Abstract
Background:
Multiple sclerosis (MS) is a demyelinating and degenerating disease which involves central nervous system. Environmental risk factors have a key role in MS susceptibility. Here we aim to investigate different risk factors effect on MS susceptibility in a large population of MS patients in Isfahan, Iran.
Materials and Methods:
This study is a cross-sectional hospital-based study, which was conducted on a large group of MS patients registered in Kashani hospital and a control group from normal healthy population. Demographic data, age at onset of the disease, history of viral infections, vaccination, history of trauma to head, recent stressful events, alimentation, familial history, method of delivery (caesarean section, normal vaginal delivery), disability score and history of smoking were gathered using a designed questionnaire.
Results:
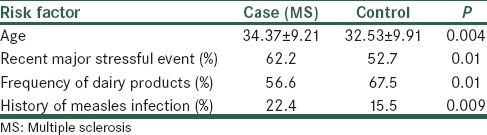
Totally 536 MS patient with the mean age of 34.37 ± 9.22 and 399 individuals from healthy population with the mean age of 32.53 ± 9.91 were recruited. Significant difference in history of measles infection (control = 15.5%, case = 22.4%, P = 0.009), consumption of dairy products (case = 56.6%, control = 67.5%, P = 0.01) and major stressful life events (case = 62.2%, control = 52.7%, P < 0.05) between these two groups were demonstrated.
Conclusion:
A significant relation between stress, history of infection and milk consumption was reached that highlights the importance of environmental risk factors in MS pathogenesis.
Keywords: Dairy products, environmental risk factors, infection, multiple sclerosis, stress
INTRODUCTION
Multiple sclerosis (MS) is a chronic autoimmune demyelinating and disabling disease of central nervous system (CNS) which predominantly affects young females.[1] Both environmental and genetic factors are considered to play a significant role in MS susceptibility.[2]
Although recurrence rate of MS in families points toward the importance of inheritable determinant in susceptibility of the disease,[3] the variation of MS frequency in different geographical parts and also change in risk of the disease development in migration studies can be explained by the strong impact of environmental risk factors in the disease susceptibility.[4]
Several line of evidences has demonstrated the important influence of environmental risk factors in MS pathogenesis.[5] Epidemiological studies have linked higher prevalence of MS to latitude, Vitamin D deficiency, Epstein–Barr virus infection and smoking among other factors.[6,7,8]
Given the fact of rising high prevalence of MS in Iran, especially in Isfahan and Tehran[9,10] here we aim to shed light on the environmental risk factors with a possible involvement in MS pathogenesis in the large population of MS patients in Isfahan, Iran.
MATERIALS AND METHODS
This study is a cross-sectional hospital registry-based study, which was conducted on a large population of patients who had a definite diagnosis of MS according to 2005 McDonald's criteria or laboratory supported MS who from April 2012 to June 2013 referred to MS clinic of Kashani hospital, Isfahan University of Medical Sciences, Isfahan, Iran, also a randomized selection of normal healthy individuals were recruited as our control group. Our database is a hospital registry database in which all patients who came to the hospital are automatically entered; nearly half of MS population of Isfahan province is now registered.
Diagnostic test and treatments were done according to accepted guidelines.[11] Patients were examined by a neurologist at least once or twice a year. Demographic data, age at onset of the disease, history of viral infections, vaccination, history of trauma to head, recent stressful events, alimentation, familial history, method of delivery (caesarean section, normal vaginal delivery), disability score and history of smoking were gathered using a designed questionnaire. This study was approved by Local Ethics Committee and all patients in this study gave a written informed consent form to access and review their medical records.
Kurtzke disability status scale was used for assessment of neurological disability in each visit.[12] Data were analyzed using SPSS19 software (SPSS Inc., Chicago, IL, USA).
RESULTS
In our study the relapsing remitting (RR) MS had higher prevalence in comparison to other forms [Table 1]. In RRMS patients, the time to develop secondary progressive MS was 6.47 ± 4.65 years. Majority of patients (73.7%) reported involvement of one system at the time of diagnosis, with higher prevalence in sensory system (45.6%) and motor system (28.6%) involvements (optic = 16.3%, cerebral = 9.5%). During summer, patients reported more relapses compared with other seasons (summer = 37%, winter = 29.6%, spring = 8.4%, autumn = 5.9%).
Table 1.
General characteristic of subject according to sex
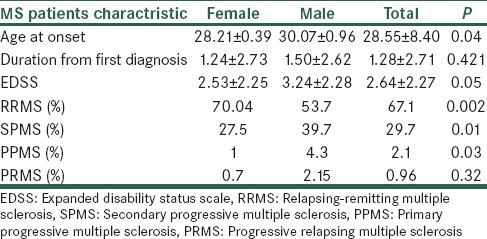
Family history of MS was reported in 19.4% of patients in which majority reported just one relative with MS (one = 84.2% two = 14.9% three = 1%).
