Summary
Vertebrate ancestors had only cone-like photoreceptors. The duplex retina evolved in jawless vertebrates with the advent of highly photosensitive rod-like photoreceptors. Despite cones being the arbiters of high-resolution color vision, rods emerged as the dominant photoreceptor in mammals during a nocturnal phase early in their evolution. We investigated the evolutionary and developmental origins of rods in two divergent vertebrate retinae. In mice, we discovered genetic and epigenetic vestiges of short wavelength cones in developing rods and cell lineage tracing validated the genesis of rods from S-cones. Curiously, rods did not derive from S-cones in zebrafish. Our study illuminates several questions regarding the evolution of duplex retina and supports the hypothesis that, in mammals, the S-cone lineage was recruited via the Maf-family transcription factor NRL to augment rod photoreceptors. We propose that this developmental mechanism allowed the adaptive exploitation of scotopic niches during the nocturnal bottleneck early in mammalian evolution.
Graphical Abstract

eTOC Blurb
The evolution of rod-dominant retinae was a critical adaptation, allowing mammalian ancestors to survive a nocturnal bottleneck. Kim et al. provide evidence suggesting that this evolutionary transition was driven by molecular innovations in the Maf-family protein NRL, which led to the recruitment of rods from S-cones in early mammals.
Introduction
The duplex retina in vertebrates consists of specialized cone and rod photoreceptors (Fain et al., 2010; Lamb et al., 2007; Luo et al., 2008). Cones mediate non-quenching, rapid responses to photons with high acuity in daylight, whereas rods allow maximum sensitivity and energy conservation at the expense of spatial and temporal resolution. Cone photoreceptors also enable color discrimination by combining outputs of visual pigments (opsins) having distinct peak wavelength sensitivity (Nathans, 1999). How and when the duplex retina evolved, however, remains a long-standing mystery (Schultze, 1866; Walls, 1942).
Short wavelength sensitive (S) and long wavelength sensitive (L) cone opsins were present in the last common ancestor of jawed and jawless vertebrates (Okano et al., 1992), while rod visual pigment Rh1 (rhodopsin) and other cone opsin classes emerged subsequently by duplication of an ancestral, likely short wavelength, opsin gene (Pisani et al., 2006). In concordance, rods first appear as an intermediate form in agnathan species (i.e., jawless vertebrates) such as lampreys and become more evident in gnathostomes (i.e., jawed vertebrates) (Lamb, 2013) (Figure 1A). Despite functional specialization, rod morphology and phototransduction machinery are similar to those of cones (Fain et al., 2010; Morshedian and Fain, 2015), and rod signals piggyback on cone pathways in retinal circuitry (Oesch et al., 2011; Strettoi et al., 1992). On the basis of phylogenetic and anatomical analyses, rod photoreceptors were proposed to have originated from ancestral cone-like photoreceptors (Collin et al., 2009; Lamb et al., 2007).
Figure 1. Developmentally Conserved Vestiges during Rod Photoreceptor Evolution.
(A) Co-emergence of rod photoreceptors and Nrl during vertebrate evolution. True functional rod photoreceptors exist only among jawed vertebrates in the evolutionary tree of Chordata (Ebrey and Koutalos, 2001), but Nrl-like Maf gene is encoded in the lamprey genome (Figure 7B) and may be expressed in RhA (rod visual pigment) expressing rod-like cone photoreceptors (Morshedian and Fain, 2015). Red bar indicates the time period that corresponds to nocturnal bottleneck during mammalian evolution. The tapering down and thickening of second and third rows of the table indicates a decrease in cone abundance and an increase in rod abundance observed in most mammals, respectively. Time calibrated tree was redrawn from Hedges et al. (2015). mya, million years ago; Monotremes, egg-laying mammals; Marsupials, pouched mammals; Eutherians, placental mammals. (B) Hypothesis being tested in the present study. Green and purple curves represent cumulative rod and cone number, respectively. The respective shades indicate opsin expression. X-axis indicates developmental stages of mouse retina. E, embryonic day; B, birth; P, postnatal day.
In the mammalian retina, the generation of rods and cones is controlled by the combined actions of two transcription factors, Maf-family leucine zipper protein NRL and thyroid hormone receptor TRβ2 (Ng et al., 2011; Swaroop et al., 2010), and S-cones are proposed to be the default fate of post-mitotic photoreceptor precursors (Hunt and Peichl, 2014; Swaroop et al., 2010). In mice, loss of NRL results in a retina with predominantly S-cones in place of rods (Mears et al., 2001), and ectopic expression of NRL in cone precursors is sufficient to induce rod differentiation (Oh et al., 2007). NRL is expressed in rod photoreceptors shortly after the final mitosis, as indicated by GFP expression directed by a 2.5-kb Nrl promoter (Akimoto et al., 2006), consistent with antibody immunostaining studies (Ng et al., 2011). Furthermore, mice lacking thyroid hormone receptor β2 (TRβ2) have S-cones but no M-cones (Ng et al., 2001), and replacement of Nrl by Trb2 produces M-cones instead of rods (Ng et al., 2011). Transcriptional control of rod photoreceptor birth is less well resolved in cone-dominant vertebrates such as teleosts (Stenkamp, 2011), especially with respect to nrl, whose expression is not limited to rods in zebrafish (Nelson et al., 2008). Notably, the loss of T-box transcription factor tbx2b in zebrafish results in rod generation from UV cone precursors (Alvarez-Delfin et al., 2009; Duval et al., 2014). In addition, thyroid hormone treatment affects photoreceptor development in trout (Allison et al., 2006), and thrb2 modulates the generation of different cone subtypes in zebrafish (Suzuki et al., 2013; Yoshimatsu et al., 2014).
A typical mammalian retina accommodates a great abundance of rods with a paucity of cones; few exceptions include rare cone-dominant retinae of a subset of diurnal species and highly specialized regions (such as fovea) in primate retinae (Gerkema et al., 2013). On the contrary, most non-mammalian vertebrates possess abundant cone photoreceptors in multiple spectral sub-classes. This stark difference in retinal composition may reflect a “nocturnal bottleneck” hypothesized to have occurred early in the evolutionary history of mammals, wherein early mammals adapted to novel scotopic (dim light) niches (Gerkema et al., 2013; Heesy and Hall, 2010). We were curious as to how a rod-dominant duplex retina evolved in a relatively short time span. We hypothesized that the abundance of rod photoreceptors in the mammalian retina originated from, and at the expense of, S-cones with the co-emergence of an NRL-centered, rod-specific, regulatory network and that developing rods would therefore carry vestiges (“footprints”) of S-cones (Figure 1B). Here, we provide multiple lines of evidence that developing rods in mouse retinae show a well-defined molecular “footprint” of S-cones, whereas rods in zebrafish, representing an outgroup to tetrapods and having a cone-rich retina, do not. While alternative explanations are possible, our studies argue in favor of the recruitment of Scones to augment rods in mammalian retinae and suggest a developmental mechanism involving NRL that facilitated the adaptation to scotopic ecological niches during a “nocturnal bottleneck” experienced by the ancestors of extant mammals.
