispanic/Latino national background, field center, education, age, and gender, health insurance status, oral hypoglycemic agent and insulin use, years since diabetes diagnosis, family support, depression, healthcare utilization in the past year, poor physical functioning, prevalent cardiovascular disease, and body mass index
Group 4, with higher levels of learning, memory, or executive function, served as the reference
Abbreviations: HbA1c, glycosylated hemoglobin; SEVLT, Spanish English Verbal Learning Test
Figure 1.
Cognitive function, family support and uncontrolled diabetes among Hispanics/Latinos over age 45-years with diagnosed diabetes, 2008–2011 (n=1,661). P-trend values test for a significant linear trend in the outcome across quartiles of cognitive dysfunction, within family support strata; models were adjusted for Hispanic/Latino national background, field center, education, age, and gender, health insurance status, oral hypoglycemic agent and insulin use, years since diabetes diagnosis, family support, depression, healthcare utilization in the past year, poor physical functioning, prevalent cardiovascular disease, and body mass index (BMI; kg·m−2).
Abbreviations: DSST, Digit Symbol Substitution Test; HbA1c, glycosylated hemoglobin; OR, odds ratio; SEVLT, Spanish English Verbal Learning Test
We also examined the association between family support and glycemic control within categories of executive functioning. Among those with the lowest level of executive function (group 1), high family support was associated with a trend for lower odds of uncontrolled diabetes (versus low family support, OR: 0.58; 95% CI: 0.30, 1.10; P=0.09). However, among individuals with high executive functioning (group 4), high family support was associated with significantly higher odds of poor glycemic control (versus low family support, OR: 2.46; 95% CI: 1.40, 4.34).
Further analyses in which cognitive scores were coded as continuous did not materially alter the results (data not shown). In addition, we did not observe effect measure modification by medication use, age, or gender, across all measures of cognitive functioning treated as either categorical or continuous (data not shown). Further adjustment for language of cognitive test administration in multivariable models did not appreciably alter effect estimates and sensitivity analyses excluding those who were administered the tests in English did not alter the results (data not shown). Of note, sensitivity analyses excluding participants previously diagnosed with diabetes but who were not taking OHGA or insulin within 4 months prior to the clinic visit yielded qualitatively similar results with somewhat increased magnitude of effect (data not shown). Results presented here included these individuals in order to avoid biases related to selective inclusion of those receiving medications.
4. Discussion
A cross-sectional analysis of diabetes control in community-dwelling Hispanic/Latino adults revealed complex relationships among cognitive function, family support, and glycemic control. We found that low levels of both a composite global cognitive function index and an index containing measures thought to represent executive functioning were both associated with increased odds of uncontrolled diabetes, but only among those with low family support. Statistically significant interaction or trends toward interaction with family support were consistent across multiple measures of cognitive functioning. In addition, while family support was not associated with glycemic control in the aggregate, there was a trend toward a protective association in those with low levels of executive functioning and a detrimental effect in those with high executive functioning.
Okura, et al. found a similar support-dependent association between cognitive functioning and glycemic control among the HRS participants with diabetes, suggesting social support may buffer the effect of cognitive function on glycemic control.[15] The present study lends support to a buffering role of social networks, and extends this finding to U.S. Hispanics with diabetes.
The relationship between type 2 diabetes prevalence and onset of cognitive impairment or dementia is well-established in prospective observational studies.[7,42] Additionally, evidence suggests that better cognition is associated with improved diabetes management. In one prospective study,[6] DSST was associated with diabetes control, a finding consistent with DSST results presented here. We speculate that the consistent association shown here and in previous analyses of diabetes control with DSST, but not with other cognitive measures when considered individually, may be representative of DSST performance as a measure involving multiple neuropsychological domains including attention, psychomotor speed, and executive function.[43]
Several cross-sectional studies have found that better cognitive function in persons with diabetes is associated with control of HbA1c.[12–15] A strong dose-dependent association was observed between glycemic control and executive function in an urban clinic-based study, as well as increased odds of inadequate control among patients with memory impairment.[13] In light of this finding, the lack of an effect of memory in the present study was unexpected. However, the older age and higher prevalence of co-morbid disease in the previous study, and the use of different cognitive tests may have contributed to the observed differences. Indeed, several of the cross-sectional studies finding an effect between cognitive function and diabetes control were conducted in older populations.[12,14,15]
A more recent prospective analysis of a largely Hispanic sample of elderly adults with diabetes failed to observe an effect of mild cognitive dysfunction on diabetes control, pointing to other contextual factors such as the social environment that could moderate the effect of cognitive decline on diabetes management.[16] The present study illustrates the importance of considering family support in such studies. Informal support with diabetes self-care may be of particular importance for older Hispanics/Latinos with diabetes. Findings presented here suggest that large family networks may buffer the effect of cognitive decline on impairment of diabetes control that has been observed in other populations.
