Abstract
Background
Industrial biotechnology that is able to provide environmentally friendly bio-based products has attracted more attention in replacing petroleum-based industries. Currently, most of the carbon sources used for fermentation-based bioprocesses are obtained from agricultural commodities that are used as foodstuff for human beings. Lignocellulose-derived sugars as the non-food, green, and sustainable alternative carbon sources have great potential to avoid this dilemma for producing the renewable, bio-based hydrocarbon fuel precursors, such as microbial lipid. Efficient bioconversion of lignocellulose-based sugars into lipids is one of the critical parameters for industrial application. Therefore, the fed-batch cultivation, which is a common method used in industrial applications, was investigated to achieve a high cell density culture along with high lipid yield and productivity.
Results
In this study, several fed-batch strategies were explored to improve lipid production using lignocellulosic hydrolysates derived from corn stover. Compared to the batch culture giving a lipid yield of 0.19 g/g, the dissolved-oxygen-stat feeding mode increased the lipid yield to 0.23 g/g and the lipid productivity to 0.33 g/L/h. The pulse feeding mode further improved lipid productivity to 0.35 g/L/h and the yield to 0.24 g/g. However, the highest lipid yield (0.29 g/g) and productivity (0.4 g/L/h) were achieved using an automated online sugar control feeding mode, which gave a dry cell weight of 54 g/L and lipid content of 59 % (w/w). The major fatty acids of the lipid derived from lignocellulosic hydrolysates were predominately palmitic acid and oleic acid, which are similar to those of conventional oilseed plants.
Conclusions
Our results suggest that the fed-batch feeding strategy can strongly influence the lipid production. The online sugar control feeding mode was the most appealing strategy for high cell density, lipid yield, and lipid productivity using lignocellulosic hydrolysates as the sole carbon source.
Electronic supplementary material
The online version of this article (doi:10.1186/s13068-016-0542-x) contains supplementary material, which is available to authorized users.
Keywords: Lignocellulosic hydrolysates, Lipid production, Fed-batch feeding strategy, Rhodosporidium toruloides, Automated online sugar control system
Background
Climate change and the increasing threat of global warming, which are most commonly caused by greenhouse gas (GHG) emissions, have drawn the attention of the world. Due to the GHG emissions from transportation, largely caused by the combustion of petroleum-based products [1], increased demand for renewable biofuels has gained tremendous attention in the last decade on the basis of their non-toxic, sustainable, and energy-efficient properties [2–4]. The current interest in renewable energy resources as alternatives to fossil fuels focuses around their capability to reduce the demand of crude oil and GHG emissions. Lipid is a potential feedstock for producing renewable hydrocarbon fuels [5]. However, using edible and industrial lipids derived from agricultural commodities results in competition with foodstuff necessary for the rising global population. Thus, microbial lipids from oleaginous microorganisms with high microbial lipid content in excess of 50 % (w/w) have been considered as alternatives to agricultural commodities for producing lipids and biofuels. Rhodosporidium toruloides is an industrially promising red oleaginous yeast, capable of converting pure glucose efficiently for microbial lipid production (Table 1) in terms of high lipid content, yield, and productivity [6–10].
Table 1.
Comparison of lipid production by various oleaginous yeasts using different carbon substrates in batch and fed-batch cultures
| Carbon substrate | Yeast strain | Culture mode | Lipid contenta (%) | YbL/S (g/g) | PrcL (g/L/h) | Refs. |
|---|---|---|---|---|---|---|
| Spent yeast | Cryptococcus curvatus | Repeated-batch | 37 | 0.12 | 0.07 | [14] |
| Sludge | Cryptococcus curvatus | Pulse-FB | 23 | – | 0.09 | [15] |
| Glycerol | Cryptococcus curvatus | DO-FB | 25 | 0.11 | 0.59 | [16] |
| Glycerol | Cryptococcus curvatus | Pulse-FB | 53 | 0.20 | 0.06 | [29] |
| Glycerol | Yarrowia lipolytica | Pulse-FB | 31 | 0.08 | 0.18 | [17] |
| Pure glucose | Cryptococcus curvatus | Pulse-FB | 53 | 0.14 | 0.21 | [30] |
| Pure glucose | R. toruloides | Pulse-FB | 67 | 0.23 | 0.54 | [6] |
| Pure glucose | R. toruloides | Pulse-FB | 59 | 0.20 | 0.36 | [7] |
| Lignocellulosic glucose | Cryptococcus sp. | Batch | 60 | 0.13 | 0.05 | [24] |
| Pure sugar mixture | Rhodococcus opacus | Pulse-FB | 54 | 0.18 | 0.29 | [31] |
| Pure sugar mixture | R. toruloides | Pulse-FB | 58 | 0.07 | 0.15 | [8] |
| Lignocellulosic sugar mixture | Lipomyces kononenkoae | Repeated-batch | 59 | 0.16 | 0.18 | [10] |
| Lignocellulosic sugar mixture | Rhodococcus opacus | Batch | 54 | 0.13 | 0.13 | [25] |
| Lignocellulosic sugar mixture | R. toruloides | Repeated-batch | 61 | 0.16 | 0.13 | [10] |
| Lignocellulosic sugar mixture | R. toruloides | DO-FB | 56 | 0.14 | 0.33 | [9] |
| Lignocellulosic sugar mixture | R. toruloides | Batch | 59 | 0.19 | 0.28d | This work |
| Lignocellulosic sugar mixture | R. toruloides | DO-FB | 60 | 0.23 | 0.33d | This work |
| Lignocellulosic sugar mixture | R. toruloides | Pulse-FB | 62 | 0.24 | 0.35d | This work |
| Lignocellulosic sugar mixture | R. toruloides | Online-FB | 59 | 0.29 | 0.40d | This work |
aLipid content, g lipid produced/g dry cell weight
bYL/S: Lipid yield, g lipid produced/g carbon source consumed
cPrL: Lipid productivity, g lipid produced/h/L
dLipid productivity was calculated at 72 h when most of the sugars were consumed for lipid production
Although lipid production by oleaginous microorganisms provides a large-scale alternative method, the carbon source is another obstacle to the broader commercialization of the biofuel production. Currently, the fermentation-based bioprocess mainly relies on the carbon sources (glucose) derived from food (e.g., grain and corn). Recently published OECD-FAO Agricultural Outlook stated that more than 10 % of food was used for the production of biofuel globally [11]. Therefore, much effort has been focused on exploring new alternative carbon sources, including food waste [12, 13], wastewater [14], sludge [15], and glycerol [16, 17], among others. However, glucose appears to be the favorite carbon source for lipid production in terms of lipid yield and productivity compared with other alternative carbon substrates (Table 1). Lignocellulosic feedstocks, being the most abundant and sustainable biomass in the world [18, 19], can be used as the carbon substrates for fermentation processes after the appropriate pretreatment process. At the National Renewable Energy Laboratory (NREL), an integrated lignocellulosic ethanol process was successfully demonstrated at pilot scale using dilute acid pretreatment [20–22]. For microbial lipid production, we used a variation of the NREL lignocellulosic ethanol process. As shown in Fig. 1, the pretreated solids were first washed with water to separate the xylose-rich liquor and glucose-rich solid. The solids were converted to the lignocellulosic hydrolysates (glucose-rich stream) by enzymatic hydrolysis and then used as the carbon source for producing lipids. Hydrocarbon fuel (biofuel) derived from lignocellulosic feedstocks has the potential to be carbon neutral (as shown in Fig. 1), meaning that the loss of carbon dioxide (CO2) to the atmosphere caused by burning them is offset by the absorption of CO2 by the biofuel feedstocks when they are growing. Farell et al. [23] estimated that first generation biofuel (corn ethanol) can reduce GHG emissions by 18 %, while the second generation biofuel from lignocellulosic feedstocks is expected to reduce emissions by 88 % relative to petroleum-based fuels. The lignocellulosic hydrolysates derived from different biomass feedstocks have been investigated for lipid production in batch cultures [10, 24–27]. Although promising results were achieved from these studies, the lipid productivity was not sufficient for industrial application. It has been proposed that a minimum lipid productivity of 1 g/L/h is required to make microbial lipids economically competitive at industrial scale [28]. However, the lignocellulosic feedstock-derived carbon substrates can dramatically reduce raw material cost as well as the production cost to clear the critical demand of lipid productivity. Exploring other culture strategies may also help solve this obstacle leading to a better production performance.
