Abstract
Ligand binding causes the EGF receptor (EGFR) to become ubiquitinated by Cbl upon association with the adaptor protein Grb2. We have investigated the role of ubiquitin and Grb2 in ligand-induced endocytosis of the EGFR. Incubation of cells with EGF on ice caused translocation of Grb2 and Cbl from the cytosol to the rim of coated pits. Grb2 with point mutations in both SH3 domains inhibited recruitment of the EGFR to clathrin-coated pits, in a Ras-independent manner. On overexpression of the Cbl-binding protein Sprouty, ubiquitination of the EGFR was inhibited, the EGFR was recruited only to the rim of coated pits, and endocytosis of the EGFR was inhibited. Conjugation-defective ubiquitin similarly inhibited recruitment of EGF-EGFR to clathrin-coated pits. Even though this does not prove that cargo must be ubiquitinated, this indicates the importance of interaction of ubiquitinated protein(s) with proteins harboring ubiquitin-interacting domains. We propose that Grb2 mediates transient anchoring of the EGFR to an Eps15-containing molecular complex at the rim of coated pits and that Cbl-induced ubiquitination of the EGFR allows relocation of EGFR from the rim to the center of clathrin-coated pits.
INTRODUCTION
Activation of the EGF receptor (EGFR) initiates signal transduction important for gene expression as well as for endocytosis and lysosomal sorting. The EGFR is endocytosed from clathrin-coated pits, but mechanisms involved in its recruitment to coated pits are incompletely understood. On ligand-induced activation of the EGFR kinase, Grb2 is rapidly complexed with the EGFR. The pathway whereby recruitment of Grb2 activates Ras has been understood for years (Chardin et al., 1993; Li et al., 1993; Olivier et al., 1993). However, more recently, it was reported that recruitment of Grb2 was also important in initiating EGFR endocytosis. This was based on the finding that microinjecting a recombinant SH2 domain of Grb2 inhibited EGFR endocytosis (Wang and Moran, 1996). Also, EGFR mutants lacking Grb2 binding sites did not efficiently colocalize with the endocytosis adaptor complex AP-2, and depletion of Grb2 from cells expressing wild-type EGFR by RNA interference substantially inhibited clathrin-dependent EGFR endocytosis (Jiang et al., 2003). Grb2 can link the multiadaptor protein and ubiquitin ligase Cbl to the EGFR and thereby increase ligand-induced ubiquitination of EGFR (Waterman et al., 2002). This suggested that recruitment of the complex of Grb2 and Cbl to the EGFR was important in order to initiate clathrin-dependent endocytosis.
The exact role of Cbl in EGFR endocytosis has not been resolved. Overexpression of wild-type Cbl increased, whereas overexpression of Cbl mutants with abolished ubiquitin ligase activity decreased the rate of endocytosis (Thien et al., 2001). Fluorescence energy transfer studies demonstrated that localization of Cbl-YFP to endosomes depended on a proline-rich domain of Cbl that interacts with Grb2. Also, although direct binding of Cbl to phosphotyrosine 1045 of the EGFR was required for maximal EGFR ubiquitination, this direct binding was not essential for localization of Cbl to EGFR-containing endosomes. These data suggested that binding of Cbl to EGFR through Grb2 is necessary and sufficient for Cbl function in clathrin-mediated endocytosis. However, these data were interpreted to mean that ubiquitination of the EGFR is not essential (Jiang and Sorkin, 2003). It has further been reported that EGFR endocytosis is independent of Cbl's ubiquitin ligase activity, but that Cbl is required to link the EGFR to CIN85, and thereby recruit endophilin to EGFR-Cbl complexes (Soubeyran et al., 2002). The requirement for Cbl-induced ubiquitination of EGFR was, however, supported by the finding that human Sprouty 2 (hSpry2) inhibited ligand-induced EGFR endocytosis (Fong et al., 2003). hSpry2 binds Cbl both constitutively and in an EGF-dependent manner and inhibits Cbl-dependent ubiquitination of the EGFR (Wong et al., 2002; Fong et al., 2003).
Eps15 as well as epsin 1 and 2 are proteins involved in early steps of endocytosis that harbor ubiquitin-interacting motifs (UIMs; Tebar et al., 1996; Benmerah et al., 1998; Chen et al., 1998; Polo et al., 2002). UIMs of epsins and Eps15 bind directly to ubiquitin (Polo et al., 2002; Shih et al., 2002; Aguilar et al., 2003). In yeast, it was recently demonstrated that Ent1, Ent2, and Ede1 (the homologues of human epsins 1 and 2 and Eps15, respectively) have redundant functions in internalization of the pheromone receptor Ste2 (Shih et al., 2002). The endocytic defect of a triple Ent1-Ent2-Ede1 knockout was rescued by wild-type Ent1, but not by an Ent1 mutant in which the UIMs were deleted (Shih et al., 2002). This, together with the findings that a chimeric Ste2-ubiquitin molecule, where the whole cytoplasmic tail of Ste2 was replaced by ubiquitin, could be efficiently internalized (Shih et al., 2002), strongly suggest that intracellular ubiquitin-binding proteins can work efficiently as endocytic cargo adaptors in yeast. However, the same has so far not been demonstrated in mammalian cells.
In this study we set out to dissect the precise function of Grb2 and the role of ubiquitination and proteins with ubiquitin-interacting domains in clathrin-mediated endocytosis of EGFR. Our findings suggest that the Grb2-Cbl complex recruits the EGFR to forming clathrin-coated pits by interacting with an Eps15-containing macromolecular complex. Our findings further indicate that Cbl-induced ubiquitination, as well as interaction of conjugated ubiquitin with endocytosis proteins harboring ubiquitin interacting domains, is required for EGF-induced endocytosis of the EGFR.
MATERIALS AND METHODS
Materials
Human recombinant EGF was from Bachem AG (Budendorf, Switzerland). 125I- Na and 125I-EGF were from Amersham Biosciences (Buckinghamshire, United Kingdom). Alexa Fluor 488–conjugated EGF (Alexa488-EGF), rhodamine-conjugated EGF (Rhodamine-EGF), Alexa Fluor 488 or 594–conjugated transferrin (Tf; Alexa488-Tf or Alexa594-Tf), and Texas Red–conjugated Tf (TexasRed-Tf) were from Molecular Probes (Eugene, OR). Dako fluorescent mounting medium was from Dako Corporation (Copenhagen, Denmark). Fugene was from Roche Molecular Biochemicals (Indianapolis, IN), and Effectene was from Qiagen GmbH (Hilden, Germany). The pcDNA3 vector, PCR amplification primers and oligonucleotides were from Invitrogen (San Diego, CA). Amplitaq DNA Polymerase and the QuickChange site-directed mutagenesis kit were from Stratagene (La Jolla, CA). Tris-Tricine gels, 10–20%, were from Bio-Rad (Hercules, CA). Other chemicals were from Sigma-Aldrich (St. Louis, MO) unless noted otherwise.
Cell Culture and Treatment
The laryngeal carcinoma cell line Hep2 and HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM) with penicillin-streptomycin mixture, and l-glutamine (2 mM), all from BioWhittaker (Walkersville, MD). FCS (5% vol/vol; PAA Innovations, Linz, Austria) was routinely used. The cells were plated at a density of 15,000 cells/cm2 48 h before experiments. In pulse-chase experiments, cells were incubated with ligand in MEM without bicarbonate with 0.1% BSA on ice for 15 min, followed by washing three times in ice-cold PBS to remove unbound ligand and subsequent chase in ligand-free MEM without bicarbonate at 37°C.
Plasmids and Transient Transfection of Cells
HeLa cells were transiently transfected with d.n. Grb2, H-Ras17N, wt UbRGG, UbR, UbR L8A/I44A, and hSpry2 using Fugene or Effectene 48 h upon plating. Transfected cells were analyzed 24 or 48 h upon transfection. D.n. Grb2 (W36, 193K; nonfunctional N- and C- terminal SH3 domains) was provided by Robin M. Scaife (University of Western Australia, Nedlands, Australia). This construct was originally made by Lyuba Varticovski (Tufts University Scholl of Medicine, Boston, MA; Tanaka et al., 1995; Jain et al., 1997). D.n. Grb2 was amplified by PCR, and a Myc fusion protein of d.n. Grb2 was made by subcloning into a pRK5Myc vector provided by Alan Hall (UCL, London, United Kingdom). Hemagglutinin (HA)-tagged dominant negative H-Ras (S17N) in pcDNA3.1 was purchased from Guthrie cDNA Resource Center (Guthrie Research Institute, Sayre, PA). The pMT123 plasmid encoding HA-Ubiquitin × 8 was obtained from Dirk Bohmann (University of Rochester, Rochester, NY). From this plasmid the constructs pcDNA3-Myc-UbRGG, pcDNA3-Myc-UbR, and pcDNA3-Myc-UbR L8A/I44A were made by PCR mutagenesis of the ubiquitin sequence and subcloning behind the Myc epitope of pcDNA3-Myc (Raiborg et al., 2001). GFP-tagged Sprouty (hSpry2; in pEGFP) was obtained from Graeme Guy (Institute of Molecular and Cell Biology, Singapore).
