Abstract
Vascularized composite allografts (VCAs) are technically feasible. Similar to other organ transplants, VCAs are hampered by the toxicity and incomplete efficacy associated with conventional immunosuppression. Complications attributable to calcineurin inhibitors remain prevalent in the clinical cases reported to date, and these loom particularly large given the non-lifesaving nature of VCAs. Additionally, acute rejection remains almost ubiquitous, albeit controllable with current agents. Costimulation blockade offers the potential to provide prophylaxis from rejection without the adverse consequences of calcineurin-based regimens. In this study, we used a non-human-primate model of VCA in conjunction with immunosuppressive regimens containing combinations of B7-specific costimulation blockade with and without adhesion blockade with LFA3-Ig to determine what adjunctive role these agents could play in VCA transplantation when combined with more conventional agents. Compared to tacrolimus, the addition of belatacept improved rejection free allograft survival. The combination with LFA3-Ig reduced CD2hi memory T cells, however did not provide additional protection against allograft rejection and hindered protective immunity. Histology paralleled clinical histopathology and Banff grading. These data provide the basis for the study of costimulation blockade in VCA in a relevant preclinical model.
Introduction
To date over 150 patients have received VCAs (1). Similar to other transplants, recipients require long-term immunosuppression incurring lifelong risks of infection and metabolic disturbances. Given that VCAs are used to enhance quality of life, these risks impose a major concern for the appropriate use of VCAs. Typical complications reported for VCAs are attributable to calcineurin inhibitors (CNIs) and steroids (2, 3) Additionally, rejection rate in VCA is 85-90% within the first year (4, 5). Thus, the optimal regimen for VCA transplantation remains to be defined.
Costimulation blockade (CoB) has emerged as a promising strategy to prevent allograft rejection and avoid the deleterious side effects of CNIs. Belatacept (LEA29Y) is a second-generation CTLA-4Ig molecule blocking the CD28:CD80/CD86 pathway, with increased affinity for the B7 molecules and more potent immunosuppression.(6) Calcineurin and steroid free CD28 blockade-based therapies can prolong allogeneic islet and kidney survival in non-human primates (NHPs).(7, 8) Studies using CoB in NHPs have typically used the mTOR inhibitor sirolimus rather than CNIs, based on mechanistic synergies shown in lower species.(9)
Clinical experience using belatacept instead of CNIs has had higher rates of acute rejection attributable to memory T cells as a major component in CoB-resistant rejection.(10) The most alloreactive subset of effector memory T cells in humans is characterized as CD8+CD2hiCD28− cells,(10) and application of the CD2-specific LFA-3Ig (alefacept) has been demonstrated to improve renal allograft survival in NHPs by depleting this population.(10, 11) This is thought to be because T effector memory cells express higher concentrations of CD2 than naïve T cells, with LFA3-CD2 signaling as an essential component of activation. Alefacept interrupts this interaction on the T cell reducing activation and exerting a depletional effect on the memory T cell compartment via its Fc region.(12) For skin-containing VCAs, this effect may be particularly beneficial given its proven efficacy in eliminating autoimmune dermatopathology. (12) Additionally, this agent can act in conjunction with CD28 CoB agents by abrogating the memory T cell compartment to prevent graft rejection (11).
Materials and Methods
Animals
All procedures conformed to principles within the Guide for the Care and Use of Laboratory Animals, were approved by the Institutional Animal Care and Use Committee, and were conducted in accordance with Yerkes National Primate Research Center guidelines. Rhesus macaques (Macaca mulatta) were obtained from either the Yerkes National Research Center field station (Lawrenceville, GA) or AlphaGenesis, Inc (Yemassee, SC), quarantined, and housed under standards condition at the Yerkes National Primate Research Center (Atlanta, GA). Animals were tuberculin non-reactive and seronegative for SIV, STLV, SRV, and Herpes B virus.
Procedure
Pairs were selected based on ABO compatibility and MHC Class I non-identity. A radial forearm graft was performed as previously described excluding the bone.(13)
Groups
Animals in all treatment groups received methylprednisolone 15 mg/kg × 3 days with a taper over one month. Experimental groups had the following agents added: Group I tacrolimus (T); Group II CTLA-4Ig, alefacept, and sirolimus; Group III CTLA-4Ig, alefacept, and tacrolimus (CAT) with conversion to sirolimus; Group IV belatacept (replacing CTLA4-Ig), in addition to alefacept, and tacrolimus (BAT) with conversion to sirolimus; and Group V belatacept and tacrolimus (BT) with conversion to sirolimus (Figure 1). Intramuscular tacrolimus dosing was adjusted for target trough levels of 8-12 ng/mL and intramuscular sirolimus dosing was targeted for a range of 8-12 ng/mL with weekly monitoring of drug levels(13,24).
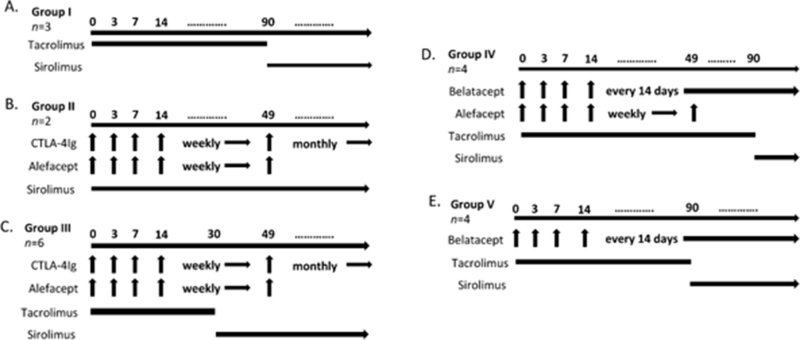
Figure 1.
Immunosuppression treatment groups.
A. Group I – Tacrolimus (T, n=3). Tacrolimus given twice daily intramuscularly.
B. Group II – CTLA-4 Ig, alefacept, sirolimus (CAS, n=2). CTLA-4 Ig 20 mg/kg and alefacept 1 mg/kg intravenously on days -1, 3, 7, and weekly thereafter. Alefacept was last administered on day 49 and CTLA-4Ig converted to monthly dosing at that time. Intramuscular sirolimus given daily.