Fig. 1.

Simplified process flow diagram of lipid production from lignocellulosic feedstock. CHP represents combined heat and power
Fed-batch cultivation is a batch culture mode with continuous or sequential feeding of the substrate, which is superior to batch cultivation in terms of eliminating substrate-associated growth inhibition and/or achieving high cell density culture (HCDC) [32]. Moreover, the culture operation of fed-batch is as simple and reliable as batch cultivation [33]. However, the fed-batch fermentation performance is highly dependent on the feeding strategy applied during the cultivation.
Several fed-batch strategies have been demonstrated in the literature for improving the fermentation performance of various desired products [9, 31, 33–35]. Simple indirect feedback control methods that couple substrate feeding with the measurement of pH or dissolved oxygen (DO) have been used for years, because they are relatively simple and inexpensive. However, the slow response of cells to pH or DO changes due to substrate utilization can cause over- or under-feeding, which could affect production [34]. In direct feedback control, monitoring the concentration of the residual growth-associated substrate in the fermentor is used for controlling feed. This can be accomplished manually (pulse feeding) or automatically (online control feeding) [33, 34]. Thus, to reduce the substrate-associated growth inhibition and achieve a high lipid yield and productivity, developing the fed-batch culture process is essential and necessary. Wiebe et al. [8] reported a fed-batch cultivation using a pulse feeding mode for producing lipids by R. toruloides. In that study, the lipid production was remarkably improved by intermittently feeding a pure glucose solution; lipid yield and productivity increased from 0.1 to 0.22 g/g and 0.08 to 0.21 g/L/h, respectively. As far as we know, however, very few studies have been conducted to date on developing different fed-batch feeding strategies for HCDC and efficient lipid production using lignocellulosic hydrolysates as the sole carbon source [9, 10].
In this paper, several effective high-cell-density fed-batch cultivation systems, including DO-stat feeding mode, pulse feeding mode, and online sugar control feeding mode, were developed to enhance lipid production by R. toruloides to achieve high lipid content, yield, and productivity. Finally, the fatty acid composition of microbial lipids produced in various fed-batch bioprocesses was analyzed and compared. Results from this study are useful for scaling up the microbial lipid production by R. toruloides for industrial applications.
Results and discussion
Lipid production in the batch cultivation using lignocellulosic hydrolysates as the sole carbon source
To develop a suitable lipid production fermentation process, a batch culture of R. toruloides was performed to acquire baseline results for estimating the performance of lipid production in terms of lipid concentration, yield, and productivity. The batch cultivation was implemented using the aforementioned lignocellulosic hydrolysates with an initial concentration of 110 g/L, derived directly from the C6 cellulosic solids stream after enzymatic hydrolysis and hydrolysate clarification. The lignocellulosic hydrolysate sugar is composed of 95 % glucose and 5 % xylose. As shown in Fig. 2, dry cell weight (DCW) of 36.2 g/L was obtained from 96-h batch cultures after consuming all sugars (Fig. 2), which is similar to the previous report using pure glucose in the batch culture of R. toruloides giving a DCW of 37 g/L [8]. Our result indicates that the hydrolysates used in this study exhibited similar behavior as the pure sugar due to the processing step after pretreatment to produce the lignocellulosic sugars. The lipid content (50 %) and lipid productivity (0.19 g/L/h) from that work were lower than what we achieved from this study, which was 58.7 % and 0.28 g/L/h, respectively (Table 2). Our batch cultivation also gave a lipid yield of 0.19 g/g (g lipid produced/g carbon source consumed) on lignocellulosic hydrolysates, which was only 50 % of the theoretical yield (0.36 g/g [36]). This may be due to the inhibition effect of the high concentration of lignocellulosic hydrolysates over this batch cultivation and the nature of the batch cultivation. Li et al. observed that lipid yield dropped significantly when glucose concentration increased from 90 to 400 g/L [6]. It should be noted that the lipid yield from our batch cultures was higher than some previous literature (Table 1), which may be due to the difference in culture condition, microorganism, and carbon substrate used for lipid production. However, under the same culture conditions, a better lipid production can be achieved in fed-batch cultures compared with batch cultures [6, 8, 30, 37]. Therefore, several fed-batch feeding strategies were developed to obtain a better lipid production performance from lignocellulosic hydrolysates in terms of higher lipid yield and productivity.
Fig. 2.

Cell growth and lipid production in batch cultures of R. toruloides. Total sugar consumption was 110 g/L (100 g/L glucose and 10 g/L xylose). Data points are the average of duplicate cultures. There was less than 5 % variation between each data point
Table 2.
