Abstract
Enteropathogenic Escherichia coli (EPEC) are deadly contaminants in water and food and induce protrusion of actin-rich membrane pedestals beneath themselves upon attachment to intestinal epithelia. EPEC then causes intestinal inflammation, diarrhea, and, among children, death. Here, we show that EPEC uses multiple tyrosine kinases for formation of pedestals, each of which is sufficient but not necessary. In particular, we show that Abl and Arg, members of the Abl family of tyrosine kinases, localize and are activated in pedestals. We also show that pyrido[2,3-d]pyrimidine (PD) compounds, which inhibit Abl, Arg, and related kinases, block pedestal formation. Finally, we show that Abl and Arg are sufficient for pedestal formation in the absence of other tyrosine kinase activity, but they are not necessary. Our results suggest that additional kinases that are sensitive to inhibition by PD also can suffice. Together, these results suggest that EPEC has evolved a mechanism to use any of several functionally redundant tyrosine kinases during pathogenesis, perhaps facilitating its capacity to infect different cell types. Moreover, PD compounds are being developed to treat cancers caused by dysregulated Abl. Our results raise the possibility that PD may be useful in treating EPEC infections, and because PD affects host and not bacterium, selecting resistant strains may be far less likely than with conventional antibiotics.
INTRODUCTION
Pathogenic Escherichia coli are a significant public health concern. In developing countries, enteropathogenic E. coli (EPEC) contaminates the water supply and causes infantile diarrhea (Goosney et al., 2000). The resulting dehydration contributes to as many as 1 million infant deaths per year (Mead et al., 1999). EPEC is closely related to enterohemmorhagic E. coli (EHEC), which causes “raw hamburger” disease, a condition comprising bloody diarrhea and hemorrhagic colitis, which can lead to hemolytic uremic syndrome and death (Riley et al., 1983). A hallmark feature of EPEC infections is the formation of attaching and effacing lesions on the host intestinal epithelia cells (Knutton et al., 1989). These lesions comprise a loss of intestinal microvilli and the formation of an actin-filled membranous pedestal that protrudes beneath the adherent bacterium. Pedestal formation is highly correlated with development of disease, and mutations in bacterial virulence factors associated with pedestal formation render the bacteria avirulent (Rosenshine et al., 1992; Foubister et al., 1994).
To form pedestals, EPEC initially attach loosely to epithelial cells through an adhesin (Frankel et al., 1996; Sinclair and O'Brien, 2002) and express several proteins associated with virulence, including a type III secretion apparatus (Jarvis et al., 1995) and a bacterial outer membrane protein called intimin (Donnenberg et al., 1993). The type III secretion system facilitates translocation of virulence factors into the host cytoplasm and plasma membrane (Goosney et al., 2000). One such factor, called translocated intimin receptor (Tir) (Kenny et al., 1997, 1999), spans the host plasma membrane, binds intimin on the bacterial surface (de Grado et al., 1999), and adheres the bacterium tightly to the host cell.
Phosphorylation of EPEC Tir on Y474 (Kenny, 1999) by an unknown host cell tyrosine kinase is crucial for formation of actin pedestals. Tir phosphorylation and pedestal formation are blocked by mutation of Y474 in Tir (Kenny, 1999; Gruenheid et al., 2001) or by genistein, a relatively nonspecific tyrosine kinase inhibitor (Rosenshine et al., 1996). Once phosphorylated, Tir facilitates recruitment and activation of host cell proteins, including Nck, N-WASP, and Arp2/3 complex (Kalman et al., 1999b; Gruenheid et al., 2001; Lommel et al., 2001), which initiate actin polymerization (Kalman et al., 1999b; Rohatgi et al., 1999) to construct and brace the pedestal.
Although much information is available on the molecules and mechanisms that trigger actin polymerization in the pedestals, much less is known about tyrosine kinases that either phosphorylate Tir directly or catalyze its phosphorylation indirectly. Moreover, no information is available on whether phosphorylation of other pedestal proteins regulates formation of actin pedestals. Notably, of the 90 tyrosine kinases in mammals (Robinson et al., 2000), only a small number regulate cytoskeletal rearrangements. One of these, c-Abl, is a member of a gene family that also includes the Abl-related gene Arg (Laneuville, 1995; Lanier and Gertler, 2000; Pendergast, 2002; Smith and Mayer, 2002). Although Abl and Arg are expressed in a broad range of tissues, homozygous deletion of Abl causes immune cell defects (Schwartzberg et al., 1991; Tybulewicz et al., 1991), and deletion of Abl and Arg causes embryonic lethality and neural defects (Koleske et al., 1998). The Abl protein contains catalytic (src homology [SH]1), SH2, and SH3 domains, and polyproline-rich regions, all of which are involved with inter- and intramolecular interactions (Laneuville, 1995). Abl also contains nuclear import and export signals and F- and G-actin–binding domains (Woodring et al., 2001). Mutant forms of Abl (e.g., the BCR-Abl oncoproteins; Raitano et al., 1997) have been identified in a variety of human cancers, most notably chronic myelogenous leukemia (CML; Druker et al., 2001).
Several observations indicate that Abl regulates actin cytoskeletal rearrangements that underlie membrane protrusions. Abl activity is stimulated by growth factors such as platelet-derived growth factor (PDGF) and fibronectin, which cause membrane ruffling and pseudopod formation, respectively (Plattner et al., 1999; Woodring et al., 2001). Whereas Abl activity increases during microspike and lamellipodia formation (Woodring et al., 2002), activity is inhibited by actin filaments (Woodring et al., 2001). Abl also interacts with Nck (Smith et al., 1999), Crk (Evans et al., 1997; Ren et al., 1994), and Grb2 (Tanaka et al., 1995), proteins found in EPEC pedestals (Goosney et al., 2001). One of these proteins, Crk, is phosphorylated in membrane ruffles in response to PDGF (Ting et al., 2001). Together, these data indicate that Abl activity is localized to cytoskeletal structures and dynamically regulated during cytoskeletal rearrangements.
Although ATP-binding sites of tyrosine kinases are highly conserved, use of structural information obtained from x-ray crystallography, and computer-assisted modeling based on kinase domain homology, has led to the development of relatively selective inhibitors. For Abl and BCR-Abl, STI-571 (also called imatinib or Gleevec) is one such inhibitor with reasonably high specificity that has proven clinically useful in treating CML (Druker et al., 2001; Goldman and Druker, 2001). The tendency of patients to develop resistance to STI-571 has led to the search for more potent inhibitors. Pyrido[2,3-d]pyrimidine (PD) compounds have this capacity (Dorsey et al., 2000; Kraker et al., 2000; Schindler et al., 2000; Wisniewski et al., 2002), although they differ in substrate specificity somewhat from STI-571 and can inhibit additionally Src-family kinases, PDGF receptor, epidermal growth factor receptor, and fibroblast growth factor receptor kinases (Dorsey et al., 2000; Kraker et al., 2000).
The availability of potent and relatively specific Abl kinase inhibitors together with cells deficient in Abl and Arg has allowed us to test the hypothesis that Abl or Arg participate in the formation and maintenance of EPEC pedestals. We describe here experiments demonstrating that the Abl and Arg tyrosine kinases localize and are activated in EPEC pedestals. Surprisingly, however, we show that Abl, Arg, and likely other unknown kinases are all sufficient to cause direct or indirect phosphorylation of Tir and possibly other pedestal proteins, and to catalyze pedestal formation. Our results suggest that rather than using a specific signaling pathway, the bacterium has adapted to use any of several functionally redundant cellular kinases. Our results also suggest that inhibitors of Abl currently being developed to treat cancer may be useful in treating bacterial infections caused by pathogenic E. coli.
