Abstract
Microtubule nucleation is the best known function of centrosomes. Centrosomal microtubule nucleation is mediated primarily by γ tubulin ring complexes (γ TuRCs). However, little is known about the molecules that anchor these complexes to centrosomes. In this study, we show that the centrosomal coiled-coil protein pericentrin anchors γ TuRCs at spindle poles through an interaction with γ tubulin complex proteins 2 and 3 (GCP2/3). Pericentrin silencing by small interfering RNAs in somatic cells disrupted γ tubulin localization and spindle organization in mitosis but had no effect on γ tubulin localization or microtubule organization in interphase cells. Similarly, overexpression of the GCP2/3 binding domain of pericentrin disrupted the endogenous pericentrin–γ TuRC interaction and perturbed astral microtubules and spindle bipolarity. When added to Xenopus mitotic extracts, this domain uncoupled γ TuRCs from centrosomes, inhibited microtubule aster assembly, and induced rapid disassembly of preassembled asters. All phenotypes were significantly reduced in a pericentrin mutant with diminished GCP2/3 binding and were specific for mitotic centrosomal asters as we observed little effect on interphase asters or on asters assembled by the Ran-mediated centrosome-independent pathway. Additionally, pericentrin silencing or overexpression induced G2/antephase arrest followed by apoptosis in many but not all cell types. We conclude that pericentrin anchoring of γ tubulin complexes at centrosomes in mitotic cells is required for proper spindle organization and that loss of this anchoring mechanism elicits a checkpoint response that prevents mitotic entry and triggers apoptotic cell death.
INTRODUCTION
The centrosome is the primary microtubule-organizing center in animal cells. At the centrosome core is a pair of barrel-shaped microtubule assemblies, the centrioles (Doxsey, 2001). Centrioles are capable of self-assembly (Marshall et al., 2001; Khodjakov et al., 2002) and can serve as templates for recruitment and organization of the surrounding pericentriolar matrix (Bobinnec et al., 1998; Kirkham et al., 2003). The pericentriolar material or centrosome matrix contains a high proportion of coiled coil proteins and is the site of microtubule nucleation. Within the matrix are large protein complexes of γ tubulin and associated proteins that have a ring-like structure and mediate the nucleation of microtubules called γ tubulin ring complexes or γ TuRCs (Moritz et al., 1995a; Zheng et al., 1995). Other proteins may share the ability to nucleate microtubules because centrosomes can organize microtubules in the absence of functional γ tubulin (Sampaio et al., 2001; Strome et al., 2001; Hannak et al., 2002).
During cell cycle progression, centrosomes “mature” by recruiting additional γ TuRCs and several other proteins, resulting in an increase in the nucleation capacity of the centrosome (reviewed in Blagden and Glover, 2003). However, we still know very little about proteins that directly anchor γ TuRCs to centrosomes in vertebrate cells. In the budding yeast, a small γ tubulin complex composed of γ tubulin (Tub4p), Spc97p, and Spc98p (∼700 kDa) is bound to the nuclear side of the spindle pole body (the centrosome equivalent) through an interaction with Spc110p (Knop and Schiebel, 1997) and to the cytoplasmic side of the spindle pole body through Spc72p (Knop and Schiebel, 1998). Spc97p and Spc98p mediate binding of the complex to Spc110p and Spc72p (Knop and Schiebel, 1997; Knop and Schiebel, 1998; Nguyen et al., 1998). Although there is no apparent homology between their SPC97/98 interacting domains, chimeras formed by fusing the binding domain of one with the localization domain of the other can rescue knockouts of the proteins encoding the localization domains, suggesting that the two binding domains are functionally homologous (Knop and Schiebel, 1998).
γ TuRCs in vertebrate cells and Drosophila contain orthologues of the three yeast proteins (γ tubulin and γ complex proteins 2 and 3 [GCP2, 3]) as well as several additional components (Zheng et al., 1995; Martin et al., 1998; Moritz et al., 1998; Murphy et al., 1998, 2001; Oegema et al., 1999; reviewed in Job et al., 2003). In vertebrates, the centrosome protein pericentrin (pericentrin A) forms a large complex with γ tubulin in the cytoplasm, and the two proteins are also in proximity at the centrosome (Dictenberg et al., 1998). Recent evidence suggests there may be as many as 10 isoforms of pericentrin in human cells (Flory and Davis, 2003). A large isoform (pericentrin B/kendrin; Flory and Davis, 2003) and another centrosome protein called AKAP450/GC-NAP share homology with the calmodulin binding domain of Spc110p (Flory et al., 2000; Gillingham and Munro, 2000; Li et al., 2001). Other potential Spc110p orthologues have been identified in Schizosacharomyces pombe, Aspergillus nudulans, and Drosophila based on sequence homology (Flory et al., 2002; Kawaguchi and Zheng, 2003) and in vertebrates (Xenopus and human) based on immunological cross-reactivity with Spc110p-specific antibodies (Tassin et al., 1997). All proposed vertebrate orthologues of Spc110p localize to the centrosome and coimmunoprecipitate with γ TuRCs (Tassin et al., 1997; Dictenberg et al., 1998; Takahashi et al., 2002). No Spc72p orthologues have been identified in other species.
In vertebrate cells, pericentrin B and AKAP450 have recently been shown to bind GCP2 in vitro (Takahashi et al., 2002). Antibody inhibition and immunodepletion studies demonstrated a role for pericentrin isoforms and AKAP450 in microtubule nucleation in vertebrates and Drosophila (Doxsey et al., 1994; Takahashi et al., 2002; Kawaguchi and Zheng, 2003; Keryer et al., 2003), perhaps by localizing the small Ran GTPase to centrosomes (AKAP450) (Keryer et al., 2003). However, other studies show that antibody depletion of pericentrin B or reduction of pericentrin A and B do not affect aster formation, microtubule organization, or centrosome-associated γ tubulin (Li et al., 2001; Takahashi et al., 2002; Dammermann and Merdes, 2002). Moreover, loss of AKAP450 from centrosomes does not affect centrosomal γ tubulin localization, even though microtubule organization is disrupted (Keryer et al., 2003). Another potential centrosomal γ TuRC-anchoring protein has recently been identified in vertebrate cells called ninein-like protein (Nlp), which can bind γ TuRC complexes, inhibit nucleation when neutralized with antibodies, and enhance nucleation when overexpressed (Casenghi et al., 2003). However, we know little about the role of these putative scaffold proteins in centrosomal anchoring of γ TuRCs during the cell cycle and the cellular consequences of specifically disrupting their interactions with γ TuRCs at centrosomes.