Lipid production by R. toruloides using lignocellulosic sugar mixture as the carbon source in batch, DO-stat fed-batch (DO-FB), pulse fed-batch (Pulse-FB), and online sugar monitoring fed-batch (Online-FB) cultures
| Culture mode | Lipid content (%) (w/w) | Lipid yield (g/g) | Lipid productivitya (g/L/h) |
|---|---|---|---|
| Batch | 58.67 ± 2.28 | 0.19 ± 0.01 | 0.28 ± 0.01 |
| DO-FB | 59.81 ± 2.09 | 0.23 ± 0.01 | 0.33 ± 0.01 |
| Pulse-FB | 61.54 ± 2.87 | 0.24 ± 0.02 | 0.35 ± 0.01 |
| Online-FB | 58.76 ± 1.13 | 0.29 ± 0.01 | 0.40 ± 0.01 |
aLipid productivity was calculated at 72 h when most of the sugars were consumed for lipid production
DO-stat fed-batch cultivation for lipid production using lignocellulosic hydrolysates
The fed-batch culture mode proved to be one of the most efficient bioprocesses to reduce growth inhibition caused by the high concentration of the carbon source. The process of fed-batch cultivation had been applied for the production of various bioproducts, including, biopolyesters (PHAs), fatty acids, single cell proteins, etc. [32, 38, 39]. Three different fed-batch cultures of R. toruloides using DO-stat feeding, pulse feeding, and automated online feeding were carried out using C6-enriched lignocellulosic hydrolysates as the sole carbon sources.
DO-stat feeding strategy is an indirect feedback control that is the simplest and most inexpensive fed-batch culture system [40]. When sugar is supplied, the DO decreases rapidly due to the active metabolic pathways of cell growth and lipid production. Once the sugar is depleted, the DO begins to rise. Using these phenomena, a DO-stat fed-batch cultivation, in which DO was coupled with feeding sugars, was carried out. When DO reached more than 55 %, which was a preset value for this DO-stat fed-batch culture, the concentrated lignocellulosic hydrolysates were fed into fermentors automatically till the DO dropped to 50 % to prevent sugar depletion. Li et al. [6] found that the specific growth of R. toruloides decreased when glucose concentration was higher than 40 g/L. Therefore, the initial sugar concentration of lignocellulosic hydrolysates used for all the fed-batch cultures was 33 g/L hydrolysates (30 g/L glucose and 3 g/L xylose). The rest of the 77 g/L hydrolysate substrates were fed using different feeding strategies. The total sugar fed in fed-batch cultures corresponded to that in batch cultures. The time courses of DO, DCW, and sugar utilization in the DO-stat fed-batch cultivation are shown in Fig. 3. The abrupt increase and decrease of DO signals indicate the response to sugar exhaustion followed by addition of the lignocellulosic hydrolysates to the fermentors. Although the lipid content only improved by 1 % in this DO-stat fed-batch culture compared with our batch cultures, DCW and lipid productivity were increased more than 16 %, which corresponds to 42 g/L and 0.33 g/L/h, respectively (Fig. 3 and Table 2). Moreover, the lipid yield on lignocellulosic hydrolysates increased to 0.23 g/g (Table 2), which was a 21 % increase over what we observed in the batch cultivation. These results suggest that the DO-stat feeding strategy enhanced the efficiency of converting hydrolysates into lipid by R. toruloides. However, due to the nature of the indirect feedback control, the sugar concentration cannot be controlled precisely, which resulted in the under-feeding issue as can be seen at 24 and 44 h during this fed-batch cultivation. The sugar depletion over the cultivation may have an influence on both cell growth and lipid production [6], suggesting that better lipid production could be expected after solving this issue. Thus, developing the direct sugar control feeding strategy, which monitors the concentration of the residual substrate during the cultivation, is essential and necessary.
Fig. 3.

Cell growth and lipid production in DO-stat fed-batch (FB) cultures of R. toruloides. Total sugar consumption was 110 g/L (100 g/L glucose and 10 g/L xylose). Data points are the average of duplicate cultures. There was less than 5 % variation between each data point
Pulse feeding fed-batch cultivation for lipid production using lignocellulosic hydrolysates
The direct sugar feedback control is one of the most efficient feeding strategies to control commercial-scale bioprocesses [34, 41]. By measuring the sugar concentration either offline or online, the under-feeding of sugar during the cultivation can be prevented. Due to the growth inhibition effect when glucose concentration is greater than 40 g/L [6, 7], the sugar concentration in the pulse feeding fed-batch cultures was controlled below 30 g/L by manually feeding the concentrated lignocellulosic hydrolysates as shown in Fig. 4. The lignocellulosic hydrolysate feed was intermittently fed five times over 60 h to give a total sugar concentration of 110 g/L. A slightly higher DCW of 43 g/L and lipid content of 62 % were obtained in this pulse fed-batch culture (Fig. 4; Table 2). Because of the benefit of direct sugar feedback control, the sugar was maintained above 5 g/L throughout the entire cultivation, which improved the overall lipid yield and productivity to 0.24 g/g and 0.35 g/L/h, respectively (Table 2). Anschau et al. [42] applied a pulse feeding mode for lipid production by intermittently feeding concentrated sugar solution eight times in an 144 h cultivation. In this study, the lipid productivity in this pulse feeding fed-batch cultivation was improved by 20 % compared with the batch cultivation. Nevertheless, overfeeding appears to be another potential issue from this pulse feeding mode, even though a wide range of sugar concentration (5–30 g/L) did not affect the cell growth and lipid production compared to batch and DO-stat fed-batch cultures. It has been reported that glucose concentration has significant effects on both cell growth and lipid production in batch cultures of different oleaginous yeasts [6, 43]. However, overfeeding could be easily overcome by increasing sampling to maintain a desired level, but the intensive and extensive labor is not suitable or economical for an industrial-scale application. From this point of view, developing an online sugar control feeding mode, which can maintain the sugar at an optimal level automatically, will provide a great advantage over other fed-batch modes in terms of lipid production and scale-up for commercialization.
Fig. 4.