Antibodies
Rabbit anti-epsin antibody was a gift from Linton Traub (University of Pittsburgh School of Medicine, Pittsburgh, PA). Rabbit anti-clathrin light chain antibody was a gift from Frances M. Brodsky (University of California, San Francisco, CA). Mouse anti-Myc antibody was from the 9E10 hybridoma (Evan et al., 1985). Rabbit anti-Myc (ab9106) and rabbit anti-GFP were from Abcam Ltd. (Cambridge, United Kingdom). Rabbit anti-EGF, rabbit anti-Grb2, rabbit anti-Cbl, mouse anti-EGFR (sc-120), and mouse anti-ubiquitin antibody (P4D1) were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-EGFR (antibody-3) was from Neomarkers (Fremont, CA). Rabbit anti-Eps15 (C-term) was from BAbCO (Richmond, CA), and rabbit anti-human transferrin receptor antibody from HybriDomus (Hellebaek, Denmark). Mouse anti-human dynamin antibody (Hudy 1) was a gift from Sandy Schmid (Scripps Research Institute, San Diego, CA). Mouse anti-HA antibody was from Zymed Laboratories Inc. (South San Francisco, CA). Mouse anti-FLAG and peroxidase-conjugated goat anti-rabbit antibodies were from Sigma-Aldrich. Rabbit anti-mouse IgG was from Cappel, ICN Biomedicals (Costa Mesa, CA). Rhodamine Red-X–conjugated donkey anti-mouse and Rhodamine Red-X–conjugated donkey anti-rabbit antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Alexa Fluor 488–conjugated goat anti-rabbit and anti-mouse antibodies were from Molecular Probes. Rabbit anti-pMAPK antibody was from Cell Signaling Technology (Beverly, MA).
Immunocytochemistry and Confocal Microscopy
Cells were plated on 12-mm coverslips (Menzel-Gläser, Braunschweig, Germany). On experiments, cells were washed twice in PBS and fixed with paraformaldehyde (4% wt/vol; Riedel-deHaën AG, Hannover, Germany) in Soerensen's phosphate buffer for 20 min on ice. Cells were washed three times with PBS before permeabilization with Triton X-100 (0.1% wt/vol in PBS) for 10 min. The fixed and permeabilized cells were preincubated with BSA (1% wt/vol in PBS) for 30 min before incubation with primary antibodies for 1 h. The coverslips were washed with PBS and incubated with secondary antibodies for 30 min before washing and mounting with Dako fluorescent mounting medium or Mowiol. The cells were examined using a Leica TSC XP confocal microscope (Leica Microsystems AG, Wetzlar, Germany) or a Zeiss LSM510 Meta confocal microscope (Carl Zeiss Microscopy, Göttingen, Germany).
Immunoelectronmicroscopy
Cells, treated as described in legends to figures, were fixed with paraformaldehyde (4% wt/vol) and glutaraldehyde (0.1% wt/vol) in Soerensen's phosphate buffer and processed for cryosectioning and immunolabeling (Griffiths et al., 1984). Bound antibodies were visualized using protein A gold (purchased from G. Posthuma, Utrecht, The Netherlands). When the primary antibody was mouse IgG, incubation with rabbit anti-mouse IgG was used as an intermediate reagent between the primary antibody and protein A gold. The sections were examined using a Philips CM 120 electron microscope (Eindhoven, The Netherlands).
125I-EGF Internalization Experiments
Cells were incubated as described in legends to figures. The radioactivity in the medium was measured in a gamma-counter (1470 Wizard; Wallac, Turku, Finland). The cells were washed three times with PBS and treated with sodium acetate buffer (0.2 M) containing NaCl (0.5 M) adjusted to pH 4.5 or 7.4 (SAB pH 4.5/SAB pH 7.4) on ice for 10 min. The 125I-EGF associated with the cells was precipitated using TCA-phosphotungstic acid. Finally, the precipitated material was dissolved in NaOH (1 M), and the radioactivity was measured. The radioactivity associated with cells treated with SAB pH 4.5 represents internalized 125I-EGF, and radioactivity from cells treated with SAB pH 7.4 represents total cell-associated 125I-EGF.
125I-Tf Interaction Experiments
Human Tf (iron-saturated, 10 μg in 40 μl of PBS) was labeled with 0.5 mCi of I125 (as NaI, carrier-free) by incubation with Iodogen (Pierce, Rockford, IL). Iodinated Tf was separated from the free iodide on a Sephadex G-25 column (PD-10 G25M; Amersham Biosciences). The specific activity of iodinated Tf was 7.5 × 107 cpm/ng. Internalization of Tf was measured after pronase treatment of cells, as described (Ciechanover et al., 1983). The medium was removed, and the cells were washed three times with ice-cold PBS and treated with 0.3% pronase in cold MEM containing BSA (1%), on ice for 60 min. On centrifugation, the radioactivity in the supernatant and pellet fractions was measured in a gamma-counter. The radioactivity in the pellet fraction represents internalized 125I-Tf.
RESULTS
The EGFR Is Recruited to Clathrin-coated Pits upon Incubation of Cells with EGF on Ice
The reported colocalization of EGF and AP-2 (Jiang et al., 2003) suggested that these proteins were recruited to clathrin-coated pits upon incubation with EGF. To study the plasma membrane localization more closely, immunoelectron microscopy (immuno-EM) studies were performed in Hep2 cells harboring more EGFR than HeLa cells. Because clathrin-coated pits quickly bud at 37°C, we incubated Hep2 cells with EGF for 60 min on ice, where no endocytosis occurs. High concentrations of EGF (60 ng/ml) were used to optimize labeling efficiency and to thereby visualize relocalization of activated EGFR at the plasma membrane. Labeling for both EGF and for EGFR demonstrated that activated EGFR indeed localized to coated pits on ice, as has previously been suggested (Sorkina et al., 2002; see Figure 1). Quantitation showed that ∼25% of the labeling of EGF and the EGFR at the plasma membrane was within coated areas compared with 2–3% EGFR in coated pits in control cells (unpublished data). Most coated areas observed upon incubation with EGF on ice were flat, and only a limited fraction (∼10%) of the labeling of EGF and EGFR was associated with invaginated coated pits. Double-labeling for EGF and clathrin or for EGFR and clathrin confirmed that the coated areas indeed contained clathrin.
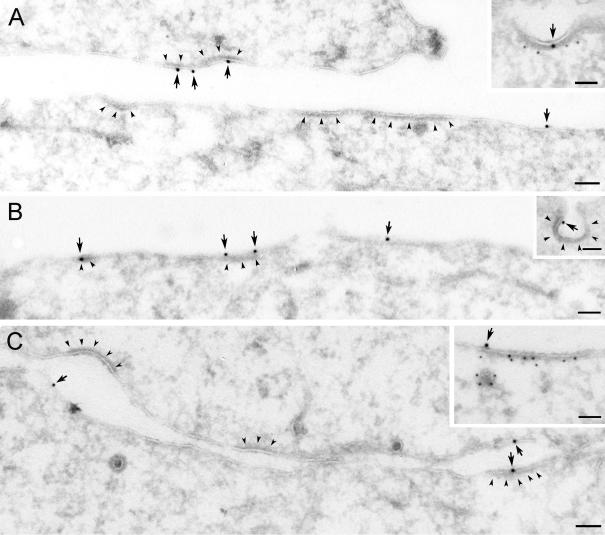
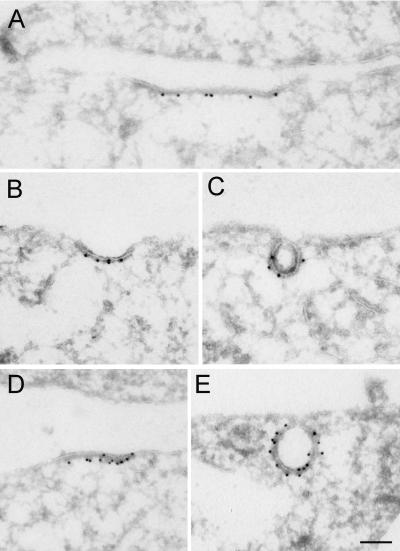
Figure 1.
Recruitment of EGF-EGFR to clathrin-coated plasma membrane areas upon incubation with EGF on ice. Serum-starved Hep2 cells, incubated with EGF (60 ng/ml) on ice for 60 min, were prepared for immuno-EM and labeled for EGFR (A) or EGF (B and C). Labeling for EGFR and EGF (arrows) was found both at smooth and coated plasma membrane areas (coat indicated by arrowheads). That the coated areas represent clathrin-coated domains was confirmed by double-labeling for EGFR (large gold particles) and clathrin (small gold particles) shown as inset in A or EGF (large gold particles) and clathrin (small gold particles; inset in C). Although most of the EGF-EGFR positive coated areas were flat, some labeling localized to invaginated coated pits (inset in B). Bars, 100 nm.
Mutant Grb2 Inhibits EGF-induced Relocalization of the EGFR to Clathrin-coated Pits
It was previously demonstrated that Grb2 incapable of binding proline-rich sequences due to amino acid substitutions in the SH3 domains inhibited the endocytosis of the EGFR (Jiang et al., 2003). To investigate in more detail at what step of endocytosis a dominant negative (d.n.) Grb2 inhibited EGF-induced clathrin-dependent endocytosis, we overexpressed Myc-tagged W36, 193K Grb2 in Hep2 cells and analyzed the cells upon incubation with 60 ng/ml EGF on ice for 60 min by immuno-EM. In nontransfected cells, the EGF-EGFR complex was found in coated areas of the plasma membrane, as demonstrated in Figure 1. However, in cells overexpressing d.n. Grb2, labeling for the EGF-EGFR complex was restricted to smooth areas of the plasma membrane, and no labeling was found in coated pits (Figure 2A). The d.n. Grb2 mutant was further overexpressed in HeLa cells, which are more readily transfected, and the effect on endocytosis and localization to coated pits was again studied by immuno-EM. In nontransfected cells (Figure 2B), the EGF-EGFR complex was efficiently endocytosed upon incubation with 60 ng/ml EGF at 37°C for 10 min, and labeling for EGF was found in multivesicular endosomes. It should be noted that such high concentrations of EGF induce both clathrin-dependent and -independent endocytosis as EGF-induced endocytosis of EGFR through clathrin-coated pits is saturable (Lund et al., 1990; Wiley et al., 1998). In cells overexpressing d.n. Grb2, endocytosis of EGF-EGFR was strongly inhibited, consistent with a potential inhibitory effect of d.n. Grb2 on both clathrin-dependent and -independent endocytosis. No labeling was found in coated pits, and the labeling for EGF was restricted to smooth areas of the plasma membrane (Figure 2, C–E). Endocytosis of the Tf-receptor (TfR) was not inhibited by overexpression of d.n. Grb2 (Figure 2, F–H), and labeling for TfR was found both in coated pits and in coated vesicles. In accordance with the data published by Jiang et al. (2003), these data indicate that Grb2 is involved in recruiting the activated EGFR to clathrin-coated pits, whereas Grb2 is not involved in endocytosis of the TfR.