In this study, we show that siRNAs targeting both pericentrin isoforms (A and B) induced specific loss of γ tubulin from spindle poles in mitosis, reduction of astral microtubules, and formation of monopolar spindles. This phenotype seemed to be specific for the smaller isoform of pericentrin because it was not observed when the larger pericentrin isoform was specifically reduced. A region at the C terminus of pericentrin interacted with both GCP2 and GCP3 in vitro as shown by coimmunoprecipitation and two-hybrid analysis. Expression of the GCP2/3 binding domain of pericentrin produced a phenotype similar to that observed in cells with reduced pericentrin. It disrupted the interaction between endogenous pericentrin and γ TuRCs, and adsorbed γ TuRCs from cell extracts. It reduced astral microtubules and centrosomal γ tubulin in mitotic cells and induced formation of small spindles and monopolar spindles. No effect on interphase microtubules was observed. When added to Xenopus extracts this domain dissociated γ tubulin from mitotic centrosomes and rapidly induced mitotic aster disassembly. The loss of γ tubulin from centrosomes in cells with reduced pericentrin levels or in cells expressing the GCP2/3 binding domain of pericentrin ultimately triggered a checkpoint inducing G2/antephase arrest and apoptosis in somatic cells. These phenotypes were not observed after specific reduction in the levels of the larger pericentrin isoform, expression of a mutant pericentrin defective in GCP2/3 binding, or expression of a homologous region of pericentrin B. We conclude that the smaller isoform of pericentrin provides a molecular scaffold for centrosomal anchoring γ TuRCs during mitosis in both embryonic and somatic cell systems.
MATERIALS AND METHODS
Molecular Cloning
All pericentrin constructs used in this study were cloned into pcDNA vectors (Invitrogen, Carlsbad, CA) with amino terminal hemagglutinin (HA) tags (Purohit et al., 1999; Purohit et al., 2001), except those used in two-hybrid studies (see below). Fragments of pericentrin and other genes were polymerase chain reaction (PCR) amplified from cDNAs by using primers with NotI and XbaI restriction sites. PCR products were digested with the appropriate enzymes, cloned into the vector, and sequences were confirmed. In some cases, EcoR1 and XhoI restriction sites were used (peri B1826-2117, 1572-1816, 1572-1816m). GCP2-, GCP3-, and γ tubulin-containing constructs were obtained from Dr. Tim Stearns (Stanford University, Stanford, CA).
Small Interfering RNA (siRNA)
Twenty-one nucleotide RNAs were chemically synthesized by Dharmacon Reasearch (Lafayette, CO). and introduced to cells using Oligofectamine (Invitrogen, Carlsbad, CA.) in accordance with the manufacturer's instructions. The target sequences used were AAUUGGAACAGCUGCAGCAGA against pericentrin A and B in human (Dammermann and Merdes, 2002), AAUGAGGUUGUCCACAGGAGA against pericentrin A and B in mouse, and AAGCUCUGAUUUAUCAAAAGA against the PACT domain of pericentrin B in human. AACUGGACUUCCAGAAGAACA, which targets human lamin A and is nonspecific in mouse, was used as a control for all siRNA studies. Crude cell lysates were analyzed for protein silencing. Cells were treated with 2 mM thymidine for 18 h starting 24 h post-siRNA treatment. Six hours after thymidine release, cells were harvested and lysed in phosphate-buffered saline (PBS) supplemented with 1% Triton X-100, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml chymotripsin,10 μg/ml phenylmethylsulfonyl fluoride, 2.0 μg/ml p-amino-benzamidine, 5 mM iodoacetamide, and 5 mg/ml N-ethylmaleimide. Cell lysates were clarified at top speed in a Microfuge for 15 min at 5°C. Protein concentration for each lysate was determined using Bio-Rad protein dye reagent, loads were adjusted, proteins were resolved by SDS-PAGE, and analyzed by Western Blot.
Antibodies
Anti-myc, anti-γ-tubulin, and anti-tubulin antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Phosphohistone H3 rabbit polyclonal antibody was purchased from Upstate Biotechnology (Lake placid, NY). M30 Cytodeath and anti-HA rat monoclonal antibody 3F10 was obtained from Roche Diagnostics (Indianapolis, IN) Anti-human lamin A/C antibody was purchased from Cell Signaling Technology (Beverley, MA). Other antibodies included M8 anti-pericentrin antibody, (Dictenberg et al., 1998), human autoimmune serum 5051 that recognizes centrosome proteins (Doxsey et.al., 1994), anti-pericentrin B/kendrin-specific antibody (Flory et.al., 2000) (obtained from Trisha Davis, University of Washington, Seattle, WA), anti-GCP2 antibody (Murphy et al., 1998) (obtained from Dr. Tim Stearns), and anti-GCP3 antibody (a gift from Michel Bornens, Institut Curie, Paris, France).