MATERIALS AND METHODS
Cell Culture
3T3 cells or 3T3 cells derived from Abl-/-/Arg-/- mice (Koleske et al., 1998) were grown on glass coverslips in DMEM containing serum and incubated for 6 to 8 h at 37°C with wild-type (WT) EPEC (strain 2389/69) at an multiplicity of infection of 10. For some experiments, cells were transfected 2 d before infection with plasmid vectors using FuGENE 6 (Roche Diagnostics, Indianapolis, IN). Abl-T315I, Arg-T314I, and N-Src-346M were made using QuikChange site-directed mutagenesis technology (Stratagene, La Jolla, CA). PD compounds PD166326, SKI-DRV-1–10 (Supplementary Figure 4) were synthesized as described previously (Kraker et al., 2000; Nagar et al., 2002) and were indistinguishable in their effects in all assays. PD compounds, STI-571, and PP2 (Calbiochem) were dissolved in 100% dimethyl sulfoxide (DMSO). For pretreatment experiments, PD, PP2, STI-571, or DMSO was added to cells 1 h before infection with bacteria. For “reversal” experiments, compound or DMSO was added to cells 5 h after addition of bacteria, and the cells fixed 15 min to 2 h subsequently. Although the Ki value of PD for Abl is ∼5–10 nM as measured by in vitro kinase assay, micromolar concentrations of PD are required to block pedestal formation in cells. We surmise that this discrepancy arose because the drug, when applied to the medium, may not achieve the same concentration in the cell. In this regard, we have now synthesized more soluble derivatives of PD, which have the same Ki value for WT Abl but that block pedestals at slightly lower concentrations (i.e., 1 μM; our unpublished data). Additionally, the ATP concentration within the cell may increase the effective Ki. In vitro kinase assays are carried out at micromolar ATP concentrations, whereas the free ATP concentration inside the cell has been estimated to be ∼2–5 mM. Because ATP and PD compete for the same binding site, it is not at all unreasonable to expect that the effective Ki increases. In vitro kinase with high concentrations of ATP seems consistent with this prediction. Moreover, in systems where Src-family kinases have been implicated, PP1 concentrations in the micromolar range are routinely used despite a measured Ki in vitro of 5–50 nM, depending on the kinase.
Crystal Structure, Western Analysis, Immunoprecipitations, and Kinase Assays
Crystal Structure of PD in the ATP-binding pocket of Abl was done essentially as described previously (Nagar et al., 2002). For Western analysis, uninfected cells or cells infected with EPEC were washed three times with cold phosphate-buffered saline and lysed for 30 min at 4°C in 20 mM Tris, pH 7.2, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10% glycerol, 1 mM sodium ortho-vanadate, and protease inhibitors (Complete protease inhibitor mix; Roche Diagnostics). Samples were centrifuged for 20 min at 10,000 × g, and 30–50 μg of the supernatant was analyzed by SDS-PAGE and Western analysis. For immunoprecipitation experiments, samples were prepared as described above and incubated with primary antibody (α-Tir or α-Abl) for 2 h at 4°C, and for an additional hour with protein G beads. The beads were washed with lysis buffer and analyzed by immunoblotting or used in in vitro kinase assays. For in vitro kinase assays, glutathione S-transferase (GST)-Crk was used as a substrate and incubated with 20 U of Abl kinase (NEB, Beverly, MA) or immunoprecipitated Abl, and 10 μM ATP in 20 μl of kinase assay buffer (25 mM Tris, 10 mM MgCl2, and 1 mM dithiothreitol) for 30 min at 23°C. Samples were then subjected to SDS-PAGE, transferred to polyvinylidene difluoride membrane, and immunoblotted with α-phosphotyrosine antibody 4G10 or α-Abl monoclonal antibody (mAb) AB3. Assays for Src kinase activity were carried out similarly except a fusion of green fluorescent protein with a peptide (IYGEF) was used as a substrate (Liu et al., 1999).
Immunofluorescence Staining
For immunofluorescence analysis, cells were fixed in 2% formaldehyde and permeabilized in Triton-X-100 as described previously (Kalman et al., 1999a). EPEC was recognized by staining with 4,6-diamidino-2-phenylindole (DAPI; 1 μg/ml; Sigma-Aldrich, St. Louis, MO), and pedestals were recognized by staining with fluorescein isothiocyanate (FITC)-phalloidin (1 μg/ml; Molecular Probes, Eugene, OR). Before staining, some polyclonal antibodies (pAbs) were preincubated with EPEC or EPEC-Δ-Tir previously fixed in formaldehyde and then centrifuged. This procedure removed serum contaminants that nonspecifically bound EPEC. The primary antibodies and concentrations used in this study were as follows: α-WASP pAb (affinity purified, 1:200 dilution), α-hemagglutinin A (HA) mAb (3F10; 0.01 μg/ml; Roche Diagnostics), α-Nck mAb (1 μg/ml; Oncogene Science, Cambridge, MA), α-Abl mAb (AB3; 0.5 μg/ml for overexpressed Abl proteins; 50 μg/ml for endogenous Abl proteins; 8E9; 0.05 μg/ml; BD PharMingen, San Diego, CA), α-Tir pAb (1:2000 for microscopy; 1:50,000 for Western analysis; from Jim Kaper, University of Maryland, College Park, MD), and α-Src pAb (0.1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), and α-Abl-pY412 and α-Abl-pY245 pAbs (0.1 μg/ml; Cell Signaling Technology, Beverly, MA). Cells expressing exogenous c-Abl-WT were distinguished by relatively high fluorescence intensity with lower α-Abl mAb concentrations. Thus, images were acquired with much shorter exposures than those used to detect endogenous c-Abl–like protein. Secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Microscopy
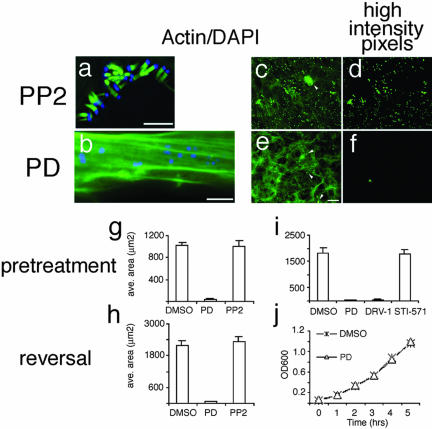
Images were acquired with a scientific-grade cooled charge-coupled device (Cool-Snap HQ with ORCA-ER chip) on a multi-wavelength, wide-field, three-dimensional microscopy system (Intelligent Imaging Innovations) (Denver, CO), based on a 200M inverted microscope using a 63× numerical aperture 1.4 lens (Carl Zeiss, Thornwood, NY). Immunofluorescent samples were imaged at room temperature by using a standard Sedat filter set (Chroma Technology, Rockingham, UT), in successive 0.25-μm focal planes through the samples, and out-of-focus light was removed with a constrained iterative deconvolution algorithm (Swedlow et al., 1997). To quantitate the effects of PD and PP2, we noted that pedestals stained more intensely with FITC-phalloidin than actin filaments. Images were segmented on the basis of intensity (e.g., Figure 3d for PP2 and 3f for PD). The correspondence of the highest intensity pixels to pedestals was confirmed visually (compare Figure 3, c and d). Cases where high pixel intensity and pedestals failed to coincide were adjusted accordingly (e.g., Figure 3, c and e, arrowheads). The area occupied by the highest intensity pixels was then calculated. Each bar represents the mean and SE calculated from 15 images. For each condition, data were acquired from cells plated and infected identically on the same day. Each experiment was repeated five times. The data in each graph are from a single experiment but are representative. Some variance in maximal pedestal area was evident between experiments due to variation in plating density and infection efficiency.
Figure 3.
