Findings suggest that the bacterium has role in febrile episodes, is contagious, and has an epidemic character.
Key words: Tropheryma whipplei, fever, epidemic fever, bacteremia, Whipple disease, bacteria, Senegal
Abstract
The bacterium Tropheryma whipplei, which causes Whipple disease in humans, is commonly detected in the feces of persons in Africa. It is also associated with acute infections. We investigated the role of T. whipplei in febrile patients from 2 rural villages in Senegal. During June 2010–March 2012, we collected whole-blood finger-prick samples from 786 febrile and 385 healthy villagers. T. whipplei was detected in blood specimens from 36 (4.6%) of the 786 febrile patients and in 1 (0.25%) of the 385 apparently healthy persons. Of the 37 T. whipplei cases, 26 (70.2%) were detected in August 2010. Familial cases and a potential new genotype were observed. The patients’ symptoms were mainly headache (68.9%) and cough (36.1%). Our findings suggest that T. whipplei is a cause of epidemic fever in Senegal.
Determining the etiologic causes of febrile illness in tropical settings provides public health and local community benefits. In the context of a decline in malaria cases in many parts of sub-Saharan Africa, the few studies that have been conducted in recent years to analyze the burden of bacterial infections used traditional blood cultures and identified typhoid fever and Streptococcus pneumoniae as the leading documented causes of nonmalarial bloodstream infections (1–3). However, this method does not enable the identification of intracellular organisms, and most causes of fever remain unknown. In 2008, we initiated a study of the etiologies of fevers of unknown origin in Africa, particularly in Senegal. Our preliminary studies showed the presence of previously known pathogenic microorganisms, such as Borrelia crocidurae, Rickettsia felis, R. conorii, and Coxiella burnetii, and the unexpected presence of Tropheryma whipplei (4–9).
T. whipplei was first considered to be an uncommon bacterium that causes Whipple disease, a rare chronic disease (10). However, T. whipplei is in fact a common bacterium associated with various conditions, such as acute infections (pneumonia and gastroenteritis) and chronic infections (classic Whipple disease and other infections without digestive involvement, including endocarditis and encephalitis) (10–19). T. whipplei can also be carried in human feces and, less commonly, in the saliva (20–23); carriage prevalence varies by the age and exposure of the population and by geographic area (21–30).
T. whipplei is highly prevalent in rural Senegal, where carriage rates reach 75% among children <2 years of age, and overall seroprevalence is 72% (21–26). In our preliminary study in Senegal, which was conducted in 2 villages (Dielmo and Ndiop) during December 2008–July 2009, we detected T. whipplei bacteremia in 6.4% of the analyzed specimens (8). Bacteremia was significantly associated with cough, but no link to feces carriage was observed (8). However, our study had several limitations, such as a small number of febrile patients, no local control group of afebrile persons, and a short study period. In this same area, we recently showed that humans comprise the only source of T. whipplei among the populations in whom the bacterium is highly prevalent. Moreover, our findings showed that limited access to toilets and exposure to human feces was associated with the high prevalence of T. whipplei, suggesting that these conditions may facilitate fecal–oral transmission of the bacterium (31). To better characterize T. whipplei bacteremia, we extended our analysis, beginning in 2010, in this same area of rural Senegal to include the collection of >1,000 blood samples from healthy persons and ambulatory patients with acute fever.
Materials and Methods
We conducted the study during June 2010–March 2012 in Senegal’s rural Sine-Saloum area, a dry sahelian ecosystem with 2 typical seasons: dry (November–May) and rainy (June–October). We obtained written consent for every person included in the study. The National Ethics Committee of Senegal approved the study.
Participants
Study participants included 786 febrile patients at the healthcare center for the villages of Dielmo and Ndiop; 78% of the patients were <15 years of age, and the sex ratio was 1:1. For all patients with fever (defined as axillary temperature of >37.5°C), we conducted a medical examination, completed a questionnaire, and collected a whole-blood finger-prick sample (200-μL [4 drops]) (8). In parallel, we collected blood samples from a control group of 385 healthy, afebrile villagers; 62.5% of these study participants were <15 years of age, and the sex ratio was 1:1.