Yeast Two-Hybrid Cloning/Methods
Direct yeast two-hybrid interactions were performed essentially as described previously (Gromley et al., 2003). Pericentrin, γ tubulin, GCP2, and GCP3 coding sequences were amplified from plasmid DNA by PCR by using Pfu Turbo (Stratagene, La Jolla, CA), cloned into either pGBKT7 or pGADT7 (BD Biosciences Clontech, Palo Alto, CA) and completely sequenced. Yeast strains AH109 and Y187 were transformed with GAL4 DNA binding domain (GAL4-DBD) or GAL4 transactivation domain (GAL4-TAD) expression constructs, respectively, and diploid strains generated by mating. Interactions between pericentrin and members of the γ tubulin ring complex were tested for by streaking yeast onto synthetic defined (SD) medium lacking leucine, tryptophan, histidine, and adenine.
Biochemical Techniques
Immunoprecipitations from Xenopus extracts were performed as described previously (Dictenberg et al., 1998) by using the antibodies to the pericentrin amino terminus (M8) (Doxsey et al., 1994) and γ tubulin (Zheng et al., 1995). For disruption of γ TuRCs from pericentrin in coimmunoprecipitations, active or heat denatured pericentrin fractions were added directly to Xenopus highspeed extracts before immunoprecipitation. Protein affinity experiments to recruit γ TuRCs (Figure 2) were performed using partially purified fractions of pericentrin domains (see below). Proteins were bound to anti-HA beads, added to extracts for 60 min, washed in extract buffer (Murray, 1991), run on SDS gels, and probed with the indicated antibodies.
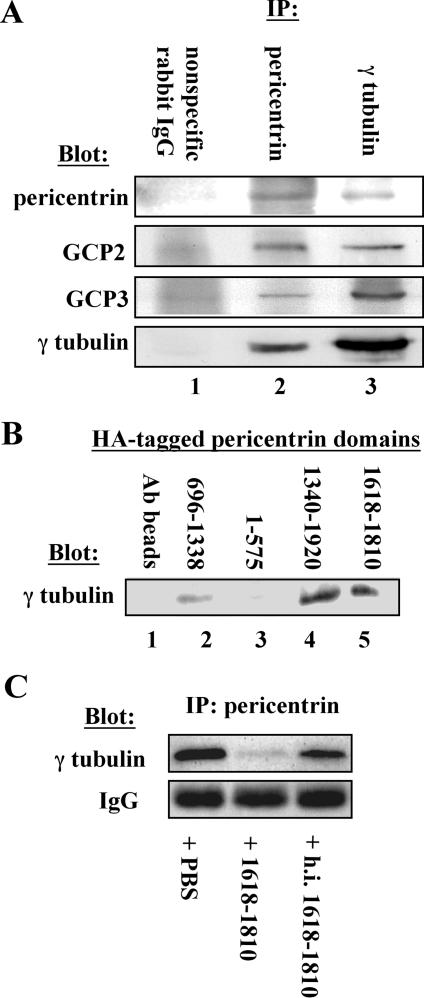
Figure 2.
Pericentrin interacts with the γ TuRC in Xenopus extracts. (A) Immunoprecipitation of endogenous pericentrin pulls down γ TuRC proteins (γ tubulin, GCP2, and GCP3) from Xenopus extracts (lane 2) and immunoprecipitation of γ tubulin pulls down pericentrin (lane 3), whereas nonspecific rabbit IgG precipitates none of these proteins (lane 1). (B) HA-tagged C-terminal domains of pericentrin bound to anti-HA beads pull down endogenous γ tubulin from Xenopus extracts (lanes 4 and 5), whereas beads alone and HA-tagged central and amino-terminal domains do not pull down significant γ tubulin (lanes 1–3). (C) A C-terminal domain of pericentrin (1618–1810) disrupts the interaction between endogenous pericentrin and the γ TuRC in extracts as shown by immunoprecipitation with anti-pericentrin antibodies, whereas heat-inactivated protein (h.i. 1618–1810) and phosphate-buffered saline (PBS) have no effect. Numbers in B and C represent amino acid numbers of pericentrin.
Proteins for recruitment of γ TuRCs (Figure 2) and for aster inhibition assays (Figures 4 and 5) were produced in COS cells and purified as follows. Confluent COS cells were transiently transfected with 3 μg of DNA/60-mm dish by using LipofectAMINE Plus reagent (Invitrogen). Transfected cells were maintained for 3 d in DMEM with 5% serum and then collected with 5 mM EDTA in PBS. Cells were lysed in PBS supplemented with protease inhibitor cocktail (#1836153; Roche Diagnostics, Basel, Switzerland), 1% Triton X-100, and 5 mg/ml N-ethylmaleimide. For recruitment of γ tubulin from extracts, HA beads were prepared by pretreating Dynabeads 450 (#110.05; Dynal, Lake Success, NY) with a saturating amount of anti-HA antibody 12CA5 (Covance, Denver, PA). Anti-HA IgG beads were treated with COS cell lysate containing an excess of the indicated HA-tagged pericentrin polypeptides, washed three times in PBS lysis buffer, two times in PBS, and two times in extract buffer, before addition of Xenopus extracts for γ TuRC recruitment experiments. For preparation of soluble HA-tagged pericentrin, 12CA5 antibody was cross-linked to protein A beads (Bio-Rad, Hercules, CA) by using standard methods (Harlow and Lane, 1988). HA-tagged pericentrin was batch depleted from COS lysates by incubation with HA cross-linked beads at 5°C with gentle agitation for 1 h. Treated beads (configured as a column) were washed with 10 column volumes of lysis buffer, 10 volumes of PBS with protease inhibitors, and 10 volumes of 10 mM Tris, pH 8.0. HA-tagged pericentrin was eluted with 2 volumes of 150 mM glycine, pH 2.5, into 1/4 volume of 1 M Tris, pH 8.0, and dialyzed against PBS overnight.