Formation and maintenance of EPEC pedestals is blocked by PD166326 and related kinase inhibitors. (a and b) Images of 3T3 cells treated with Src kinase inhibitor PP2 (25 μM; a) or with PD166326 (10 μM; b) and then infected with EPEC for 6 h. Cells were stained with DAPI to recognize bacteria (blue) and FITC-phalloidin to recognize actin (green). (c–f) Representative images taken with 20× objective from cells pretreated with 10 μM PP2 (c) or 10 μM PD 166326 (e) and infected with EPEC. Cells were stained with FITC-phalloidin (green) to recognize actin, and DAPI (our unpublished data) and quantitated (see MATERIALS AND METHODS). Spatial distribution of the highest intensity pixels are exemplified in d for PP2 and f for PD and correspond to the actin pedestals in c and e. Arrowheads denote high-intensity pixels that did not correspond to pedestals and were excluded from the analysis. (g–i) Area occupied by the highest intensity pixels for EPEC treated according to the pretreatment (g and i) or reversal (h) regimens with DMSO, 10 μM PD166326, or 10 μM PP2. For i, PD analogs (SKI-DV-1-10 [DRV-1]; 10 μM) blocked EPEC pedestal formation but STI-571 (25 μM) did not. (j) Growth of EPEC was unaffected by treatment with PD166326. EPEC were cultured with either 0.1% DMSO (X) or 25 μM PD (Δ) and the OD 600 measured at the times indicated. Bar, 5 μm (a and b). Bar in e represents 40 μm and applies to c, d, and f as well.
RESULTS
Abl and Arg Localize in EPEC Pedestals
To test the hypothesis that Abl and related tyrosine kinases participate in pedestal formation, we first determined whether endogenous proteins resembling Abl localized within pedestals. 3T3 cells were exposed to EPEC and then stained with the α-Abl mAb 8E9, which recognizes an Abl kinase domain epitope (Wang, 1988). Pedestals are seen as intense actin staining (Figure 1a) directly apposed to the bacterium. An endogenous protein recognized by α-Abl-8E9 mAb was enriched in pedestals relative to the cytoplasm (Figure 1b). Identical results were obtained with α-Abl-AB3 mAb (our unpublished data). An endogenous protein recognized by the antibodies against the Abl-related kinase Arg also was enriched in pedestals relative to the cytoplasm (Figure 1, c and d). The α-Abl mAb and α-Arg pAb were specific: no staining with either antibody was evident in cells lacking Abl and Arg (Supplementary Figure 1, a–d). Moreover, in Abl-/-/Arg-/- cells, transfected Abl was not recognized by α-Arg pAb, and transfected Arg-yellow fluorescent protein (YFP) was not recognized by the α-Abl mAbs 8E9 or AB3 (Supplementary Figure 1, e–h). Staining with α-Abl and α-Arg antibodies also was evident in pedestals on HT29 cells (our unpublished data), a human colon epithelial cell line that retains many characteristics in culture of colonic epithelium, a tissue with which EPEC interacts in vivo. The localization of Abl and Arg to pedestals was specific but not unique (see below). We could find no evidence for localization of many tyrosine kinases, including c-Src (Supplementary Figure 2, a–d), Fyn, PDGF receptor, fibroblast growth factor receptor, Lck, FAK, Ntk, Lyn, Jak1, Csk, Tyk2, and Pyk2 (our unpublished data).
Figure 1.
Abl and Arg localize in EPEC pedestals. (a–d) Images of 3T3 cells (a–d) exposed to EPEC and stained with DAPI to identify EPEC, FITC-phalloidin to visualize actin, and α-Abl mAb 8E9 (a and b) or α-Arg pAb (c and d). In these merged images and in h and l, EPEC are pseudocolored blue, Abl or Arg red, and actin green. Note that α-Abl mAb and α-Arg pAb recognize proteins beneath EPEC in 3T3 cells. (e–h) Images of 3T3 cells expressing exogenous HA-Abl and exposed to EPEC and stained with DAPI (e), FITC-phalloidin (f), and α-HA (g). (i–l) Images of 3T3 cells expressing exogenous YFP-Arg (k) and exposed to EPEC and stained with DAPI (i) and Alexa 594-phalloidin (j). Together, these results suggest that both exogenous and endogenous Abl and Arg localize in pedestals. Supplementary Figure 1 shows that α-Abl mAb and α-Arg pAb are specific and recognize only Abl or Arg, respectively. Bars, 5 μm.
We next determined whether exogenously expressed HA-tagged Abl (HA-Abl) or yellow fluorescent protein-tagged Arg (Arg-YFP) localized in pedestals. The HA-tagged Abl protein detected with the α-HA-3F10 mAb (Figure 1, e–h), or Arg-YFP (Figure 1, i–l) localized in pedestals. The localization of proteins resembling Abl in pedestals was somewhat specific: neither exogenously expressed c-Src protein (Supplementary Figure 2, e–h) nor activated v-Src (our unpublished data), both detected with α-Src pAb, nor green or yellow fluorescent protein fluorescence, were enriched in pedestals relative to the cytoplasm (Supplementary Figure 2, i–l). However, N-Src, a splice variant of c-Src, did localize when overexpressed (Supplementary Figure 2, m–p), although the significance of such localization for EPEC infections in vivo is obscure because endogenous N-Src expression is restricted to brain. Together, these results suggest that Abl and Arg localize in pedestals but that other tyrosine kinases also can localize.
Abl and Arg Are Activated in EPEC Pedestals
To determine whether Abl and Arg were active in pedestals, we stained with an antibody that recognizes the phosphorylated Y412 (α-PY412) in the Abl activation loop domain (Pluk et al., 2002). We could not detect changes in Abl activity after EPEC infection by Western analysis of Abl autophosphorylation with α-PY412, by in vitro kinase assay, or by changes in phosphorylation of the Abl substrate CrkII (our unpublished data), presumably because the effect is local and the amount of activated Abl in the pedestal is only a small percentage of the total Abl in the cell. However, staining with α-PY412 was evident in the pedestals (Figure 2, a–h; e–h represents an enlargement of a–d). Similar results were obtained with α-PY245 pAb, another antibody that recognizes activated Abl (our unpublished data). Staining with α-PY412 was specific for Abl or Arg and was not evident on pedestals in cells lacking Abl and Arg (Figure 2, i–l). However, because the activation loop epitope recognized by the α-PY412 pAb is identical in Abl and Arg, the antibody likely cannot discriminate between the two proteins in a fluorescence experiment. To determine whether Abl, Arg, or both are active in the pedestal, we transfected either HA-Abl or Arg-YFP into Abl-/-/Arg-/- cells. In cells overexpressing either HA-Abl (Figure 2, m–p) or Arg-YFP (Figure 2, q–t), we observed staining with α-pY412 pAb in pedestals, indicating that both kinases were likely activated.
Figure 2.
Abl and Arg are activated in EPEC pedestals. (a–h) Images of 3T3 cells exposed to EPEC and stained with DAPI (a), FITC-phalloidin (b), and α-pY412 pAb (c). In the merged images (d and h), EPEC are pseudocolored blue, actin green, and pY412 red. Note the presence of α-pY412 staining in the pedestals. Images in e–h are enlargements of pedestals found in a–d, as indicated by the white box in b. (i–l) Images of Abl-/-/Arg-/- cells stained with DAPI (i), FITC-phalloidin (j), α-HA (k), and α-pY412 (l). Note that although pedestals form in Abl-/-/Arg-/- cells, no pY412 staining was evident. (m–p) Images of Abl-/-/Arg-/- cells expressing HA-Abl and stained with DAPI (m), α-HA (n), α-pY412 (o), and FITC-phalloidin (p). (q–t) Images of Abl-/-/Arg-/- cells expressing Arg-YFP and stained with DAPI (q) and α-pY412 pAb(s). The YFP channel is shown in r. Note that transfected Abl or Arg colocalize with PY412 staining. Together, these data suggest that α-pY412 pAb recognizes phosphorylated Abl or Arg but not other pedestal proteins and that both Abl and Arg are activated in pedestals. Bars, 5 μm.