Results
Developing Mouse Rod Photoreceptors Have a Molecular Footprint of S-Cones
To test our hypothesis, we examined whether rod photoreceptors showed a signature of gene expression similar to S-cones during development. We took advantage of an Nrlp-GFP transgene to obtain pure populations of rods from wild-type mouse retinae and of S-cone-like photoreceptors from Nrl−/− retinae (Akimoto et al., 2006; Ng et al., 2011). Transcriptome profiling, using RNA-seq, of fluorescence activated cell sorting (FACS)-purified rods from Nrlp-GFP retinae revealed a progressive increase in the expression of rod-specific phototransduction genes, including rhodopsin (Rho), as the rods reached anatomical and functional maturity (Figure 2A). As predicted, absolutely no known rod-specific gene was expressed in Nrlp-GFP photoreceptors from the rod-less Nrl−/− retinae (Figure 2A). Interestingly, we noted strong expression of S-opsin (Opn1sw), but not M-opsin (encoded by Opn1mw), and of many cone-specific phototransduction genes, well above the median gene expression values, in the developing rods from wild-type Nrlp-GFP retinae (Figure 2B). The expression of cone genes diminished with rod maturation in wild-type Nrlp-GFP retinae but not in photoreceptors of Nrl−/− retinae (Figure 2B). Expression of several progenitor genes was also detected in rods at postnatal day (P) 2 and P4, but rapidly declined as rod birth was completed and maturation began (Figure S1), reflecting the likely contribution of GFP-positive rods that just exited terminal mitosis (Akimoto et al., 2006). Genes specifically expressed in other retinal cell types (ganglion, bipolar, amacrine and horizontal cells) were barely detectable in the rod transcriptome data (Figure S1). As a low level of opsin expression was recently reported in cultured human epidermal skin cells (Haltaufderhyde et al., 2015), we examined S-opsin expression in ENCODE datasets (Consortium, 2012; Yue et al., 2014) to ensure the specificity of Opn1sw expression as a S-cone marker. S-opsin expression was not detected in any non-retinal human/mouse tissue or cell type (including epidermal cells) for which expression data were available (Figure S2).
Figure 2. Molecular ‘Footprint’ of S-cones in Developing Mouse Rod Photoreceptors.
(A and B) Gene expression profiles of selected (A) rod- and (B) cone-specific phototransduction genes in the rod photoreceptors purified from Nrlp-GFP mouse retina. Developmental time points are indicated on the X-axis. Y-axis shows FPKM (fragments per kilobase of exon per million fragments mapped) ± SEM (standard error of the mean) from RNA-seq data. Gene expression in photoreceptors from Nrlp-GFP;Nrl−/− retina is shown as mean in dark grey lines and range of five indicated genes in grey shades. The six time points selected for RNA-seq analyses represent key stages of mouse rod development: P2, peak of post-mitotic rod generation; P4, robust increase in phototransduction gene expression; P6, rod outer segment discs beginning to form; P10, outer segment elongation and synaptogenesis; P14, eye opening and outer segment/synapse formation completed; P28, rods fully mature. (C) qRT-PCR validation of Rho and Opn1sw expression from 50 manually collected GFP(+) rods. Data are normalized to P2 expression level and presented as mean of fold change (FC) ± SEM.
To validate RNA-seq results, we performed quantitative PCR analysis using RNA purified from 50 manually collected Nrlp-GFP(+) cells each at P2 (peak of newborn rods) and P28 (mature rods). As predicted, rhodopsin expression increased dramatically in P28 rods compared to P2, whereas S-opsin expression was undetectable in mature rods (Figure 2C).
We then examined the expression of S-opsin in dissociated Nrlp-GFP(+) retinal cells at P2 and P28 (Figure 3A and Figure S3). Consistent with the RNA-seq and previously published P0 immunohistochemistry data (Ng et al., 2011), S-opsin immunostaining was clearly detectable in GFP(+) rods at P2 but not at P28. Quantification by FACS analysis demonstrated that 13.8% of GFP(+) cells were also positive for S-opsin at P2, but double positive cells were negligible at P28 (Figure 3B). In addition, FACS analysis of NRL and S-opsin double immunostained wild type retinal cells revealed 26.4% NRL(+) cells being S-opsin(+) at P2, while double positive cells decreased to almost none by P28 (Figure 3C). We then estimated the number of S-opsin(+) and S-opsin/GFP double positive cells at different developmental stages. P2 retina contained a greater number of total S-opsin(+) cells compared with P28 retina (Figure 3D). A majority (72%) of S-opsin(+) cells at P2 (on an average ~ 141,000) were also positive for GFP; however, the number of S-opsin/GFP double positive cells was negligible by P28. The number of cells that were positive for only S-opsin remained remarkably constant (Figure 3D).
Figure 3. S-opsin Expression in Developing Mouse Rod Photoreceptors.
(A) Immunocytochemistry of dissociated Nrlp-GFP retinal cells. S-opsin (purple) and GFP (green) double positive cells were detected only at P2, but not at P28. (B) Quantification of S-opsin positive rod photoreceptors by flowcytometry. Percentage of cells in each quadrant relative to total counted cells is indicated in the dot plots, and percentage of S-opsin positive cells among GFP positive rods is shown in parenthesis. (C) Quantification of S-opsin and NRL double positive cells by immunostaining followed by flow cytometry. Percentage of cells in each subpopulation is indicated. Percentage of S-opsin positive cells among NRL positive population is shown in parenthesis. (D) Quantification of S-opsin positive and GFP/S-opsin double positive photoreceptors per retina. Percentage of each cell population determined by flowcytometry of Nrlp-GFP retina was multiplied by total retinal cell number at the corresponding stage. Y-axis indicates mean cell number (x105) ± SEM. (E) Quantification of NRL-expressing S-cones at indicated time points by flowcytometry of Opn1swp-Venus mouse retina. Proportion of cells in each subpopulation is indicated as a percentage relative to total cells, and percentage of Venus positive cells among NRL-positive rods is shown in parenthesis.
To further validate the preceding results, we performed FACS analysis of photoreceptors from an independent Opn1swp-Venus transgenic mouse line where the S-opsin promoter was used to drive the expression of the Venus reporter gene. Consistent with the above data, NRL immunostaining was detectable in 85.6% of Venus(+) cells at P2, and Venus and NRL double positive cells accounted for 12% of NRL(+) cells in the developing retina; however, the number of double positive cells was negligible in the P28 retina (Figure 3E).
Taken together, our findings exclude the possibility of S-opsin positive rods being an artifact of the transgenic mouse line. Our data thus demonstrate the expression of S-opsin and other cone phototransduction genes in mouse rod photoreceptors during early stages of development.
Chromatin State in Developing Mouse Rods Favors Cone Gene Expression
Our hypothesis that mammalian rods derive from cells fated as S-cones further predicts that developing rods would exhibit a chromatin state concordant with S-cone characteristics. To address this, we performed reduced representation bisulfite sequencing (RRBS) and histone modification chromatin immunoprecipitation sequencing (ChIP-seq) using flow-sorted rod photoreceptors at three key stages of development - P2, P10 and P28 (see Figure 2 legend). We also analyzed DNase I hypersensitivity-sequencing (DNase-seq) data from developing mouse retinae (Vierstra et al., 2014). In P2 rods, chromatin state was unfavorable for active Rho transcription as indicated by the high degree of DNA methylation (meDNA), limited enrichment of active histone mark H3 lysine 4 trimethylation (H3K4me3) at the promoter and low chromatin accessibility (indicated by lack of DNase-seq signal) (Figure 4). In contrast, Opn1sw promoter exhibited hallmarks of active chromatin state in P2 rods; these include the absence of meDNA, accumulation of H3K4me3, chromatin accessibility, and a low level of repressive H3K27me3 marks (Figure 4). Rod-specific epigenetic architecture was acquired by P10 and maintained thereafter with concurrent accumulation of repressive chromatin marks in Opn1sw (Figure 4). The observed chromatin dynamics were specific to opsin gene loci and other rod and cone-specific genes (data not shown). The promoters of photoreceptor-specific transcription factors including Nrl (Figure 4) and of constitutively expressed genes possessed active chromatin architecture at all stages of rod development, whereas genes specifically expressed in non-neuronal tissues exhibited repressed chromatin state (data not shown). Overall, the state of chromatin in newly post-mitotic rods, but not in mature rods, is favorable for expression of S-opsin and other cone genes.