Figure 2.
Grb2 with mutations in both SH3 domains (d.n. Grb2) inhibits endocytosis of EGF, but not Tf. (A) Hep2 cells transiently transfected with d.n. Grb2 were incubated with EGF (60 ng/ml) for 60 min on ice and prepared for immuno-EM. Thawed cryosections were double-labeled for EGFR (15-nm gold particles) and d.n. Grb2 (rabbit anti-Myc; 10-nm gold particles). Labeling for EGFR (large arrows) was restricted to smooth plasma membrane areas, no labeling was found in flat or invaginated coated plasma membrane areas (coat indicated by small arrows). (B–H) HeLa cells transiently transfected with d.n. Grb2 were incubated with EGF (60 ng/ml) for 10 min at 37°C before preparation for immuno-EM. Thawed cryosections were double-labeled for EGF (B–E) or TfR (F–H; 15-nm gold particles) and d.n. Grb2 (mouse anti-Myc; 5-nm gold particles, arrowheads). In nontransfected cells (B) the EGF-EGFR complex was efficiently endocytosed, and labeling for EGF was found in multivesicular endosomes. In transfected cells (C–H) labeling for EGF (large arrows) was restricted to smooth areas of the plasma membrane (C–E). No labeling was found in coated pits (D) or in endosomes (E). Endocytosis of TfR (F–H) was not inhibited by overexpression of d.n. Grb2, and labeling for TfR was found both in shallow (F) and fully invaginated coated pits (G) as well as in coated vesicles (H). Bars, 100 nm.
Ras Activity Is not Required for Clathrin-dependent EGFR Endocytosis
Grb2 appears to be constitutively associated with Cbl as well as with the Ras GDP/GTP exchange factor Sos (Jiang et al., 2003). On activation of the EGFR, Grb2-Sos is recruited to the EGFR, and this results in activation of Ras. Because activation of Ras has been demonstrated to be essential for endocytosis of the EGFR (Barbieri et al., 1998; Tall et al., 2001) and has further been demonstrated to be involved in initiation of macropinocytosis (Amyere et al., 2002), we wanted to investigate whether the inhibitory effect of d.n. Grb2 on clathrin-dependent endocytosis of EGF-EGFR could be explained by inhibited Ras activity. To investigate the role of Ras activity in EGF-induced EGFR endocytosis, we studied the effect of overexpressing d.n. Ras (H-Ras17N) on endocytosis of EGF and of Tf. By confocal microscopy studies, we observed no inhibition of endocytosis of Alexa488-EGF in transfected HeLa cells compared with in nontransfected cells when 15 ng/ml EGF was added (Figure 3A). However, when high concentrations of EGF (60–150 ng/ml) were used, a slight inhibition was observed (unpublished data). This probably reflects the inhibitory effect of H-Ras17N on EGF-induced macropinocytosis (Amyere et al., 2002). Immuno-EM confirmed that EGF was endocytosed in H-Ras17N transfected cells incubated with 60 ng/ml EGF (unpublished results).
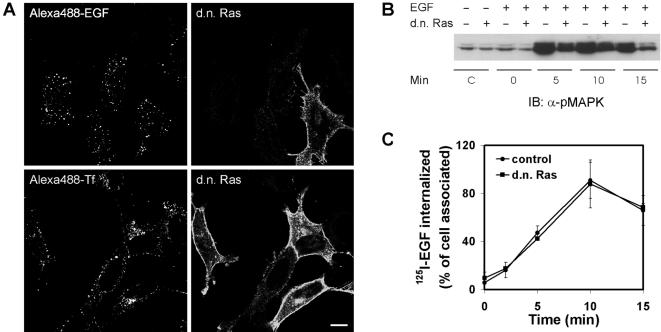
Figure 3.
Dominant negative Ras does not inhibit endocytosis of EGF or of Tf. HeLa cells were transiently transfected with HA-tagged H-Ras17N (d.n. Ras). (A) Cells were incubated with Alexa488-EGF (15 ng/ml) on ice for 15 min followed by a 15-min chase at 37°C (top panel) or with Alexa488-Tf (20 μg/ml) at 37°C for 20 min (bottom panel). Transfected cells were identified by fluorescence labeling using anti-HA antibody followed by secondary Rhodamine Red-X–conjugated antibodies. Bar, 10 μm. (B) Mock-transfected HeLa cells or HeLa cells transiently overexpressing H-Ras17N (transfection efficiency 30–35%) were incubated with EGF (60 ng/ml) on ice for 15 min and chased at 37°C for the indicated times. Cell lysates were subjected to SDS-PAGE and immunoblotting with antibody to phospho-MAPK (pMAPK). SDS-PAGE and immunoblotting was as previously described (Stang et al., 2000). (C) Mock-transfected HeLa cells (•) or HeLa cells transiently overexpressing H-Ras17N (▪) were incubated with 125I-EGF (1 ng/ml) on ice for 15 min and chased at 37°C for the indicated time periods. Analysis of internalized EGF was performed as described in MATERIAL AND METHODS. The data represent the mean of two independent experiments with three parallels ± SEM.
We also investigated the effect of overexpressing H-Ras17N on endocytosis of Tf. As demonstrated in Figure 3A, there was no effect of H-Ras17N on endocytosis of Alexa488-Tf. Overexpressing H-Ras17N significantly reduced phosphorylation of MAPK upon addition of EGF (Figure 3B), demonstrating that the expression of H-Ras17N affected intracellular signaling. Because EGF-induced endocytosis of EGFR through clathrin-coated pits is saturable (Lund et al., 1990; Wiley et al., 1998), we additionally performed an internalization assay using a low concentration of 125I-EGF (1 ng/ml) in order to quantify the effect of H-Ras17N on clathrin-dependent endocytosis of the EGFR. As demonstrated (Figure 3C), overexpression of H-Ras17N did not inhibit the clathrin-dependent endocytosis of 125I-EGF. Altogether, this argues that Ras activity is not important in EGF-induced clathrin-dependent endocytosis and that the inhibitory effect of d.n. Grb2 on clathrin-dependent endocytosis occurring at low concentrations of EGF cannot be explained by inhibited Ras activity.
Grb2 and Cbl Localize at the Rim of Clathrin-coated Pits in an EGF-dependent Manner
Grb2-mediated interaction of Cbl with the EGFR has been demonstrated to be required for efficient ubiquitination of the EGFR (Waterman et al., 2002; Jiang et al., 2003). A potential explanation for the requirement of Grb2 for recruitment of EGFR to coated pits is that Cbl-dependent ubiquitination is required for the EGFR to enter coated pits. It was reported that Grb2 colocalized with the β2 subunit of AP-2 and EGF upon incubation of PAE cells with EGF for 1 h at 4°C (Jiang et al., 2003). We have previously demonstrated that the EGFR colocalizes with Cbl at the plasma membrane under similar conditions (Longva et al., 2002), and it has also been shown that Eps15 is recruited to the plasma membrane when cells are incubated with EGF on ice (Torrisi et al., 1999). As expected, we found by immunofluorescence and confocal microscopy that incubation of Hep2 cells with EGF on ice for 60 min recruited Grb2, Cbl, and Eps15 to the plasma membrane (Figure 4A). The recruitment of these molecules was more efficient upon incubation with EGF for 60 min than for 15 min on ice (unpublished data).
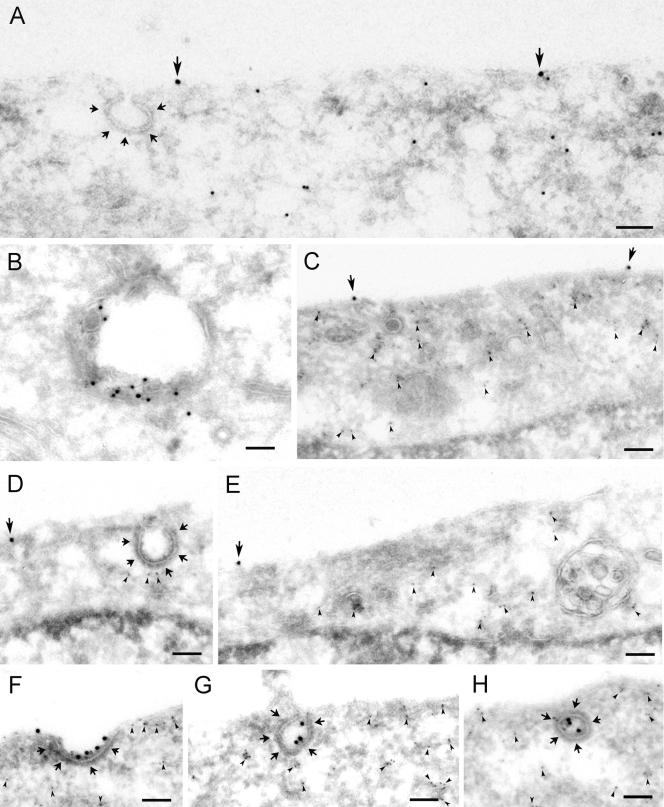
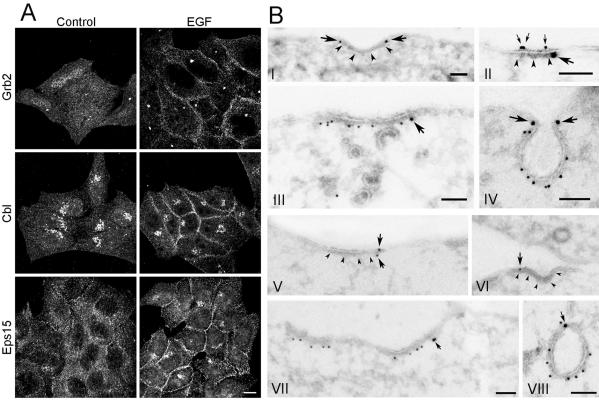
Figure 4.