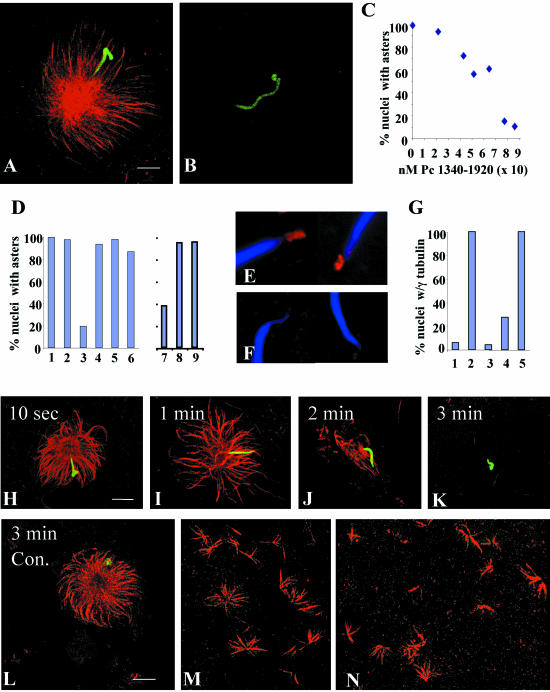
Figure 4.
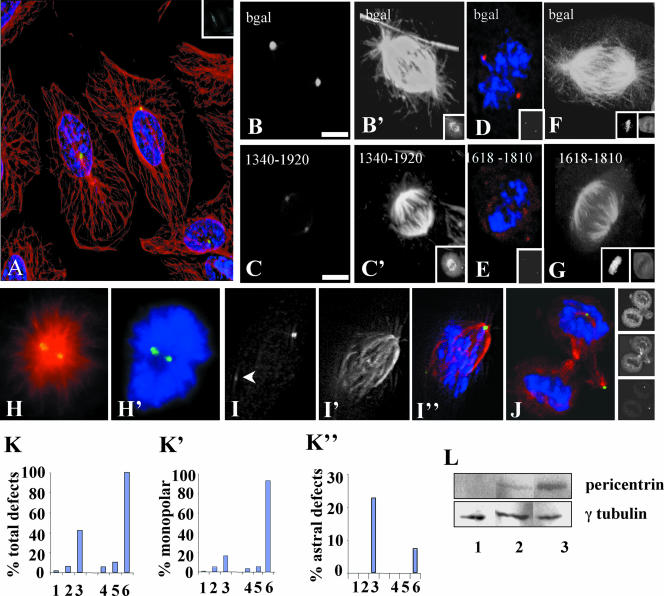
C-terminal fragments of pericentrin disrupt aster formation and stability and γ tubulin assembly onto centrosomes in Xenopus mitotic extracts. (A and B) Mitotic asters assembled in the presence of equal amounts of pericentrin 1340–1920 (B) or heat inactivated (h.i.) 1340–1920 (A). (C) Mitotic aster assembly in the presence of increasing concentrations of pericentrin (Pc) 1340–1920. (D) Quantification of aster assembly in mitotic extracts in the presence of pericentrin domains. Amount of protein added per 10 μl of extract is indicated. (1, PBS; 2, 33 ng of 1–535; 3, 12 ng of 1340–1920 (p < 0.0001); 4, 12 ng of h.i. 1340–1920; 5, 10 ng of peri B1826–2117; 6, 10,000 ng of BSA). Quantification of aster assembly in interphase extracts (D7–9) by using a pericentrin domain (1618–1810) that inhibits aster assembly in mitotic extracts (7. p < 0.0001) and is inactivated by heat (8), but has no activity in interphase extracts in the same experiment (9). (E and F) γ Tubulin assembly onto nascent centrosomes in the presence of h.i. 1340–1920 (E) or 1340–1920 (F, p < 0.0001). (G) Quantification of γ tubulin assembly onto centrosomes in the absence of mitotic extract (1), in extracts with 1–595 (2), 1340–1920 (3, p < 0.0001), 1618–1810 (4, p < 0.0001), or heat inactivated 1618–1810 (5). For C, D, and G, 200 sperm nuclei were counted per bar or point. (H–K) Rapid disassembly of preassembled mitotic asters over time after addition of 1340–1920. (L) h.i. Pc 1340–1920 has no effect on preassembled asters. (M and N) Ran-mediated aster assembly in extracts in the presence of h.i 1618–1810 (M) or 1618–1810 (N). A–D, H–N, microtubules or γ tubulin, red; nuclei, green. Bar (A), 10 μm for A and B; in L, 10 μm for H–N.
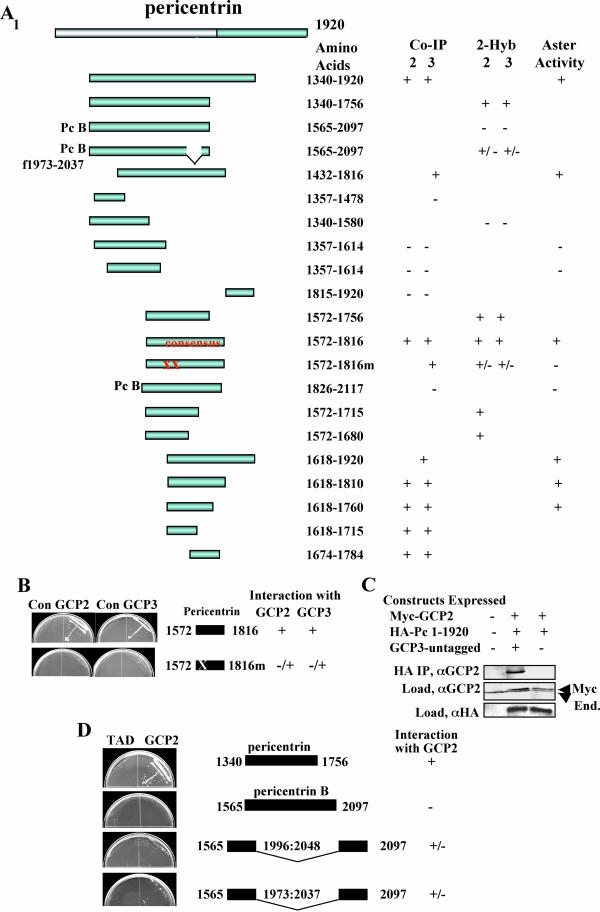
Figure 5.