Figure 4. Epigenetic Vestiges of Cones in the Developing Rod Photoreceptors in Mouse Retina.
Epigenetic state on Rho, Opn1sw and Nrl genes were revealed by reduced representation bisulfite sequencing (RRBS; DNA methylation profiling), H3K4me3 and H3K27me3 ChIP-seq, and DNase I hypersensitivity sequencing (DNase-seq) at indicated developmental stages. Data were generated using flow-sorted rod photoreceptors from Nrlp-GFP mouse retina, except for retina DNase-seq data that were downloaded from mouse ENCODE project (Vierstra et al., 2014). Percentage ± SEM of methylated cytosine in the promoter regions is indicated as bar graphs. Aligned sequencing reads for mRNA expression (dark blue), H3K4me3 (green, active mark) and H3K27me3 (red, repressive mark) enrichment, and DNase hypersensitivity sites (orange) are shown as histogram. Promoter of each gene, defined as −1 kb to +1 kb from the transcription start site, is highlighted with a gray shade.
Lineage Tracing Shows History of S-opsin Expression in Mouse Rods
To further test whether rod photoreceptors in mice originated from S-cones, we performed lineage tracing using the Opn1swp-Cre mouse (Akimoto et al., 2004) by breeding it to ROSA26-iAP reporter line (Badea et al., 2009) (Figure 5A). Here, active Opn1sw promoters permanently tag the cell by inducing alkaline phosphatase (AP) expression (Figure 5A), allowing inference of lineage history. Cell bodies of most, but not all, rods and cones in the outer nuclear layer (ONL) showed heavy AP activity in the P28 retinae (Figure 5B and Figure S4), demonstrating the expression of S-opsin in rods at an early stage in their development. Immunostaining with antibodies against markers of mature photoreceptors revealed stronger AP staining in S-cones and further confirmed the history of S-opsin promoter activity in rods (Figure S4D and E). Similar experiments using Opn1mwp-Cre mouse, which expresses Cre-recombinase under the control of medium wavelength (M) opsin promoter (Akimoto et al., 2004), demonstrated no overlap between rod and M-cone lineage (Figure 5C and Figure S4C). Occasional ganglion cell staining was detected in both Opn1sw and Opn1mw lineage tracing experiments (Figure S4C). Detection of the history of Opn1sw expression in most mature mouse rods was further validated by an independent lineage tracing experiment using BAC transgenic Opn1swp-Cre and Z/EG fluorescence reporter lines (Figure 5D). In accordance with the results above, GFP lineage tracer was detected in both cones and most rods in mature retinae even though Cre and S-opsin immunoreactivity was observed only in mature cones (Figure 5E and 5F). Robust lineage tracer labeling of S-cones in most but not all rods, and not of any other retinal neuron, in the BAC transgenic Opn1swp-Cre mice strongly argues in favor of the derivation of rods from Scone lineage.
Figure 5. History of S-opsin Expression in Mature Mouse Rod Photoreceptors.
(A) Lineage tracing strategy in mouse. Opn1swp-Cre mouse line was crossed with ROSA26-iAP line. In this line, cells with active Opn1sw promoter express Cre recombinase, which brings alkaline phosphatase (AP) gene back to the correct configuration by inverting the DNA piece surrounded by two loxP sites in head-to-head orientation. Once recombined, a constitutive promoter in the ROSA locus drives AP reporter gene expression even when cells no longer have Opn1sw promoter activity. (B and C) Lineage tracing of S-opsin-expressing cells using Opn1swp-Cre;ROSA26-iAP (B) or tracing of M-opsin lineage cells using Opn1mwp-Cre;ROSA26-iAP mouse lines (C). Shown is AP staining (purple) in the photoreceptor layer of adult (P28) retina of respective genotype. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; Cone, outer portion of ONL where cone nuclei are located; Rod, inner ONL with rod nuclei. Scale bar: 20 μm. (D) Lineage tracing strategy using BAC transgenic mouse line. Opn1swp-Cre BAC transgenic mouse line was crossed with Z/EG reporter line. Cells with active Opn1sw promoter express Cre recombinase, which permanently activates GFP expression in all progeny of the cells that once expressed Opn1sw. (E and F) Lineage tracing of S-opsin-expressing cells using Opn1swp-Cre (BAC tg); Z/EG mouse line. (E) CRE (green) and S-opsin (magenta) immunoreactivity in the outer portion of ONL. Note that S-opsin signal is strong in outer segments, and only weak S-opsin immunoreactivity can be detected in inner segments and cell bodies (merged image). (F) GFP positive cells (green) are found both in outer and inner layers of ONL (i.e., cone and rod nuclei layers, the upper and lower half of ONL in the micrograph, respectively), while S-opsin positive cones (red) are located only in the outer portion of ONL. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; Cone, outer portion of ONL where cone nuclei are located; Rod, inner ONL with rod nuclei. Scale bar: 20 μm.
Rods Do Not Show UV Cone Opsin sws1 Lineage in Zebrafish
Next we examined whether rods in a cone-dominated, teleost retina were also specified from a cone lineage. Teleosts branched earlier than mammals in the evolutionary history of vertebrates. To address this, we engineered a lineage tracing zebrafish line with two genetic constructs that constituted a positive-feedback mechanism (“Kaloop” technology, Figure 6A). We used this line to permanently label cells with the history of expression of UV cone opsin sws1, a zebrafish homolog of mouse Opn1sw. We characterized 4,024 cells that had expressed sws1 opsin at some point in development from 10 retinas of 4 dpf (days post-fertilization) zebrafish larvae. The vast majority of lineage-traced cells (4,021 of 4,024; 99.92%) unambiguously lacked immunolabeling by 4C12 (a rod marker), whereas 3 cells (0.08%) found amongst two individuals were ambiguously labelled (i.e., they probably were not rods, though we cannot formally exclude this possibility) (Figure 6B). Lineage tracing of an mCherry transgene in each cell, where image resolution permitted assessment, was tightly associated with immunolabeling by the UV cone marker 10C9.1 antibody (Figure 6B). Although sws1 is the closest homolog of mouse Opn1sw, we also traced the lineage of zebrafish blue cone opsin sws2 to exclude the possibility that rods may have derived from the other short wavelength sensitive cone class. As with sws1 lineage-traced cells, we did not find any rods with sws2 lineage (Figure S5). Therefore, the cells with a history of sws1 or sws2 expression exclusively differentiate into UV or blue-sensitive cones, respectively.
Figure 6. Absence of sws1 Opsin Expression History in Zebrafish Rod Photoreceptors.