Cell growth and lipid production in pulse fed-batch (FB) cultures of R. toruloides. Total sugar consumption was 110 g/L (100 g/L glucose and 10 g/L xylose). Data points are the average of duplicate cultures. There was less than 5 % variation between each data point
Automated online sugar control fed-batch cultivation for lipid production using lignocellulosic hydrolysates
To maintain the desired sugar concentration during cultivation, an automated online sugar control feeding mode was developed using an automated cell-free sampling system coupled with an online sugar analyzer (details can be found in Methods and Materials). The automated sampling system will start feeding lignocellulosic hydrolysates based on the sugar concentration measured by the YSI and adjust the speed of the feeding pump to maintain a desired glucose concentration. It was suggested that maintaining a lower glucose concentration at 5 g/L in the cultivation of R. toruloides provided a 46 % increase in lipid productivity, compared with a 30 g/L glucose concentration [7]. Therefore, the glucose concentration was controlled at 10 g/L by this automated online sugar feeding system. As shown in Fig. 5, the glucose concentration measured by the online automated system via YSI (the dotted line) perfectly corresponded to the one measured offline by high performance liquid chromatography (HPLC) (the solid line: offline FB-Glucose). This automated system was able to control glucose around 10 ± 2 g/L, which provided a lipid yield of 0.29 g/g (Table 2) or 80 % of the theoretical yield (0.36 g/g [36]). The highest DCW of 54 g/L along with a lipid content of 59 % was achieved using this online sugar control fed-batch cultivation. Finally, the maximum lipid concentration of 32 g/L and lipid productivity of 0.4 g/L/h obtained from this fed-batch culture (Fig. 5 and Table 2) corresponded to a 52 and 42 % improvement, respectively, compared to our batch cultivation (Table 2). Slininger et al. [10] recently achieved lipid concentration of 30 g/L with lipid productivity of 0.22 g/L/h and lipid yield of 0.15 g/g in a two-stage process, which are unprecedented in any lignocellulosic hydrolysates. The results from our automated fed-batch system provided a higher lipid concentration, productivity, and yield compared with Slininger’s study [10] and exhibited the best lipid production performance achieved from lignocellulosic hydrolysates. This is likely because the R. toruloides cells converted the hydrolysate sugar more efficiently for cell mass generation and lipid production by controlling the glucose concentration around 10 g/L. Our result agrees with a previous report [44] that a higher sugar utilization rate and cell growth were achieved by maintaining glucose in a range of 12–17 g/L. This online sugar control fed-batch cultivation revealed that the highest cell density, lipid yield, and lipid productivity were obtained by maintaining an appropriate substrate level, indicating that this feeding mode efficiently converts lignocellulosic hydrolysates for lipid production by R. toruloides. Our findings suggest that using the proper culture mode, lignocellulosic sugar substrates can substitute for food-based sugars for producing lipid and biofuel, which will help to solve the food vs. fuel issue dramatically.
Fig. 5.
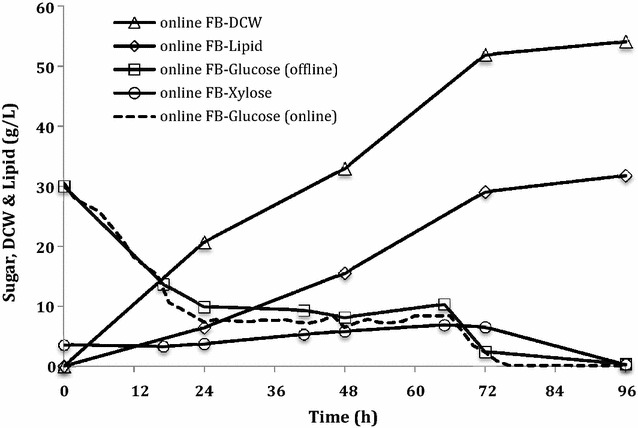
Cell growth and lipid production in online sugar monitoring fed-batch (FB) cultures of R. toruloides. Total sugar consumption was 110 g/L (100 g/L glucose and 10 g/L xylose). Data points are the average of duplicate cultures. There was less than 5 % variation between each data point
Composition analysis of fatty acid produced by R. toruloides in various culture modes using lignocellulosic hydrolysates
The influence of different culture modes on fatty acid composition was investigated at the end of this study. The fatty acid profiles from four different culture modes are compared in Table 3. Although the lipid content from different culture modes is slightly different, fatty acid composition is very similar based upon the relative amount of total fatty acids. The fatty acids produced by R. toruloides in this study were predominantly 16 and 18 carbons (98 % of the total) and consisted of myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3). Our result agreed with previous reports that similar distributions of fatty acid were found in the cultivation of R. toruloides for lipid production [8, 45, 46]. These fatty acids are major precursors for biodiesel production [47]. The cetane number (CN), which measures the readiness of the diesel fuel to auto-ignite when injected into the engine [48, 49], is one of the most important properties to specify the quality of various biofuels used in a diesel engine, specifically the ignition quality that is one of the properties of biodiesel that is determined by the structure of the FAME component [50, 51]. According to an estimation equation [52], fatty acids produced with lignocellulosic hydrolysates in this work were expected to be in the upper 50 s, which are slightly lower than palm-based fatty acids, but higher than sesame and maize-based fatty acids (Table 3). Summarizing the result, fatty acids produced by R. toruloides using lignocellulosic hydrolysates as carbon substrates are promising raw materials for biodiesel production based upon the biofuel standards of the US and European Organizations, which require the minimal CN value to be about 50 [47].
Table 3.
Comparison of fatty acid composition of lipids from R. toruloides and different feedstocks
| Fatty acid source | Relative amount of total fatty acids (%) | CNa | |||||
|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C18:0 | C18:1n9 | C18:2n6 | C18:3n3 | ||
| Batch | 1.3 ± 0.1 | 25.1 ± 1.1 | 10.1 ± 0.3 | 45.9 ± 2.1 | 10.5 ± 0.5 | 3.3 ± 0.1 | 57.7 ± 1.9 |
| DO-FB | 1.5 ± 0.1 | 26.5 ± 0.9 | 11.0 ± 0.2 | 46.4 ± 1.5 | 9.9 ± 0.3 | 3.0 ± 0.2 | 58.9 ± 2.1 |
| Pulse-FB | 1.3 ± 0.1 | 25.5 ± 1.2 | 10.9 ± 0.5 | 46.1 ± 2.2 | 10.1 ± 0.3 | 3.9 ± 0.2 | 58.2 ± 2.5 |
| Online-FB | 1.4 ± 0.1 | 26.8 ± 1.0 | 11.2 ± 0.5 | 45.8 ± 1.9 | 9.8 ± 0.2 | 4.1 ± 0.1 | 59.1 ± 2.2 |
| Palmb | N.D. | 44.8 | 4.6 | 38.9 | 9.5 | 0.4 | 61.4 |
| Sesameb | N.D. | 9.6 | 6.7 | 41.1 | 41.2 | 0.7 | 48.5 |
| Maizeb | N.D. | 11.6 | 2.5 | 38.7 | 44.7 | 1.4 | 47.0 |
N.D. not detected
aCN: The cetane number was calculated as described by Gopinath et al. [52]: CN = 62.2 + 0.074X1 + 0.115X2 + 0.177X3 -0.103X4 − 0.279X5 − 0.366X6, where X1 to X6 indicates the weight percentages of methyl myristate (C14:0), palmitate (C16:0), palmitoleate (C16:1), stearate (C18:0), oleate (C18:1), linoleate (C18:2), and linolenate (C18:3) in lipids, respectively
bData obtained from the literature [53]
Conclusions
In this study, we successfully demonstrated the improvement in cell density, lipid yield, and lipid productivity from lignocellulosic hydrolysate by R. toruloides using an online sugar control feeding mode when compared with batch cultivation and other fed-batch feeding strategies. The online sugar control feeding mode produced a lipid yield of 0.29 g/g and a lipid productivity of 0.4 g/L/h, which correspond to a 52 and 42 % increase over batch cultivation, respectively. To the best of our knowledge and previous reports [10, 54], the lipid yield and productivity from this type of fermentation appears to be the highest reported yield achieved from lignocellulosic hydrolysates. Also, the online sugar control feeding mode yielded a maximum DCW and lipid concentration of 54 and 32 g/L, respectively. Two other fed-batch strategies tested also showed improvement over batch cultivation. The DO-FB and pulse-FB produced similar lipid production, resulting in a lipid yield of 0.23–0.24 g/L and lipid productivity of 0.33–0.35 g/L/h, which corresponds to a 20 % increase over batch cultivation. Our results indicate that the online sugar control feeding mode can greatly enhance the efficiency of converting the hydrolysates into lipids by R. toruloides. Furthermore, this is the first report comparing an automated fed-batch system with other typical fed-batch systems for bioconversion of lignocellulosic-based sugars for producing hydrocarbon fuel. The results achieved in this work provide valuable foundational knowledge that can be applied to future research to further optimize fed-batch cultivation for lipid production.