Summary of GCP2/3 binding and aster inhibitory activity of pericentrin domains. (A) Binding and aster activity of various pericentrin constructs. CoIP, coimmunoprecipitation; 2-hyb, yeast two-hybrid; aster, aster inhibitory activity; consensus, smallest domain identified in both coIP and 2-hyb that has high-affinity binding activity to both GCP2 and GCP3; XX, E to A mutations in 1613 and 1615, Pc B φ 1973–2037 lacks an exon encoding the indicated amino acids. Although variable, interactions of all affinities were scored as + unless they were at the limit of detection (then scored as +/-). No markings such as + or - denote data not acquired for these parameters. Proteins were considered positive in the aster inhibition assay if they showed at least 31% reduction in aster assembly relative to control activity. For clarity, the pericentrin B constructs are arbitrarily sized and aligned with homologous regions of pericentrin. (B) Yeast two-hybrid data showing significantly reduced binding of mutant pericentrin domain for GCP2 and GCP3. (C) Cooverexpression, coimmunoprecipitation data showing enhanced binding of GCP2 to pericentrin 1–1920 in the presence of GCP3 (see Figure 2 legend for details). Con, control. (D) Yeast two-hybrid data showing binding of GCP2 by a pericentrin A fragment and lack of GCP2 binding by the homologous pericentrin B fragment as well as mutants lacking a pericentrin B specific exon (see MATERIALS AND METHODS for details).
Coimmunoprecipitations
Coimmunnoprecipitation of pericentrin isoforms and γ TuRC components (Figure 3) was performed in COS cells 40–48 h after transient cotransfection of the indicated constructs by using LipofectAMINE Plus reagent. Cells were collected using 5 mM EDTA in PBS. Cell pellets were lysed with 1% NP-40, 1 mM dithiothreitol, 10% glycerol in buffer C (100 mM PIPES, pH 6.9, 6 mM MgCl2, 0.5 mM EGTA, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml chymotripsin, 10 μg/ml phenylmethylsulfonyl fluoride, 2.0 μg/ml p-aminobenzamidine, 5 mM iodoacetamide). Lysates were clarified 15 min at top speed in a Microfuge at 5°C and then applied to HA Dyna beads (see above). Beads were treated for 1 h at 5°C with end-over-end agitation and washed two times in lysis buffer (see above) and two times in wash buffer (buffer C with 100 mM Na acetate, pH 6.9). Loads and treated beads (immunoprecipitates) were analyzed by SDS gel electrophoresis and Western blot by using the indicated antibodies.
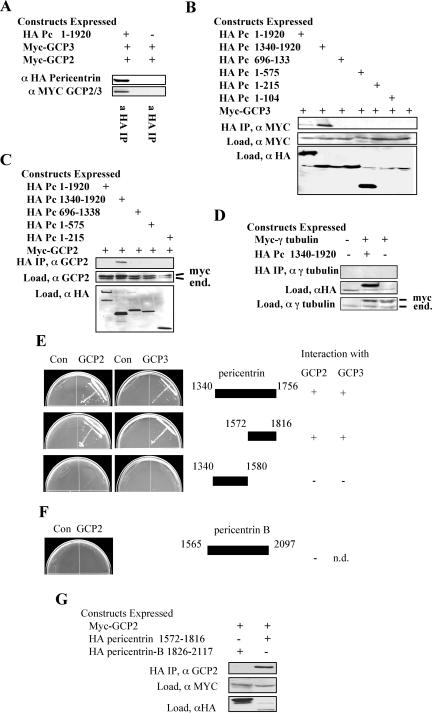
Figure 3.
C-terminal domains of pericentrin interact with γ TuRC proteins GCP3 and GPC2 in vitro. (A) When coexpressed in vertebrate cells myc-tagged GCP2 and/or myctagged GCP3 coimmunoprecipitate with HA-tagged pericentrin (similar mobility of GCP2 and GCP3 prevents their individual identification in this experiment). (B–D) A C-terminal domain of pericentrin (amino acids 1340–1920) interacts with GCP3 (B) and GCP2 (C) but not γ tubulin (D) when coexpressed in vertebrate cells. Immunoprecipitation and immunoblotting performed as indicated. End., endogenous protein. (E) Two-hybrid analysis confirms the interaction of the pericentrin C terminus with GCP2 and GCP3 but not with γ tubulin (data not shown). (F and G) Segments of pericentrin-B corresponding to the C terminal region of pericentrin do not interact with GCP2 in two-hybrid (F) or coimmunoprecipitation experiments (G).
Xenopus Extracts
Cytostatic factor (CSF)-arrested Xenopus extracts were prepared, and aster assembly assays were preformed as described previously (Murray, 1991; Stearns and Kirschner, 1994). For purpose of quantization, two hundred sperm were counted and scored for the presence of assembled microtubules. In some cases, the standard fix [0.3 volume of 37% formaldehyde, 0.6 volumes of 80% (wt/vol) glycerol, 0.1 volume 10× MMR, 1 μg/ml 4,6-diamidino-2-phenylindole (DAPI)] was modified by the addition of 0.05% Oligreen (Molecular Probes, Eugene, OR) to facilitate visualization of sperm nuclei with a scanning confocal microscope. Centrosome assembly in the presence of nocodazole was preformed using published methods (Stearns and Kirschner, 1994). Treated nuclei were prefixed in 5% formaldehyde, spun onto coverslips through a 20% sucrose cushion by using a JS13.1 rotor at 8000 rpm 15 min, and postfixed in methanol (-20°C) before staining for immunofluorescence. Ran-mediated asters were prepared using constitutively active RanL43E as described previously (Wilde and Zheng, 1999). Centrosome-dependent and -independent Xenopus mitotic asters were fixed in formaldehyde on coverslips as described previously (Murray, 1991; Wilde and Zheng, 1999).