Molecular Analyses
DNA Extraction
For DNA extraction, we used a BioRobot EZ1 Workstation (QIAGEN, Courtaboeuf, France) according to the manufacturer’s instructions. Extraction was performed in Senegal, and specific quantitative real-time PCR (qPCR) was performed in France.
Specific qPCR
We used a 7900HT-thermocycler (Applied Biosystems, Foster City, CA, USA) with the QuantiTect-Probe PCR Kit (QIAGEN) to perform qPCR. First, we analyzed specimens for T. whipplei by using the primer pair Twhi3F (5′-TTG TGT ATT TGG TAT TAG ATG AAA CAG-3′)/Twhi3R (5′-CCC TAC AAT ATG AAA CAG CCT TTG-3′) and the specific Twhi3 probe (6-FAM-GGG ATA GAG CAG GAG GTG TCT GTC TGG-TAMRA). For specimens with positive results, we ran a second, confirmatory qPCR with the Twhi2F (5′-TGA GGA TGT ATC TGT GTA TGG GAC A-3′)/Twhi2R (5′-TCC TGT TAC AAG CAG TAC AAA ACA AA-3′) primer pair and the specific Twhi2 probe (6-FAM-GAG AGA TGG GGT GCA GGA CAG GG-TAMRA) (8,21). To validate the assays, we included positive (T. whipplei) and negative (PCR mix) controls in each run, as previously reported (8,21).
We considered samples to be T. whipplei–positive if qPCR results for the 2 specific genes were positive at a log-based fluorescence cycle threshold (Ct) of <38. We used qPCR for the β-actin housekeeping gene, as previously described (7), to check the quality of DNA handling and blood specimen extraction; only positive samples were considered reliable.
Genotyping
We performed genotyping of T. whipplei as previously described (32). We attempted to amplify and sequence each of 4 multispacer sequences (TW133, ProS, SecA, and Pro184) from positive specimens. When sequences were obtained, we compared them with those available in the GenBank database and our internal laboratory database to determine their corresponding genotype.
Statistical Analyses
We performed statistical analyses by using Epi Info 6 software (http://www.cdc.gov/epiinfo/index.html); results with p<0.05 were considered statistically significant. The corrected χ2 test or the Fisher exact test was used where indicated.
Results
Prevalence of T. whipplei Bacteremia
A total of 786 febrile patients and 385 healthy controls were included in the study, among whom 36 (4.6%) and 1 (0.25%), respectively, were positive for T. whipplei DNA (p<0.00007). The positive control participant was a 13-year-old boy who had low concentrations of T. whipplei DNA (Ct of 36.85 and 37.99). The Ct for febrile patients ranged from 26.10 to 36.41 (mean ISD 33.40 ± 2.53).
Age Distribution
The prevalence of T. whipplei bacteremia was 4% (3/75) for febrile patients <12 months of age, 4.8% (12/250) for those 1–3 years of age, 4.2% (5/119) for those 4–6 years of age, 5.4% (9/167) for those 7–15 years of age, 2.7% (2/75) for those 16–29 years of age, and 5.2% (5/97) for those >30 years of age. Age data were not available for 3 patients. No significant differences in age distribution were observed.
Clinical Manifestations
Clinical data were available for 786 febrile patients (Table 1). The main symptoms in the 36 T. whipplei–positive febrile patients were headache (23 [68.9%]), cough (13 [36.1%]), rhinorrhea (8 [22.2%]), nausea (5 [13.9%]), vomiting (4 [11.1%]), and diarrhea (3 [8.3%]). No significant clinical differences were observed by Ct level.