Methods
Microorganism, media, and chemicals
The red yeast R. toruloides DSMZ 4444 used in this study was purchased from Leibniz Institute DSMZ (German collection of microorganisms and cell cultures). The strain was maintained at −80 °C on yeast peptone dextrose (YPD) medium (20 g/L glucose, 20 g/L peptone, and 10 g/L yeast extract) with 20 % glycerol (v/v) and subcultured biweekly on YPD agar plate (15 % agar). The first seed culture medium (pH 5.2) consisted of 50 g/L glucose, 20 g/L peptone, and 10 g/L yeast extract. When the first seed culture reached the exponential growth phase, 10 % inocula were transferred to the second seed culture in the YNB culture medium (Cat# 1524-100, Sunrise Science Products Inc., San Diego, CA, USA) with 50 g/L glucose and 0.2X YP (2 g/L peptone, and 1 g/L yeast extract) for 2–3 days. The YNB culture medium contained the following ingredients (per liter): potassium phosphate (1000 mg), magnesium sulfate (500 mg), sodium chloride (100 mg), calcium chloride (100 mg/L), boric acid (0.5 mg), copper sulfate (0.04 mg), potassium iodide (0.1 mg), ferric chloride (0.2 mg), manganese sulfate (0.4 mg), sodium molybdate (0.2 mg), zinc sulfate (0.4 mg), and pH 5.2. All inocula (10 % of second seed culture) were resuspended with YNB culture medium before the inoculation for batch and fed-batch cultures. Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA).
Preparation of lignocellulosic hydrolysates
The simplified scheme for preparing lignocellulosic hydrolysates is shown in Fig. 1. Deacetylation and sulfuric acid impregnation were performed in the 1900-L dynamic impregnator tank. Dry corn stover was added to the tank along with a dilute sodium hydroxide solution (0.4 %wt/wt). The slurry was heated to 80 °C and held for 2 h, and then, the liquor was discharged. Water was then added to the tank along with a dilute sulfuric acid solution to achieve a 0.8 % acid concentration for pretreatment. After thoroughly mixing the acid and solids at room temperature for 2 h, the slurry was pumped to the Vincent screw press and dewatered to approximately 40 % solids. The horizontal pretreatment reactor (Metso Inc., Norcross, GA, USA) was preheated for 1 h using steam jackets and allowed to reach steady state at the desired temperature before the runs began. Feedstock was fed through the reactor at a rate of 25 dry kg/h. The reactor was operated at 160 °C with a residence time of 10 min. After leaving the reactor, the material was discharged into an atmospheric-pressure flash tank where it separated into a high solid pretreated slurry stream and volatile flash vent stream. The pretreated slurry was then discharged into 55-gal drums and stored under refrigeration. The pretreatment slurry was washed to remove the xylose-rich hydrolysate as well as residual acids and other downstream inhibitors. This was done by centrifugation in an 80-L Western States basket centrifuge (Fairfield, OH, USA). The slurry was loaded into the basket, and water was fed through the cake until the liquid exiting the centrifuge showed negligible amounts of sugars when tested on a YSI. The cake of washed solids was then collected and stored under refrigeration.
Enzymatic hydrolysis was performed using Cellic CTec2 provided by Novozymes (Franklinton, NC, USA). To maximize the amount of monomeric glucose, enzyme was loaded at 40 mg protein/g cellulose. The washed solids were neutralized using a commercial dough mixer to approximately pH 5.2 with 15 % (w/w) ammonium hydroxide. The neutralized pretreated biomass, enzyme, and sufficient water were combined to achieve a 20 % total solids loading and placed in custom-built 8-L baffled roller bottles. The bottles were then incubated in a custom-built, incubated roller bottle apparatus at 49 °C and 15 rpm for 7 days. The resulting enzyme hydrolysate was centrifuged in bottles using a Sorvall Lynx 6000 centrifuge (Thermo Scientific, Waltham, MA USA) to remove solids and then sterile-filtered using a Sartopure 2 XLG 0.8 + 0.2 µm capsule filter (Sartorius AG, Germany). The glucose-rich stream (lignocellulosic hydrolysates) was concentrated in Buchi rotary evaporators. Evaporation took place at 72.0 °C, 17 inches Hg vacuum, for 5–8 h to bring the overall sugar concentration in the lignocellulosic hydrolysate to approximately 550 g/L with a ratio of glucose:xylose = 10:1 measured by a HPLC.
Culture condition
All seed cultures were grown in 250-ml Erlenmeyer flasks containing a 50-ml medium as specified above. The seed cultures were grown under 30 °C and 200 rpm in a rotary shaker. The exponentially growing cells were used for production cultures. All batch and fed-batch cultures were carried out in Sartorius BioStat Q-plus fermentors (Bohemia, NY, USA) with a 300 mL working volume in duplicate. Data points presented in this study are the average of duplicate cultures. The culture temperature was maintained at 30 °C, and pH was controlled at 5.2 with 4 M NaOH automatically by fermentors. The DO in the fed-batch cultures was maintained at 50 % of air saturation by adjusting the agitation speed from 300 to 700 rpm with an air flow rate of 0.4 volume per volume per minute (VVM). YNB culture medium with 0.2X YP was used for all production cultures. With the exception of the batch cultivation starting with 110 g/L lignocellulosic hydrolysates (100 g/L glucose and 10 g/L xylose), all fed-batch cultures were loaded with initial lignocellulosic hydrolysates of 33 g/L (30 g/L glucose and 3 g/L xylose) to reduce the lag phase due to less growth inhibition effect from low sugar concentration [6, 7]. Another 77 g/L of lignocellulosic hydrolysates (70 g/L glucose and 7 g/L xylose) was added during the cultivation via different feeding modes. The feeding solution was fed completely in 65 h in all fed-batch cultures, and all production cultures were terminated at 96 h when all sugars were consumed completely. The rapid increase in pH and DO values were observed after 72 h in all fed-batch cultures (see Additional files 1, 2, 3, 4, 5).