Pedestals Form on Cell Lines Deficient in a Variety of Tyrosine Kinases
To determine whether Abl-, Arg-, or Src-family kinases were necessary for pedestal formation, we infected 3T3 cells derived from mice lacking both Abl and the Abl-related kinase Arg (Koleske et al., 1998), or from mice lacking Src, Fyn, and Yes (Stein et al., 1994). EPEC was still capable of forming pedestals in Abl-/-/Arg-/- cells (e.g., Figure 2, i–l) and on Src-/-/Fyn-/-/Yes-/- cells (Supplementary Figure 3, a–d). Moreover, we could find no evidence for localization of Src-family kinases in Abl-/-/Arg-/- cells (Supplementary Figure 3, e–j). Thus, neither Abl, Arg, nor Src-family kinases alone seem necessary for EPEC pedestal formation.
Inhibitors of Abl-, Arg-, and Src-Family Kinases Block Pedestal Formation and Maintenance
The observations that Abl, and Arg localize in pedestals, that at least Abl and Arg are activated in pedestals, and that pedestals formed on cell lines derived from mice lacking these kinases suggests two alternatives. First, Abl, or Arg can each catalyze pedestal formation, but in the absence of either or both of these kinases, another functionally redundant kinase can suffice. Alternatively, localization and activation of Abl or Arg could be unrelated to pedestal formation. To test whether Abl or Arg was involved in pedestal formation, we developed a test of sufficiency based on 1) the identification of an inhibitor of tyrosine kinases, including Abl and Arg, to block pedestal formation; and 2) the capacity of Abl or Arg mutants, resistant to such an inhibitor, to support pedestal formation with the inhibitor present. To do this, we first assessed the effects of PD compounds (Supplementary Figure 4a) that competitively inhibit binding of ATP to Abl and kinases with homologous ATP-binding domains, including Arg, Src, Fyn, and Yes (Dorsey et al., 2000; Kraker et al., 2000), and that are being developed to treat cancers caused by dysregulated Abl (e.g., CML; Goldman and Druker, 2001; Wisniewski et al., 2002). In cells pretreated with 5 μM PD and then infected with EPEC, fewer attached bacterium were evident, and little or no actin was apparent beneath those that did attach, even with protracted incubation (up to 8 h; Figure 3b). Concentrations of PD <5 μM were without effect (our unpublished data).
Quantitation of pedestals showed that PD treatment reduced EPEC pedestal formation by at least 50-fold (Figure 3, g and h). Treatment of cells with PD 5 h after infection with EPEC disassembled extant pedestals within 2 h (Figure 3h, reversal). Structurally related PD compounds (e.g., SKI-DV-1-10, 10 μM) were as effective as PD in blocking pedestal formation (Figure 3i). In contrast to PD, 2-phenylamino pyrimidine compounds (e.g., STI-571; Goldman and Druker, 2001), which inhibit Abl with lower efficacy than PD (Nagar et al., 2002), had no detectable effects on pedestal formation at concentrations up to 25 μM, the highest tested (Figure 3i). PP1 and PP2 inhibit Src-family kinases with a Ki value of ∼100 nM and Abl-family kinases with a Ki value of ∼300 nM measured by in vitro kinase assay (Liu et al., 1999; Tatton et al., 2003; see also Arnaud et al., 2003). In our assay, PP1 had no effect on pedestal formation even at concentrations up to 100 μM, the highest tested (e.g., Figure 3a). DMSO (0.1%), the carrier for PD and STI-571, was also without effect. To test for functional redundancy between Abl and Src or other kinases, we tested the effects of PD, STI-571, or PP2 inhibitors in Abl-/-/Arg-/- cells, and in Src-/-/Fyn-/-/Yes-/- cells. As in wild-type cells, PD but not PP2 or STI-571 inhibited pedestals in both of these cell lines (our unpublished data). The absence of pedestals was not attributable to a bactericidal effect of PD because no effect of the drug on growth or viability of EPEC was apparent (Figure 3j). The effects of PD were also not due to nonspecific inhibition of actin polymerization: PD had no effect on the capacity of Listeria monocytogenes or Shigella flexneri to form actin comet tails (Supplementary Figure 5).
PD Blocks Recruitment of Nck, N-WASP, and Arp2/3 in Pedestals but Not Tir
We next tested whether PD affected localization of Tir, Nck, N-WASP, or Arp2/3 complex beneath attached EPEC. In agreement with previous reports (Kenny et al., 1997), Tir localized in the pedestal beneath an attached bacterium (Figure 4, h–k) and was detectable by Western analysis after ≥3 h of infection (Figure 4e). With PD treatment, Tir protein remained detectable beneath attached EPEC (Figure 4c), despite the absence of pedestals (Figure 4b). Nck, N-WASP, and Arp2/3 complex are required for EPEC pedestal formation and, like Tir, localize in the pedestal (Figure 5; Kalman, 1999b; Gruenheid et al., 2001). Recruitment of Nck, N-WASP (Figure 5), and Arp2/3 complex (our unpublished data) beneath EPEC was blocked by PD.
Figure 4.
PD blocks tyrosine phosphorylation of EPEC Tir but not Tir localization. (a–d) Images of 3T3 cells treated with PD166326 (10 μM) and exposed to EPEC. Cells were stained with DAPI (a) to recognize EPEC, with FITC-phalloidin to recognize actin (b), with α-Tir pAb (c) and with α-phosphotyrosine mAb 4G10 (d). Note that Tir is present despite absence of pedestals and that phosphotyrosine staining is not evident beneath attached EPEC. Bar, 5 μm. (e–g) Tyrosine phosphorylation of EPEC Tir can be blocked or reversed by PD. Cells were treated with DMSO (e) or PD (f) and were left uninfected (0 h) or infected with EPEC for the times indicated. Cells were lysed and subjected to Western analysis with α-phosphotyrosine mAb 4G10 (top), stripped, and then reprobed with α-Tir pAb (bottom). Note that Tir protein is evident after 3 h and becomes phosphorylated in DMSO-treated cells and that PD blocks Tir phosphorylation. (g) Cells were left uninfected (lane 1) or infected with EPEC for 6 h, treated with PD for the times indicated, and analyzed as in e and f. Note the band corresponding to Tir becomes dephosphorylated within 5 min of adding PD. (h–o) Cells were infected with EPEC and stained with DAPI to recognize bacteria (h and l), with FITC-phalloidin to recognize actin (I and m), and with α-Tir pAb (j) or α-4G10 mAb (n) to recognize phosphotyrosine. In the merged images (k and o), bacteria are pseudocolored blue, Tir or phosphotyrosine red, and actin green. Bar, 5 μm.
Figure 5.
PD166326 blocks recruitment of Nck and N-WASP. (a–h) Images of 3T3 cells treated with DMSO (a–d) or PD166326 (e–h) and concomitantly exposed to EPEC. Cells were stained with DAPI (a and e) to recognize EPEC, with FITC-phalloidin to recognize actin (b and f), and with α-Nck mAb (c and g). (i–p) Images of 3T3 cells treated as in a–h and stained with DAPI (I and m), FITC-phalloidin (j and n), and α-N-WASP pAb (k and o). Note that Nck and N-WASP localize at the tip of actin pedestals and that PD blocks Nck and N-WASP localization. Bars, 5 μm.