Recruitment of Grb2, Cbl, and Eps15 to the plasma membrane upon incubation with EGF on ice. (A) Hep2 cells, incubated without ligand (control) or with EGF (60 ng/ml) for 60 min on ice, were prepared for immunofluorescence and labeled for Grb2, Cbl, or Eps15, respectively. On incubation with EGF on ice, Grb2, Cbl, and Eps15 were recruited to the plasma membrane. Bar, 10 μm. (B) Thawed cryosections of serum-starved Hep2 cells, incubated with EGF (60 ng/ml) on ice for 60 min, were either single-labeled for Grb2 (I) or double-labeled for Grb2 (large gold particles) and either EGF (II) or clathrin (III, IV; small gold particles). Note that although coat-associated labeling for Grb2 (large arrows in I-IV) is restricted to the rim of the coated area, labeling for EGF (small arrows in II) is all along the coat. The double-labeling for Grb2 and clathrin confirmed that Grb2 is associated both with flat and invaginated clathrin-coated plasma membrane. Sections from the same specimen were either labeled only for Cbl (V–VI), or both for Cbl (large gold particles) and clathrin (small gold particles; VII and VIII). Note that the coat associated labeling for Cbl (arrows) is restricted to the rim of the coated area. The double-labeling for Cbl and clathrin confirmed that Cbl is associated with both flat and invaginated clathrin-coated plasma membrane. Bars, 100 nm.
The plasma membrane localization of Grb2 and Cbl was further studied by immuno-EM. When sections from the same specimens as used in Figure 1 were labeled with antibodies to Grb2 (Figure 4B, I–IV) or antibodies to Cbl (Figure 4B, V–VIII), both Grb2 and Cbl were found at the plasma membrane. Grb2 and Cbl localized to both smooth and coated plasma membrane areas. Approximately 15% of Grb2 at the plasma membrane and 25% of Cbl at the plasma membrane were associated with coated regions (see Figure 4B). However, although EGF and EGFR labeling appeared randomly distributed along the coat (see Figure 1), labeling for Grb2 and Cbl was restricted to the rim of the coat, both in flat and in fully invaginated clathrin-coated pits. A similar localization to the rim of clathrin-coated pits has previously been shown for Eps15 (Tebar et al., 1996; Stang et al., 2000). The observations that Grb2, Cbl, and Eps15 are not found within the coat could theoretically be caused by poor antigen accessibility to EGFR-associated proteins within the coat. This cannot formally be ruled out, but is, however, unlikely, as we have previously detected the EGFR within coated pits using antibodies directed to the intracellular domain of the EGFR (Stang et al., 2000). Also, labeling for the Eps15-interacting protein epsin was found all along the coat (Figure 5, A–C) and showed the same distribution as did dynamin (Figure 5, D and E) and the α-adaptin subunit of AP-2 (unpublished results). Interestingly, epsin, which has been found to modify membrane curvature (Ford et al., 2002), localized to both flat and to fully invaginated clathrin-coated pits, showing that recruitment of epsin does not per se cause invagination of coated pits. The finding that incubation with EGF recruits Grb2 and Cbl to the rim of coated pits where also Eps15 has been demonstrated to localize (Tebar et al., 1996; Stang et al., 2000), suggests that these proteins cooperate in the recruitment of EGFR to clathrin-coated pits. The finding that the EGFR dissociates from Grb2 and Cbl when the EGFR enters central parts of clathrin-coated pits is consistent with our previous finding that the EGFR is transiently dephosphorylated upon recruitment to coated pits (Stang et al., 2000). The described dephosphorylation could either be due to transient inhibition of the EGFR kinase or to transient activation of a phosphatase dephosphorylating the EGFR in coated pits. Clarification of this requires further studies.
Figure 5.
Localization of epsin and dynamin to coated plasma membrane areas. Thawed cryosections of serum-starved Hep2 cells, incubated with EGF (60 ng/ml) on ice for 60 min, were labeled for epsin (A–C) or for dynamin (D and E). Note that both epsin and dynamin are localized all along the coated plasma membrane area, both in flat coats and in invaginated coated pits. Bar, 100 nm.
Differential Localization of EGFR and TfR in Clathrincoated Pits at the Plasma Membrane
Labeling for epsin revealed that the labeling density in coated plasma membrane domains varied. Although some coats showed a strong labeling, the labeling of others were weak or negative (unpublished results). This could suggest that epsin is either recruited at a late stage of coated pit formation or that epsin is recruited only to a limited set of coated pits. It has been suggested that epsin is an adaptor protein that recruits ubiquitinated receptors to coated pits (Shih et al., 2002). To investigate whether epsin was restricted to coated pits containing ubiquitinated receptors, we compared the colocalization of EGFR and epsin with the colocalization of TfR and epsin using Hep2 cells and immuno-EM. It has been debated whether the EGFR and the TfR are recruited to different coated pits or whether the two receptors share common coated pits. We therefore initially quantified the extent of colocalization of EGFR and TfR. In cells incubated with EGF on ice for 60 min, we found a limited colocalization of the two receptors. Double-labeling for EGFR and TfR showed that although 15% of the labeled coated areas were positive for both receptors, 19% were positive for the EGFR only, and the remaining 66% were positive for the TfR only (Table 1A). Double-labeling for EGF and TfR gave similar results with 14% double positive coated areas, 32% positive for EGF only, and 54% positive for the TfR only (Table 1 A). Double-labeling for EGF and epsin showed that not all EGF-containing coats were epsin positive (Table 1B). Although 23% of the positive coats labeled for both EGF and epsin, 31% labeled for EGF only and 46% for epsin only. A similar double-labeling for TfR and epsin showed that as much as 42% of the positive coats labeled for both TfR and epsin, 22% labeled for TfR only and 36% for epsin only (Table 1B). This argues that epsin does not preferentially associate with EGFR positive clathrin-coated pits.
Table 1.
Colocalization of EGFR, TfR, and epsin in coated pits
| A. TfR and EGFR or EGF label
|
||||
|---|---|---|---|---|
| EGFR+ | EGFR– | EGF+ | EGF– | |
| TfR+ | 15 | 66 | 14 | 54 |
| TfR– | 19 | — | 32 | — |
| B. Epsin and EGF or TfR
|
||||
|---|---|---|---|---|
| EGF+ | EGF– | TfR+ | TfR– | |
| Epsin+ | 23 | 46 | 42 | 36 |
| Epsin– | 31 | — | 22 | — |
Thawed cryosections of Hep2 cells, incubated with EGF on ice for 60 min, were double-labeled either for TfR and the EGFR or EGF (A), or for epsin and EGF or the TfR (B). The number of coated pits showing labeling for at least one of the molecules were counted, and the degree of colocalization was calculated. The results are shown as % of the total number of labeled coated pits in each double-labeling.
Overexpression of hSpry2 Inhibits EGF-induced Relocalization of the EGFR into Clathrin-coated Pits
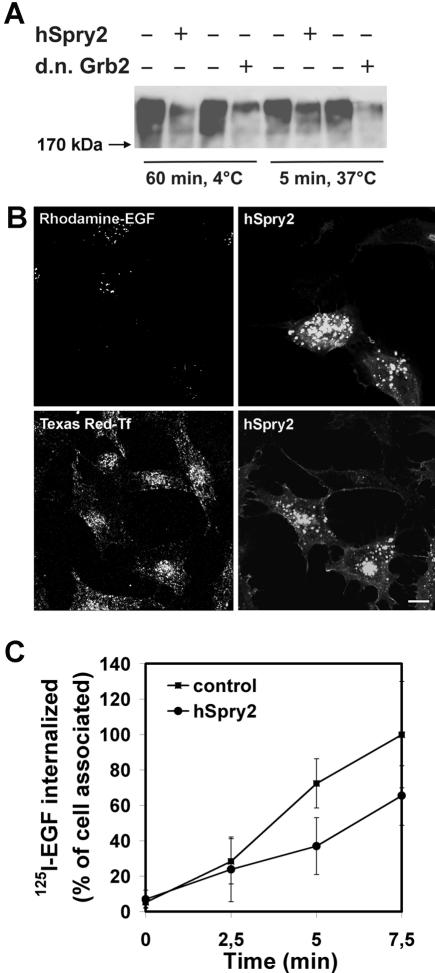
hSpry2 has been demonstrated to bind Cbl and to inhibit ligand-induced ubiquitination and endocytosis of the EGFR (Wong et al., 2002; Fong et al., 2003). To address the potential impact of Cbl-dependent ubiquitination on EGFR endocytosis, we overexpressed GFP-tagged hSpry2 in HeLa cells. We first confirmed that overexpression of hSpry2-inhibited ubiquitination of the EGFR. HeLa cells were transfected with a plasmid encoding HA-Ubiquitin and with or without plasmids encoding hSpry2 or d.n. Grb2. As demonstrated in Figure 6A, both hSpry2 and d.n. Grb2 efficiently inhibited ubiquitination of EGFR upon incubation of transfected cells with EGF for 60 min on ice and for 5 min at 37°C. When cells were incubated at 37°C for 15 min upon binding fluorescing EGF, the transfected cells were observed to inefficiently endocytose EGF, in the sense that hSpry2 reduced the number of endocytic vesicles containing fluorescent EGF, whereas the number of endocytic vesicles containing fluorescent Tf was unchanged (Figure 6B). The sensitivity of this assay is limited by the intensity of the vesicle fluorescence, and it is potentially possible that small early endosomes could be below the detection limit of the confocal microscope. Furthermore, hSpry2-dependent reduction in ubiquitination of the EGFR will affect the endosomal sorting of EGFR. We therefore directly assayed the clathrin-dependent endocytosis of EGF-EGFR using low concentrations of 125I-EGF and short times of incubation at 37°C upon binding. As demonstrated in Figure 6C, the internalization assay demonstrated that endocytosis as such was inhibited.