C. Group III – CTLA-4 Ig, alefacept, tacrolimus conversion to sirolimus (CAT, n=6). CTLA-4 Ig 20 mg/kg and alefacept 1 mg/kg intravenously on days -1, 3, 7, and weekly thereafter. Alefacept was last administered on day 49 and CTLA-4Ig converted to monthly dosing at that time. Intramuscular tacrolimus given twice daily for 30 days, followed by daily sirolimus.
D. Group IV – Belatacept, alefacept, tacrolimus conversion to sirolimus (BAT, n=4). Belatacept 20 mg/kg intravenously on days 0, 3, 7, 14, and every other week thereafter. alefacept 1 mg/kg was administered intravenously on days -1, 3, 7, and continued weekly until last dose on day 49. Intramuscular tacrolimus given twice daily for 90 days, followed by daily sirolimus.
E. Group V – Belatacept, tacrolimus conversion to sirolimus (BT, n=4). Belatacept 20 mg/kg intravenously on days 0, 3, 7, 14, and every other week thereafter. Intramuscular tacrolimus given twice daily for 90 days, followed by daily sirolimus.
Monitoring
Allografts were inspected for wound healing, and changes in epidermal and hair growth. Skin biopsies were performed with visual changes and monthly. Viral reemergence was observed in the initial transplants, which prompted cytomegalovirus (CMV) prophylaxis with oral valganciclovir and weekly monitoring of CMV levels by polymerase chain reactions (PCR) testing (Table 1). Animals received intramuscular ganciclovir at 6mg/kg when levels exceeded 10,000 copies/mL of whole blood.
Table 1. Cytomegalovirus monitoring.
Cytomegalovirus (CMV) levels (copies/mL) were monitored in animals on a weekly basis, the table depicts the maximum level detected during the time course of the allograft. Not all animals received valganciclovir prophylaxis; this was instituted after deficits were noted in protective immunity affecting experimental endpoints. CAT1 and CAT2 did not have CMV levels consistently monitored and therefore are not shown.
| Group | Animal ID | Highest CMV level (copies/mL) | On prophlyasix (Y/N) |
|---|---|---|---|
| Group I | T1 | 500 | Y |
| T2 | 1500 | Y | |
| T3 | 1000 | Y | |
| Group III | CAT3 | 20,176,950 | N |
| CAT4 | 13,050 | N | |
| CAT5 | 230,225 | N | |
| CAT6 | 350 | N | |
| Group IV | BAT1 | 23,000,000 | N |
| BAT2 | 3,070,050 | N | |
| BAT3 | 850 | Y | |
| BAT4 | 14,150 | Y | |
| Group V | BT1 | 2800 | Y |
| BT2 | 6000 | Y | |
| BT3 | 1500 | Y | |
| BT4 | 200 | Y |
Polychromatic flow cytometry (PFC)
PFC was used to monitor the immunologic profile of the peripheral blood. Surface staining for CD2, CD3, CD4, CD8, CD20, CD28, and CD95 was carried out weekly with commercially available anti-human or anti-rhesus antibodies (BD Biosciences, San Jose, CA, USA). Cytometry was performed on a LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software version 9 (Tree Star, Ashland, OR, USA).
Donor-specific antibody (DSA) detection
DSA was measured at baseline and weekly for one month after transplant, then monthly. Donor lymphocytes were blocked with goat IgG (Jackson Immuno Research Laboratories, West Grove, PA, USA).Cells were then incubated with recipient serum followed by FITC-labeled goat anti-monkey IgG (KPL, Gaithersburg, MD, USA) and analyzed using flow cytometry. Positive control samples were obtained from historic NHP serum sensitized from allogeneic skin grafts. A positive DSA assay was determined to be alloantibody mean fluorescent intensity (MFI) twice the level of baseline control.
Statistical analysis
To eliminate lag-time bias due to variations from visual changes to biopsy, the time point of initial visual changes prior to a biopsy with confirmed rejection was used as the time point for analysis. Kaplan-Meier death censored rejection-free survival analysis was performed using a log-rank (Mantel-Cox) test using GraphPad Prism® version 6 (San Diego, CA).
Results
We have shown that untreated VCAs reject promptly and that CNIs can marginally extend rejection free allograft survival (Table 2B) (13). As has been seen clinically, most VCAs are preformed on CNI-based regimens and nevertheless have early rejection requiring rescue therapy. In order to revalidate these findings with contemporaneous controls, we performed three VCAs in NHPs using a tacrolimus-based regimen as described in the methods.
Table 2.