Strengths of our study include the large population-based sampling design of HCHS/SOL allowing adequate control of several potential confounders and the uniform assessment of cognitive measures. However, there are some limitations, most notable being the nature of our family support measure. Hispanic individuals are generally believed to have closer ties with extended families than some other ethnic groups, which may have accentuated both the benefits and costs of large family networks. Our finding among individuals in the lowest quartile for some measures of cognitive function, in which increased family support tended to be associated with uncontrolled diabetes, likely reflects the quantitative rather than qualitative nature of our measure. We did not measure whether family members actually supported diabetes care. Broadhead and colleagues posit that an increase in social contacts may produce increased demands for reciprocal support and therefore have a detrimental impact on health in some settings.[44] The observation of this phenomenon in the present context highlights the fact that family members may be a burden on self-sustaining older adults with diabetes, and that efforts should be made to educate family members, regardless of mental and physical comorbidities of the patient.
Secondly, the relatively low household-level response rate in the study may have resulted in selection biases related to diabetes status, cognitive functioning, and family support. However, HCHS/SOL study employed a probability sampling design. Even though the response rate is not optimal, a widely accepted statistical adjustment protocol was followed to reduce the potential bias of effect estimates due to study non-participation.
Finally, the cross sectional design of the study precludes conclusions about the causal direction of the association between glycemic control and cognition. One case-control study found that mild cognitive impairment was associated with increased duration and severity of diabetes,[5] implying that increased exposure to unregulated glucose levels leads to cognitive impairment. Several biological mechanisms have been proposed to support this argument.[42,45] On the other hand, individuals with diabetes who have evidence of cognitive impairment are significantly less likely to be involved in diabetes self-care and diabetes monitoring.[46] If self-care is the only option due to lack of family support and access to healthcare resources, then cognitive dysfunction could lead to inadequate control. This may be particularly true in Hispanics/Latinos, a population with an increased reliance on informal healthcare support structures.[18,19] The buffering role of family connections in the present study supports this view.
Further work is necessary to understand the causal relationships between glycemic control, cognitive function, and family support among middle-aged and older Hispanics/Latinos. Our findings suggest that targeting diabetes control resources to individuals with low cognitive functioning and education programs aimed at family members of middle-aged and older adults with diabetes may help improve glycemic control in the population.
Acknowledgments
The authors thank the more than 16,000 participants who generously gave of their time and provided the study data. The authors also thank the more than 250 staff and investigators for their dedication and expertise. A complete list has been provided by Sorlie P., et al. in Ann Epidemiol. 2010 Aug;20: 642–649 and is also available on the study website: http://www.cscc.unc.edu/hchs/.
Funding
The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. Drs. Daviglus, Gonzalez, Kaplan, and Lipton also received funding from the National Institute on Aging (R01-AG48642).
Abbreviations
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- CESD
Center for Epidemiologic Studies Depression Scale
- CVD
cardiovascular disease
- DSST
Digit Symbol Substitution Test
- HCHS/SOL
Hispanic Community Health Study / Study of Latinos
- HRS
Health and Retirement Study
- OHGA
oral hypoglycemic agents
- SEVLT
Spanish English Verbal Learning Test
- WF
word fluency
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
G.S. analyzed the data and drafted the manuscript; R.C.K., H.M.G., and R.B.L. contributed to interpretation of data and manuscript preparation; E.G. created the analytic approach, designed the analysis, and contributed to interpretation of the data and manuscript preparation; M.L.D., A.L.G., and Y.T. contributed to interpretation of the data and made critical revisions to the manuscript. R.C.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. All authors have reviewed the current version and given approval to be published.