PD Blocks and Reverses Tir Phosphorylation
Because pedestal formation and recruitment of Nck, N-WASP, and Arp2/3 depend on phosphorylation of EPEC Tir (Kenny, 1999), we next determined whether PD affected EPEC Tir phosphorylation. Lysates from cells infected with EPEC and treated with DMSO or PD were subjected to Western analysis with α-phosphotyrosine mAb 4G10. As seen in Figure 4, the band corresponding to Tir is tyrosine phosphorylated in the absence of PD (Figure 4e) but not in its presence (Figure 4f). Accordingly, phosphotyrosine staining was evident at the tip of pedestals in cells infected with EPEC (Figure 4n; Rosenshine and Finlay, 1993), but not beneath adherent EPEC in cells treated with PD (Figure 4d). We next treated cells with PD 5 h after infection with EPEC. Within 5 min, neither phosphotyrosine staining of Tir measured by Western analysis (Figure 4g) nor phosphotyrosine staining in pedestals (Supplementary Figure 6d) was detectable, although actin structures persisted for up to an hour (Supplementary Figure 6b). Thus, sustained activity of a PD-sensitive kinase is required to maintain Tir in a phosphorylated state, and phosphorylation/dephosphorylation reactions are rapid and dynamic. Together, these data provide evidence that PD compounds specifically inhibit Tir phosphorylation and formation and maintenance of actin pedestals, whereas other compounds such as STI-571, which only inhibits Abl-family kinases, or PP2, which inhibits both Src- and Abl-family kinases, were without effect. These results suggest that a PD-sensitive tyrosine kinase regulates pedestal formation. However, these results do not rule out the possibility that Abl, Arg, or other PD-sensitive kinases can act in a redundant manner to regulate pedestal formation.
Abl or Arg Is Sufficient among Tyrosine Kinases for Pedestal Formation and Maintenance
To determine whether Abl or Arg is sufficient among PD-sensitive kinases for tyrosine phosphorylation of Tir or pedestal formation, we assessed whether each kinase could support pedestals in the absence of other kinase activity. Specifically, we tested whether expression of a PD-resistant Abl or Arg allele allowed pedestals to form or persist in the presence of PD. To develop PD-resistant alleles of Abl and Arg, we took advantage of a mutation in BCR-Abl, T315I, acquired by CML patients who develop resistance to STI-571 (Gorre et al., 2001). The T315I mutation is in the ATP-binding domain of Abl and prevents inhibition of Abl by STI-571 by disrupting a hydrogen bond between T315 and STI-571 (Gorre et al., 2001; Nagar et al., 2002). To test whether PD-166326 interacted with Abl by the same mechanism as STI-571, we crystalized PD together with the ATP-binding domain of Abl. Although no hydrogen bond was evident, PD showed a strong van der Waals interaction with T315 in Abl (Figure 6A). Substitution of T for I at position 315 showed pronounced steric hindrance that disrupted the van der Waals interactions with PD (Figure 6B). Together, the structural data indicate that Abl-T315I binds PD less effectively that Abl-WT.
Figure 6.
PD blocks activity of Abl-WT but not Abl-T315I. (A and B) Crystal structure of PD-166326 in the ATP-binding pocket of Abl-WT (A) and Abl-T315I (B). Structure shows van der Waals interactions between T315 (blue in A) and PD. Ile substitution for Thr at position 315 (red in B) results in disruption of van der Waals interactions, steric hindrance, and loss of binding. (C) PD-166326 blocks kinase activity of Abl-WT but not Abl-T315I. Analysis of kinase activity in untransfected 293 cells (lanes 1 and 2) or in 293 cells transiently transfected with Abl-WT (lanes 3 and 4) or Abl-T315I (lanes 5 and 6). Transfected Abl was immunoprecipitated with α-Abl mAb AB3 and incubated with GST-Crk and ATP, together with DMSO (lanes 1, 3, and 5) or 10 nM PD-166326 (lanes 2, 4, and 6). No kinase activity from endogenous Abl was detectable in these cells. An equivalent amount of Abl immunoprecipitated from cells transfected with Abl-WT or Abl-T315I (see below). PD blocked kinase activity of precipitates from Abl-WT cells (lane 4), but not Abl-T315I cells.
To test whether lower efficacy of binding affects the capacity of PD to inhibit kinase activity, we assessed the kinase activity of Abl-WT and Abl-T315I in the presence of the drug. We expressed Abl-WT or Abl-T315I in 293 cells and then isolated the overexpressed Abl proteins by immunoprecipitation with α-Abl mAb 8E9. 293 cells have little precipitable endogenous Abl protein (Figure 6C, lanes 1 and 2), so the activity of the transfected WT and mutant Abl proteins can be readily evaluated. We incubated the immunoprecipitated proteins with ATP; GST-CrkII, a substrate for Abl; and either PD or DMSO. Kinase activity was assessed by Western analysis of GST-CrkII phosphorylation with α-phosphotyrosine mAb 4G10 (Ren et al., 1994). Transfected Abl-WT protein was capable of phosphorylating GST-CrkII but not GST-CrkII-Y221F (our unpublished data), a nonphosphorylatable mutant (Ren et al., 1994). As seen in Figure 6C (lanes 3 and 4), PD completely blocked exogenous Abl-WT. By contrast, PD had little effect on the activity of Abl-T315I (Figure 6C, lanes 5 and 6), although high concentrations of PD (>500 nM) did partially block activity (our unpublished data). Stripping and reprobing the blot with α-Abl mAb 8E9 confirmed that the amount of precipitated Abl-WT or Abl-T315I protein was equivalent (Figure 6C, bottom). Thus, in accordance with the structural predictions, Abl-T315I activity was much less susceptible to block by PD than Abl-WT.
To test whether Abl was sufficient for pedestal formation, we expressed Abl-T315I in 3T3 cells cultured in PD and then infected the cells with EPEC. As seen in Figure 7, i–l, pedestal formation as well as localization of phosphotyrosine beneath attached bacterium (Figure 7, i–l) was evident in PD-treated cells expressing Abl-T315I, but not in cells expressing endogenous Abl (Figure 7, a–d). We next tested the possibility that overexpression of Abl-T315I could, via a low-affinity interaction with PD, reduce the effective concentration of PD. To do this, we overexpressed Abl-WT, which has a higher affinity for PD than Abl-T315I. However, no pedestals or phosphotyrosine staining was evident in cells expressing Abl-WT and cultured in PD (Figure 7, e–h), suggesting that no such titration of the drug by overexpressed protein occurred.
Figure 7.
Expression of PD-resistant mutant Abl-T315I permits pedestal formation and tyrosine phosphorylation in the presence of PD. (a–l) Images of 3T3 cells transfected with vector (a–d), Abl-WT (e–h), or Abl-T315I (i–l), treated with 10 μM PD, and infected with EPEC for 5 h. Cells were stained with DAPI to recognize EPEC, FITC-phalloidin to recognize actin, α-Abl at low concentration to only detect transfected Abl, and α-phosphotyrosine mAb 4G10. Note the p-Tyr staining (l) and pedestals (j) are present in cells transfected with Abl-T315I, but not with vector (b and d) or Abl-WT (f and h). Note also that transfected Abl-T315I (k) localizes in the pedestal. Thus, Abl is sufficient among tyrosine kinases for tyrosine phosphorylation and pedestal formation. Bars, 5 μm.
When PD was added after pedestals had formed, expression of c-Abl-T315I also prevented loss of tyrosine phosphorylation in the pedestal (Supplementary Figure 6, i–l). Overexpression of c-Abl, even at high levels, was not sufficient to block loss of phosphorylation induced by PD (Supplementary Figure 6, e–h). In Supplementary Figure 6, cells were fixed 15 min after PD treatment. At this time point, pedestals were still evident in cells expressing endogenous Abl, Abl-WT, and Abl-T315I. After an hour, however, pedestals were only evident in cells expressing Abl-T315I.