Table 1. Clinical manifestations observed in 786 febrile Tropheryma whipplei–positive or –negative patients in 2 villages, Dielmo and Ndiop in the Sine-Saloum area of Senegal, June 2010–March 2012.
| Clinical manifestation | T. whipplei–positive patients, no. (%), n = 36 | T. whipplei–negative patients, no. (%), n = 750 | p value by χ2 test |
|---|---|---|---|
| Headache | 23 (68.9) | 439 (58.5) | 0.52 |
| Arthralgia | 0 | 19 (2.5) | 0.46 |
| Myalgia | 0 | 53 (7.0) | 0.07 |
| Diarrhea | 3 (8.3) | 39 (5.2) | 0.3 |
| Vomiting | 4 (11.1) | 94 (12.5) | 0.56 |
| Nausea | 5 (13.9) | 100 (13.3) | 0.53 |
| Abdominal pain | 1 (2.8) | 21 (2.8) | 0.68 |
| Cough | 13 (36.1) | 274 (36.5) | 0.95 |
| Expectoration | 2 (5.6) | 42 (5.6) | 0.67 |
| Otalgia | 1 (2.8) | 28 (3.7) | 0.61 |
| Otorrhea | 0 | 2 (0.3) | 0.91 |
| Rhinorrhea | 8 (22.2) | 229 (30.5) | 0.28 |
| Burning urination | 1 (2.8) | 33 (4.4) | 0.53 |
| Rash | 0 | 10 (1.3) | 0.62 |
| Meningeal signs | 2 (5.5) | 25 (3.3) | 0.35 |
Seasonality
All 36 T. whipplei cases detected among the 786 febrile patients were in the 466 patients tested during the June–October rainy season; no cases were detected among the 320 febrile patients sampled during the November–May dry season (p = 0.0000001). Moreover, 33 (92%) of these 36 cases were diagnosed during the 2010 rainy season, and the other 3 were diagnosed during August 2011 (2 cases) and October 2011 (1 case) (Figure). The highest prevalence of T. whipplei bacteremia cases was detected during August, when 28 (30%) of 93 febrile patients were found to be positive (19 [28%] of 73 patients in Dielmo and 9 [45%] of 20 patients in Ndiop). In fact, the data were affected by the high prevalence of cases observed in August 2010, which seemed to be indicative of an outbreak.
Figure.
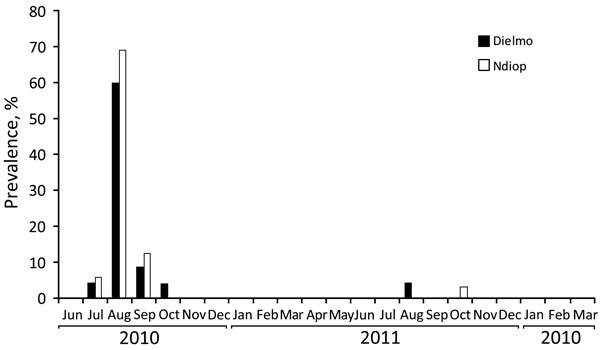
Monthly prevalence of Tropheryma whipplei bacteremia in Dielmo and Ndiop, Senegal, June 2010–March 2012. These 2 rural villages are located in the Sine-Saloum area, a dry sahelian ecosystem.
In July 2010, T. whipplei infection was detected in 2 febrile patients, an 18-year-old boy in Dielmo (case detected July 24) and a 15-year-old girl in Ndiop (case detected July 27). In August 2010, a total of 29 febrile patients from Dielmo were tested; 17 (58.5%) of the 29 patients had samples (18 total samples) positive for T. whipplei bacteremia. During the same month in Ndiop, 9 (69%) of 13 febrile patients had positive samples. In September 2010, 2 patients were positive in Dielmo and 1 in Ndiop, and in October, 2 patients were positive in Dielmo and none in Ndiop. For almost 1 year, all specimens from febrile patients were negative for T. whipplei. Then, in August 2011, only 2 patients were positive in Dielmo, and in October 2011, only 1 patient was positive in Ndiop.