(A) Lineage tracing strategy in zebrafish. Lineage tracing in zebrafish was accomplished using a feed-forward system that has been previously designated as “Kaloop” (Distel et al., 2009) (see Figure S6). Two constructs Tg[sws1:KalTA4] and Tg[UAS:nfsB-mCherry-V2A-KalTA4] (ua3139 and ua3137, respectively) were independently inserted to generate the transgenic zebrafish. In ua3137, a fluorescent reporter protein mCherry is fused in-frame to a bacterial nitroreductase gene (nfsB), and the fusion protein is connected via the labile linker peptide V2A to a second copy of the KalTA4 transcription factor. (B) Lineage tracing of sws1-expressing cells using sws1:KalTA4; UAS:nfsB-mCherry-V2A-KalTA4 zebrafish line. The UV-sensitive cones (cyan, 10C9.1) and rods (green, 4C12) of 4 dpf zebrafish were labeled using immunohistochemistry. Representative images from 1 of 10 left eyes. D, dorsal; V, ventral; N, nasal; T, temporal. Scale bar: 50 μm in low magnification images and 20 μm in high magnification image.
Advent of Nocturnal Mammals Coincides with the Acquisition of Novel Regulatory Elements, Rod-Specific Expression and Deep Conservation of NRL
Our data describe a novel developmental origin of rod abundance in mouse (a representative placental mammal) as compared to zebrafish (an earlier branching, non-amniotic vertebrate with a cone-dominant retina), encouraging us to determine a mechanistic explanation. We first compared genomic sequences spanning the Nrl gene in selected vertebrates. DNA sequences in the coding region of Nrl are highly conserved in all vertebrates (Figure 7A). Strikingly however, we observed extensive conservation of genomic sequences upstream and downstream of Nrl in mammals as well as in the intronic and untranslated regions of selected loci (Figure 7A). We also noted the gradual evolution of conserved genomic sequences spanning Nrl from the egg-laying platypus to the pouched and placental mammals, consistent with the appearance of rod-dominant duplex retina and the pivotal role of NRL in mammalian rod cell fate determination.
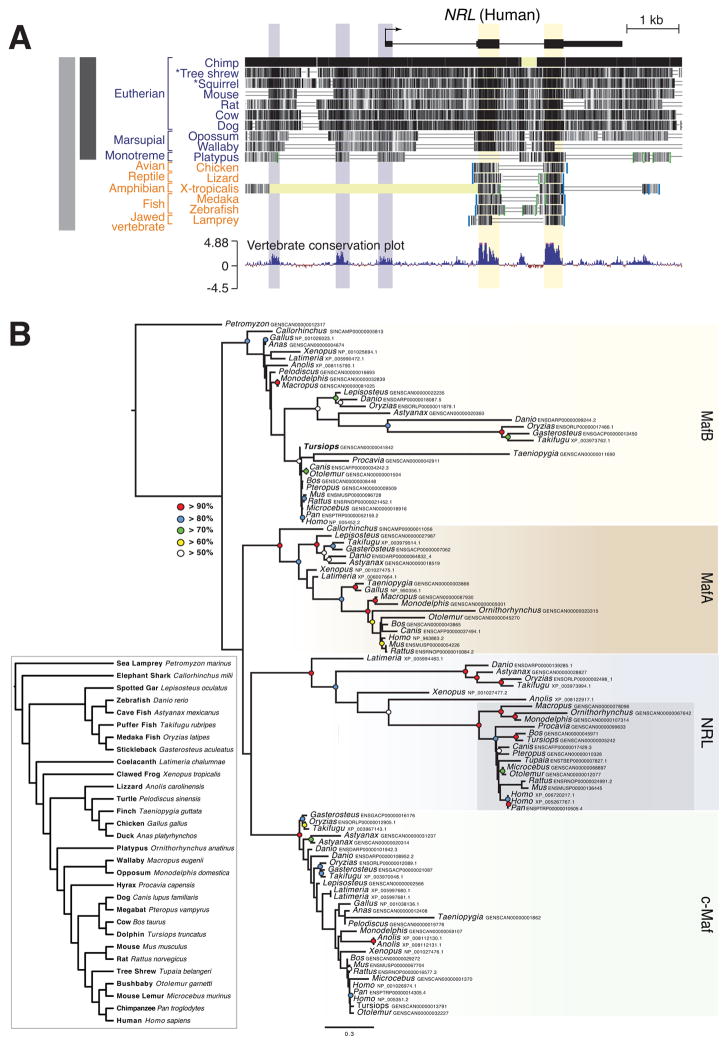
Figure 7. Advent of Nocturnal Mammals Coincides with the Acquisition of Novel Regulatory Elements and Rod-specific Expression of NRL.
(A) Comparison of genomic sequences within and flanking areas of Nrl. Yellow shades highlights a high degree of DNA sequence homology across all vertebrate species in the protein-coding region of Nrl, purple shades indicate additional conserved domains including upstream regulatory sequences and untranslated regions. (B) Phylogenomic analysis of Nrl and related Maf genes among vertebrates. A single homolog is present in the genome of the agnathan Petromyzon while other Maf paralogs, including Nrl, originated in the lineage leading to gnathostomes. Mammalian Nrl loci are shaded in grey. Colored circles indicate bootstrap support for nodes greater than 50%. Inset: species relationships of the 30 vertebrates genomes included in this analysis.
Next, we explored the phylogeny of NRL and related Maf proteins from a range of vertebrate genomes including jawless agnathans, cartilaginous and bony fishes, terrestrial vertebrates and representatives of each major lineage of mammals (Figure 7B). Our results show that NRL and other closely related Maf paralogy classes have each originated among the jawed vertebrates, with a single distant ortholog being present in the earlier branching agnathan (Petromyzon) genome. Further, each of these Maf paralogy classes, including NRL, demonstrates dynamic histories of loss among taxa. Strikingly, while we infer that NRL has been lost from several non-mammalian taxa, NRL loci are retained in all mammalian genomes included, in contrast to each of the other paralogy classes. Further, the mammalian NRL genes are separated from non-mammalian paralogs by the longest internal branch in our tree, indicating a burst of molecular evolution. Together, our results suggest an evolutionary history for mammalian NRL whereby rapid molecular innovations in the lineage leading to mammals are followed by strong conservation and gene retention.
We also evaluated NRL and other Maf protein sequences across vertebrate species (Figure S7). Transactivation and DNA binding domains were highly conserved in all vertebrate species including zebrafish and mouse. Protein sequence comparison revealed a high degree of conservation between all chicken long MAFs and vertebrate NRLs in both transactivation and DNA binding domains (Figure S7). To test for functional conservation, we introduced the chicken MAFA gene, which is expressed in avian rods and a few other retinal neuronal types but not in cones (Ochi et al., 2004), in cone-only Nrl−/− mouse retina (Mears et al., 2001) (Figure 8). Chicken MAFA, but not GFP empty vector, induced the expression of rhodopsin (Figure 8B) and characteristic single synaptic ribbon observed in rods (Carter-Dawson and LaVail, 1979), as opposed to cone opsin expression and multiple ribbons present in cones (Figure 8C and Figure 8D). Chicken MAFA-induced cone-to-rod transformation was confirmed by expression of rod-specific genes including Nr2e3, Gnat1 and Cnga1, but not cone genes such as Arr3 and Gnat2, in MAFA-expressing Nrl−/− mouse retina (Figure 8E). Thus, chicken MAFA induces rod development akin to the actions of Mouse Nrl when expressed ectopically in Nrl −/− retinae.