Figure 6.
Endocytosis of EGFR, but not of TfR, is inhibited in cells where ubiquitination of the EGFR is inhibited because of overexpression of hSpry2. (A) HeLa cells transfected with HA-ubiquitin and with or without either GFP-tagged hSpry2 or d.n. Grb2 were incubated with 60 ng/ml EGF as indicated. The EGFR was immunoprecipitated, and the precipitate was subjected to Western blotting with antibody to HA. (B) HeLa cells on coverslips were transfected with GFP-tagged hSpry2 for 24 h. Rhodamine-EGF (15 ng/ml; top panel) or TexasRed-Tf (20 μg/ml; bottom panel) was added at 37°C for 15 min before the cells were fixed. Bar, 10 μm. (C) Mock-transfected HeLa cells (▪) or HeLa cells transiently overexpressing GFP-tagged hSpry2 (•) for 24 h were incubated with 125I-EGF (1 ng/ml) on ice for 15 min and chased at 37°C for the indicated time periods. Analysis of internalized EGF was performed as described in MATERIALS AND METHODS. The data represent one typical experiment with six parallels ± SD.
The EGF-induced localization of hSpry2 and EGFR in HeLa cells incubated with EGF on ice was further investigated by immuno-EM. At the plasma membrane, overexpressed hSpry2 (anti-GFP labeling) localized mainly to smooth membrane areas. However, a limited amount of the labeling was associated with coated membrane areas (Figure 7). Double-labeling for GFP-hSpry2 and clathrin confirmed that the coats contained clathrin (Figure 7C), and interestingly, hSpry2 was found to localize to the rim and not to the central parts of the clathrin coats. Most strikingly, double-labeling for GFP-hSpry2 and EGF, or the EGFR, showed that, compared with in nontransfected cells, in which EGF and EGFR were found to localize to central parts of coated pits (see Figure 1), EGF (Figure 7, D and E) and EGFR (Figure 7F) were found at the rim of coated pits in cells overexpressing hSpry2. Double-labeling for GFP-hSpry2 and Grb2 (Figure 7G) or Cbl (unpublished results) showed that all three proteins localized to the rim of coated pits also in cells overexpressing hSpry2. This strongly suggests that ubiquitination, induced by plasma membrane-localized Cbl, is essential in guiding the EGFR into coated pits, but that overexpression of hSpry2 counteracts this Cbl-mediated guiding of activated EGFR.
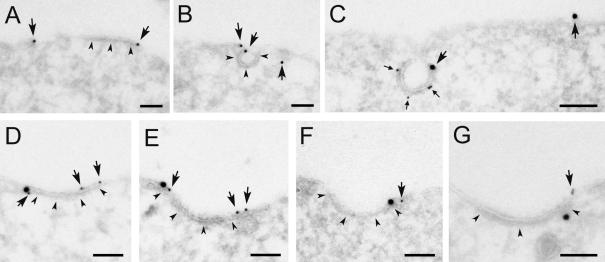
Figure 7.
Recruitment of EGFR into coated pits is inhibited in cells overexpressing hSpry2. HeLa cells transfected with GFP-tagged hSpry2 for 24 h were incubated with EGF (60 ng/ml) on ice for 60 min and prepared for immuno-EM. (A and B) Thawed cryosections were single-labeled using anti-GFP antibodies and 15-nm protein A gold. Labeling for GFP-tagged hSpry2 (arrows) was found both at smooth and coated plasma membrane areas (coat indicated by arrowheads). (C) Double-labeling using anti-GFP antibodies (large gold particles) followed by anti-clathrin antibodies (small gold, small arrows). (D–F) Double-labeling using either anti-EGF (D and E) or anti-EGFR (F) antibodies (large gold), followed by anti-GFP antibodies (small gold, arrows). (G) Double-labeling using anti-Grb2 antibodies (large gold) followed by anti-GFP antibodies (small gold). Bars, 100 nm.
Monoubiquitin-binding Proteins Are Essential for Endocytosis of EGFR
The finding that Cbl seems to be important for recruiting activated EGFR to clathrin-coated pits suggests that Cbl-induced ubiquitination is essential in this translocation process. An explanation for the potential requirement for ubiquitination is the ability of ubiquitinated proteins to interact with proteins harboring UIMs or other ubiquitin-binding domains. To directly address the impact of interaction of ubiquitin with ubiquitin-binding domains on endocytosis of activated EGFR, we transiently transfected Hep2 cells and HeLa cells with a plasmid encoding ubiquitin lacking C-terminal glycines (residues 75–76 deleted). This mutant ubiquitin (UbR) cannot be conjugated to substrates, but binds noncovalently to ubiquitin interacting domains. Therefore, upon overexpression, UbR will block interaction of ubiquitinated proteins with proteins harboring sequence motifs known to bind directly to monoubiquitin. To counteract interaction of the overexpressed ubiquitin with such motifs, a plasmid encoding ubiquitin with the L8A/I44A mutations was created in the context of the deletion mutant UbR (UbR L8A/I44A). The double mutation (L8A/I44A) and the single mutation (I44A) have both been demonstrated to efficiently inhibit interaction of ubiquitin with UIMs (Beal et al., 1996; Shih et al., 2002) as well as with other ubiquitin-binding domains (Kang et al., 2003; Alam et al., 2004; Shiba et al., 2004). All the ubiquitin constructs were Myc-tagged in order to facilitate detection of exogenously expressed protein. On transfection and overexpression in Hep2 cells (unpublished data) and HeLa cells (Figure 8), conjugation of Myc-ubiquitin to cellular proteins could be observed by Western blotting with antibody recognizing Myc. As demonstrated in Figure 8A (top panel), conjugation was absent upon expression of UbR, but was observed in the case of full-length ubiquitin (UbRGG). Consistently, a pool of Myc-tagged nonconjugated ubiquitin was observed by Western blotting lysate from cells transfected with UbR (Figure 8A, top panel). When the same lysates were immunoblotted with antibody recognizing monomeric and conjugated ubiquitin (Figure 8A, bottom panel), it was observed that the amount of free nonconjugated ubiquitin was significantly increased in cells transfected with UbR. Because only 40% of the cells were transfected, the amount of free ubiquitin in the transfected cells is underestimated. This is further demonstrated by immunostaining of untransfected and UbR-transfected cells with antibodies to Myc and to ubiquitin (Figure 8B), directly demonstrating overexpression of UbR in single cells.
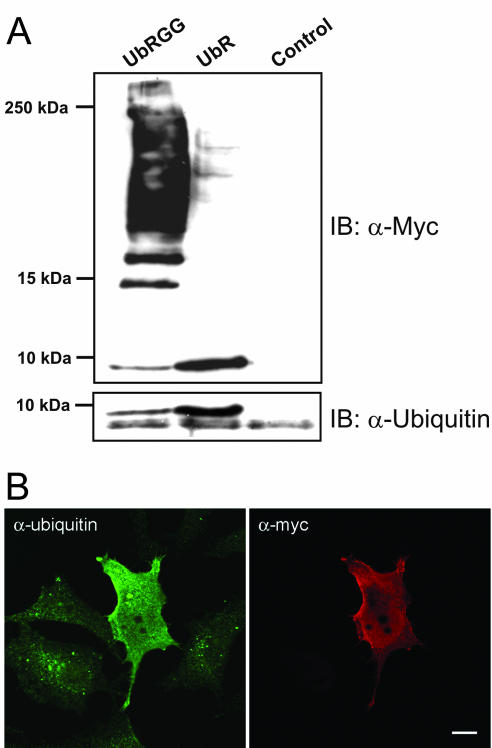
Figure 8.
Conjugation of Myc-tagged ubiquitin to cellular proteins is blocked when the C-terminal glycine residues (75–76) are deleted. (A) HeLa cells were transfected with Myc-tagged UbRGG or Myctagged UbR for 24 h as described in MATERIALS AND METHODS. The cells were lysed, and the lysates were subjected to SDS-PAGE (10–20% Tris-Tricine) and immunoblotting with a rabbit anti-Myc antibody (top panel) or with an antibody recognizing both monomeric and conjugated ubiquitin (bottom panel). In the transfected cells two monomeric ubiquitin bands are visualized by the antibody to ubiquitin. The top band represents the Myc-tagged ubiquitin. Control = nontransfected cells. (B) HeLa cells grown on coverslips were transfected with Myc-tagged UbR for 24 h. The cells were fixed and immunostained, using a rabbit anti-Myc antibody and a mouse anti-ubiquitin antibody recognizing monomeric and conjugated ubiquitin. The secondary antibodies were Alexa488- and Rhodamine Red-X conjugated, respectively.