Treatment and Follow-Up
Data about antimicrobial drug therapy was available for 33 patients, 23 of whom benefited from treatment with amoxicillin (18 patients), metronidazole (3 patients), or cotrimoxazole (2 patients). In Dielmo, 24 specimens from 23 patients were positive for T. whipplei; 1 patient was sampled twice 15 days apart, and both specimens were positive. For 17 patients, blood specimens were also sampled during other febrile episodes. Nine specimens from 5 patients were sampled from 15 days to 13 months before the positive sample was detected, and 43 specimens from 17 patients were sampled from 3 weeks to 16 months after the positive sample was detected; all of these samples were negative. Moreover, our previously published data (8) included test results for a 4-year-old boy who was diagnosed with T. whipplei bacteremia in January 2009 (19 months before August 2010). Four other blood specimens from this patient were tested 1 month before (1 sample) or 4, 11, and 15 months after (3 samples) the positive specimen was detected, and all were negative for T. whipplei.
In Ndiop, 12 specimens from 12 patients were positive. For 8 of these patients, blood specimens were sampled during other febrile episodes. The specimen for 1 patient was sampled 1 month before the positive sample, and 9 specimens from 6 patients were sampled from 7 weeks to 18 months after the positive samples; all of these specimens were negative. No data were available for these patients about antibody response against T. whipplei.
Genotyping
Because of the lack of specimens available for genotyping and the low sensitivity of genotyping, we could obtain multispacer sequences for only 8 patients at the time of the 2010 peak in T. whipplei bacteremia cases (Table 2). The T. whipplei genotype corresponds to the concatenation of the 4 spacers (TW133-ProS-SecA-Pro184); however, TW133 sequencing was not successful, so the corresponding spacer was not available (NA) for any of the patients. ProS sequence was obtained for 5 patients, SecA for 6 patients, and Pro184 for all patients. For 4 patients, 3 spacers were available, enabling the detection of the same multispacer sequence combination (NA-7-2-1) for the 4 patients. For another 4 patients, 2 spacers were available, enabling the detection of the NA-7-NA-1 combination for 2 of the patients and the NA-NA-2-1 combination for the other 2 patients. None of the potential combinations has previously been sequenced in Senegal. Moreover, the NA-7-2-1 combination has also not previously been detected in any other area of the world and is thus a new genotype. Overall, our data suggest that the same genotype was detected in Dielmo and Ndiop during the summer of 2010. However, T. whipplei genotyping was performed (sometimes only partially) for only 8 of 36 patients, so we can only suspect, but not confirm, that an epidemic clone was present and that an outbreak was ongoing at that time.
Table 2. Tropheryma whipplei multispacer typing results for 8 patients in the Sine-Saloum area of Senegal, 2010*.
| Patient no. | Age, y/sex | Sampling date | Village | Household no. | Spacers |
|||
|---|---|---|---|---|---|---|---|---|
| TW133 | ProS | SecA | Pro184 | |||||
| 1 | 1/M | 2010 Aug 4 | Dielmo | 14 | NA | NA | 2 | 1 |
| 2 | 1/M | 2010 Aug 10 | Dielmo | 39 | NA | 7 | 2 | 1 |
| 3 | 5/M | 2010 Aug 16 | Dielmo | 19 | NA | NA | 2 | 1 |
| 4 | 1/F | 2010 Aug 22 | Dielmo | 6 | NA | 7 | NA | 1 |
| 5 | 4/M | 2010 Aug 24 | Dielmo | 39 | NA | 7 | 2 | 1 |
| 6 | 13/F | 2010 Jul 27 | Ndiop | 2 | NA | 7 | 2 | 1 |
| 7 | 2/F | 2010 Aug 6 | Ndiop | 38 | NA | 7 | 2 | 1 |
| 8 | 2/M | 2010 Aug 13 | Ndiop | 10 | NA | 7 | NA | 1 |
*NA, not available.