Figure 8. Cone-to-rod Transformation in Nrl−/− Mouse Retina by Chicken MAFA.
(A) Chicken MAFA expression vector design. GFP or FLAG-tagged chicken MAFA-T2A-GFP was placed under CMV promoter. MAFA expression vector or empty control vector was introduced by in vivo electroporation into dividing progenitor cells in P2 Nrl−/− mouse retina. (B) GFP signal (green; marks electroporated cells) and rhodopsin (RHO) immunoreactivity (red) of electroporated Nrl−/− retina at P21. DAPI counterstains all nuclei. Merged images are shown on the rightmost column. Scale bar: 50 μm (C) GFP signal (green) and RIBEYE immunoreactivity (red) in electroporated Nrl−/− retina at P21. DAPI counterstains all nuclei. Insets in each micrograph show a high magnification image of synaptic area of the transfected cells as outlined with dotted line. Merged image is shown in the rightmost micrograph. Scale bar: 20 μm (D) Bar graph summarizing the number of ribbons per chicken MAFA expressing cell (indicated by GFP). Error bars indicate SEM. (E) Quantitative RT-PCR analysis of the transfected retina. Expression of selected cone- (Arr3 and Gnat2) and rod specific genes (Rho, Nr2e3, Gnat1 and Cnga1) in chicken MAFA-expressing Nrl−/− retina was assayed by qRT-PCR and compared to their respective expression in Nrl−/− retina transfected with an empty control vector. Error bars indicate SEM.
Discussion
Current concepts regarding the behavioral and organismal ecology of early mammals propose that the evolution of a rod-dominant retina was a landmark event, central to the persistence and success of the lineage. Extant mammalian lineages first diverged during the Jurassic when dinosaurs were dominant and diurnal predators in most terrestrial habitats. During this period, predation pressures forced early mammals to adapt to a nocturnal lifestyle (termed the nocturnal bottleneck), permitting them to exploit a novel scotopic ecological niche (Davies et al., 2012; Heesy and Hall, 2010; Menaker et al., 1997; Walls, 1942). How these early mammals survived and prospered during this nocturnal bottleneck, and attained the capacity for high sensitivity photon capture in night vision, has defied explanation. Here, we provide strong evidence in support of the hypothesis that a subset of short wavelength sensitive S-cones was transformed to rods by ectopic expression of NRL early in the evolutionary history of mammals. We suggest that this extreme dominance of rods is retained in the retinae of most extant mammals.
Our understanding of photoreceptor evolution has been continuously revised since Schultze (1867) initially considered cones to be a more derived photoreceptor type than rods based on their capacity to detect highly specific wavelengths of light. A more detailed histological examination (Dickson and Graves, 1979) and phylogenetic analysis of opsin genes (Okano et al., 1992; Pisani et al., 2006) predicted the opposite scenario. Inspired by recent technical advances, we revisited the question of rod origins, specifically their vast abundance in mammals. Global transcriptome and epigenome analysis of pure photoreceptor populations enabled us to detect prominent footprints of S-cones in developing mouse rods. Further validation at single cell type resolution and fate mapping analyses allowed us to conclude that most, but not all rod photoreceptors in mice are derived from the S-cone lineage. Conversely, lack of direct evidence supporting the origin of rods from the S-cone lineage in zebrafish indicates that the derivation of most rods from S-cones might be a developmental innovation of mammals, one that serves to contrast two distinct phases of rod origins: 1) the initial origin of unambiguous rods in early gnathostomes and 2) the later shift to rod-dominated retinae in mammalian species. It remains feasible that this lack of direct evidence supporting the origin of rods from S-cones in zebrafish represents loss of this character in fish taxa, or could be attributable to the developmental phase examined; e.g., lineage tracing to examine rod development in post-larval zebrafish may be warranted. Importantly, the rod population without a detectable S-cone footprint (Figure 5B and 5F) in the mouse retina may represent a more ancient cellular lineage of rods homologous to those of earlier branching, non-mammalian taxa including zebrafish.
Our finding of two distinct rod populations in the mouse retina is intriguing and requires further explanation. Although not as explicit as the distinct rod populations reported in amphibian retinas (Sherry et al., 1998), some evidence indicates that mouse rods are not a homogeneous cell type. For example, a subpopulation of rods forms direct synaptic contacts with cone bipolar cells, whereas others only synapse with rod bipolar cells (Tsukamoto et al., 2001). In addition, two phases of rod differentiation are reported in developing rat retinas (Morrow et al., 1998). Although there is no evidence of physiological or morphological difference between early- and late-born rods, rods born prior to embryonic day 19 (E19) exhibit the onset of rhodopsin expression that is further lagged and more synchronized than those born on and after E19 (Morrow et al., 1998). Whether the rod subgroups with distinct synaptic pathways and differential temporal expression of rhodopsin can be related to the rods with or without a history of Opn1sw expression would be an interesting and testable future direction.
Multiple lines of evidence validate the expression of Opn1sw and other cone genes in developing rods. Though not emphasized as such, Nrlp-GFP cells in P0 mouse retina are documented to express S-opsin (Ng et al., 2011). Both transcriptome and epigenome analyses demonstrate the robustness of co-expression of Nrl and Opn1sw in isolated mouse rod photoreceptors. We used flow-sorted rods of purity of over 96% (>99% for most samples) for all next generation sequencing (NGS) experiments; thus, S-cone contamination in purified rod samples can’t explain the expression values (FPKM) in our samples. We note that the genes associated with inner retina show no to negligible expression in flow-sorted rods (see Figure S1). Although some progenitor genes were detected in early developing rods (P2 and P4), it is not surprising given that Nrl expression commences during or immediately after the final mitosis (Akimoto et al., 2006). Furthermore, qRT-PCR analysis of manually picked GFP-positive cells, where no contamination is allowed, confirmed high Opn1sw expression in P2 rods compared to P28 (see Figure 2C). Multiple independent experiments, including immunocytochemistry and flowcytometry using distinct combinations of reporter mouse lines (see Figure 3), point to Opn1sw expression in immature rods. Lineage tracing experiments using two different S-opsin Cre-lines and phylogenetic analysis provide additional strong support of our hypothesis.
Evolutionary innovations are enabled by changes in gene regulatory networks that consist of transcriptional regulators and their cognate DNA binding elements (Koentges, 2008). Comparative genomic analysis of Nrl, a hub of the transcriptional regulation circuits regulating rod development (Swaroop et al., 2010), provides insights into the underlying mechanism of rod recruitment from S-cones. At the level of protein sequence, functional domains of NRL are highly conserved throughout vertebrates (see Figure S7) and functional equivalency exists among different Maf paralogs when expressed in a suitable context. For example, ectopic expression of mouse Nrl (Ng et al., 2011; Oh et al., 2007) or chicken MAFA (see Figure 8) is sufficient to convert cones to rods in the mouse retina. Notably, ectopic expression of human NRL led to an increased number of rods at the expense of cones in the Xenopus retina, whereas Xenopus Nrl induced the differentiation of both rods and lens fiber cells (McIlvain and Knox, 2007). Thus, the function of NRL as a rod differentiation factor seems to be well conserved across amniotes despite additional function(s) Nrl-homologs may have outside of mammals. Given that rod photoreceptors, broadly defined, likely originated in ancient (jawless) vertebrates, it is reasonable to hypothesize that the development of these early rods involved a long Maf homolog, such as the single locus present in the Lamprey (Figure 7B). In contrast, novel regulatory sequences at the Nrl locus later evolved within the mammalian lineage (see Figure 7A) giving rise to a novel gene regulatory network and a second, independent origin of rods. Additional independent origins of rods have also been noted in nocturnal birds (Martin et al 2004) and deep sea fish (Landgren et al., 2014), however the developmental basis for these transitions remains an open question.