To test whether Abl-T315I was unique in its capacity to rescue pedestal formation and tyrosine phosphorylation in the presence of PD, we constructed and tested a PD-resistant mutant of Arg. As seen in Figure 8 localization of phosphotyrosine beneath attached bacterium colocalized with Arg-YFP expression (Figure 8, a–c). Phosphotyrosine staining was evident beneath bacteria in PD-treated cells expressing Arg-T314I-YFP (Figure 8, g–i), but not in nontransfected cells (Figure 7, a–d) nor in cells overexpressing Arg-WT-YFP (Figure 8, d–f).
Figure 8.
Expression of PD-resistant mutant Arg-T314I permits tyrosine phosphorylation in the presence of PD. (a–i) Images of 3T3 cells transfected with Arg-YFP or Arg-T314I-YFP. Cells were infected with EPEC for 5 h and then either treated with carrier or 10 μM PD for 1 h postinfection. Cells were stained with DAPI to recognize EPEC and α-4G10-Cy5 to recognize phosphotyrosine. Note that P-Tyr staining colocalizes with Arg-YFP (c). Note also that P-Tyr staining is no longer evident upon addition of PD to cells overexpressing Arg-YFP(f) but that P-Tyr staining persists in cells expressing Arg-T314I-YFP (i). Thus, like Abl, Arg is sufficient among tyrosine kinases for tyrosine phosphorylation. Bars, 5 μm.
We next tested whether rescue of pedestal formation in PD by Abl-T315I or Arg-T314I was due to nonspecific phosphorylation by an overexpressed tyrosine kinase. To do this, we generated PD-resistant alleles of other tyrosine kinases, particularly those in the Src family. Shokat and colleagues (Liu et al., 1999) found that mutations at position 338 or its equivalent affect the sensitivity of Src-family kinases to PP2. Accordingly, we found that v-Src, which contains I at position 338, was resistant to PD (Supplementary Figure 7a). Substitution of T with M at position 346 in N-Src likewise rendered this protein resistant to PD (our unpublished data). Both mutants localized identically to their wild-type counterparts. Thus, N-Src-T346M localized in pedestals, whereas v-Src-338I did not (our unpublished data). However, neither v-Src-338I nor N-Src-T346M was capable of maintaining phosphotyrosine staining under the bacteria (Supplementary Figure 7, b–i), or rescuing pedestal formation (our unpublished data) in the presence of PD. These results suggest that overexpression of a tyrosine kinase per se, even one that localizes in the pedestal, is not sufficient to support pedestal formation or attendant tyrosine phosphorylation. Together, these results suggest that either Abl or Arg is sufficient among tyrosine kinases for pedestal formation and maintenance.
DISCUSSION
Our results indicate that Abl and Arg localize and are activated in EPEC pedestals and that Abl and Arg are sufficient among tyrosine kinases for maintenance and formation of pedestals. Our results additionally suggest that Abl and Arg contribute either directly or indirectly to the phosphorylation of EPEC Tir. To our knowledge, these are the first results describing any tyrosine kinase involved in EPEC signaling. Our conclusion of sufficiency is based on the capacity of overexpressed PD-resistant alleles of Abl and Arg to support pedestal formation in the presence of drug. Although we cannot exclude the possibility that overexpression of Abl-T315I or Arg-T314I results in nonspecific phosphorylation of targets critical for pedestal formation, this possibility seems unlikely because PD-resistant alleles of other kinases (e.g., v-Src-338I), including those which localize in pedestals (e.g., N-Src-T346M), fail to rescue pedestal formation in the presence of PD.
Our results with cells deficient in Abl and Arg indicate that other redundant kinases are also capable of supporting formation of actin pedestals. The subset of additional tyrosine kinases capable of supporting pedestal formation is defined by 1) susceptibility to inhibition by PD, and 2) the capacity to localize and become activated in the pedestal. Notably, PP2 or STI-571 fails to block pedestals in Abl-/-/Arg-/- cells or Src-/-/Fyn-/-/Yes-/- cells. These data suggest that there exist some additional unknown kinase or kinases that are capable of localizing in and supporting pedestals and that are sensitive to PD but resistant to PP2 and STI-571. Our results do not rule out the possibility that a hierarchy exists and that one kinase is preferentially used or that multiple kinases are required, only some of which are sensitive to PD. Tests of these hypothesis await the identification of additional kinases. Experiments to isolate these additional kinases using the differential sensitivity to PD, STI-571, and PP2 are currently under way.
Although redundancy among tyrosine kinases and other molecules is well documented (Imamoto et al., 1994; Stein et al., 1994), signaling pathways in mammalian cells are generally considered to maintain a high degree of specificity. Even in loss of function experiments that result in a lack of phenotype, dosage compensation mechanisms maintain general specificity by up-regulating one family member when another is removed. Thus, removal of Rac2 results in up-regulation of Rac1 (Li et al., 2002), and whereas targeted gene deletions of either Src or Fyn, alone, pairwise, or with Yes cause lethality, the frequency increases in mice harboring multiple deletions (Imamoto et al., 1994; Stein et al., 1994). Nevertheless, evidence from knock-in experiments with chimeric PDGFαβ receptors suggests that whereas some cellular phenotypes require a specific receptor (e.g., PDGFβ receptor in vascular genesis), in many cell types the intracellular signaling systems initiated by the two receptors are completely interchangeable (Klinghoffer et al., 2001).
Such a redundancy mechanism in EPEC signaling may have evolved to ensure phosphorylation of Tir or other targets required for pathogenesis and to broaden the range of cell types susceptible to infection to include those lacking particular kinases. Mechanisms that activate host signaling pathways to broaden host range or facilitate replication are certainly not unprecedented among bacterial or viral pathogens. For example, the polyoma virus protein middle t dysregulates the activity of c-Src, c-Fyn, and c-Yes to facilitate transformation (Ichaso and Dilworth, 2001). Likewise, the Yersinia-encoded phosphatase YOP-H dephosphorylates a variety of host proteins, including FAK, paxillin, and p130Cas to prevent engulfment (Cornelis, 2000). Abl and Arg have been implicated in entry of Shigella into the host cell (Burton et al., 2003), although the contributions of Abl or Arg alone were not assessed. Notably, entry of Shigella still occurred in ∼10% of Abl-/-/Arg-/- cells, suggesting either that another entry mechanism exists, or that a redundant kinase participates. Finally, EPEC itself takes advantage of redundancy in signaling molecules. Thus, homozygous deletion of both Nck1 and Nck2 are required to block pedestal formation (Gruenheid et al., 2001). It is not unreasonable to speculate that host mechanisms that effectively prevent use of host proteins by the pathogen may select for novel virulence strategies by the pathogen, such as adoption of a host kinase or phosphatase as a virulence factor.
Together, our results suggest a model of EPEC signaling that contributes to pedestal formation. Phosphorylation of EPEC Tir after recruitment of Abl, Arg, or another redundant kinase beneath the bacterium seems to initiate a signaling cascade at the cell surface that includes recruitment and activation of Nck (Gruenheid et al., 2001), N-WASP (Kalman et al., 1999b; Lommel et al., 2001), and the Arp2/3 complex (Kalman et al., 1999b), culminating in actin polymerization and pedestal formation. We cannot rule out the possibility that Abl or Arg do not directly phosphorylate Tir, but rather act through an intermediary, or that other signaling molecules in the pathway are targets of Abl or Arg. Recent work suggests that clustering of Tir via coupling of the bacterial outer membrane protein intimin is required for initiating Tir phosphorylation and thereby activating the signaling cascade leading to actin polymerization (Campellone et al., 2004). It is possible that clustering of Tir by intimin creates a binding site that facilitates recruitment and activation of Abl or Arg. Alternatively, clustering may induce conformational changes within Tir that permit access of Abl or Arg to its phosphorylation site. Blocking Abl or Arg activity with PD results in rapid dephosphorylation of Tir (Figure 4), indicating that kinase activity is likely high in pedestals (Figure 2) and that phosphorylation of Tir is dynamic and tightly regulated. In the absence of Abl or Arg, other redundant kinases can suffice. Frischknecht et al. (1999) have reported that phosphorylation of the A36R protein by Src-family tyrosine kinases mediates actin motility of vaccinia virus. Given the similarity of the phosphorylation site in Tir and A36R, it is not unreasonable to speculate that additional kinases also may participate in vaccinia motility.