Affected Households
In Dielmo during the peak of the August 2010 outbreak, multiple persons in several households were positive for T. whipplei bacteremia: 4 of 6 persons in household no. 19, 3 of 4 persons in household no. 39, 2 of 2 persons in household no. 9, and 2 of 3 persons in household no. 14. In Ndiop, 2 of 2 persons in household no. 3 and 2 of 3 persons in household no. 8 were positive for T. whipplei bacteremia. Of note, during this time, the family in household no. 39 had a furnace in which they baked bread that they marketed locally. In December 2010, most of the family left the village and the furnace was shut down; no additional T. whipplei bacteremia cases were subsequently observed.
Discussion
We report the detection of T. whipplei DNA in the blood of patients in Dielmo and Ndiop, Senegal. The validity of our data is based on strict experimental procedures and controls, including rigorous positive and negative controls, used to validate test results. In addition, we confirmed each positive PCR result by the successful amplification of an additional specific DNA sequence, and we performed T. whipplei genotyping on several specimens. We also showed that the presence of T. whipplei in blood is significantly linked to the presence of fever; T. whipplei DNA was detected (at a low level) in the blood of only 1 afebrile person in the study area. Moreover, we included a control group of afebrile persons from the same area, thereby reinforcing the validity of our data. Indeed, several well-known pathogens have been detected in recently analyzed specimens from healthy persons. For example, Plasmodium falciparum has been detected in 32% of blood specimens from healthy, afebrile persons in Senegal (33); respiratory viruses, including influenza virus, have been detected in 12% of nasopharyngeal samples from symptom-free Hajj pilgrims (34); and S. pneumoniae has been detected in 6.3% of blood specimens from afebrile children in Tanzania (35). Thus, because of the significantly higher prevalence of T. whipplei among febrile patients compared with healthy controls, we suspect that this microorganism is a pathogenic agent.
The overall prevalence of T. whipplei bacteremia is 4.6%. However, in August 2010, we observed a peak in T. whipplei bacteremia cases in Dielmo and Ndiop, where T. whipplei was involved in more than half of the observed cases of fever. This peak corresponds to a short outbreak of T. whipplei bacteremia with 1 potential genotype. A similar new genotype was observed for the patients from Dielmo and Ndiop for whom genotyping was available at the time of the outbreak. To date, 35 different T. whipplei genotypes have been detected in Senegal, but only 1 common genotype has been detected in Dielmo and Ndiop, even though the villages are 5 km apart (25). All of the other genotypes detected in the Sine-Saloum area were specific to each village, including the 2 that were more prevalent: genotype 52 was detected in 54% of feces samples in Dielmo, and genotype 49 was detected in 28% of feces samples from Ndiop (25).
Several familial cases also occurred during this outbreak. The family in household no. 39 in Dielmo was 1 of the most affected families: 3 of 4 persons living in the home had fever and T. whipplei bacteremia. Genotyping was available for 2 of these patients, both of whom exhibited the same potential genotype. The family in household no. 39 was involved in the management of a traditional oven for preparing bread, which was thoroughly cooked and sold directly to other residents. Since the departure of the baker and his family, no other outbreaks have been observed, and the prevalence of T. whipplei bacteremia has dramatically decreased. Thus, this family may have contributed to spread of the outbreak on a daily basis in Dielmo and possibly on a weekly basis at traditional markets, which served as the main contact between villagers from Dielmo and Ndiop. Also of note, no toilet facilities were present in household no. 39, and a link between a lack of toilet facilities and the high detection of T. whipplei, mainly in feces, has previously been reported (31). Thus, we hypothesize that T. whipplei was transmitted to customers who bought bread contaminated with infectious feces (31). Overall, all of our data confirm human-to-human transmission of the bacterium (22,23,26,31).
One of the main symptoms among febrile patients with T. whipplei bacteremia is cough (36.1%). In our preliminary study of T. whipplei bacteremia, cough was also the main manifestation observed (36). Thus, T. whipplei could be involved in respiratory infections (13,14,36,37). However, the presence of cough in ≈36% of febrile patients who were either T. whipplei–positive or –negative may also suggest that this symptom was poorly specific.