Sponsor’s role
The NHLBI participated in the design, methods, subject recruitment, and data collection of HCHS/SOL. However, the funding agencies were not involved in the planning, execution, or interpretation of the analyses presented here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirkman SM, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342–56. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noble J, Manly J, Schupf N, Tang M, Luchsinger J. Type 2 diabetes and ethnic disparities in cognitive impairment. Ethn Dis. 2012;22:38–44. [PMC free article] [PubMed] [Google Scholar]
- 3.Gurland B, Wilder D, Lantigua R, Stern Y, Chen J, Killeffer E, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;493:481–93. [PubMed] [Google Scholar]
- 4.Umpierrez GE, Gonzalez A, Umpierrez D, Pimentel D. Diabetes mellitus in the Hispanic/Latino population: an increasing health care challenge in the United States. Am J Med Sci. 2007;334:274–82. doi: 10.1097/MAJ.0b013e3180a6efe3. [DOI] [PubMed] [Google Scholar]
- 5.Roberts RO, Geda YE, Knopman DS, Christianson TJH, Pankratz VS, Boeve BF, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066–73. doi: 10.1001/archneur.65.8.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaffe K, Falvey C, Hamilton N, Schwartz AV, Simonsick EM, Satterfield S, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012;69:1170–5. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamport DJ, Lawton CL, Mansfield MW, Dye L. Impairments in glucose tolerance can have a negative impact on cognitive function: a systematic research review. Neurosci Biobehav Rev. 2009;33:394–413. doi: 10.1016/j.neubiorev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Strachan MWJ, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–14. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 9.Smolina K, Wotton CJ, Goldacre MJ. Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998–2011: a retrospective national record linkage cohort study. Diabetologia. 2015;58:942–50. doi: 10.1007/s00125-015-3515-x. [DOI] [PubMed] [Google Scholar]
- 10.Silliman R, Bhatti S, Khan A, Dukes K, Sullivan L. The care of older persons with diabetes mellitus: families and primary care physicians. J Am Geriatr Soc. 1996;44:1314–21. doi: 10.1111/j.1532-5415.1996.tb01401.x. [DOI] [PubMed] [Google Scholar]
- 11.Crandall J. Type 2 Diabetes: Geriatric Considerations. In: Goldstein BJ, Mueller-Vieland D, editors. Type 2 Diabetes Princ Pract. 2. New York: Informa Healthcare; 2008. pp. 479–88. [Google Scholar]
- 12.Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009;32:221–6. doi: 10.2337/dc08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grober E, Hall CB, Hahn SR, Lipton RB. Memory Impairment and Executive Dysfunction are Associated with Inadequately Controlled Diabetes in Older Adults. J Prim Care Community Health. 2011;2:229–33. doi: 10.1177/2150131911409945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munshi M, Capelson R, Grande L, Lin S, Hayes M, Milberg W, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29:1794–9. doi: 10.2337/dc06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okura T, Heisler M, Langa KM. Association between cognitive function and social support with glycemic control in adults with diabetes mellitus. J Am Geriatr Soc. 2009;57:1816–24. doi: 10.1111/j.1532-5415.2009.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palta P, Golden SH, Teresi J, Palmas W, Weinstock RS, Shea S, et al. Mild Cognitive Dysfunction Does Not Affect Diabetes Mellitus Control in Minority Elderly Adults. J Am Geriatr Soc. 2014 doi: 10.1111/jgs.13129. n/a – n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabogal F, Marin G, Otero-Sabogal R, Marin B, Perez-Stable E. Hispanic Familism and Acculturation: What Changes and What Doesn’t? Hisp J Behav Sci. 1987;9:397–412. doi: 10.1177/07399863870094003. [DOI] [Google Scholar]
- 18.Miranda A, Bilot J, Peluso P, Berman K, Van Meek L. Latino Families: The Relevance of the Connection Among Acculturation, Family Dynamics, and Health for Family Counseling Research and Practice. Fam J. 2006;14:268–73. doi: 10.1177/1066480706287805. [DOI] [Google Scholar]
- 19.Angel RJ, Angel JL, Himes CL. Minority Group Status, Health Transitions, and Community Living Arrangements among the Elderly. Res Aging. 1992;14:496–521. doi: 10.1177/0164027592144004. [DOI] [Google Scholar]
- 20.Lavange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–9. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: Results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol. 2014 Dec 1; doi: 10.1093/arclin/acu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hispanic Community Health Study. About the Study/Public Manual and Docs, Manual 9: Neurocognitive Function. 2008 v1.0. https://www2.cscc.unc.edu/hchs/sites/default/files/public_docs/09Neurocognitivev1002182008.pdf.