Summary of outcomes. (A) Study groups—all immunosuppression treated groups included a steroid taper over a course of 1 month. Animals that were sacrificed due to viral illness and failure to thrive did not demonstrate gross abnormalities on pathologic evaluation. (B) Historical controls previously published (13)
| (A) Group and regimen* | Animal ID | Initial visual changes prior to biopsy (POD) |
Biopsy (POD) |
Banff grade |
Study endpoint | DSA (Y/N) |
|---|---|---|---|---|---|---|
| Allograft control | Allo1 | 2 | rejection | N/A | ||
| No immunosupresion | Allo2 | 5 | rejection | N/A | ||
| Autograft control | Auto1 | negative | N/A | – | survival | N/A |
| No immunosuppression | Auto2 | negative | N/A | – | survival | N/A |
| Auto3 | negative | N/A | – | survival | N/A | |
| Group I | T1 | 14 | 15 | 1 | rejection | Y |
| Tacrolimus | T2 | 7 | 7 | 1 | rejection | N |
| T3 | 13 | 14 | 2 | rejection | N | |
| Group II | CAS1 | 14 | 14 | N/A | graft failure | N |
| CTLA-4Ig, alefacept, sirolimus | CAS2 | 5 | 9 | N/A | graft failure | N |
| Group III | CAT1 | 56 | 62 | 1 | rejection | N |
| CTLA-4Ig, alefacept, tacrolimus with conversion to sirolimus |
CAT2 | 63 | 63 | 1 | rejection | N |
| CAT3 | none | 41 | 0 | failure to thrive (cuthanasia) |
N | |
| CAT4 | none | 65 | 0 | failure to thrive (cuthanasia) |
N | |
| CAT5 | 84 | 92 | 3 | rejection | N | |
| CAT6 | 42 | 49 | 3 | rejection | N | |
| Group IV | BAT1 | none | 56 | 0 | failure to thrive (cuthanasia) |
N |
| Belatacept, alefacept, tacrolimus with sirolimus conversion |
BAT2 | 85 | 90 | 3 | rejection | N |
| BAT3 | none | 73 | 0 | failure to thrive (cuthanasia) |
N | |
| BAT4 | 21 | 21 | 2 | rejection | N | |
| Group V | BAT1 | 28 | 28 | 2 | rejection | N |
| Belatacept, tacrolimus with sirolimus conversion |
BAT2 | none | 77 | 1 | failure to thrive (cuthanasia) |
N |
| BAT3 | 98 | 133 | 3 | rejection | N | |
| BAT4 | 112 | 140 | 3 | rejection | N |
| (B) Group and regimen* | Animal ID | Day of rejection | Donor specific antibody (Y/N) |
|---|---|---|---|
| 1. DSA, donor-specific antibody; MMF, mycophenolate mofetil; POD, postoperative day. | |||
| Allograft controls without immunosuppression | Hist1 | 3 | Y |
| Hist2 | 4 | N | |
| Hist3 | 4 | N | |
| Hist4 | 3 | N/A | |
| Hist5 | 7 | Y | |
| Hist6 | 4 | Y | |
| Hist7 | 8 | Y | |
| Triple therapy (tacrolimus, MMF, and steroid taper) | Hist8 | 15 | Y |
| Hist9 | 5 | N | |
| Hist10 | 17 | Y | |
| Hist11 | 24 | Y | |
| Hist12 | 8 | Y | |
| Hist13 | 73 | Y | |
| Hist14 | 76 | Y | |
Three animals received VCAs using the basic methylprednisolone and tacrolimus regimen. All animals rejected within the first 15 days after transplant. One animal developed DSAs. All animals received CMV prophylaxis and serum viral loads did not approach treatment threshold.
To mirror prior studies using CoB in kidney transplantation in lieu of tacrolimus, we performed two transplants using CTLA4-Ig, alefacept, and sirolimus.(10, 11) Both allografts failed to heal at the skin edges and the deeper tissues lasting 14 days, and wound failure was seen in native and allograft skin (Figure 2) indicating that this was a non-allospecific wound failure. Histology demonstrated infiltrates consisting predominately of monocytes with fewer lymphocytes, supporting a generalized inflammatory response and inconsistent with rejection. Thus, while this regimen might be efficacious in preventing rejection, the use of sirolimus, with its known impediments related to wound healing, were impractical for VCAs. Consequently, we modified succeeding regimens to start with tacrolimus and convert to sirolimus.
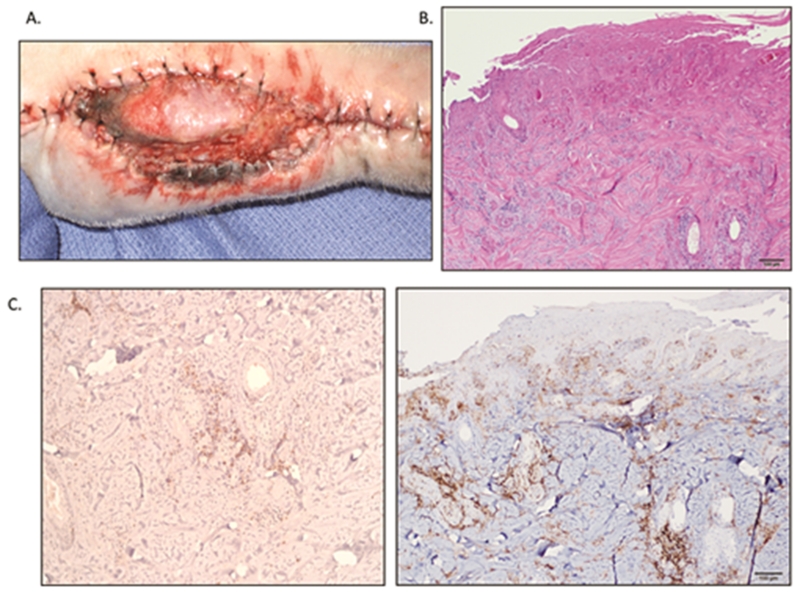
Figure 2.
Wound healing complications of a VCA treated with sirolimus.
A. Macroscopic appearance of CAS1 on POD 14. Note the poorly healed skin edges and edema not only on the graft-native border, but also between the native skin edges as well. There is poor granulation of the exposed tissues in the open wound.
B. Biopsies taken from (A) demonstrate significant infiltrates on skin H&E stain (5× magnification).
C. Further immunohistochemistry analysis of the biopsy revealed a far greater macrophage (left image) than T-cell (right image) response, suggesting a generalized inflammatory response as opposed to rejection.
We performed six transplants using CTLA4-Ig, alefacept, and tacrolimus with sirolimus conversion for long-term survivors. Two transplants CAT1 and CAT2, remained rejection free until day 62 and 63 respectively. Histology for both biopsies revealed Banff 1. Serum CMV levels were not obtained in these animals.
Two transplants, CAT3 and CAT4, were sacrificed on days 41 and 65 for weight loss due to viral illness. Peak CMV viral loads were 2 × 107 and 1.3 × 104 copies/mL respectively (Table 1). Both allografts were free of macroscopic signs of rejection and demonstrated Banff 0.
One transplant (CAT5) remained without gross changes for over two months, with a protocol biopsy on POD 77 demonstrating Banff 0. A petechial rash arose on POD 84. This progressed to a more severe lesion including erythema, induration, petichiae, scaling/flaking, epidermal necrosis, and hair loss. Biopsy at this time on POD 92 revealed a Banff III.
One transplant (CAT6) began developing a diffuse scaly rash on POD 42. The gross appearance progressed to include edema and mild epidermal sloughing, biopsy performed on POD 49 demonstrated Banff III.