Of note, a 4-year-old patient had 2 febrile episodes associated with T. whipplei bacteremia 18 months apart (8); however, it was not possible to make a distinction between relapse and reinfection because genotyping was not available (38). Blood specimens from this patient that we tested for T. whipplei before and after the last febrile episode were negative, confirming that the infection was acute. Thus, these data suggest that some patients may have several febrile episodes linked to T. whipplei.
T. whipplei bacteremia cannot be diagnosed in tropical regions that lack the proper laboratory facilities or in industrialized countries that lack or do not routinely perform molecular biology–based diagnostics due to the specific training, expensive reagents, and excessive time required to perform such tests. Moreover, even recent studies that have looked for causes of nonmalarial fevers, including by performing molecular detection in blood for intracellular bacteria, such as R. felis, have not included the molecular detection of T. whipplei (39). Thus, it is currently difficult to estimate the prevalence of T. whipplei bacteremia. In conclusion, the results of our large-scale study clearly confirm the role of T. whipplei in febrile episodes as well as its contagiousness and epidemic character.
Acknowledgments
We thank all villagers who participated in the study, and we thank Aliou Diallo, Khadim Leye, Malick Diop, and Annick Bernard for technical support.
This research was financially supported through the Agence National de Recherche (ANR) grant 2010 MALEMAF (research on emergent pathogens in Africa) and the Foundation Mediterranée Infection.
Biography
Mr. Bassene is a PhD student at the Unité de Recherche sur les Maladies Infectieuses et Tropicales Emergentes (URMITE) in Dakar, Senegal. His main research interests include Tropheryma whipplei, Mansonella spp., and the microbiota of mosquitoes.
Footnotes
Suggested citation for this article: Bassene H, Mediannikov O, Socolovschi C, Ratmanov P, Keita AK, Sokhna C, et al. Tropheryma whipplei as a cause of epidemic fever, Senegal, 2010–2012. Emerg Infect Dis. 2016 Jul [date cited]. http://dx.doi.org/10.3201/eid2207.150441
References
- 1.Brent AJ, Ahmed I, Ndiritu M, Lewa P, Ngetsa C, Lowe B, et al. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya: community-based observational study. Lancet. 2006;367:482–8 . 10.1016/S0140-6736(06)68180-4 [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412 . 10.1371/journal.pntd.0000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–32 . 10.1016/S1473-3099(10)70072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, et al. Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis. 2011;17:883–5 . 10.3201/eid1705.100573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Socolovschi C, Mediannikov O, Sokhna C, Tall A, Diatta G, Bassene H, et al. Rickettsia felis–associated uneruptive fever, Senegal. Emerg Infect Dis. 2010;16:1140–2 . 10.3201/eid1607.100070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mediannikov O, Diatta G, Fenollar F, Sokhna C, Trape JF, Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis. 2010;4:e821 . 10.1371/journal.pntd.0000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Bassene H, Molez JF, et al. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis. 2010;4:e654 . 10.1371/journal.pntd.0000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenollar F, Mediannikov O, Socolovschi C, Bassene H, Diatta G, Richet H, et al. Tropheryma whipplei bacteremia during fever in rural West Africa. Clin Infect Dis. 2010;51:515–21 . 10.1086/655677 [DOI] [PubMed] [Google Scholar]