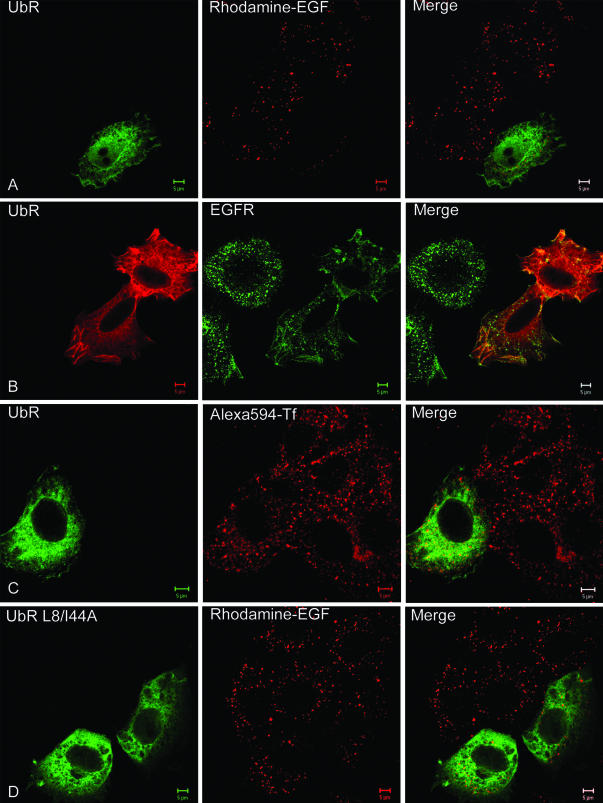
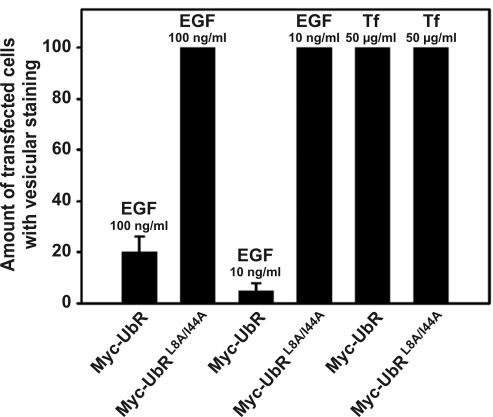
The effect of overexpressing UbR and UbR L8A/I44A in Hep2 cells on endocytosis of the EGFR was studied using Rhodamine-EGF. As demonstrated, although UbR efficiently inhibited endocytosis of EGF compared with nontransfected cells (Figure 9A), there was no observable effect on endocytosis of EGF by overexpressing UbR L8A/I44A (Figure 9D). The same was observed when studying localization of the EGFR in EGF-activated cells by immunofluorescence (Figure 9B). When the same experiment was performed with Alexa594-Tf, no effect on endocytosis was observed upon overexpression of UbR (Figure 9C), nor of UbR L8A/I44A (see Figure 10). To quantify the effect of overexpressing UbR on endocytosis of the EGFR, transfected cells with or without fluorescing vesicles were counted, and the percentage of transfected cells with vesicular staining was estimated. As demonstrated in Figure 10, only overexpression of UbR significantly inhibited the endocytosis of EGF-EGFR. The inhibitory effect was stronger when 10 ng/ml fluorescent EGF was used than upon incubation with 10-fold more EGF. These experiments indicate that monoubiquitin-interacting proteins are involved in the ligand-induced clathrin-dependent endocytosis of the EGFR, but not the constitutive clathrin-dependent endocytosis of the TfR. The experiments further indicate that macropinocytic uptake of EGFR induced upon addition of high concentrations of EGF (100 ng/ml; Figure 10) is not ubiquitin dependent.
Figure 9.
Endocytosis of EGF and EGFR is inhibited upon overexpression of ubiquitin that cannot be conjugated to endogenous proteins. Hep2 cells on coverslips were transfected with Myc-tagged UbR (A–C) or with Myc-tagged UbR L8A/I44A (D) for 48 h. Rhodamine-EGF (10 ng/ml, A and D), EGF (100 ng/ml, B), or Alexa594-Tf (50 μg/ml, C) was added to the cells at 37°C for 15 min. The cells were fixed and immunostained using mouse anti-Myc antibodies and secondary Rhodamine Red-X– or FITC-conjugated antibodies to detect transfected cells (left columns). Cells in B were additionally immunostained with anti-EGFR antibodies, followed by secondary FITC-conjugated antibodies. Bar, 5 μm.
Figure 10.
Mutant ubiquitin (UbR) inhibits endocytosis of EGF, but not of Tf. Hep2 cells were treated as described in legend to Figure 9. To quantify the uptake of EGF or Tf after 15 min, transfected cells with or without vesicular staining of Rhodamine-EGF or Alexa594-Tf were counted manually in the microscope. Transfected cells with vesicular staining are presented as percentage of the total number of transfected cells. Transfected cells with different expression of the transgene were picked randomly. For each transfection, 60 cells (20 cells each from 3 different coverslips) were analyzed. Error bars, SEM.
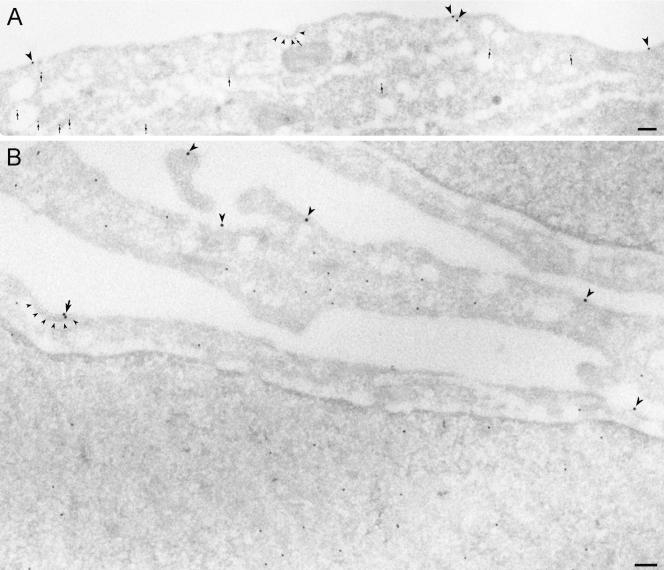
Because lack of interaction of ubiquitinated proteins with proteins harboring ubiquitin-interacting motifs could inhibit initial steps of endocytosis as well as transfer of EGFR from early small endosomes to larger endosomes detectable by confocal microscopy, we directly investigated whether overexpression of UbR affected recruitment of the EGFR into clathrin-coated pits by immuno-EM. Sections of UbR-transfected Hep2 cells incubated with EGF for 60 min on ice were double-labeled with antibodies to Myc and to EGF or EGFR. Quantitations of the labeling showed that EGF-induced recruitment of EGFR into coated pits was strongly reduced upon expression of UbR (see Figure 11). Compared with nontransfected cells within the same specimen (see also Figure 1), the amount of EGF-EGFR localizing to coated pits in UbR-positive cells was reduced by ∼65%, confirming the role of ubiquitination in recruitment of the EGFR to clathrin-coated pits. We further investigated whether localization of the UIM-containing protein epsin to clathrin-coated pits was altered in cells overexpressing UbR. Sections of UbR-transfected Hep2 cells incubated with EGF (as described above) were double-labeled with antibodies to Myc and to epsin. Compared with nontransfected cells within the same specimen, the amount of epsin localizing to coated pits appeared unaltered (unpublished data). This suggests that epsin-ubiquitin interactions are not required for recruitment of epsin to clathrin-coated pits.
Figure 11.
Mutant ubiquitin (UbR) inhibits recruitment of EGF-EGFR to coated pits. Hep2 cells transiently transfected with UbR were incubated with EGF (60 ng/ml) for 60 min on ice and prepared for immuno-EM. Thawed cryosections were double-labeled for EGF (15-nm gold particles) and UbR (rabbit anti-Myc; 10-nm gold particles, indicated by small arrows in A). In transfected cells labeling for EGF was restricted to smooth plasma membrane areas (large arrowheads in A and B). A very small proportion of 15-nm gold particles (indicating EGF, arrow in B) was found in coated plasma membrane areas (coats indicated by small arrowheads). Bars, 100 nm.
DISCUSSION
It was recently demonstrated by several authors that the adaptor protein Grb2 is involved in endocytosis of EGF-bound EGFR (Wang and Moran, 1996; Waterman et al., 2002; Yamazaki et al., 2002; Jiang et al., 2003). By immuno-EM, we now demonstrate a clear lack of EGFR-positive clathrin-coated pits upon overexpression of Grb2 with point mutations in both SH3 domains. It should be noted that even at relatively high concentrations of EGF (60 ng/ml), no EGFR-positive coated pits could be found in cells overexpressing d.n. Grb2, whereas TfR-positive coated pits were clearly observed under the same conditions. This argues that functional Grb2 is critical in recruiting the EGFR both to coated pits induced upon EGFR activation and to preformed TfR-positive clathrin-coated pits. Our data are consistent with previous reports (Waterman et al., 2002; Jiang and Sorkin, 2003) demonstrating that Grb2 is required to efficiently recruit Cbl to the EGFR and to thereby efficiently induce ubiquitination of the EGFR. This would argue that the requirement for Grb2 in endocytosis could be explained by a requirement for ubiquitination of the EGFR. Interestingly, this notion is consistent with our current results demonstrating that upon overexpressing nonconjugatable ubiquitin capable of competitively binding cellular proteins with ubiquitin interacting domains, endocytosis of EGF-EGFR and recruitment of EGF-EGFR to clathrin-coated pits was inhibited. Our current data therefore establish the importance of interactions of ubiquitin and ubiquitin-binding domains in initial steps of EGF-induced endocytosis of the EGFR in mammalian cells. However, our current data do not establish that cargo itself needs to be ubiquitinated.