Cell Lines
Cell lines (COS-7, SAOS, and U2OS) were grown as described previously (American Type Culture Collection, Manassas, VA) and prepared for transfection experiments as described previously (Purohit et al., 1999). Primary mouse embryonic fibroblasts (MEFs) were obtained from Dr. Geoffrey Wahl (Salk Institute for Biological Studies, La Jolla, CA) and used at less than six passages.
Transfection and Immunofluorescence
For transfection and immunofluorescence analysis, logarithmically growing cells were transfected as indicated by the manufacturer with 1 μg of DNA/35-mm dish of the appropriate construct by using LipofectAMINE Plus (Invitrogen) for COS cells and LipofectAMINE for SAOS and U2OS cells. The transfection efficiency for COS cells with control constructs ranged from 35 to 60%. MEFs were transfected using Superfect (QIAGEN, Valencia, CA). For immunofluorescence, cells were fixed with –20°C MeOH as described previously (Purohit et al., 1999). Data were collected as a Z series for deconvolution with 0.3 μm between planes. Images were deconvolved using MetaMorph software, no neighbors algorithm. All images were rendered two dimensional by showing maximum intensity at each point.
Microinjection Experiments
For microinjection, COS cells were synchronized by thymidine block. Cells were treated 16 h with 2 mM thymidine and released (single block), or treated for an additional 16 h with 2 mM thymidine after 8 h of release (double block). The mitotic index of synchronized cells was determined using replicate coverslips, fixed and stained with DAPI, and then counted at the indicated times postrelease from thymidine block. 1000 cells were counted for each time point. Microinjection into the nucleus of released cells was performed using an Eppendorf transjector 5246, with Eppendorf femtotips, with an injection pressure of 100 hPa, injection time of 0.4 s, and DNA at a concentration of 0.2 μg/μl in PBS.
RESULTS
Pericentrin Silencing Mislocalizes γ Tubulin from Spindle Poles and Disrupts Spindle Bipolarity
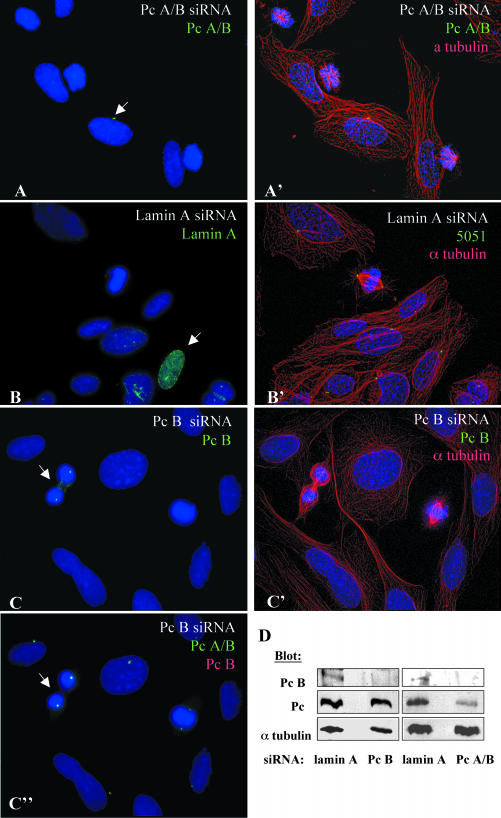
We previous showed that pericentrin interacts with the γ tubulin ring complex and that pericentrin antibodies disrupt spindle organization and function (Doxsey et al., 1994; Dictenberg et al., 1998). In this study, we address the molecular mechanism of the mitotic function of pericentrin. Initially, we used siRNAs designed to silence the two previously characterized isoforms of pericentrin (A and B/kendrin), although we cannot rule out silencing of other potential pericentrin isoforms under these conditions (Flory and Davis, 2003). We typically observed silencing in 80 to 90% of treated cells (Figure 1, A–C, G, and H). Silencing of pericentrin A/B disrupted mitotic spindle organization and reduced astral microtubules, ultimately leading to the formation of monopolar spindles in most mitotic cells (Figure 1, A, A′, E, and F). The phenotype seemed to be specific for mitotic cells because interphase microtubule organization was not detectably altered (Figure 1, A and A′). γ Tubulin was reduced at spindle poles in mitotic cells, although centrioles were present; centrosomes in adjacent interphase cells retained strong γ tubulin staining (Figure 1, G and H). Selective silencing of the larger isoform of pericentrin (B) had no effect on interphase or mitotic microtubule organization (Figure 1C, C′, C″, and D), although we cannot rule out the possibility that activity of the residual protein is sufficient to support these functions. These results suggested that the phenotype observed after pericentrin A/B silencing resulted from reduction in pericentrin A, although this isoform could not be specifically targeted because it is homologous through most of its length with pericentrin B (Flory and Davis, 2003). Control cells with reduced lamin levels showed no detectable changes in any of the parameters described above (Figure 1, B, B′, E, and F; our unpublished data).
Figure 1.