Three different feeding strategies were investigated to determine the best feeding mode in terms of cell density, lipid yield, and lipid productivity. In fed-batch cultures employing the DO-stat method, a rapid increase in the DO reading caused by the exhaustion of carbon substrates was used as an indicator for sugar feeding. The concentrated lignocellulosic hydrolysate was automatically fed with a peristaltic pump at different speeds when the DO burst to above 55 % until it dropped to below 50 %. The pulse feeding mode was carried out by feeding concentrated lignocellulosic hydrolysate manually during the cultivation after the residual glucose was lower than 10 g/L. The glucose concentration was maintained between 10 and 30 g/L for most of the culture time. An automated bioreactor sampling system was developed for the online sugar control feeding mode. The online YSI sugar analyzer (YSI 2950M, YSI Life Science, OH, USA) was coupled with an automated cell-free sampling system (Seg-Flow 1200, Flownamics Instruments Inc, Madison, WI, USA), which is capable of performing automated sampling (preset at every 2 h) and analysis during the fed-batch cultivation in response to the YSI sugar analysis data to control the substrate at an appropriate concentration. In this study, the concentrated lignocellulosic hydrolysate was automatically added in fermentors to maintain a glucose concentration of 10 g/L.
Analytical methods
Cell growth was estimated by measuring OD at 600 nm. A sample of 10-mL culture broth was transferred to a preweighed centrifuge tube and centrifuged at 5000 rpm for 20 min. After rinsing the pellet once with deionized water, the pellet was centrifuged and frozen at −80 °C for 1–2 days, and then dried for 24 h in a lyophilizer for DCW measurement. Lipid content was measured as total FAME content after a whole biomass in situ transesterification procedure. In brief, 7–10 mg of lyophilized microbial biomass was homogenized with 0.2 mL of chloroform–methanol (2:1 v/v), followed by the transesterification procedure in situ with 0.3 mL of HCl–methanol (5 %, v/v) for 1 h at 85 °C. FAMEs were analyzed by gas chromatography–flame ionization detection (GC-FID) on an Agilent 6890N; DB-WAX-MS column (Agilent, Santa Clara, CA) with dimensions 30 m × 0.25 mm i.d. and 0.25 μm film thickness [55, 56]. Sugar concentrations were measured by a YSI sugar analyzer and/or HPLC (Santa Clara, CA. USA) based upon the NREL standard analytical procedure [57].
Authors’ contributions
QF designed and performed the batch and fed-batch cultures and drafted this manuscript. ND advised on the design of the batch and fed-batch cultivation and drafted the manuscript. MO and RN carried out the pretreatment of biomass feedstock and preparation of lignocellulosic hydrolysates and drafted the manuscript. XC helped the experiment design, prepared the seed cultivation, and revised this manuscript. AL prepared the culture medium and seed cultures and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by funding (NREL/PO-5100-63912) from the US Department of Energy’s Bioenergy Technologies Office. The authors want to thank Leslee Pohle for editing this manuscript. The authors would like to extend their thanks to NREL staff members Dr. Jeff Linger for providing the Rhodosporidium toruloides DSMZ 4444; Kelsey Ramirez, Jeffrey Wolfe, and Stefanie VanWychen for their supportive help with FAME analysis; and Eric Knoshaug and Thieny Trinh for media recommendations. Authors would also like to thank YSI Life Science and Flownamics Instruments Inc for their technical support. NREL is a national laboratory of the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, operated by the Alliance for Sustainable Energy, LLC.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by funding (NREL/PO-5100-63912) from the United States Department of Energy’s Bioenergy Technologies Office.
Abbreviations
- CHP
combined heat and power
- C14:0
myristate
- C16:0
palmitate
- C16:1
palmitoleate
- C18:0
stearate
- C18:1
oleate
- C18:2
inoleate
- C18:3
linolenate
- CN
cetane number
- CO2
carbon dioxide
- DCW
dry cell weight
- DO
dissolved oxygen
- rpm
rotations per minute
- FAME
fatty acid methyl esters
- FB
fed-batch
- GHG
greenhouse gas
- HCDC
high cell density culture
- H2O
water
- HPLC
high performance liquid chromatography
- OD
optical density
- NREL
National Renewable Energy Laboratory
- VVM
volume per volume per minute
- YL/S
coefficient of lipid yield to sugar consumption
- PrL
volumetric lipid productivity
- YPD
yeast extract, peptone extract and dextrose
Additional files
10.1186/s13068-016-0542-x Time course of pH during the DO-stat fed-batch cultures.
10.1186/s13068-016-0542-x Time course of pH during the pulse fed-batch (FB) cultures.
10.1186/s13068-016-0542-x Time course of dissolved oxygen (DO) value during the pulse fed-batch (FB) cultures.
10.1186/s13068-016-0542-x Time course of pH during the online fed-batch (FB) cultures.
10.1186/s13068-016-0542-x Time course of dissolved oxygen (DO) value during the online fed-batch (FB) cultures.
Contributor Information
Qiang Fei, Email: feiqiang@mail.xjtu.edu.cn.
Marykate O’Brien, Email: Marykate.OBrien@nrel.gov.
Robert Nelson, Email: Robert.Nelson@nrel.gov.
Xiaowen Chen, Email: Xiaowen.Chen@nrel.gov.
Andrew Lowell, Email: alowell@kbibiopharma.com.
Nancy Dowe, Email: nancy.dowe@nrel.gov.
References
- 1.EPA. Clean Air Act. US Environmental Protection Agency. http://www3.epa.gov/climatechange/EPAactivities/regulatory-initiatives.html. 2015.
- 2.Ratledge C, Cohen Z. Microbial and algal oils: do they have a future for biodiesel or as commodity oils. Lipid Technol. 2008;20(7):155–160. doi: 10.1002/lite.200800044. [DOI] [Google Scholar]
- 3.Khanna M, Crago CL, Black M. Can biofuels be a solution to climate change? The implications of land use change-related emissions for policy. Interface Focus. 2011;1(2):233–247. doi: 10.1098/rsfs.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature. 2010;463(7280):559–562. doi: 10.1038/nature08721. [DOI] [PubMed] [Google Scholar]
- 5.Davis R, Tao L, Tan E, Biddy M, Beckham G, Scarlata C et al. Process design and economics for the conversion of lignocellulosic biomass to hydrocarbons: dilute-acid prehydrolysis and enzymatic hydrolysis deconstruction of biomass to sugars and biological conversion of sugars to hydrocarbons. Technical report NREL/TP-5100-60222013.