Out of this study group, only four animals were monitored for CMV infection, with all 4 developing detectable CMV viremia. Two animals were ultimately sacrificed for weight loss consistent with systemic illness. Other infectious complications included a gram-positive bacteremia in one animal, and Entomoeba histolytica diarrhea in one animal.
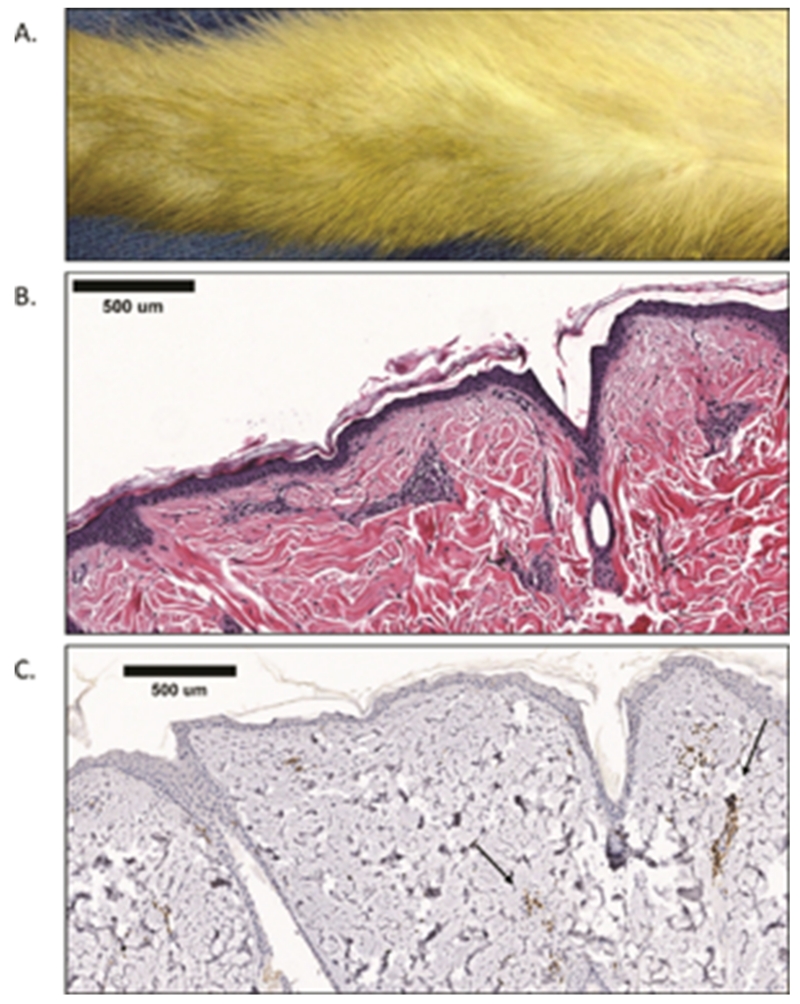
As these studies were taking place, the FDA approved belatacept for kidney transplantation. Thus, we chose to change from CTLA4-Ig to belatacept, to make our studies more clinically translatable and to mirror subsequent experience in NHP renal transplantation (8). Four animals received belatacept, alefacept and all succumbed to problems associated with control of protective immunity. The first transplant (BAT1) was sacrificed on POD 56 due to weight loss. This animal did not receive CMV prophylaxis and had a peak of 2.3 × 107 viral copies/mL at the time of sacrifice. The allograft was macroscopically similar to baseline and demonstrated Banff 0 skin histology (Figure 3).
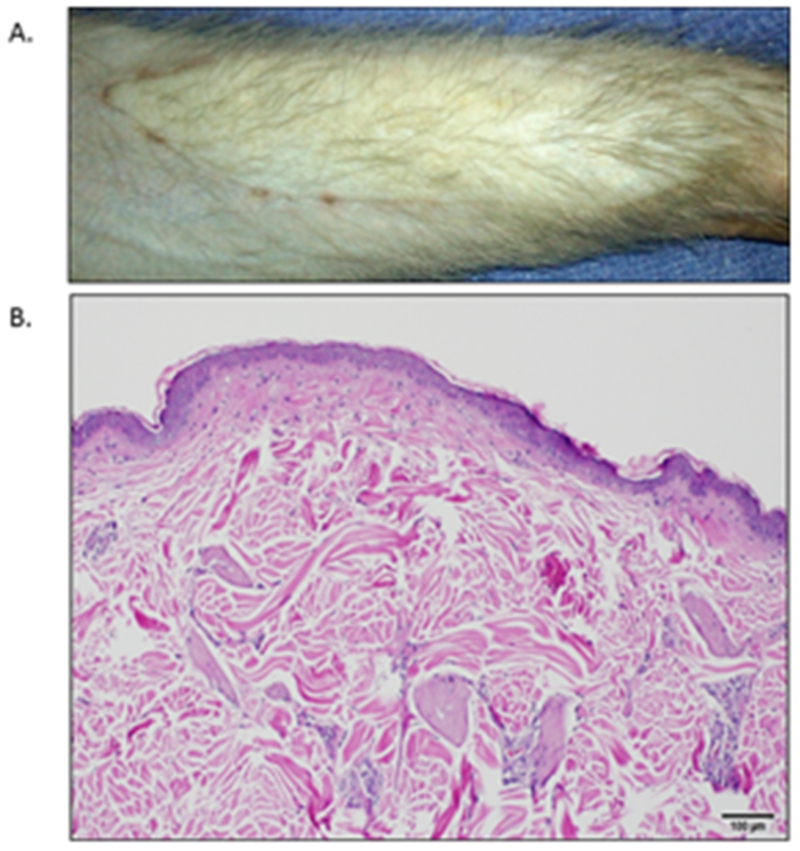
Figure 3.
Engraftment without rejection.
A. Macroscopic appearance of BAT1 on POD 56 at the time of sacrifice. This allograft has well healed skin edges with no hair loss and no gross abnormalities or rash present.
B. Skin biopsy from (A) with H&E staining shown (5× magnification). No leukocyte infiltrate and normal appearance of epithelial and dermal tissues demonstrate Banff 0.