Grb2 and Cbl were interestingly found to specifically localize to the rim of EGFR-positive clathrin coats in the same manner as previously reported for Eps15 (Torrisi et al., 1999; Stang et al., 2000). We have further demonstrated that also hSpry2 localizes to the rim of coated pits in transfected cells. hSpry2 interacts with Cbl both constitutively and inducibly, and this interaction inhibits ubiquitination of the EGFR (Wong et al., 2002; Fong et al., 2003; and the current study). We found the inhibition of EGFR ubiquitination induced upon overexpression of hSpry2 to correlate with inhibited translocation of activated EGFR to the central parts of clathrin-coated pits, and we observed that EGF-EGFR accumulated at the rim of clathrin-coated pits in cells overexpressing hSpry2. This finding clearly illustrates that ubiquitination is required for translocation of the EGFR into clathrin-coated pits.
Several investigators have concluded that Cbl is important for endocytosis of EGFR (Lill et al., 2000; Thien et al., 2001; Soubeyran et al., 2002; Waterman et al., 2002; Wong et al., 2002; Haglund et al., 2003; Jiang and Sorkin, 2003; Mosesson et al., 2003). However, the importance of ubiquitination per se in this process has been debated (Soubeyran et al., 2002; Waterman et al., 2002; Duan et al., 2003; Jiang and Sorkin, 2003; Schmidt et al., 2003). The fact that a clear conclusion has been difficult to reach on this point could possibly be explained by the experimental design of these studies. In most cases the potential importance of ubiquitination has been addressed by overexpressing catalytically inactive mutants of Cbl (Thien et al., 2001; Jiang and Sorkin, 2003) or by overexpressing deletion mutants of Cbl (Longva et al., 2002). In these cases there will likely be residual catalytic activity due to presence of endogenous Cbl and due to the fact that catalytically inactive mutants of Cbl do not act in a true dominant interfering manner. This also applies to the study where knockout mice for Cbl were created, because in these mice only the c-Cbl isoform was knocked out and Cbl-b was not targeted (Duan et al., 2003).
The ubiquitin internalization signal is distinct from linear internalization sequences (tyrosine- or di-leucine-sequences) that promote internalization by binding to AP-2. Consistently, AP-2 was found not to be required for direct interaction with the EGFR for clathrin-dependent internalization of the EGFR (Huang et al., 1999; Nesterov et al., 1999). Furthermore, it was recently reported that the EGFR was efficiently endocytosed even in the absence of AP-2 upon RNA interference knock-down (Conner and Schmid, 2003; Motley et al., 2003). This could be consistent with the possibility that ubiquinated EGFR interacts directly with an endocytosis adaptor harboring a ubiquitin-interacting domain. Epsin, which contains an ENTH domain at the N-terminus, two UIMs, and EH-, clathrin- and AP-2 interaction motifs at the C-terminus, has been suggested to function as an adaptor in endocytosis (Wendland, 2002). However, our finding that EGFR localized to the central part of clathrin-coated pits lacking epsin, suggests that such parts of coated pits contain other, yet undefined, functional ubiquitin binding proteins.
On overexpressing H-Ras17N, EGF-induced phosphorylation of MAPK was clearly inhibited. However, clathrin-dependent endocytosis of the EGFR, assayed by nonsaturating concentrations of EGF (1 ng/ml) was not inhibited, whereas overexpression of H-Ras17N to some extent inhibited endocytosis of EGFR when cells were incubated with 60–150 ng/ml EGF. This is consistent with a role for Ras in EGF-induced macropinocytosis. Data reported by Stahl and coworkers (Barbieri et al., 1998) suggested that Ras facilitates endocytosis of EGF-EGFR, possibly by activation of Rab5 upon incubation of cells with 600 ng/ml EGF. It has previously also been reported that Grb2 has a role in events associated with a macropinocytic internalization pathway for EGFR (Yamazaki et al., 2002). We therefore conclude that Grb2 seems to be involved both in clathrin-dependent and -independent endocytosis of the EGFR. The role of Grb2 in clathrin-independent endocytosis could potentially be to activate Ras, whereas the requirement for Grb2 in EGF-induced clathrin-dependent endocytosis is not explained by a requirement for Ras activity.
The EGFR may by binding Grb2 and Cbl, initially be recruited to a molecular complex containing Eps15, located at the rim of coated pits. This complex could consist of Eps15, epsin, RalBP1, POB1, and AP-2 as suggested (Nakashima et al., 1999). The fact that POB1 has been demonstrated to bind Grb2 (Ikeda et al., 1998) makes POB1 an attractive candidate for linking the EGFR-Grb2-Cbl complex to the rim of coated pits. Dynamin was found to decorate the whole cytosolic part of coated pits, and it is unlikely that dynamin is responsible for the EGF-induced recruitment of Grb2 to coated pits. Grb2 could preferentially bind dynamin in the GTP-bound form, which potentially could localize close to the rim of the coat. However, our unpublished studies have shown that Grb2 also localizes to the rim of coated pits in HeLa cells that inducibly overexpress dynamin K44A, reportedly incapable of binding GTP (Damke et al., 1994). Eps15 has further been demonstrated to bind Crk (Schumacher et al., 1995), which can again interact with the EGFR (Moran et al., 1990; Birge et al., 1992) as well as with Cbl (Fukazawa et al., 1996). In a more or less defined complex containing Grb2, Cbl, and Eps15 at the rim of coated pits, autophosphorylated EGFR becomes ubiquitinated by Cbl. We have now demonstrated that also hSpry2, in addition to Grb2, Cbl, and Eps15, localizes at the rim of clathrin-coated pits in cells transfected with GFP-tagged hSpry2. There is reason to believe that hSpry2 under physiological conditions interacts with Cbl at the rim of clathrin-coated pits and in this way modulates the speed by which the activated EGFR is translocated to clathrin-coated pits by fine-tuning the ubiquitination.
Acknowledgments
Linton Traub, Sandra L. Schmid, Robin M. Scaife, Alan Hall, Dirk Bohmann, Frances M. Brodsky, and Graeme Guy are acknowledged for gifts of valuable reagents and Randi Idsoe for technical assistance. This work was supported by The Norwegian Research Council, The Norwegian Cancer Society, Medinnova, NOVO Nordic Foundation, Anders Jahre's Foundation for the Promotion of Science, Torsted's Legacy, Blix Legacy, and Bruun's Legacy.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-01-0041. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-01-0041.
Abbreviations used: EGFR, EGF receptor; SAB, sodium acetate buffer; Tf, transferrin; TfR, transferrin receptor; UIM, ubiquitin-interacting motif.