- 24.González HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc. 2001;7:544–55. doi: 10.1017/s1355617701755026. [DOI] [PubMed] [Google Scholar]
- 25.González HM, Mungas D, Haan MN. A Verbal Learning and Memory Test for English- and Spanish-speaking Older Mexican-American Adults. Clin Neuropsychol. 2010;16:439–51. doi: 10.1076/clin.16.4.439.13908. [DOI] [PubMed] [Google Scholar]
- 26.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 27.Lezak MMD. Neuropsychological Assessment. 2. New York: Oxford University Press; 1983. [Google Scholar]
- 28.Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17:213–33. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- 29.Salthouse Ta. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19:532–45. doi: 10.1037/0894-4105.19.4.532. [DOI] [PubMed] [Google Scholar]
- 30.Miyake A, Friedman NP, Emerson MJ, Witzki a H, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 31.Baudouin A, Clarys D, Vanneste S, Isingrini M. Executive functioning and processing speed in age-related differences in memory: contribution of a coding task. Brain Cogn. 2009;71:240–5. doi: 10.1016/j.bandc.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Albinet CT, Boucard G, Bouquet CA, Audiffren M. Processing speed and executive functions in cognitive aging: how to disentangle their mutual relationship? Brain Cogn. 2012;79:1–11. doi: 10.1016/j.bandc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, et al. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517–27. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. The Preclinical Alzheimer Cognitive Composite: Measuring Amyloid-Related Decline. JAMA Neurol. 2014;71:961–70. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langbaum JB, Hendrix SB, Ayutyanont N, Chen K, Fleisher AS, Shah RC, et al. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014;10:666–74. doi: 10.1016/j.jalz.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones R, Stout JC, Labuschagne I, Say M, Justo D, Roos R, et al. The Potential of Composite Cognitive Scores for Tracking Progression in Huntington ’ s Disease. J Huntingtons Dis. 2014;3:197–207. doi: 10.3233/JHD-140101. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35:S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–4. [PubMed] [Google Scholar]
- 39.Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic Amygdala - Cortical Functional Connectivity Predicts Social Network Size in Humans. J Neurosci. 2012;32:14729–41. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andresen E, Malmgren J, Carter W, Patrick D. Screening for depression in well older adults: Evaluation of a short form of the CES-D. Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 41.Kabadi SM, Lee BK, Liu L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: Results from the NHANES 2001–2006. Diabetes Care. 2012;35:2048–54. doi: 10.2337/dc12-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–9. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 43.Wechsler D. Wechsler Adult Intelligence Scale - Third Edition (WAIS III) San Antonio, Tx: The Psychological Corporation; 1997. [Google Scholar]
- 44.Broadhead W, Kaplan B, Sherman A, Wagner E, Schoenbach V, Grimson R, et al. The epidemiologic evidence for a relationship between social support and health. Am J Epidemiol. 1983;117:521–37. doi: 10.1093/oxfordjournals.aje.a113575. [DOI] [PubMed] [Google Scholar]
- 45.Luchsinger J. Type 2 diabetes and cognitive impairment: linking mechanisms. J Alzheimer’s Dis. 2012;30:1–18. doi: 10.3233/JAD-2012-111433.Type. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. Diabetes Res Clin Pract. 2000;50:203–12. doi: 10.1016/S0168-8227(00)00195-9. [DOI] [PubMed] [Google Scholar]