- 6.Li Y, Zhao ZK, Bai F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb Technol. 2007;41(3):312–317. doi: 10.1016/j.enzmictec.2007.02.008. [DOI] [Google Scholar]
- 7.Zhao X, Hu C, Wu S, Shen H, Zhao ZK. Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies. J Ind Microbiol Biotechnol. 2011;38(5):627–632. doi: 10.1007/s10295-010-0808-4. [DOI] [PubMed] [Google Scholar]
- 8.Wiebe MG, Koivuranta K, Penttilä M, Ruohonen L. Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol. 2012;12(1):26. doi: 10.1186/1472-6750-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, Wu S, Hu C, Wang Q, Hua Y, Zhao ZK. Lipid production from Jerusalem artichoke by Rhodosporidium toruloides Y4. J Ind Microbiol Biotechnol. 2010;37(6):581–585. doi: 10.1007/s10295-010-0704-y. [DOI] [PubMed] [Google Scholar]
- 10.Slininger PJ, Dien BS, Kurtzman CP, Moser BR, Bakota EL, Thompson SR et al. Comparative lipid production by oleaginous yeasts in hydrolyzates of lignocellulosic biomass and process strategy for high titers. Biotechnol Bioeng. 2016. doi:10.1002/bit.25928. [DOI] [PubMed]
- 11.OECD-FAO . 2015–2024 OECD-FAO agricultural outlook. Paris: OECD; 2015. [Google Scholar]
- 12.Fei Q, Chang HN, Shang L, Kim N, Kang J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour Technol. 2011;102(3):2695–2701. doi: 10.1016/j.biortech.2010.10.141. [DOI] [PubMed] [Google Scholar]
- 13.Fei Q, Chang HN, Shang L, Choi J-D-R. Exploring low-cost carbon sources for microbial lipids production by fed-batch cultivation of Cryptococcus albidus. Biotechnol Bioprocess Eng. 2011;16(3):482–487. doi: 10.1007/s12257-010-0370-y. [DOI] [Google Scholar]
- 14.Ryu B-G, Kim J, Kim K, Choi Y-E, Han J-I, Yang J-W. High-cell-density cultivation of oleaginous yeast Cryptococcus curvatus for biodiesel production using organic waste from the brewery industry. Bioresour Technol. 2013;135:357–364. doi: 10.1016/j.biortech.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 15.Seo YH, Lee IG, Han JI. Cultivation and lipid production of yeast Cryptococcus curvatus using pretreated waste active sludge supernatant. Bioresour Technol. 2013;135:304–308. doi: 10.1016/j.biortech.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Meesters P, Huijberts G, Eggink G. High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl Microbiol Biotechnol. 1996;45(5):575–579. doi: 10.1007/s002530050731. [DOI] [Google Scholar]
- 17.Rakicka M, Lazar Z, Dulermo T, Fickers P, Nicaud JM. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol Biofuels. 2015;8:104. doi: 10.1186/s13068-015-0286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol. 2010;101(13):4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez OJ, Cardona CA. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol. 2008;99(13):5270–5295. doi: 10.1016/j.biortech.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Humbird D, Mohagheghi A, Dowe N, Schell DJ. Economic impact of total solids loading on enzymatic hydrolysis of dilute acid pretreated corn stover. Biotechnol Prog. 2010;26(5):1245–1251. doi: 10.1002/btpr.441. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Tao L, Shekiro J, Mohaghaghi A, Decker S, Wang W, et al. Improved ethanol yield and reduced Minimum Ethanol Selling Price (MESP) by modifying low severity dilute acid pretreatment with deacetylation and mechanical refining: 1) Experimental. Biotechnol Biofuels. 2012;5(1):60. doi: 10.1186/1754-6834-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humbird D, Davis R, Tao L, Kinchin C, Hsu D, Aden A, et al. Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol: dilute-acid pretreatment and enzymatic hydrolysis of corn stover: Technical report NREL/TP-5100-477642011.
- 23.Crago CL, Khanna M, Barton J, Giuliani E, Amaral W. Competitiveness of Brazilian sugarcane ethanol compared to US corn ethanol. Energy Policy. 2010;38(11):7404–7415. doi: 10.1016/j.enpol.2010.08.016. [DOI] [Google Scholar]
- 24.Chang Y-H, Chang K-S, Lee C-F, Hsu C-L, Huang C-W, Jang H-D. Microbial lipid production by oleaginous yeast Cryptococcus sp. in the batch cultures using corncob hydrolysate as carbon source. Biomass Bioenergy. 2015;72:95–103. doi: 10.1016/j.biombioe.2014.11.012. [DOI] [Google Scholar]
- 25.Kurosawa K, Wewetzer SJ, Sinskey AJ. Triacylglycerol production from corn stover using a xylose-fermenting Rhodococcus opacus strain for lignocellulosic biofuels. J Microb Biochem Technol. 2014;6(5):254–259. doi: 10.4172/1948-5948.1000153. [DOI] [Google Scholar]
- 26.Huang C, Wu H, Li R-F, Zong M-H. Improving lipid production from bagasse hydrolysate with Trichosporon fermentans by response surface methodology. New Biotechnol. 2012;29(3):372–378. doi: 10.1016/j.nbt.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 27.X-f Chen, Huang C, Xiong L, Ma L-L. Microbial oil production from corncob acid hydrolysate by Trichosporon cutaneum. Biotechnol Lett. 2012;34(6):1025–1028. doi: 10.1007/s10529-012-0869-8. [DOI] [PubMed] [Google Scholar]
- 28.Rs M. Physiology of lipid accumulation yeast. Single cell oil. London: Longman; 1988. [Google Scholar]
- 29.Liang Y, Cui Y, Trushenski J, Blackburn JW. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour Technol. 2010;101(19):7581–7586. doi: 10.1016/j.biortech.2010.04.061. [DOI] [PubMed] [Google Scholar]
- 30.Hassan M, Blanc PJ, Granger L-M, Pareilleux A, Goma G. Influence of nitrogen and iron limitations on lipid production by Cryptococcus curvatus grown in batch and fed-batch culture. Process Biochem. 1996;31(4):355–361. doi: 10.1016/0032-9592(95)00077-1. [DOI] [Google Scholar]
- 31.Fei Q, Wewetzer SJ, Kurosawa K, Rha C, Sinskey AJ. High-cell-density cultivation of an engineered Rhodococcus opacus strain for lipid production via co-fermentation of glucose and xylose. Process Biochem. 2015;50(4):500–506. doi: 10.1016/j.procbio.2015.01.008. [DOI] [Google Scholar]