Because PD compounds block and reverse tyrosine phosphorylation and pedestals, these compounds may prove effective as therapeutics for EPEC infections. The strategy of using PD as a drug to treat a bacterial infection suggests an important general solution to the development of drug resistance by microbes. Many bacteria develop resistance to antibiotics or drugs that inhibit growth or replication of the pathogen. Indeed the development of multidrug-resistant strains of many microbes such as vancomycin-resistant Staphylococcus aureus and multidrug-resistant-Mycobacterium tuberculosis represent an enormous public health concern. Unlike antibiotics, however, PD affects the host response to the bacterium, and not growth of the bacterium per se (Figure 3j). To develop resistance to PD, EPEC would have to alter its entire pathogenic program without strong growth selection. Thus, the likelihood of developing resistant strains with PD may be much less than with conventional antibiotics. Our approach of interfering with host cell targets such as Abl may have general utility in developing drugs to combat microorganisms that have acquired multidrug resistance.
Supplementary Material
Acknowledgments
We thank David Kalman, O. Weiner, J. Taunton, G. Benian, and K Saxe for helpful discussions; J. Kaper for Tir pAb; A. Goga and O. Witte for α-Abl mAb AB3; J. Taunton for WASP pAb; B. Meyer and J. Wang for Abl cDNAs; T. Koleske for Abl-/-/Arg-/- cells, α-Arg pAb, and Arg-YFP cDNA; and O. Weiner, and P. Jensen for commenting on the manuscript. The work was supported by grants from the University Research Council, Emory University, a National Institutes of Health Pilot Project Feasability Award and R01-AI056067–01 (all to D.K.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-02-0093. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-02-0093.
Abbreviations used: CML, chronic myelogenous leukemia; DAPI, 4,6 diamidino-2-phenylindol; EPEC, enteropathogenic E. coli O157:H6; HA, hemagglutinin A; mAb, monoclonal antibody; pAb, polyclonal antibody; PD, pyrido[2,3-d]pyrimidine; p-Tyr, phosphotyrosine; YFP, yellow fluorescent protein.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Arnaud, L., Ballif, B.A., Foerster, E., and Cooper, J.A. (2003). Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr. Biol. 13, 9-17. [DOI] [PubMed] [Google Scholar]
- Burton, E.A., Plattner, R., and Pendergast, A.M. (2003). Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 22, 5471-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone, K.G., Rankin, S., Pawson, T., Kirschner, M.W., Tipper, J., and Leong, J.M. (2004). Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J. Cell Biol. 164, 407-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis, G.R. (2000). Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 97, 8778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Grado, M., Abe, A., Gauthier, A., Steele-Mortimer, O., DeVinney, R., and Finlay, B.B. (1999). Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli. Cell Microbiol. 1, 7-17. [DOI] [PubMed] [Google Scholar]
- Donnenberg, M.S., Yu, J., and Kaper, J.B. (1993). A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J. Bacteriol. 175, 4670-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey, J.F., Jove, R., Kraker, A.J., and Wu, J. (2000). The pyrido[2,3-d]pyrimidine derivative PD180970 inhibits p210Bcr-Abl tyrosine kinase and induces apoptosis of K562 leukemic cells. Cancer Res. 60, 3127-3131. [PubMed] [Google Scholar]
- Druker, B.J., Sawyers, C.L., Capdeville, R., Ford, J.M., Baccarani, M., and Goldman, J.M. (2001). Chronic myelogenous leukemia. Hematology. Am. Soc. Hematol. Educ. Program 87-112. [DOI] [PubMed]
- Evans, E.K., Lu, W., Strum, S.L., Mayer, B.J., and Kornbluth, S. (1997). Crk is required for apoptosis in Xenopus egg extracts. EMBO J. 16, 230-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubister, V., Rosenshine, I., Donnenberg, M.S., and Finlay, B.B. (1994). The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect. Immun. 62, 3038-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel, G., Lider, O., Hershkoviz, R., Mould, A.P., Kachalsky, S.G., Candy, D.C., Cahalon, L., Humphries, M.J., and Dougan, G. (1996). The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J. Biol. Chem. 271, 20359-20364. [DOI] [PubMed] [Google Scholar]
- Frischknecht, F., Moreau, V., Rottger, S., Gonfloni, S., Reckmann, I., Superti-Furga, G., and Way, M. (1999). Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401, 926-929. [DOI] [PubMed] [Google Scholar]
- Goldman, J.M., and Druker, B.J. (2001). Chronic myeloid leukemia: current treatment options. Blood 98, 2039-2042. [DOI] [PubMed] [Google Scholar]
- Goosney, D.L., DeVinney, R., and Finlay, B.B. (2001). Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohemorrhagic Escherichia coli pedestals. Infect. Immun. 69, 3315-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosney, D.L., Gruenheid, S., and Finlay, B.B. (2000). Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell Dev. Biol. 16, 173-189. [DOI] [PubMed] [Google Scholar]
- Gorre, M.E., Mohammed, M., Ellwood, K., Hsu, N., Paquette, R., Rao, P.N., and Sawyers, C.L. (2001). Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293, 876-880. [DOI] [PubMed] [Google Scholar]
- Gruenheid, S., DeVinney, R., Bladt, F., Goosney, D., Gelkop, S., Gish, G.D., Pawson, T., and Finlay, B.B. (2001). Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 3, 856-859. [DOI] [PubMed] [Google Scholar]
- Ichaso, N., and Dilworth, S.M. (2001). Cell transformation by the middle T-antigen of polyoma virus. Oncogene. 20, 7908-7916. [DOI] [PubMed] [Google Scholar]
- Imamoto, A., Soriano, P., and Stein, P.L. (1994). Genetics of signal transduction: tales from the mouse. Curr. Opin. Genet. Dev. 4, 40-46. [DOI] [PubMed] [Google Scholar]
- Jarvis, K.G., Giron, J.A., Jerse, A.E., McDaniel, T.K., Donnenberg, M.S., and Kaper, J.B. (1995). Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92, 7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman, D., Gomperts, S.N., Hardy, S., Kitamura, M., and Bishop, J.M. (1999a). Ras family GTPases control growth of astrocyte processes. Mol. Biol. Cell 10, 1665-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman, D., Weiner, O.D., Goosney, D.L., Sedat, J.W., Finlay, B.B., Abo, A., and Bishop, J.M. (1999b). Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat. Cell Biol. 1, 389-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, B. (1999). Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol. 31, 1229-1241. [DOI] [PubMed] [Google Scholar]
- Kenny, B., DeVinney, R., Stein, M., Reinscheid, D.J., Frey, E.A., and Finlay, B.B. (1997). Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91, 511-520. [DOI] [PubMed] [Google Scholar]
- Kenny, B., Devinney, R., Stein, M., Reinscheid, D.J., Frey, E.A., and Finlay, B.B. (1999). Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91, 511-520. [DOI] [PubMed] [Google Scholar]