- 9.Mediannikov O, Socolovschi C, Edouard S, Fenollar F, Mouffok N, Bassene H, et al. Common epidemiology of Rickettsia felis infection and malaria, Africa. Emerg Infect Dis. 2013;19:1775–83 . 10.3201/eid1911.130361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenollar F, Lagier JC, Raoult D. Tropheryma whipplei and Whipple’s disease. J Infect. 2014;69:103–12 . 10.1016/j.jinf.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 11.Moos V, Schneider T. Changing paradigms in Whipple’s disease and infection with Tropheryma whipplei. Eur J Clin Microbiol Infect Dis. 2011;30:1151–8 . 10.1007/s10096-011-1209-y [DOI] [PubMed] [Google Scholar]
- 12.Harris JK, De Groote MA, Sagel SD, Zemanick ET, Kapsner R, Penvari C, et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci U S A. 2007;104:20529–33 . 10.1073/pnas.0709804104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bousbia S, Papazian L, Auffray JP, Fenollar F, Martin C, Li W, et al. Tropheryma whipplei in patients with pneumonia. Emerg Infect Dis. 2010;16:258–63 . 10.3201/eid1602.090610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenollar F, Ponge T, La Scola B, Lagier JC, Lefebvre M, Raoult D. First isolation of Tropheryma whipplei from bronchoalveolar fluid and clinical implications. J Infect. 2012;65:275–8 . 10.1016/j.jinf.2011.11.026 [DOI] [PubMed] [Google Scholar]
- 15.Raoult D, Fenollar F, Rolain JM, Minodier P, Bosdure E, Li W, et al. Tropheryma whipplei in children with gastroenteritis. Emerg Infect Dis. 2010;16:776–82 . 10.3201/eid1605.091801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagier JC, Lepidi H, Raoult D, Fenollar F. Systemic Tropheryma whipplei: clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine (Baltimore). 2010;89:337–45 . 10.1097/MD.0b013e3181f204a8 [DOI] [PubMed] [Google Scholar]
- 17.Gubler JG, Kuster M, Dutly F, Bannwart F, Krause M, Vögelin HP, et al. Whipple endocarditis without overt gastrointestinal disease: report of four cases. Ann Intern Med. 1999;131:112–6 . 10.7326/0003-4819-131-2-199907200-00007 [DOI] [PubMed] [Google Scholar]
- 18.Goldenberger D, Künzli A, Vogt P, Zbinden R, Altwegg M. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol. 1997;35:2733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rickman LS, Freeman WR, Green WR, Feldman ST, Sullivan J, Russack V, et al. Brief report: uveitis caused by Tropheryma whippelii (Whipple’s bacillus). N Engl J Med. 1995;332:363–6 . 10.1056/NEJM199502093320604 [DOI] [PubMed] [Google Scholar]
- 20.Fenollar F, Trani M, Davoust B, Salle B, Birg ML, Rolain JM, et al. Prevalence of asymptomatic Tropheryma whipplei carriage among humans and nonhuman primates. J Infect Dis. 2008;197:880–7 . 10.1086/528693 [DOI] [PubMed] [Google Scholar]
- 21.Fenollar F, Laouira S, Lepidi H, Rolain JM, Raoult D. Value of Tropheryma whipplei quantitative polymerase chain reaction assay for the diagnosis of Whipple’s disease: usefulness of saliva and stool specimens for first-line screening. Clin Infect Dis. 2008;47:659–67 . 10.1086/590559 [DOI] [PubMed] [Google Scholar]
- 22.Fenollar F, Keita AK, Buffet S, Raoult D. Intrafamilial circulation of Tropheryma whipplei, France. Emerg Infect Dis. 2012;18:949–55 . 10.3201/eid1806.111038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keita AK, Brouqui P, Badiaga S, Benkouiten S, Ratmanov P, Raoult D, et al. Tropheryma whipplei prevalence strongly suggests human transmission in homeless shelters. Int J Infect Dis. 2013;17:e67–8 . 10.1016/j.ijid.2012.05.1033 [DOI] [PubMed] [Google Scholar]