Silencing of pericentrin A and B causes mitotic defects. (A) SAOS cells with reduced pericentrin after siRNA treatment stained with a pericentrin antibody (M8, green), which recognizes both isoforms of pericentrin (Pc A/B) and DNA (DAPI, blue). (A′) Same field as in A costained for microtubules (α tubulin, red). (B) Cells with reduced lamin A stained for lamin A (green) and DNA. (B′) Same field as in B stained for centrosomes with 5051 autoimmune sera (green) and microtubules (red). (C and C′) Cells with reduced pericentrin B (targeting the C-terminal PACT domain) stained with pericentrin B-specific antibody, Pc B, (C, green), together with microtubule label (C′) or pericentrin antibody that recognizes both isoforms (C″). (A, B, C, and C″) Maximum projection of z series without deconvolution. (A′, B′, and C′) Maximum projection of deconvolved z series. Note the improvement in the resolution of the DNA. Arrows indicate cells expressing near normal levels of the targeted protein. (D) Western blots of crude cell lysates demonstrating reduction of pericentrin B (Pc B) but not pericentrin A (Pc) by using Pc B-specific siRNA or reduction of both isoforms relative to lamin A siRNA control, by siRNA targeting Pc A and Pc B (Pc A/B). Pericentrin isoforms probed with Pc A/B anti-pericentrin antibody (M8). α Tubulin loading control probed with DM1α anti-alpha tubulin antibody. (E) Graph showing percentage of mitotic SAOS cells with spindle defects (monopolar, multipolar, and reduced astral microtubules) at 48 and 72 h after siRNA treatment. One hundred to 150 mitotic cells scored per bar. (F) Graph indicating percentage of mitotic cells with monopolar spindles at 48 and 72 h. (G) Cells with reduced pericentrin A/B (Pc A/B, red) retain the centriole marker GT335 (green) in interphase and mitosis. (H) γ Tubulin in cells with reduced pericentrin A/B seems largely unchanged at centrosomes in interphase but is reduced at spindle poles. Pc A/B (red), γ tubulin (GTU88, green), and DAPI (blue). (G–H) Maximum projection of z series with no neighbor deconvolution. Arrows indicate cells with near normal staining levels of pericentrin. Asterisks indicate mitotic cells. Mitotic cells are show at higher magnification in insets.
Pericentrin Interacts with the γ TuRC in Xenopus Extracts through GCP2 and 3
We next examined the relationship of pericentrin and the γ tubulin ring complex in more detail. We found that immunoprecipitation of pericentrin from Xenopus extracts coprecipitated several components of the γ TuRC, including γ tubulin, GCP2, and GCP3 (Figure 2A). Conversely, immunoprecipitation of γ tubulin coprecipitated pericentrin in addition to GCP2 and GCP3. Additional evidence for the pericentrin-γ TuRC interaction was obtained by showing that an HA-tagged C-terminal region of pericentrin affixed to beads could be used to specifically pull out endogenous γ tubulin and associated proteins from Xenopus extracts (Figure 2B, 1340–1920; our unpublished data). Moreover, the C-terminal region of pericentrin was able to disrupt the endogenous pericentrin–γ TuRC interaction when added to extracts as shown by the loss of γ tubulin from pericentrin immunoprecipitates (Figure 2C). These results demonstrate that pericentrin interacts with the γ TuRC and that the interaction is mediated by a domain at the C-terminal region of the protein.
To determine the molecular basis of the interaction of pericentrin with the γ TuRC, we tested whether pericentrin could bind individual proteins of the complex in vitro. We found that HA-tagged full-length pericentrin and the C-terminal third of pericentrin coimmunoprecipitated myctagged GCP3 when the proteins were cooverexpressed in COS-7 cells (Figure 3, A and B). In parallel assays, the pericentrin C terminus coimmunoprecipitated myc-tagged GCP2 (Figure 3, A and C) but not myc-tagged γ tubulin (Figure 3D). GCP2/3 binding was specific for the C terminus of pericentrin because several domains comprising the amino terminal two-thirds of the molecule showed no interaction (Figure 3, B and C). Direct two-hybrid analysis confirmed the interaction of the pericentrin C terminus with both GCP2 and GCP3 (Figure 3E) and failed to detect an interaction with γ tubulin or amino terminal domains of pericentrin (our unpublished data). In addition, domains of the larger isoform (pericentrin B) that included the GCP2/3 interacting region of pericentrin as well as an additional exon not present in pericentrin (66% identical, 78% similarity) did not interact with GCP2 (or GCP3) by immunoprecipitation (Figure 3G) or two-hybrid analysis (Figure 3F, see accession numbers gi458668 and gi31296687 for more details on sequence differences). Data from these two independent assays demonstrate that pericentrin interacts specifically with at least two members of the γ TuRC, GCP2 and GCP3. The C terminus of pericentrin seemed to bind GCP2 and GCP3 more efficiently than the full-length molecule. Similar binding patterns have been observed for other pericentrin-interacting proteins such as the dynein light intermediate chain, and they could result from increased accessibility to epitopes that are masked in the full-length protein (Tynan et al., 2000).
The C Terminus of Pericentrin Disassembles Mitotic Asters and Centrosomal γ Tubulin in Xenopus Extracts
Microtubule aster formation on nascent centrosomes of sperm nuclei in Xenopus extracts is dependent on the recruitment of soluble γ TuRCs to these sites (Felix et al., 1994; Stearns and Kirschner, 1994). Previous studies implicated pericentrin in this process (Dictenberg et al., 1998; Doxsey et al., 1994). To address this issue directly, we examined the effect of the GCP2/3 interacting domain of pericentrin on microtubule aster assembly in mitotic Xenopus extracts. Addition of this domain to extracts before the aster assembly reaction significantly reduced aster formation (Figure 4, A and B). Even after extended periods (30 min), few asters were detected, and they had few microtubules and were highly disorganized, a phenotype almost never observed in controls. Half maximal aster inhibitory activity was seen at a protein concentration ∼4:1 with endogenous pericentrin (Figure 4C). No change in aster assembly was observed in the presence of the pericentrin N terminus, heat-denatured C terminus, bovine serum albumin, or buffer alone (Figure 4D, 1–6). The activity seemed to be specific for mitotic extracts as there was no detectable effect on aster assembly in interphase extracts (Figure 4D, 7–9).
The mechanism of aster inhibition was examined in more detail by monitoring recruitment of γ tubulin onto nascent centrosomes in Xenopus mitotic extracts as described previously (Doxsey et al., 1994; Felix et al., 1994; Stearns and Kirschner, 1994). The pericentrin C-terminal domain and subdomains of this protein specifically inhibited recruitment of γ tubulin onto centrosomes to the same extent and at the same concentration that prevented microtubule aster assembly and disrupted the interaction between pericentrin and the γ TuRC (Figure 4, E–G). These results suggested that the pericentrin C terminus inhibited microtubule aster formation in mitotic extracts by preventing recruitment of γ tubulin to pericentrin sites on the nascent centrosome.