High number of individuals in our MS population reported recent major stressful event, which was significantly higher than the control group.
In the control population, 65.6% individuals reported history of viral infections among them chickenpox and measles had the highest prevalence (chickenpox = 42.6%, measles = 15.5%, mumps = 6.2%, rubella = 1.7%), and also in MS population amongst the 66.1% of patients with history of these infection, infection with chickenpox predominated (chickenpox = 32.5%, measles = 22.4%, mumps = 8.9%, rubella = 2%), the prevalence of measles infection in case and control group had a significant difference.
The frequency of dairy products consumption in the control group was significantly higher than in MS patients population [Table 2]. However, there was no significant differences in the frequency of conserved food consumption between groups (case = 26.4%, control = 18.2%, P = 0.67).
Table 2.
Comparison of characteristics between case and control groups
The relation between other risk factors in MS patients and healthy control group was demonstrated in Tables 3 and 4.
Table 3.
Risk factors are compared in MS population and control group
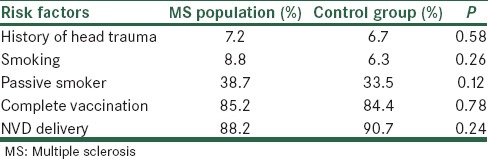
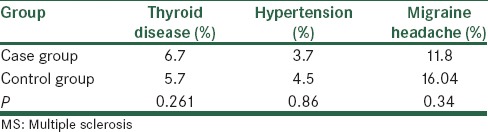
Table 4.
History of suffering from different diseases and risk of developing MS
DISCUSSION
Our findings demonstrated significant relation between major stressful life events 1-month prior the onset of the disease and subsequent MS diagnosis. A possible link between stress and onset of MS has been reported suggesting the relation of stress 6 months and 2 years prior to the exacerbation of the disease.[13,14,15] These results could point toward the fact that stress may cause the first episode of MS however, the possibility of bias in collecting data or recalling should be considered. Also an increased risk of developing MS in parents who lost their child has been demonstrated.[16]
However, in contrast to our findings a study conducted on large population of registered nurses found no significant correlation between stress at home/work with risk of developing MS,[17,18] these finding could be due to the lack of objectivity in measuring stress in this population.
An overwhelming number of different infectious agents have been proposed to play an important role in MS susceptibility.[19] The hypothesis that MS exacerbation can be triggered by late exposure to childhood infections comes from evidence in migration studies and also the positive titer of antibodies to these infections in areas with lower prevalence of MS in ecological studies.[20,21]
The result of our study showed a significant correlation between history of measles infection and subsequent MS diagnosis. Several lines of evidence have linked the risk of developing MS to childhood infection if acquired later in life such as measles and rubella.[22,23] Also a study showed that clinical measles was more frequent in MS patients however, without any significant difference,[19] This finding are due to the probable fact that viral infection can cause autoimmune reactions due to molecular mimicry which leads to CNS inflammatory disease. In contrast with our findings was the studies that showed no significant difference in infection with measles in any stage of life in MS population compared to normal population.[24,25]
The role of dietary factors in MS etiology has been considered, here our findings showed lower consumption of processed milk and dairy products in MS population compared to control group. In accordance with our results, it was reported that one of possible risk factors for MS development is linked to high milk consumption in childhood and sudden reduction of milk consumption in adolescent growth spurt.[26] However, in a large study conducted in 27 countries it was demonstrated that cow milk consumption can lead to MS exacerbation, however there was no relation between processed milk and risk of MS development. It is speculated that a possible mimicry between butyrophilin and myelin oligodendrocyte glycoprotein may explain these results.[26]
CONCLUSION
A significant relation between stress, history of infection and milk consumption was reached that highlights the importance of environmental risk factors in MS pathogenesis and that lifestyle change can play a preventive role in MS susceptibility.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Harris MK, Maghzi AH, Etemadifar M, Kelley RE, Gonzalez-Toledo E, Minagar A. Acute demyelinating disorders of the central nervous system. Curr Treat Options Neurol. 2009;11:55–63. doi: 10.1007/s11940-009-0008-6. [DOI] [PubMed] [Google Scholar]