Developmental plasticity can support the evolution of novel traits by providing the raw materials (i.e. phenotypic variation) necessary for rapid adaptation (Muller, 2007) and vertebrate photoreceptors are known to demonstrate such plasticity. In humans, opsin expression is plastic over the course of development. S-opsin expression precedes L/M-opsin expression, and the number of cones expressing both increases during development, followed by their decrease at later stages (Szel et al., 1994; Xiao and Hendrickson, 2000). Similarly, mouse genetic studies have demonstrated the developmental plasticity of postmitotic photoreceptor precursors (Ng et al., 2011; Oh et al., 2007). Thus, it is reasonable to predict that the recruitment of S-cones to augment rod photoreceptors in mammalian retinae via the plastic redeployment of NRL enabled the rapid adaptation of early mammals to the nocturnal niche. Notably, loss of Sws2 and Rh2 as well as a shift in spectral sensitivity of Sws1 also coincided with this critical period (Jacobs, 2013). Thus, multiple lines of evidence suggest a fundamental reorganization of the mammalian retina during the nocturnal bottleneck.
Despite their nocturnal origin, extant mammalian species have adapted to diverse activity patterns such as nocturnal, diurnal, crepuscular and cathemeral habits (Gerkema et al., 2013). Paradoxically and with few exceptions, rods remain the dominant photoreceptor type in most mammalian retinas (Peichl, 2005). Why is rod-dominancy retained in diurnal mammals? It is interesting to note that patients with retinal degeneration and 90% cone loss in the fovea had sufficient visual acuity (Geller and Sieving, 1993). Considering the low energy consumption rate of rods compared to cones (Okawa et al., 2008), it is conceivable that maintaining rod abundance in diurnal mammals is adaptive because it allows energy efficiency without compromising visual acuity. In addition, rods may have additional functions beyond scotopic light detection. Chemical and electrical rod-cone coupling can mediate mesopic vision (Abd-El-Barr et al., 2009; Asteriti et al., 2014; Hornstein et al., 2005; Pang et al., 2012; Ribelayga and Mangel, 2010). Rods also drive circadian photoentrainment (Altimus et al., 2010) and horizontal cell-mediated surround inhibition of cone signal (Szikra et al., 2014). Furthermore, trophic factor(s) released by rods can enhance cone survival in degenerating retina (Ait-Ali et al., 2015). Thus, the integration of numerous functions in mammalian rods may have comprised a pleiotropic constraint against a reversion to cone dominance in the retinae of diurnal mammalian taxa.
We recognize that our proposed mechanism for the transition from cone-dominant to rod-dominant retinae in early mammals does not provide a complete explanation for the developmental transformation of mammalian retinae that took place during the nocturnal bottleneck. In addition to their cellular composition, rod dominant retinae also have a greatly increased proportion of photoreceptors. Thus, an explanation is also required for the increase in progenitor proliferation and cell number. How cell number is modulated in developing retinae is largely unclear although the myoblast oncogene Myb has been implicated in determining in cone photoreceptor number (Whitney et al., 2011).
In conclusion, we propose that the evolutionary origin of the majority of mammalian rods occurred by their developmental recruitment from the S-cone lineage, a phenomenon not observed in non-mammalian vertebrates. We further hypothesize that this process was predicated on the origins of novel regulatory sequences of Nrl that restrict its expression to rod photoreceptors in mammals. We show that the origins of novel Nrl regulatory sequences coincide with a period of punctuated visual system evolution early in mammalian evolution known as the “nocturnal bottleneck”, followed by strong conservation and stasis in extant mammalian species. Our study provides critical mechanistic details underlying the rapid adaption to the scotopic light environment that was the driving force behind the profound cone-to-rod transformation that occurred during the nocturnal bottleneck and reveals how the evolutionary history of a neuronal cell type can be revealed by comparative molecular and ontogenetic analysis.
Experimental Procedures
Mouse and Zebrafish Strains
All procedures involving the use of mice were approved by Animal Care and Use Committee of the National Eye Institute (NEI), National Institutes of Health (NIH), USA. Nrlp-GFP and Nrlp-GFP;Nrl−/− were described previously (Akimoto et al., 2006). Opn1swp-Cre (BP-Cre) and Opn1mwp-Cre (RGP-Cre) (Akimoto et al., 2004) mice were crossed with ROSA26-iAP line (Badea et al., 2009) for the lineage tracing. Opn1swp-Cre (BAC tg) mice were crossed with Z/EG line (The Jackson Laboratory) to generate Opn1swp-Cre (BAC tg); Z/EG for lineage tracing. Zebrafish were maintained and bred in accordance with the approved protocols of the of the University of Alberta’s Animal Care and Use Committee:BioSciences. Details of the strains are provided in Supplemental Experimental Procedures.
FACS Isolation of Mouse Rod Photoreceptors and Next Generation Sequencing (NGS)
Postnatal day (P) 2, P4, P6, P10, P14 and P28 Nrlp-GFP transgenic mouse retinas were dissected and retinal cells were dissociated by incubating in Accutase (Life Technologies) or Papain (Worthington biochemical, NJ, USA) at 37°C f or 10 min followed by trituration. GFP-positive cells were isolated by fluorescence-activated cell sorting (FACS) using FACS Aria II (Becton Dickinson, CA, USA). After sorting, these samples were also re-analyzed to check its purity. Only samples showing over 96% purity were used for strand-specific RNA-seq, ChIP-seq and RRBS, and a majority had 99% or more GFP-positive cells. Strand-specific RNA-seq was performed as described (Brooks et al., 2012), and data quantification was performed at transcript level using Ensembl v73 transcriptome annotation (Kaewkhaw et al., 2015). Modifications were made to standard methods (Barski et al., 2007; Brooks et al., 2012) for ChIP-seq and RRBS using isolated photoreceptors. ChIP-seq and RRBS reads were aligned using the Genomatix Mapper v3.7.5 (Genomatix, GmBH, Munich Germany), and the aligned RRBS reads were further analyzed using the methylKit package in R (Akalin et al., 2012). Details of experimental methods are provided in Supplemental Experimental Procedures. The dynamics of rod transcriptome during development and network analysis will be published elsewhere. NGS data, reported here, is available under Gene Expression Omnibus accession numbers GSE74660 (RNA-seq), GSE81099 (Methyl DNA), and GSExxxxx (ChIP-seq).
Immunostaining and Flowcytometry Analysis of Dissociated Retinal Cells
Retinal cells were dissociated as described above and fixed in 1% paraformaldehyde for 15 min at room temperature. Fixed cells were incubated with anti-S-opsin or NRL antibodies. After wash with PBS, cells were incubated in 1:200 dilution of secondary antibodies. Stained cells were mounted on slide glass for fluorescence microscopy or analyzed by FACS Calibur (Becton Dickinson). To obtain total retinal cell count, one retina from Nrlp-GFP mouse was dissociated by trypsin (Life Technologies) and counted using a hemocytometer. Details are provided in Supplemental Experimental Procedures.