References
- Aguilar, R.C., Watson, H.A., and Wendland, B. (2003). The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278, 10737-10743. [DOI] [PubMed] [Google Scholar]
- Alam, S.L., Sun, J., Payne, M., Welch, B.D., Blake, B.K., Davis, D.R., Meyer, H.H., Emr, S.D., and Sundquist, W.I. (2004). Ubiquitin interactions of NZF fingers. EMBO J. 23, 1411-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyere, M., Mettlen, M., Van Der Smissen, P., Platek, A., Payrastre, B., Veithen, A., and Courtoy, P.J. (2002). Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int. J. Med. Microbiol. 291, 487-494. [DOI] [PubMed] [Google Scholar]
- Barbieri, M.A., Kohn, A.D., Roth, R.A., and Stahl, P.D. (1998). Protein kinase B/akt and rab5 mediate Ras activation of endocytosis. J. Biol. Chem. 273, 19367-19370. [DOI] [PubMed] [Google Scholar]
- Beal, R., Deveraux, Q., Xia, G., Rechsteiner, M., and Pickart, C. (1996). Surface hydrophobic residues of multiubiquitin chains essential for proteolytic targeting. Proc. Natl. Acad. Sci. USA 93, 861-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah, A., Lamaze, C., Begue, B., Schmid, S.L., Dautry-Varsat, A., and Cerf-Bensussan, N. (1998). AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140, 1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge, R.B., Fajardo, J.E., Mayer, B.J., and Hanafusa, H. (1992). Tyrosine-phosphorylated epidermal growth factor receptor and cellular p130 provide high affinity binding substrates to analyze Crk-phosphotyrosine-dependent interactions in vitro. J. Biol. Chem. 267, 10588-10595. [PubMed] [Google Scholar]
- Chardin, P., Camonis, J.H., Gale, N.W., van Aelst, L., Schlessinger, J., Wigler, M.H., and Bar-Sagi, D. (1993). Human Sos 1, a guanine nucleotide exchange factor for Ras that binds to GRB2. Science 260, 1338-1343. [DOI] [PubMed] [Google Scholar]
- Chen, H., Fre, S., Slepnev, V.I., Capua, M.R., Takei, K., Butler, M.H., Di Fiore, P.P., and De Camilli, P. (1998). Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394, 793-797. [DOI] [PubMed] [Google Scholar]
- Ciechanover, A., Schwartz, A.L., Dautry-Varsat, A., and Lodish, H.F. (1983). Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J. Biol. Chem. 258, 9681-9689. [PubMed] [Google Scholar]
- Conner, S.D., and Schmid, S.L. (2003). Differential requirements for AP-2 in clathrin-mediated endocytosis. J. Cell Biol. 162, 773-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke, H., Baba, T., Warnock, D.E., and Schmid, S.L. (1994). Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, L. et al. (2003). Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J. Biol. Chem. 278, 28950-28960. [DOI] [PubMed] [Google Scholar]
- Evan, G.I., Lewis, G.K., Ramsay, G., and Bishop, J.M. (1985). Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5, 3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, C.W., Leong, H.F., Wong, E.S., Lim, J., Yusoff, P., and Guy, G.R. (2003). Tyrosine phosphorylation of Sprouty2 enhances its interaction with c-Cbl and is crucial for its function. J. Biol. Chem. 278, 33456-33464. [DOI] [PubMed] [Google Scholar]
- Ford, M.G., Mills, I.G., Peter, B.J., Vallis, Y., Praefcke, G.J., Evans, P.R., and McMahon, H.T. (2002). Curvature of clathrin-coated pits driven by epsin. Nature 419, 361-366. [DOI] [PubMed] [Google Scholar]
- Fukazawa, T., Miyake, S., Band, V., and Band, H. (1996). Tyrosine phosphorylation of Cbl upon epidermal growth factor (EGF) stimulation and its association with EGF receptor and downstream signaling proteins. J. Biol. Chem. 271, 14554-14559. [DOI] [PubMed] [Google Scholar]
- Griffiths, G., McDowall, A., Back, R., and Dubochet, J. (1984). On the preparation of cryosections for immunocytochemistry. J. Ultrastruct. Res. 89, 65-78. [DOI] [PubMed] [Google Scholar]
- Haglund, K., Sigismund, S., Polo, S., Szymkiewicz, I., Di Fiore, P.P., and Dikic, I. (2003). Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 5, 461-466. [DOI] [PubMed] [Google Scholar]
- Huang, K.M., D'Hondt, K., Riezman, H., and Lemmon, S.K. (1999). Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 18, 3897-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, M., Ishida, O., Hinoi, T., Kishida, S., and Kikuchi, A. (1998). Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral. J. Biol. Chem. 273, 814-821. [DOI] [PubMed] [Google Scholar]
- Jain, S.K., Langdon, W.Y., and Varticovski, L. (1997). Tyrosine phosphorylation of p120cbl in BCR/abl transformed hematopoietic cells mediates enhanced association with phosphatidylinositol 3-kinase. Oncogene 14, 2217-2228. [DOI] [PubMed] [Google Scholar]
- Jiang, X., Huang, F., Marusyk, A., and Sorkin, A. (2003). Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol. Biol. Cell 14, 858-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., and Sorkin, A. (2003). Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic 4, 529-543. [DOI] [PubMed] [Google Scholar]
- Kang, R.S., Daniels, C.M., Francis, S.A., Shih, S.C., Salerno, W.J., Hicke, L., and Radhakrishnan, I. (2003). Solution structure of a CUE-ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell 113, 621-630. [DOI] [PubMed] [Google Scholar]
- Li, N., Batzer, A., Daly, R., Yajnik, V., Skolnik, E., Chardin, P., Bar-Sagi, D., Margolis, B., and Schlessinger, J. (1993). Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature 363, 85-88. [DOI] [PubMed] [Google Scholar]
- Lill, N.L., Douillard, P., Awwad, R.A., Ota, S., Lupher, M.L., Jr., Miyake, S., Meissner-Lula, N., Hsu, V.W., and Band, H. (2000). The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 275, 367-377. [DOI] [PubMed] [Google Scholar]
- Longva, K.E., Blystad, F.D., Stang, E., Larsen, A.M., Johannessen, L.E., and Madshus, I.H. (2002). Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J. Cell Biol. 156, 843-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, K.A., Opresko, L.K., Starbuck, C., Walsh, B.J., and Wiley, H.S. (1990). Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J. Biol. Chem. 265, 15713-15723. [PubMed] [Google Scholar]
- Moran, M.F., Koch, C.A., Anderson, D., Ellis, C., England, L., Martin, G.S., and Pawson, T. (1990). Src homology region 2 domains direct protein-protein interactions in signal transduction. Proc. Natl. Acad. Sci. USA 87, 8622-8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson, Y., Shtiegman, K., Katz, M., Zwang, Y., Vereb, G., Szollosi, J., and Yarden, Y. (2003). Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J. Biol. Chem. 278, 21323-21326. [DOI] [PubMed] [Google Scholar]
- Motley, A., Bright, N.A., Seaman, M.N., and Robinson, M.S. (2003). Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162, 909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, S., Morinaka, K., Koyama, S., Ikeda, M., Kishida, M., Okawa, K., Iwamatsu, A., Kishida, S., and Kikuchi, A. (1999). Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 18, 3629-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov, A., Carter, R.E., Sorkina, T., Gill, G.N., and Sorkin, A. (1999). Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 18, 2489-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier, J.P., Raabe, T., Henkemeyer, M., Dickson, B., Mbamalu, G., Margolis, B., Schlessinger, J., Hafen, E., and Pawson, T. (1993). A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell 73, 179-191. [DOI] [PubMed] [Google Scholar]
- Polo, S., Sigismund, S., Faretta, M., Guidi, M., Capua, M.R., Bossi, G., Chen, H., De Camilli, P., and Di Fiore, P.P. (2002). A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416, 451-455. [DOI] [PubMed] [Google Scholar]
- Raiborg, C., Bremnes, B., Mehlum, A., Gillooly, D.J., D'Arrigo, A., Stang, E., and Stenmark, H. (2001). FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J. Cell Sci. 114, 2255-2263. [DOI] [PubMed] [Google Scholar]
- Schmidt, M.H., Furnari, F.B., Cavenee, W.K., and Bogler, O. (2003). Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc. Natl. Acad. Sci. USA 100, 6505-6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, C., Knudsen, B.S., Ohuchi, T., Di Fiore, P.P., Glassman, R.H., and Hanafusa, H. (1995). The SH3 domain of Crk binds specifically to a conserved proline-rich motif in Eps15 and Eps15R. J. Biol. Chem. 270, 15341-15347. [DOI] [PubMed] [Google Scholar]
- Shiba, Y., Katoh, Y., Shiba, T., Yoshino, K., Takatsu, H., Kobayashi, H., Shin, H.W., Wakatsuki, S., and Nakayama, K. (2004). GAT (GGA and Tom1) domain responsible for ubiquitin binding and ubiquitination. J. Biol. Chem. 279, 7105-7111. [DOI] [PubMed] [Google Scholar]
- Shih, S.C., Katzmann, D.J., Schnell, J.D., Sutanto, M., Emr, S.D., and Hicke, L. (2002). Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4, 389-393. [DOI] [PubMed] [Google Scholar]
- Sorkina, T., Huang, F., Beguinot, L., and Sorkin, A. (2002). Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of epidermal growth factor receptor. J. Biol. Chem. 277, 27433-27441. [DOI] [PubMed] [Google Scholar]
- Soubeyran, P., Kowanetz, K., Szymkiewicz, I., Langdon, W.Y., and Dikic, I. (2002). Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416, 183-187. [DOI] [PubMed] [Google Scholar]
- Stang, E., Johannessen, L.E., Knardal, S.L., and Madshus, I.H. (2000). Polyubiquitination of the epidermal growth factor receptor occurs at the plasma membrane upon ligand-induced activation. J. Biol. Chem. 275, 13940-13947. [DOI] [PubMed] [Google Scholar]
- Tall, G.G., Barbieri, M.A., Stahl, P.D., and Horazdovsky, B.F. (2001). Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73-82. [DOI] [PubMed] [Google Scholar]
- Tanaka, M., Gupta, R., and Mayer, B.J. (1995). Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol. Cell. Biol. 15, 6829-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar, F., Sorkina, T., Sorkin, A., Ericsson, M., and Kirchhausen, T. (1996). Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 271, 28727-28730. [DOI] [PubMed] [Google Scholar]
- Thien, C.B., Walker, F., and Langdon, W.Y. (2001). RING finger mutations that abolish c-Cbl-directed polyubiquitination and downregulation of the EGF receptor are insufficient for cell transformation. Mol. Cell 7, 355-365. [DOI] [PubMed] [Google Scholar]
- Torrisi, M.R., Lotti, L.V., Belleudi, F., Gradini, R., Salcini, A.E., Confalonieri, S., Pelicci, P.G., and Di Fiore, P.P. (1999). Eps15 is recruited to the plasma membrane upon epidermal growth factor receptor activation and localizes to components of the endocytic pathway during receptor internalization. Mol. Biol. Cell 10, 417-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., and Moran, M.F. (1996). Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science 272, 1935-1939. [DOI] [PubMed] [Google Scholar]
- Waterman, H., Katz, M., Rubin, C., Shtiegman, K., Lavi, S., Elson, A., Jovin, T., and Yarden, Y. (2002). A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 21, 303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, B. (2002). Epsins: adaptors in endocytosis? Nat Rev Mol. Cell. Biol. 3, 971-977. [DOI] [PubMed] [Google Scholar]
- Wiley, H.S., Woolf, M.F., Opresko, L.K., Burke, P.M., Will, B., Morgan, J.R., and Lauffenburger, D.A. (1998). Removal of the membrane-anchoring domain of epidermal growth factor leads to intracrine signaling and disruption of mammary epithelial cell organization. J. Cell Biol. 143, 1317-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, E.S., Fong, C.W., Lim, J., Yusoff, P., Low, B.C., Langdon, W.Y., and Guy, G.R. (2002). Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J. 21, 4796-4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, T., Zaal, K., Hailey, D., Presley, J., Lippincott-Schwartz, J., and Samelson, L.E. (2002). Role of Grb2 in EGF-stimulated EGFR internalization. J. Cell Sci. 115, 1791-1802. [DOI] [PubMed] [Google Scholar]