- 2.Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: Risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9:727–39. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- 3.Compston A. Genetic epidemiology of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1997;62:553–61. doi: 10.1136/jnnp.62.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004;3:709–18. doi: 10.1016/S1474-4422(04)00933-0. [DOI] [PubMed] [Google Scholar]
- 5.Gale CR, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol. 1995;47:425–48. [PubMed] [Google Scholar]
- 6.Handel AE, Williamson AJ, Disanto G, Dobson R, Giovannoni G, Ramagopalan SV. Smoking and multiple sclerosis: An updated meta-analysis. PLoS One. 2011;6:e16149. doi: 10.1371/journal.pone.0016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 8.Haahr S, Plesner AM, Vestergaard BF, Höllsberg P. A role of late Epstein-Barr virus infection in multiple sclerosis. Acta Neurol Scand. 2004;109:270–5. doi: 10.1046/j.1600-0404.2003.00221.x. [DOI] [PubMed] [Google Scholar]
- 9.Etemadifar M, Maghzi AH. Sharp increase in the incidence and prevalence of multiple sclerosis in Isfahan, Iran. Mult Scler. 2011;17:1022–7. doi: 10.1177/1352458511401460. [DOI] [PubMed] [Google Scholar]
- 10.Sahraian MA, Khorramnia S, Ebrahim MM, Moinfar Z, Lotfi J, Pakdaman H. Multiple sclerosis in Iran: A demographic study of 8,000 patients and changes over time. Eur Neurol. 2010;64:331–6. doi: 10.1159/000321649. [DOI] [PubMed] [Google Scholar]
- 11.Flachenecker P, Hartung HP. EDMUS – A new European databank for multiple sclerosis. A brief introduction of ongoing and planned multicenter studies within the scope of the “European Concentrated Action for Multiple Sclerosis”. Nervenarzt. 1996;67:277–82. [PubMed] [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 13.Warren S, Greenhill S, Warren KG. Emotional stress and the development of multiple sclerosis: Case-control evidence of a relationship. J Chronic Dis. 1982;35:821–31. doi: 10.1016/0021-9681(82)90047-9. [DOI] [PubMed] [Google Scholar]
- 14.Grant I, Brown GW, Harris T, McDonald WI, Patterson T, Trimble MR. Severely threatening events and marked life difficulties preceding onset or exacerbation of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989;52:8–13. doi: 10.1136/jnnp.52.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Johansen C, Brønnum-Hansen H, Stenager E, Koch-Henriksen N, Olsen J. The risk of multiple sclerosis in bereaved parents: A nationwide cohort study in Denmark. Neurology. 2004;62:726–9. doi: 10.1212/01.wnl.0000113766.21896.b1. [DOI] [PubMed] [Google Scholar]
- 16.Bachmann S, Kesselring J. Multiple sclerosis and infectious childhood diseases. Neuroepidemiology. 1998;17:154–60. doi: 10.1159/000026167. [DOI] [PubMed] [Google Scholar]
- 17.Granieri E, Casetta I, Tola MR, Ferrante P. Multiple sclerosis: Infectious hypothesis. Neurol Sci. 2001;22:179–85. doi: 10.1007/s100720170021. [DOI] [PubMed] [Google Scholar]
- 18.Poskanzer DC, Sever JL, Sheridan JL, Prenney LB. Multiple sclerosis in the Orkney and Shetland Islands. IV: Viral antibody titres and viral infections. J Epidemiol Community Health. 1980;34:258–64. doi: 10.1136/jech.34.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahlgren C, Torén K, Odén A, Andersen O. A population-based case-control study on viral infections and vaccinations and subsequent multiple sclerosis risk. Eur J Epidemiol. 2009;24:541–52. doi: 10.1007/s10654-009-9367-2. [DOI] [PubMed] [Google Scholar]
- 20.Alter M, Zhen-xin Z, Davanipour Z, Sobel E, Min Lai S, LaRue L. Does delay in acquiring childhood infection increase risk of multiple sclerosis? Ital J Neurol Sci. 1987;(Suppl 6):11–6. [PubMed] [Google Scholar]
- 21.Hernán MA, Zhang SM, Lipworth L, Olek MJ, Ascherio A. Multiple sclerosis and age at infection with common viruses. Epidemiology. 2001;12:301–6. doi: 10.1097/00001648-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Bansil S, Singhal BS, Ahuja GK, Riise T, Ladiwala U, Behari M, et al. Multiple sclerosis in India: A case-control study of environmental exposures. Acta Neurol Scand. 1997;95:90–5. doi: 10.1111/j.1600-0404.1997.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 23.Bager P, Nielsen NM, Bihrmann K, Frisch M, Hjalgrim H, Wohlfart J, et al. Childhood infections and risk of multiple sclerosis. Brain. 2004;127:2491–7. doi: 10.1093/brain/awh283. [DOI] [PubMed] [Google Scholar]
- 24.Butcher PJ. Milk consumption and multiple sclerosis – An etiological hypothesis. Med Hypotheses. 1986;19:169–78. doi: 10.1016/0306-9877(86)90057-5. [DOI] [PubMed] [Google Scholar]
- 25.Malosse D, Perron H, Sasco A, Seigneurin JM. Correlation between milk and dairy product consumption and multiple sclerosis prevalence: A worldwide study. Neuroepidemiology. 1992;11:304–12. doi: 10.1159/000110946. [DOI] [PubMed] [Google Scholar]
- 26.Stefferl A, Schubart A, Storch2 M, Amini A, Mather I, Lassmann H, et al. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J Immunol. 2000;165:2859–65. doi: 10.4049/jimmunol.165.5.2859. [DOI] [PubMed] [Google Scholar]