The second transplant (BAT2) experienced weight loss and viral illness with immunosuppression adjusted with reduction by POD 78. CMV viral copies/mL peaked to 3×106. No visual skin changes were observed until after POD 84. Due to progression of weight loss, the animal was sacrificed on POD 90, with histology demonstrating Banff III. The third transplant (BAT3) was sacrificed on day 73 due to significant weight loss, with an allograft devoid of skin changes and Banff 0.
The fourth transplant (BAT4) had a rash on POD 21 with a Banff II rejection (Figure 4). Adjustment of tacrolimus dosing to target the upper therapeutic limit resulted in improvement of the rash similar to BT1. The graft did not demonstrate any visual changes until day 105. At this time the animal was sacrificed due to significant weight loss and a CMV infection with a maximum of 1.4×104 viral copies/mL. Macroscopically the skin demonstrated small areas of light pink rash with few scattered petichiae and preserved hair growth. Histology revealed Banff II rejection with prominent perivascular inflammation with notable endarteritis and fibrinoid change.
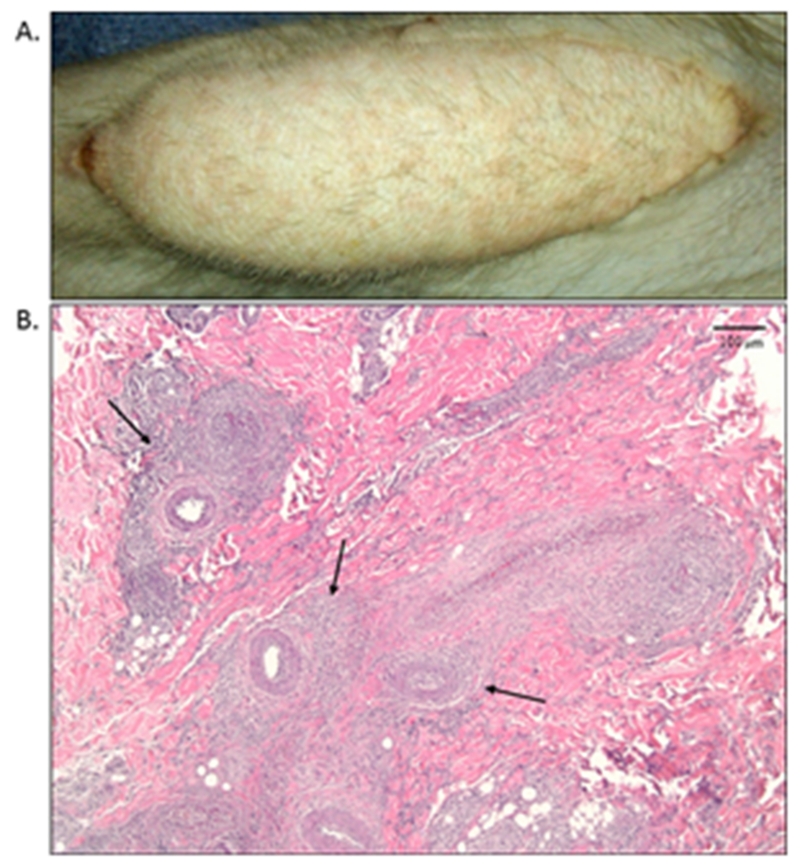
Figure 4.
Rejection of a VCA treated with dual costimulation blockade.
A. Macroscopic appearance of BAT4 on POD 21. A petechial rash is newly present, however no significant hair loss is noted from baseline.
B. Skin biopsy from image (A) with H&E staining shown (5× magnification). Significant perivascular infiltration is noted (arrows) with no epithelial involvement, demonstrating a Banff II.
To reduce problems with protective immunity we excluded alefacept from four animals that received VCAs with methylprednisolone, tacrolimus and belatacept, with sirolimus conversion for long-term survivors. Graft survival was significantly prolonged compared to Group I (mean rejection free survival = 78.8 days, p =0.01). These grafts proceeded as follows. The first transplant (BT1) presented a patchy erythematous rash on POD 28, biopsy demonstrated Banff I. Tacrolimus trough level was 3.6 mg/dL, subsequently the dose was adjusted to target levels and the rash disappeared. Significant weight loss led to the sacrifice of the animal on POD 44 with and excisional biopsy demonstrating Banff 0.
The second transplant (BT2) had immunosuppression withdrawn and graft excision on POD 77 due to weight loss from viral infection. No visual changes were observed, histology demonstrated Banff I. (Figure 5).
Figure 5.
VCA treated with belatacept and tacrolimus.
A. Macroscopic appearance of BT2 on POD77 at time of sacrifice. No significant changes were noted on gross inspection, minimal hair loss, well healed skin edges, and no rash was observed.
B. Protocol skin biopsy was taken from (A) with H&E staining shown (10× magnification). Mild perivascular infiltration is present with no significant epithelial abnormalities noted demonstrating a Banff I.
C. Immunohistochemistry from biopsy (B) using a CD3 stain confirms lymphocyte infiltration as demonstrated by arrows.
The third transplant (BT3) had a macroscopically normal appearing allograft up to 4 months post-transplant. At the time of graft excision on day 133, the graft was swollen with erythematous, indurated skin and significant hair loss. Histology demonstrated significant lymphocytic infiltration with epithelial dyskeratosis - a Banff III rejection.
The fourth transplant (BT4) had sparse light pink macules on allograft skin on day 28, which was accompanied by a similar rash at the ipsilateral axilla. Skin biopsy on POD 35 was Banff 0. The rash resolved without treatment. A protocol biopsy on POD 100 was Banff 0, with no gross abnormalities of the skin present. On POD 112 the graft developed areas of erythema and induration with epidermal scabbing. The graft was excised on POD 140, histology demonstrated Banff III.
A summary is depicted in Table 2.