Lineage Tracing and Immunohistochemistry
Enucleated eyes from the indicated lineage tracing mouse lines were pre-immersed in 4% paraformaldehyde for 15 min at room temperature. After alkaline phosphatase (AP) staining, tissues were washed three times in PBS with 0.1% Tween 20 for 20 min each and post-fixed in 4% paraformaldehyde overnight. The AP stained retinas were sectioned at 70 μm and processed for immunofluorescent staining as needed. See Supplemental Experimental Procedures for details.
Phylogenomic Analyses
Whole genomes from 30 taxa representing the major lineages of vertebrates were selected for analysis (Table S1). Protein models for each genome searched using BLAST (Altschul et al., 1997) and known NRL query sequences. Redundant hits were removed using cd-hit (Li and Godzik, 2006), sequences were aligned in MAFFT (Katoh and Standley, 2013) and regions of the alignment that contained less than 20% sequence coverage were removed using trimAl (Capella-Gutierrez et al., 2009). Phylogenetic analysis was conducted using RAxML 8.2 (Stamatakis, 2014) under the bestfit model using 20 random starts and 1000 bootstrap replicates. Reconciled tree analysis was conducted using NOTUNG 2.6 (Chen et al., 2000).
Supplementary Material
Highlights.
Rod photoreceptors in mouse retina show genetic and epigenetic vestiges of Scones
Rod photoreceptors in mice but not zebrafish exhibit S-cone lineage
NRL locus of early mammals accrued regulatory elements driving rod-dominant retina
Recruitment of S-cones to rods in mammals helped mitigate the nocturnal bottleneck
Acknowledgments
We are grateful to Douglas Forrest for advice and discussions, Tudor Badea for Rosa26-iAP mouse, Rafael Vilasmil for flowcytometry, and Robert Farris for imaging support. We thank Alexis Boleda, Sam Perez and Genomatix (Claudia Gugenmus, Tanja Drüke, Martin Seifert) for assistance in some of the next generation sequencing data analysis. We acknowledge Hao Wang and Michèle DuVal for generating transgenic zebrafish. This research was supported by intramural research program of the National Eye Institute, a Discovery Accelerator Grant from Natural Sciences & Engineering Research Council of Canada, and the National Research Foundation of Korea (NRF-2015 R1C1A1A01055466). This work utilized the high performance computational resources of the HPC Biowulf cluster at NIH (http://hpc.nih.gov).
Footnotes
Author Contributions
Overall Conceptualization, J.K. and A.S.; Methodology and Investigation (mouse), J.K., H.Y. and L.J.; Conceptualization, Methodology and Investigation (zebrafish), A.P.O. and W.T.A.; Resource Generation (Opn1swp-Venus transgenic mouse), L.J. and W.L.; Data Analysis, J.K., H.Y., M.J.B., A.P.O., W.T.A. and A.S.; Data Curation, M.J.B.; Initial Analysis for Evolution, J.K. and M.J.B.; NGS Dataset Analysis, J.K., H.Y., M.J.B and D.C.P.; Phylogenomic Analysis, D.C.P.; Writing – Original Draft, H.Y., J.K.; Writing – Review & Editing, J.K., H.Y., M.J.B., A.P.O., D.C.P., W.L., W.T.A. and A.S.; Funding Acquisition, A.S., W.T.A., J.K., W.L., D.C.P.; Supervision, A.S. and W.T.A.; Project Administration, A.S.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd-El-Barr MM, Pennesi ME, Saszik SM, Barrow AJ, Lem J, Bramblett DE, Paul DL, Frishman LJ, Wu SM. Genetic dissection of rod and cone pathways in the dark-adapted mouse retina. J Neurophysiol. 2009;102:1945–1955. doi: 10.1152/jn.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Ali N, Fridlich R, Millet-Puel G, Clerin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC, et al. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161:817–832. doi: 10.1016/j.cell.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 2012;13:R87. doi: 10.1186/gb-2012-13-10-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto M, Cheng H, Zhu D, Brzezinski JA, Khanna R, Filippova E, Oh EC, Jing Y, Linares JL, Brooks M, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci USA. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto M, Filippova E, Gage PJ, Zhu X, Craft CM, Swaroop A. Transgenic mice expressing Cre-recombinase specifically in M- or S-cone photoreceptors. Invest Ophthal Vis Sci. 2004;45:42–47. doi: 10.1167/iovs.03-0804. [DOI] [PubMed] [Google Scholar]
- Allison WT, Dann SG, Veldhoen KM, Hawryshyn CW. Degeneration and regeneration of ultraviolet cone photoreceptors during development in rainbow trout. J Comp Neurol. 2006;499:702–715. doi: 10.1002/cne.21164. [DOI] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Delfin K, Morris AC, Snelson CD, Gamse JT, Gupta T, Marlow FL, Mullins MC, Burgess HA, Granato M, Fadool JM. Tbx2b is required for ultraviolet photoreceptor cell specification during zebrafish retinal development. Proc Natl Acad Sci USA. 2009;106:2023–2028. doi: 10.1073/pnas.0809439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asteriti S, Gargini C, Cangiano L. Mouse rods signal through gap junctions with cones. Elife. 2014;3:e01386. doi: 10.7554/eLife.01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Brooks MJ, Rajasimha HK, Swaroop A. Retinal transcriptome profiling by directional next-generation sequencing using 100 ng of total RNA. Methods Mol Biol. 2012;884:319–334. doi: 10.1007/978-1-61779-848-1_23. [DOI] [PubMed] [Google Scholar]
- Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Chen K, Durand D, Farach-Colton M. NOTUNG: a program for dating gene duplications and optimizing gene family trees. J Comp Biol. 2000;7:429–447. doi: 10.1089/106652700750050871. [DOI] [PubMed] [Google Scholar]
- Collin SP, Davies WL, Hart NS, Hunt DM. The evolution of early vertebrate photoreceptors. Philos Trans R Soc Lond B Biol Sci. 2009;364:2925–2940. doi: 10.1098/rstb.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WI, Collin SP, Hunt DM. Molecular ecology and adaptation of visual photopigments in craniates. Mol Ecol. 2012;21:3121–3158. doi: 10.1111/j.1365-294X.2012.05617.x. [DOI] [PubMed] [Google Scholar]
- Dickson DH, Graves DA. Fine structure of the lamprey photoreceptors and retinal pigment epithelium (Petromyzon marinus L.) Exp Eye Res. 1979;29:45–60. doi: 10.1016/0014-4835(79)90165-9. [DOI] [PubMed] [Google Scholar]
- Distel M, Wullimann MF, Koster RW. Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc Natl Acad Sci USA. 2009;106:13365–13370. doi: 10.1073/pnas.0903060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval MG, Oel AP, Allison WT. gdf6a is required for cone photoreceptor subtype differentiation and for the actions of tbx2b in determining rod versus cone photoreceptor fate. PloS one. 2014;9:e92991. doi: 10.1371/journal.pone.0092991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Curr Biol. 2010;20:R114–124. doi: 10.1016/j.cub.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AM, Sieving PA. Assessment of foveal cone photoreceptors in Stargardt’s macular dystrophy using a small dot detection task. Vision Res. 1993;33:1509–1524. doi: 10.1016/0042-6989(93)90144-l. [DOI] [PubMed] [Google Scholar]