- Klinghoffer, R.A., Mueting-Nelsen, P.F., Faerman, A., Shani, M., and Soriano, P. (2001). The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Mol. Cell 7, 343-354. [DOI] [PubMed] [Google Scholar]
- Knutton, S., Shaw, R., McNeish, A.S., Philips, A., Price, E., and Watson, P. (1989). Diagnosis of enteropathogenic Escherichia coli. Lancet 2, 218. [DOI] [PubMed] [Google Scholar]
- Koleske, A.J., Gifford, A.M., Scott, M.L., Nee, M., Bronson, R.T., Miczek, K.A., and Baltimore, D. (1998). Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron 21, 1259-1272. [DOI] [PubMed] [Google Scholar]
- Kraker, A.J., Hartl, B.G., Amar, A.M., Barvian, M.R., Showalter, H.D., and Moore, C.W. (2000). Biochemical and cellular effects of c-Src kinase-selective pyrido[2,3-d]pyrimidine tyrosine kinase inhibitors. Biochem. Pharmacol. 60, 885-898. [DOI] [PubMed] [Google Scholar]
- Laneuville, P. (1995). Abl tyrosine protein kinase. Semin. Immunol. 7, 255-266. [DOI] [PubMed] [Google Scholar]
- Lanier, L.M., and Gertler, F.B. (2000). From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility. Curr. Opin. Neurobiol. 10, 80-87. [DOI] [PubMed] [Google Scholar]
- Li, S., Yamauchi, A., Marchal, C.C., Molitoris, J.K., Quilliam, L.A., and Dinauer, M.C. (2002). Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J. Immunol. 169, 5043-5051. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Bishop, A., Witucki, L., Kraybill, B., Shimizu, E., Tsien, J., Ubersax, J., Blethrow, J., Morgan, D.O., and Shokat, K.M. (1999). Structural basis for selective inhibition of Src family kinases by PP1. Chem. Biol. 6, 671-678. [DOI] [PubMed] [Google Scholar]
- Lommel, S., Benesch, S., Rottner, K., Franz, T., Wehland, J., and Kuhn, R. (2001). Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2, 850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead, P.S., Slutsker, L., Dietz, V., McCaig, L.F., Bresee, J.S., Shapiro, C., Griffin, P.M., and Tauxe, R.V. (1999). Food-related illness and death in the United States. Emerg. Infect. Dis. 5, 607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar, B., Bornmann, W.G., Pellicena, P., Schindler, T., Veach, D.R., Miller, W.T., Clarkson, B., and Kuriyan, J. (2002). Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res. 62, 4236-4243. [PubMed] [Google Scholar]
- Pendergast, A.M. (2002). The Abl family kinases: mechanisms of regulation and signaling. Adv. Cancer Res. 85, 51-100. [DOI] [PubMed] [Google Scholar]
- Plattner, R., Kadlec, L., DeMali, K.A., Kazlauskas, A., and Pendergast, A.M. (1999). c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 13, 2400-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluk, H., Dorey, K., and Superti-Furga, G. (2002). Autoinhibition of c-Abl. Cell. 108, 247-259. [DOI] [PubMed] [Google Scholar]
- Raitano, A.B., Whang, Y.E., and Sawyers, C.L. (1997). Signal transduction by wild-type and leukemogenic Abl proteins. Biochim. Biophys. Acta 1333, F201-F216. [DOI] [PubMed] [Google Scholar]
- Ren, R., Ye, Z.S., and Baltimore, D. (1994). Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 8, 783-795. [DOI] [PubMed] [Google Scholar]
- Riley, L.W., et al. (1983). Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308, 681-685. [DOI] [PubMed] [Google Scholar]
- Robinson, D.R., Wu, Y.M., and Lin, S.F. (2000). The protein tyrosine kinase family of the human genome. Oncogene 19, 5548-5557. [DOI] [PubMed] [Google Scholar]
- Rohatgi, R., Ma, L., Miki, H., Lopez, M., Kirchhausen, T., Takenawa, T., and Kirschner, M.W. (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221-231. [DOI] [PubMed] [Google Scholar]
- Rosenshine, I., Donnenberg, M.S., Kaper, J.B., and Finlay, B.B. (1992). Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 11, 3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine, I., and Finlay, B.B. (1993). Enteropathogenic Escherichia coli Epec activates protein tyrosine kinase Tpk in infected epithelial cells characterization of the major Tpk substrate. Abstr. Gen. Meet. Am. Soc. Microbiol. 93, 73. [Google Scholar]
- Rosenshine, I., Ruschkowski, S., Stein, M., Reinscheid, D.J., Mills, S.D., and Finlay, B.B. (1996). A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 15, 2613-2624. [PMC free article] [PubMed] [Google Scholar]
- Schindler, T., Bornmann, W., Pellicena, P., Miller, W.T., Clarkson, B., and Kuriyan, J. (2000). Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289, 1938-1942. [DOI] [PubMed] [Google Scholar]
- Schwartzberg, P.L., Stall, A.M., Hardin, J.D., Bowdish, K.S., Humaran, T., Boast, S., Harbison, M.L., Robertson, E.J., and Goff, S.P. (1991). Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell 65, 1165-1175. [DOI] [PubMed] [Google Scholar]
- Sinclair, J.F., and O'Brien, A.D. (2002). Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O 157, H7. J. Biol. Chem. 277, 2876-2885. [DOI] [PubMed] [Google Scholar]
- Smith, J.M., Katz, S., and Mayer, B.J. (1999). Activation of the Abl tyrosine kinase in vivo by Src homology 3 domains from the Src homology 2/Src homology 3 adaptor Nck. J. Biol. Chem. 274, 27956-27962. [DOI] [PubMed] [Google Scholar]
- Smith, J.M., and Mayer, B.J. (2002). Abl: mechanisms of regulation and activation. Front. Biosci. 7, d31-d42. [DOI] [PubMed] [Google Scholar]
- Stein, P.L., Vogel, H., and Soriano, P. (1994). Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 8, 1999-2007. [DOI] [PubMed] [Google Scholar]
- Swedlow, J.R., Sedat, J.W., and Agard, D.A. (1997). Deconvolution in optical microscopy. In: Deconvolution of Images and Spectra, ed. P.A. Jansson, San Diego: Academic Press, 284-307.
- Tanaka, M., Gupta, R., and Mayer, B.J. (1995). Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol. Cell Biol. 15, 6829-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton, L., Morley, G.M., Chopra, R., and Khwaja, A. (2003). The Src-selective kinase inhibitor PP1 also inhibits Kit and Bcr-Abl tyrosine kinases. J. Biol. Chem. 278, 4847-4853. [DOI] [PubMed] [Google Scholar]
- Ting, A.Y., Kain, K.H., Klemke, R.L., and Tsien, R.Y. (2001). Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc. Natl. Acad. Sci. USA 98, 15003-15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz, V.L., Crawford, C.E., Jackson, P.K., Bronson, R.T., and Mulligan, R.C. (1991). Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153-1163. [DOI] [PubMed] [Google Scholar]
- Wang, J.Y. (1988). Negative regulation of c-abl tyrosine kinase by its variable N-terminal amino acids. Oncogene Res. 3, 293-298. [PubMed] [Google Scholar]
- Wisniewski, D., et al. (2002). Characterization of potent inhibitors of the Bcr-Abl and the c-kit receptor tyrosine kinases. Cancer Res. 62, 4244-4255. [PubMed] [Google Scholar]
- Woodring, P.J., Hunter, T., and Wang, J.Y. (2001). Inhibition of c-Abl tyrosine kinase activity by filamentous actin. J. Biol. Chem. 276, 27104-27110. [DOI] [PubMed] [Google Scholar]
- Woodring, P.J., Litwack, E.D., O'Leary, D.D., Lucero, G.R., Wang, J.Y., and Hunter, T. (2002). Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J. Cell Biol. 156, 879-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.