- 32.Shang L, Jiang M, Chang HN. Poly (3-hydroxybutyrate) synthesis in fed-batch culture of Ralstonia eutropha with phosphate limitation under different glucose concentrations. Biotechnol Lett. 2003;25(17):1415–1419. doi: 10.1023/A:1025047410699. [DOI] [PubMed] [Google Scholar]
- 33.Salehmin M, Annuar M, Chisti Y. High cell density fed-batch fermentations for lipase production: feeding strategies and oxygen transfer. Bioprocess Biosyst Eng. 2013;36(11):1527–1543. doi: 10.1007/s00449-013-0943-1. [DOI] [PubMed] [Google Scholar]
- 34.Kim BS, Lee SC, Lee SY, Chang HN, Chang YK, Woo SI. Production of poly (3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol Bioeng. 1994;43(9):892–898. doi: 10.1002/bit.260430908. [DOI] [PubMed] [Google Scholar]
- 35.Ding S, Tan T. L-lactic acid production by Lactobacillus casei fermentation using different fed-batch feeding strategies. Process Biochem. 2006;41(6):1451–1454. doi: 10.1016/j.procbio.2006.01.014. [DOI] [Google Scholar]
- 36.Huang W-D, Zhang Y-HP. Analysis of biofuels production from sugar based on three criteria: thermodynamics, bioenergetics, and product separation. Energy Environ Sci. 2011;4(3):784–792. doi: 10.1039/C0EE00069H. [DOI] [Google Scholar]
- 37.Kitcha S, Cheirsilp B. Enhancing lipid production from crude glycerol by newly isolated oleaginous yeasts: strain selection, process optimization, and fed-batch strategy. Bioenergy Res. 2013;6(1):300–310. doi: 10.1007/s12155-012-9257-4. [DOI] [Google Scholar]
- 38.Doelle HW, Kirk L, Crittenden R, Toh H, Doelle MB. Zymomonas mobilis—science and industrial application. Crit Rev Biotechnol. 1993;13(1):57–98. doi: 10.3109/07388559309069198. [DOI] [PubMed] [Google Scholar]
- 39.Schell DJ, Dowe N, Chapeaux A, Nelson RS, Jennings EW. Accounting for all sugars produced during integrated production of ethanol from lignocellulosic biomass. Bioresour Technol. 2016. [DOI] [PubMed]
- 40.Kim BS, Lee SC, Lee SY, Chang YK, Chang HN. High cell density fed-batch cultivation of Escherichia coli using exponential feeding combined with pH-stat. Bioprocess Biosyst Eng. 2004;26(3):147–150. doi: 10.1007/s00449-003-0347-8. [DOI] [PubMed] [Google Scholar]
- 41.Johnson A. The control of fed-batch fermentation processes—a survey. Automatica. 1987;23(6):691–705. doi: 10.1016/0005-1098(87)90026-4. [DOI] [Google Scholar]
- 42.Anschau A, Xavier MC, Hernalsteens S, Franco TT. Effect of feeding strategies on lipid production by Lipomyces starkeyi. Bioresour Technol. 2014;157:214–222. doi: 10.1016/j.biortech.2014.01.104. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X, Kong X, Hua Y, Feng B, Zhao ZK. Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur J Lipid Sci Technol. 2008;110(5):405–412. doi: 10.1002/ejlt.200700224. [DOI] [Google Scholar]
- 44.Tsakona S, Skiadaresis AG, Kopsahelis N, Chatzifragkou A, Papanikolaou S, Kookos IK, et al. Valorisation of side streams from wheat milling and confectionery industries for consolidated production and extraction of microbial lipids. Food Chem. 2016;198:85–92. doi: 10.1016/j.foodchem.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Guo F-J, Rong Y-J, Chi Z-M. Lipid production from hydrolysate of cassava starch by Rhodosporidium toruloides 21167 for biodiesel making. Renew Energy. 2012;46:164–168. doi: 10.1016/j.renene.2012.03.002. [DOI] [Google Scholar]
- 46.Wu S, Zhao X, Shen H, Wang Q, Zhao ZK. Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Bioresour Technol. 2011;102(2):1803–1807. doi: 10.1016/j.biortech.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 47.Azam MM, Waris A, Nahar N. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy. 2005;29(4):293–302. doi: 10.1016/j.biombioe.2005.05.001. [DOI] [Google Scholar]
- 48.Van Gerpen J, editor. Cetane number testing of biodiesel. In: Proceedings, third liquid fuel conference: liquid fuel and industrial products from renewable resources; 1996.
- 49.Puhan S, Saravanan N, Nagarajan G, Vedaraman N. Effect of biodiesel unsaturated fatty acid on combustion characteristics of a DI compression ignition engine. Biomass Bioenergy. 2010;34(8):1079–1088. doi: 10.1016/j.biombioe.2010.02.017. [DOI] [Google Scholar]
- 50.Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol. 2005;86(10):1059–1070. doi: 10.1016/j.fuproc.2004.11.002. [DOI] [Google Scholar]
- 51.Bamgboye A, Hansen A. Prediction of cetane number of biodiesel fuel from the fatty acid methyl ester (FAME) composition. Int Agrophys. 2008;22(1):21. [Google Scholar]
- 52.Gopinath A, Puhan S, Nagarajan G. Relating the cetane number of biodiesel fuels to their fatty acid composition: a critical study. Proc Inst Mech Eng Part D J Automob Eng. 2009;223(4):565–583. doi: 10.1243/09544070JAUTO950. [DOI] [Google Scholar]
- 53.Kamal-Eldin A, Andersson R. A multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. J Am Oil Chem Soc. 1997;74(4):375–380. doi: 10.1007/s11746-997-0093-1. [DOI] [Google Scholar]
- 54.Jin M, Slininger PJ, Dien BS, Waghmode S, Moser BR, Orjuela A, et al. Microbial lipid-based lignocellulosic biorefinery: feasibility and challenges. Trends Biotechnol. 2015;33(1):43–54. doi: 10.1016/j.tibtech.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Laurens LM, Quinn M, Van Wychen S, Templeton DW, Wolfrum EJ. Accurate and reliable quantification of total microalgal fuel potential as fatty acid methyl esters by in situ transesterification. Anal Bioanal Chem. 2012;403(1):167–178. doi: 10.1007/s00216-012-5814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laurens L, Nagle N, Davis R, Sweeney N, Van Wychen S, Lowell A, et al. Acid-catalyzed algal biomass pretreatment for integrated lipid and carbohydrate-based biofuels production. Green Chem. 2015;17(2):1145–1158. doi: 10.1039/C4GC01612B. [DOI] [Google Scholar]
- 57.Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D. Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Golden: National Renewable Energy Laboratory; 2006. [Google Scholar]