- Gerkema MP, Davies WI, Foster RG, Menaker M, Hut RA. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc Biol Sci. 2013;280:20130508. doi: 10.1098/rspb.2013.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol. 2015;91:117–123. doi: 10.1111/php.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesy CP, Hall MI. The nocturnal bottleneck and the evolution of mammalian vision. Brain Behav Evol. 2010;75:195–203. doi: 10.1159/000314278. [DOI] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Li PH, Schnapf JL. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci. 2005;25:11201–11209. doi: 10.1523/JNEUROSCI.3416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DM, Peichl L. S cones: Evolution, retinal distribution, development, and spectral sensitivity. Vis Neurosci. 2014;31:115–138. doi: 10.1017/S0952523813000242. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Losses of functional opsin genes, short-wavelength cone photopigments, and color vision--a significant trend in the evolution of mammalian vision. Vis Neurosci. 2013;30:39–53. doi: 10.1017/S0952523812000429. [DOI] [PubMed] [Google Scholar]
- Kaewkhaw R, Kaya KD, Brooks M, Homma K, Zou J, Chaitankar V, Rao M, Swaroop A. Transcriptome Dynamics of Developing Photoreceptors in Three-Dimensional Retina Cultures Recapitulates Temporal Sequence of Human Cone and Rod Differentiation Revealing Cell Surface Markers and Gene Networks. Stem cells. 2015;33:3504–3518. doi: 10.1002/stem.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koentges G. Evolution of anatomy and gene control. Nature. 2008;451:658–663. doi: 10.1038/451658a. [DOI] [PubMed] [Google Scholar]
- Lamb TD. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retin Eye Res. 2013;36:52–119. doi: 10.1016/j.preteyeres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN., Jr Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren E, Fritsches K, Brill R, Warrant E. The visual ecology of a deep-sea fish, the escolar Lepidocybium flavobrunneum (Smith, 1843) Philos Trans R Soc Lond B Biol Sci. 2014;369:20130039. doi: 10.1098/rstb.2013.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Luo DG, Xue T, Yau KW. How vision begins: an odyssey. Proc Natl Acad Sci USA. 2008;105:9855–9862. doi: 10.1073/pnas.0708405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvain VA, Knox BE. Nr2e3 and Nrl can reprogram retinal precursors to the rod fate in Xenopus retina. Dev Dyn. 2007;236:1970–1979. doi: 10.1002/dvdy.21128. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Menaker M, Moreira LF, Tosini G. Evolution of circadian organization in vertebrates. Braz J Med Biol Res. 1997;30:305–313. doi: 10.1590/s0100-879x1997000300003. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Belliveau MJ, Cepko CL. Two phases of rod photoreceptor differentiation during rat retinal development. J Neurosci. 1998;18:3738–3748. doi: 10.1523/JNEUROSCI.18-10-03738.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedian A, Fain GL. Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr Biol. 2015;25:484–487. doi: 10.1016/j.cub.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller GB. Evo-devo: extending the evolutionary synthesis. Nat Rev Genet. 2007;8:943–949. doi: 10.1038/nrg2219. [DOI] [PubMed] [Google Scholar]
- Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Frey RA, Wardwell SL, Stenkamp DL. The developmental sequence of gene expression within the rod photoreceptor lineage in embryonic zebrafish. Dev Dyn. 2008;237:2903–2917. doi: 10.1002/dvdy.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Saltó C, Vennström B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Ng L, Lu A, Swaroop A, Sharlin DS, Swaroop A, Forrest D. Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. J Neurosci. 2011;31:11118–11125. doi: 10.1523/JNEUROSCI.1709-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi H, Sakagami K, Ishii A, Morita N, Nishiuchi M, Ogino H, Yasuda K. Temporal expression of L-Maf and RaxL in developing chicken retina are arranged into mosaic pattern. Gene Expr Patterns. 2004;4:489–494. doi: 10.1016/j.modgep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Kothmann WW, Diamond JS. Illuminating synapses and circuitry in the retina. Curr Opin Neurobiol. 2011;21:238–244. doi: 10.1016/j.conb.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Khan N, Novelli E, Khanna H, Strettoi E, Swaroop A. Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc Natl Acad Sci USA. 2007;104:1679–1684. doi: 10.1073/pnas.0605934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Kojima D, Fukada Y, Shichida Y, Yoshizawa T. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc Natl Acad Sci USA. 1992;89:5932–5936. doi: 10.1073/pnas.89.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Paul DL, Wu SM. Rod, M-cone and M/S-cone inputs to hyperpolarizing bipolar cells in the mouse retina. J Physiol. 2012;590:845–854. doi: 10.1113/jphysiol.2011.224113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1001–1012. doi: 10.1002/ar.a.20262. [DOI] [PubMed] [Google Scholar]
- Pisani D, Mohun SM, Harris SR, McInerney JO, Wilkinson M. Molecular evidence for dim-light vision in the last common ancestor of the vertebrates. Curr Biol. 2006;16:R318–319. doi: 10.1016/j.cub.2006.03.090. author reply R320. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. Identification of a circadian clock-controlled neural pathway in the rabbit retina. PloS one. 2010;5:e11020. doi: 10.1371/journal.pone.0011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M. Zur Anatomie und Physiologie der Retina. Archiv für Mikroskopische Anatomie. 1866;3:215–247. [Google Scholar]
- Sherry DM, Bui DD, Degrip WJ. Identification and distribution of photoreceptor subtypes in the neotenic tiger salamander retina. Vis Neurosci. 1998;15:1175–1187. doi: 10.1017/s0952523898156201. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL. The rod photoreceptor lineage of teleost fish. Prog Retin Eye Res. 2011;30:395–404. doi: 10.1016/j.preteyeres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Suzuki SC, Bleckert A, Williams PR, Takechi M, Kawamura S, Wong RO. Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc Natl Acad Sci USA. 2013;110:15109–15114. doi: 10.1073/pnas.1303551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010;11:563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szel A, van Veen T, Rohlich P. Retinal cone differentiation. Nature. 1994;370:336. doi: 10.1038/370336a0. [DOI] [PubMed] [Google Scholar]
- Szikra T, Trenholm S, Drinnenberg A, Juttner J, Raics Z, Farrow K, Biel M, Awatramani G, Clark DA, Sahel JA, et al. Rods in daylight act as relay cells for cone-driven horizontal cell-mediated surround inhibition. Nat Neurosci. 2014;17:1728–1735. doi: 10.1038/nn.3852. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra J, Rynes E, Sandstrom R, Zhang M, Canfield T, Hansen RS, Stehling-Sun S, Sabo PJ, Byron R, Humbert R, et al. Mouse regulatory DNA landscapes reveal global principles of cis-regulatory evolution. Science. 2014;346:1007–1012. doi: 10.1126/science.1246426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls GL. The vertebrate eye and its adaptive radiation. Bloomfield Hills, Mich: Cranbrook Institute of Science; 1942. [Google Scholar]
- Whitney IE, Raven MA, Lu L, Williams RW, Reese BE. A QTL on chromosome 10 modulates cone photoreceptor number in the mouse retina. Invest Ophthal Vis Sci. 2011;52:3228–3236. doi: 10.1167/iovs.10-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. J Comp Neurol. 2000;425:545–559. [PubMed] [Google Scholar]
- Yoshimatsu T, Williams PR, D’Orazi FD, Suzuki SC, Fadool JM, Allison WT, Raymond PA, Wong RO. Transmission from the dominant input shapes the stereotypic ratio of photoreceptor inputs onto horizontal cells. Nat Commun. 2014;5:3699. doi: 10.1038/ncomms4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.