The addition of costimulation blockade delays rejection in VCA
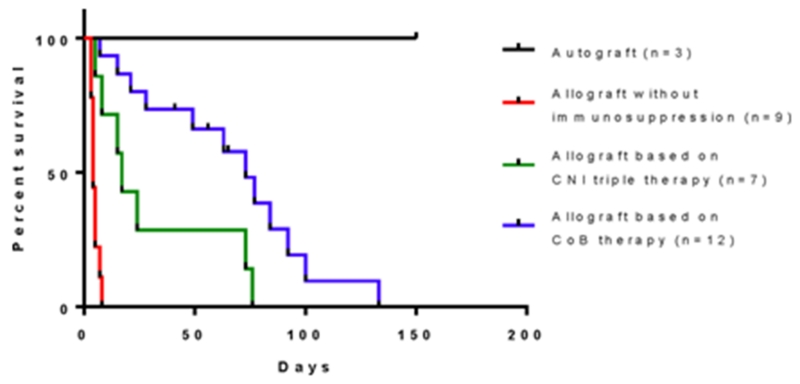
Overall comparison of the four treatment groups revealed that the addition of CoB improved rejection free survival compared to tacrolimus with a steroid taper alone (T vs BT p=0.01, vs BAT p=0.01, vs CAT p<0.01). Alefacept combined with belatacept did not benefit costimulation blockade when compared to belatacept alone (BT vs BAT p=0.60, BT vs CAT p=0.34). When adding the historic control data the p-value for the log rank comparing all animals treated with CNI’s based therapy to all animals treated with CoB was .0143 (Figure 6).
Figure 6.
Overall rejection-free survival of vascular composite allografts. Rejection in this analysis was determined by time of initial visual changes leading to a biopsy proven rejection to minimize variability from time of visual changes to biopsy. Addition of CoB to tacrolimus resulted in improved rejection-free survival when compared to historic controls (p=0.0143 vs combined CoB).
Alefacept diminishes CD2hi CD8+ T cells long term but only transiently reduces CD2+ memory effector T cells when added to belatacept
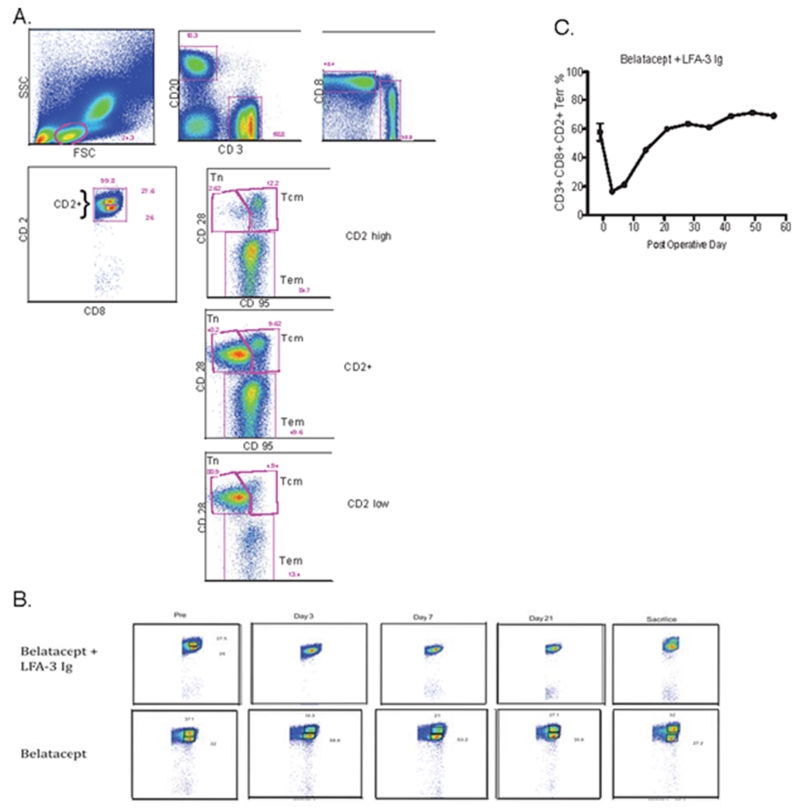
Belatacept therapy without alefacept demonstrated a bimodal distribution of CD2lo and CD2hi CD8+ T cells (Figure 7). The administration of alefacept resulted in the loss of a distinct CD2hi CD8+ T cell population. Despite this observation, longitudinal monitoring of CD8+CD28−CD95+CD2+ Tem cells demonstrated a transient decrease which was most profound on POD 3, with prompt reconstitution to baseline levels despite continued treatment. This suggests that although CD2+CD8+ T cells were effectively targeted by alefacept, the CD8+ Tem population may not have been greatly reduced in the long term.
Figure 7.
Alefacept in combination with belatacept abrogates CD2hiCD8+ T cells without long term effect on the CD2+ effector memory T-cell compartment. Polychromatic flow cytometry was used to evaluate the effect of alefacept on peripheral blood CD2+CD8+ T-cells, representative findings depicted here.
A. PFC gating algorithm to identify the memory T-cell compartment.
B. Distinct CD2hi and CD2low populations within CD8+ T-cells can be identified throughout the entire course in animals receiving belatacept without alefacept. Contrary to this, alefacept administration results in decrease of CD2hiCD8+ that is sustained throughout treatment
C. Effector memory T-cells (CD8+CD28−CD95+CD2+) nadir 3 days after administration of alefacept and subsequently rebounds to pre-treatment levels thereafter.
Costimulation blockade regimens prevented DSA formation
No alloantibody formation was observed in CoB groups. One of three animals treated with tacrolimus and steroids and six out of seven historic controls treated with CNIs based regimens developed DSA (Figure 8). T2’s graft was excised on POD 7 with Banff I and was not found to have positive alloantibody. This may be due to early cellular rejection prior to DSA formation by the experimental endpoint. T3 did not develop DSA by graft excision on POD 14.
Figure 8.