- 24.Fenollar F, Trape JF, Bassene H, Sokhna C, Raoult D. Tropheryma whipplei in fecal samples from children, Senegal. Emerg Infect Dis. 2009;15:922–4 . 10.3201/eid1506.090182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keita AK, Bassene H, Tall A, Sokhna C, Ratmanov P, Trape JF, et al. Tropheryma whipplei: a common bacterium in rural Senegal. PLoS Negl Trop Dis. 2011;5:e1403 . 10.1371/journal.pntd.0001403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keita AK, Raoult D, Fenollar F. Tropheryma whipplei as a commensal bacterium. Future Microbiol. 2013;8:57–71 . 10.2217/fmb.12.124 [DOI] [PubMed] [Google Scholar]
- 27.Dutly F, Altwegg M. Whipple’s disease and “Tropheryma whippelii.”. Clin Microbiol Rev. 2001;14:561–83 . 10.1128/CMR.14.3.561-583.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amsler L, Bauernfeind P, Nigg C, Maibach RC, Steffen R, Altwegg M. Prevalence of Tropheryma whipplei DNA in patients with various gastrointestinal diseases and in healthy controls. Infection. 2003;31:81–5 . 10.1007/s15010-002-3083-0 [DOI] [PubMed] [Google Scholar]
- 29.Ramharter M, Harrison N, Bühler T, Herold B, Lagler H, Lötsch F, et al. Prevalence and risk factor assessment of Tropheryma whipplei in a rural community in Gabon: a community-based cross-sectional study. Clin Microbiol Infect. 2014;20:1189–94 . 10.1111/1469-0691.12724 [DOI] [PubMed] [Google Scholar]
- 30.Keita AK, Dubot-Pérès A, Phommasone K, Sibounheuang B, Vongsouvath M, Mayxay M, et al. High prevalence of Tropheryma whipplei in Lao kindergarten children. PLoS Negl Trop Dis. 2015;9:e0003538 . 10.1371/journal.pntd.0003538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keita AK, Mediannikov O, Ratmanov P, Diatta G, Bassene H, Roucher C, et al. Looking for Tropheryma whipplei source and reservoir in rural Senegal. Am J Trop Med Hyg. 2013;88:339–43 . 10.4269/ajtmh.2012.12-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Fenollar F, Rolain JM, Fournier PE, Feurle GE, Müller C, et al. Genotyping reveals a wide heterogeneity of Tropheryma whipplei. Microbiology. 2008;154:521–7 . 10.1099/mic.0.2007/011668-0 [DOI] [PubMed] [Google Scholar]
- 33.Males S, Gaye O, Garcia A. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin Infect Dis. 2008;46:516–22 . 10.1086/526529 [DOI] [PubMed] [Google Scholar]
- 34.Gautret P, Charrel R, Benkouiten S, Belhouchat K, Nougairede A, Drali T, et al. Lack of MERS coronavirus but prevalence of influenza virus in French pilgrims after 2013 Hajj. Emerg Infect Dis. 2014;20:728–30 . 10.3201/eid2004.131708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundgren IS, Heltshe SL, Smith AL, Chibwana J, Fried MW, Duffy PE. Bacteremia and malaria in Tanzanian children hospitalized for acute febrile illness. J Trop Pediatr. 2015;61:81–5 . 10.1093/tropej/fmu069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenollar F, Mediannikov O, Socolovschi C, Bassene H, Diatta G, Richet H, et al. Tropheryma whipplei bacteremia during fever in rural West Africa. Clin Infect Dis. 2010;51:515–21 . 10.1086/655677 [DOI] [PubMed] [Google Scholar]
- 37.Stein A, Doutchi M, Fenollar F, Raoult D. Tropheryma whipplei pneumonia in a patient with HIV-2 infection. Am J Respir Crit Care Med. 2013;188:1036–7 . 10.1164/rccm.201304-0692LE [DOI] [PubMed] [Google Scholar]
- 38.Lagier JC, Fenollar F, Lepidi H, Raoult D. Evidence of lifetime susceptibility to Tropheryma whipplei in patients with Whipple’s disease. J Antimicrob Chemother. 2011;66:1188–9 . 10.1093/jac/dkr032 [DOI] [PubMed] [Google Scholar]
- 39.Mayxay M, Castonguay-Vanier J, Chansamouth V, Dubot-Pérès A, Paris DH, Phetsouvanh R, et al. Causes of non-malarial fever in Laos: a prospective study. Lancet Glob Health. 2013;1:e46–54 . 10.1016/S2214-109X(13)70008-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