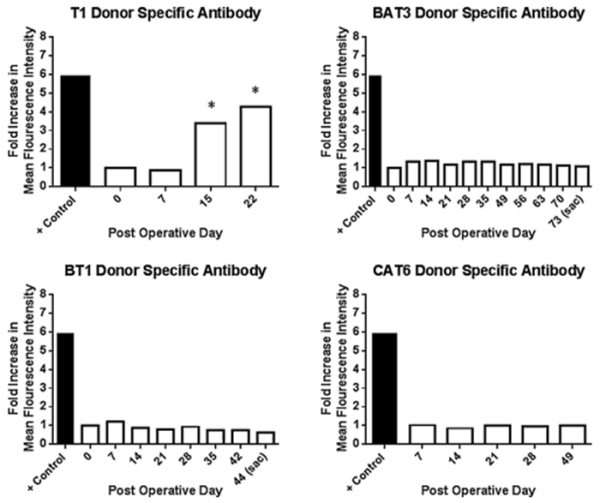
Costimulation blockade prevents formation of donor specific antibodies. Representative data shown from each study group. No alloantibody was observed in any costimulation blockade based regimen, however it was observed in T1 by POD 15 when the graft was excised for rejection. Alloantibody persisted one week after excision as noted on POD 22.
Discussion
VCA has the potential to provide life-improving function to people suffering from tissue loss unable to be corrected through traditional reconstructive techniques. Severe complications and mortality have been reported after VCA (2,(14, 15). These complications loom particularly large given the non-life saving nature of VCA. In this study, we used our established model of VCA (13). These studies represent a judicious, extensive initial testing of CoB-based regimens in VCA, which draw from other successful experiences using CoB in NHPs to test both B7 blockade and CD2 blockade (8, 11, 16). Rejection was diagnosed using the 2007 VCA-Banff working classification,(17) which was comparative to clinical VCA rejection. Based on our observations and similar to clinical cases, CNI’s are ineffective in preventing rejection. Although rejection may have been reversed, treatment of rejection was outside the scope of this study.
Our first study regimen included sirolimus starting on the day of transplant. Both grafts experienced wound healing complications ultimately leading to engraftment failure. These healing related complications are well-documented in other surgical settings but have not been specifically tested VCAs. Thus, these studies provide objective documentation that de novo sirolimus might be a particular risk for VCAs (18-22). As opposed to other solid organ transplants, in a skin-containing VCA, the skin is a component of the transplant and the wound healing complications prevent engraftment. Thus, our results with sirolimus in VCA may be clinically relevant and demonstrate that sirolimus is not an appropriate agent to be used immediately after a skin containing-VCA. The conversion of tacrolimus to sirolimus avoided these healing problems in our studies, thus providing a clinically relevant alternative to consider.
The combination of CTLA-4Ig and alefacept provided additional protection to the allograft and delayed rejection significantly compared to tacrolimus and steroids alone. However, the combination of these agents led to a failure to thrive in several animals ultimately requiring euthanasia. The necropsy reports described no gross abnormality. Significantly elevated CMV in euthanized animals suggests that the compromise of protective immunity led to death due to a viral illness. This diminution of protective immunity from alefacept has been previously reported in the NHP islet and kidney transplantation experience(8, 16). Modification of this regimen to use belatacept with alefacept and tacrolimus instead of CTLA-4Ig resulted in similar outcomes. With animals that succumbed to viral illness having histology devoid of rejection in both these study groups, it is likely that the combination of alefacept and CD28 blockade was too effective at preventing rejection with a significant cost to protective immunity.
Withdrawal of alefacept and treatment with belatacept and tacrolimus alone resulted in similar protection against allograft rejection. However, the impact in protective immunity did not differ when compared to either CoB group using alefacept. Thus, in our studies, alefacept did not provide additional benefit to B7 CoB in VCA despite the risks. Additionally, alefacept was recently withdrawn from the market. Study groups using CoB did not develop detectable DSA, which is consistent with prior reports (6, 23). Our rationale was to continue with a group consisting of belatacept and steroids. Our preliminary data from one NHP VCA recipient resulted in a rejection free survival of 28 days and a Banff III (data not shown).Further results on this group are forthcoming.
Summary
CoB are capable of improving rejection free survival of VCAs when compared to a standard CNI-based therapy. Alefacept, although effective at reducing the memory T cell compartment, did not improve outcomes and ultimately hindered protective immunity. Sirolimus immediately post-transplant of VCAs led to immediate post-operative wound healing complications. DSA development was not observed in CoB groups, which correlates to current experience with CoB in other solid organ transplants. These data provide initial results of CoB-based regimens in NHPs in VCA. Further refinements of how best to use belatacept in VCA require more experimentation.
Acknowledgments
The authors wish to acknowledge Jennifer Cheeseman, Sebastian Perez, Stephanie Monday, Benjamin Kramer, Andrew Page, and Adriana Gibby of the Emory Transplant Center for their technical assistance.
This study was supported by a grant from the Department of Defense administered through the Navy Bureau of Medicine and Surgery’s Medical Development Program, by the American Society of Transplant Surgeons, by the Melina Nakos Foundation, and by resources at the Atlanta VA Medical Center.
Abbreviations
- VCA
vascularized composite allograft
- NHP
non-human primate
- CMV
cytomegalovirus
- PCR
polymerase chain reaction
- CoB
costimulation blockade
- PFC
polychromatic flow cytometry
- DSA
donor-specific antibody
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Diaz-Siso JR, Bueno EM, Sisk GC, Marty FM, Pomahac B, Tullius SG. Vascularized composite tissue allotransplantation--state of the art. Clinical transplantation. 2013 May-Jun;27(3):330–7. doi: 10.1111/ctr.12117. PubMed PMID: 23581799. Pubmed Central PMCID: 3724233. Epub 2013/04/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petruzzo P, Lanzetta M, Dubernard JM, Landin L, Cavadas P, Margreiter R, et al. The International Registry on Hand and Composite Tissue Transplantation. 2010 Dec 27;90(12):1590–4. doi: 10.1097/TP.0b013e3181ff1472. PubMed PMID: 21052038. [DOI] [PubMed] [Google Scholar]
- 3.Landin L, Cavadas PC, Rodriguez-Perez JC, Garcia-Bello MA, Garcia-Cosmes P, Thione A, et al. Improvement in renal function after late conversion to sirolimus-based immunosuppression in composite tissue allotransplantation. Transplantation. 2010 Sep 27;90(6):691–2. doi: 10.1097/TP.0b013e3181ebf7ae. PubMed PMID: 20847634. [DOI] [PubMed] [Google Scholar]
- 4.Petruzzo P, Dubernard JM. The International Registry on Hand and Composite Tissue allotransplantation. Clinical transplants. 2011:247–53. PubMed PMID: 22755418. [PubMed] [Google Scholar]
- 5.Schneeberger S, Landin L, Jableki J, Butler P, Hoehnke C, Brandacher G, et al. Achievements and challenges in composite tissue allotransplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2011 Aug;24(8):760–9. doi: 10.1111/j.1432-2277.2011.01261.x. PubMed PMID: 21554424. [DOI] [PubMed] [Google Scholar]
- 6.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005 Mar;5(3):443–53. doi: 10.1111/j.1600-6143.2005.00749.x. PubMed PMID: 15707398. [DOI] [PubMed] [Google Scholar]
- 7.Adams AB, Shirasugi N, Durham MM, Strobert E, Anderson D, Rees P, et al. Calcineurin inhibitor-free CD28 blockade-based protocol protects allogeneic islets in nonhuman primates. Diabetes. 2002 Feb;51(2):265–70. doi: 10.2337/diabetes.51.2.265. PubMed PMID: 11812731. [DOI] [PubMed] [Google Scholar]
- 8.Lo DJ, Anderson DJ, Weaver TA, Leopardi F, Song M, Farris AB, et al. Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 Feb;13(2):320–8. doi: 10.1111/j.1600-6143.2012.04342.x. PubMed PMID: 23311611. Pubmed Central PMCID: 3558532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature medicine. 1999 Nov;5(11):1298–302. doi: 10.1038/15256. PubMed PMID: 10545997. [DOI] [PubMed] [Google Scholar]
- 10.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011 Jan;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x. PubMed PMID: 21070604. Pubmed Central PMCID: 3057516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nature medicine. 2009 Jul;15(7):746–9. doi: 10.1038/nm.1993. PubMed PMID:19584865. Pubmed Central PMCID: 2772128. Epub 2009/07/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama H, McCormick TS, Cooper KD, Korman NJ. Alefacept in the treatment of psoriasis. Clinics in dermatology. 2008 Sep-Oct;26(5):503–8. doi: 10.1016/j.clindermatol.2007.10.028. PubMed PMID: 18755368. [DOI] [PubMed] [Google Scholar]
- 13.Cendales LC, Xu H, Bacher J, Eckhaus MA, Kleiner DE, Kirk AD. Composite tissue allotransplantation: development of a preclinical model in nonhuman primates. Transplantation. 2005 Nov 27;80(10):1447–54. doi: 10.1097/01.tp.0000183292.57349.27. PubMed PMID: 16340790. Epub 2005/12/13. eng. [DOI] [PubMed] [Google Scholar]
- 14.Lantieri L, Hivelin M, Audard V, Benjoar MD, Meningaud JP, Bellivier F, et al. Feasibility, reproducibility, risks and benefits of face transplantation: a prospective study of outcomes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011 Feb;11(2):367–78. doi: 10.1111/j.1600-6143.2010.03406.x. PubMed PMID: 21272240. [DOI] [PubMed] [Google Scholar]
- 15.Hui-Chou HG, Nam AJ, Rodriguez ED. Clinical facial composite tissue allotransplantation: a review of the first four global experiences and future implications. Plastic and reconstructive surgery. 2010 Feb;125(2):538–46. doi: 10.1097/PRS.0b013e3181c722a8. PubMed PMID: 20124840. [DOI] [PubMed] [Google Scholar]
- 16.Lowe MC, Badell IR, Turner AP, Thompson PW, Leopardi FV, Strobert EA, et al. Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 Feb;13(2):312–9. doi: 10.1111/j.1600-6143.2012.04341.x. PubMed PMID:23279640. Pubmed Central PMCID: 3558637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cendales LC, Kanitakis J, Schneeberger S, Burns C, Ruiz P, Landin L, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008 Jul;8(7):1396–400. doi: 10.1111/j.1600-6143.2008.02243.x. PubMed PMID: 18444912. Epub 2008/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 18.Devries JG, Collier RC, Niezgoda JA, Sanicola S, Simanonok JP. Impaired lower extremity wound healing secondary to sirolimus after kidney transplantation. The journal of the American College of Certified Wound Specialists. 2009 Jul;1(3):86–91. doi: 10.1016/j.jcws.2009.06.001. PubMed PMID: 24527122. Pubmed Central PMCID: 3478904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn D, Spearman CW, Mall A, Shepherd E, Engelbrecht G, Lotz Z, et al. Effect of rapamycin on the healing of the bile duct. Transplantation proceedings. 2005 Mar;37(2):832–3. doi: 10.1016/j.transproceed.2004.12.164. PubMed PMID: 15848547. [DOI] [PubMed] [Google Scholar]
- 20.Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012 Sep 27;94(6):547–61. doi: 10.1097/TP.0b013e3182551021. PubMed PMID: 22941182. [DOI] [PubMed] [Google Scholar]
- 21.Tabibian JH, Girotra M, Yeh HC, Segev DL, Gulsen MT, Cengiz-Seval G, et al. Sirolimus based immunosuppression is associated with need for early repeat therapeutic ERCP in liver transplant patients with anastomotic biliary stricture. Annals of hepatology. 2013 Jul-Aug;12(4):563–9. PubMed PMID: 23813134. [PubMed] [Google Scholar]
- 22.Zakliczynski M, Nozynski J, Kocher A, Lizak MK, Zakliczynska H, Przybylski R, et al. Surgical wound-healing complications in heart transplant recipients treated with rapamycin. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007 May-Jun;15(3):316–21. doi: 10.1111/j.1524-475X.2007.00232.x. PubMed PMID: 17537118. [DOI] [PubMed] [Google Scholar]
- 23.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012 Jan;12(1):210–7. doi: 10.1111/j.1600-6143.2011.03785.x. PubMed PMID: 21992533. [DOI] [PubMed] [Google Scholar]
- 24.Lo DJ, Farris AB, Song M, et al. Inhibition of αvβ6 promotes acute renal allograft rejection in nonhuman primates. Am J Transplant. 2013 Dec;13(12):3085–93. doi: 10.1111/ajt.12467. [DOI] [PubMed] [Google Scholar]