To more directly test whether pericentrin anchored γ TuRCs to nascent centrosomes, we examined the effect of the pericentrin C-terminal polypeptide on asters preassembled in extracts. Within 60 s after addition of the protein, the focus of microtubules in preassembled asters was disrupted, and free microtubules were observed in the region surrounding the aster (Figure 4, H–K). By 2 min after addition of the protein, most microtubules seemed to have lost their attachment to the centrosome; the remaining microtubules were of normal length and often formed bundles. By 3–5 min, no microtubules were detected at most centrosomes. In contrast, preassembled asters exposed to heat-inactivated pericentrin C terminus (Figure 4L), other pericentrin domains, the pericentrin B homology domain, or buffer alone showed no detectable loss of centrosomal microtubules, no change in microtubule organization, and few to no free microtubules in the vicinity of the aster. Pericentrin C-terminal peptide was just as effective at disrupting preexisting asters as it was at inhibiting their assembly, through a range of test concentrations. Pericentrin C terminal peptide caused loss of γ tubulin from preassembled centrosomes within the same time frame that it caused loss of microtubules from asters (90% reduction in 5 min). These results indicate that the pericentrin C terminus disrupts the interaction of γ TuRCs with centrosomes releasing the complexes and attached microtubules.
Microtubule asters can form in Xenopus extracts by a centrosome-independent pathway that requires the Ran GTPase (reviewed in Dasso, 2002). Ran-mediated aster assembly can be inhibited by a dominant negative form of Ran and enhanced by a dominant active form of the protein. Under conditions that resulted in rapid disassembly of mitotic asters, the pericentrin C terminus did not significantly affect Ran-mediated aster assembly even after extended periods of incubation (Figure 4, M and N). Thus, although formation of Ran asters requires γ tubulin (Wilde and Zheng, 1999), it seems to be less dependent on pericentrin than does centrosome-mediated aster assembly.
Mapping the GCP2/3 Binding Domain and Aster-disrupting Activity of Pericentrin
We further defined the pericentrin–GCP2/3 interaction site and made point mutants that inhibited the pericentrin–GCP2/3 interaction. Using directed two-hybrid and coimmunoprecipitation analyses, we identified a subdomain of the C terminus that was required for strong GCP2/3 binding in both assays (Figure 5A, consensus). We identified a point mutation in this domain with significantly reduced binding to GCP2 in vitro and lacked aster activity in Xenopus extracts. GCP2 and GCP3 bind cooperatively to pericentrin with in the consensus region because myc-tagged GCP2 showed cooperative binding to HA-tagged pericentrin in the presence of GCP3 (Figure 5C). In functional assays, pericentrin domains that bound GCP2/3 showed aster inhibitory activity in Xenopus extracts (Figure 5A). Those that did not interact with GCP2/3 lacked aster inhibitory activity, including the pericentrin mutant, a domain of pericentrin B containing all pericentrin sequences required for activity (Figure 5A), and several pericentrin domains outside the GCP2/3 interacting domain (Figure 5A, consensus). The strong correlation between regions of pericentrin that interacted with GCP2/3 and those that showed mitotic aster and γ TuRC-disrupting activity indicated that pericentrin was required for anchoring γ TuRCs to centrosomes in Xenopus mitotic extracts.
To further address differences in GCP2/3 binding between pericentrin (A) and pericentrin B, we excised most of an extra exon (and some additional sequences) that is present in the homologous region of pericentrin B. Truncated pericentrin B proteins lacking the amino acids encoded by these sequences had weak GCP2 binding activity (Figure 5, A and D), suggesting that pericentrin B binding to the γ TuRC in this region may be blocked by incorporation of an extra exon.
GCP2/3 Interacting Domains of Pericentrin Disrupt Mitotic Asters and Spindles in Vertebrate Cells
We next tested whether the GCP2/3-interacting domains of pericentrin affected microtubule organization in vertebrate cells. We found that these domains had no detectable effect on the organization or nucleation of microtubules or the organization of centrosomes in interphase SAOS cells (Figure 6A). However, the same domains disrupted microtubule structures in mitotic cells (Figure 6, B–K). The most common phenotype was monopolar spindles, which represented ∼15% of all mitotic cells at early times posttransfection (20–22 h) and increased to ∼90% at later times (44 h post-transfection, Figure 6, H, H′, and M). Most monopolar spindles had two duplicated and separated centrosomes. We also observed spindles with reduced numbers of centrosome-associated astral microtubules (Figure 6, C′, G, J, and K′), bipolar spindles with shortened pole-to-pole axes (Figure 6, C′ and G, minispindles) and half spindles with single focused poles (Figure 6, I–I″). In many spindles, we observed a decrease in the centrosome level of γ tubulin (Figure 6, E and I) and other centrosome proteins (Figure 6C), although the proteins were never reduced to undetectable levels. Pericentrin domains that bound both GCP2 and GCP3 induced the same defects, and those that did not interact had little or no effect (Figure 6, K–K″). Moreover, we were unable to detect aster inhibitory activity in Xenopus extracts (Figures 4D and 5A), disruption of spindle organization (Figure 6, M–M′) or apoptosis (below) associated with the homologous region of pericentrin B, suggesting that these two molecules may not be functionally analogous. Together, these results suggest that uncoupling of the pericentrin A–γ tubulin interaction in mitotic cells caused a reduction in the centrosome-associated γ TuRCs and disrupted astral microtubules and spindle organization, ultimately producing monopolar spindles.
Figure 6.
GCP2/3 binding domain of pericentrin affects astral microtubules and spindle organization in vertebrate cells. (A) Interphase cell expressing pericentrin 1680–1810 (inset, top right) shows no difference in microtubule organization compared with surrounding control cells (red, microtubules; blue, DNA stained with DAPI; yellow, 5051 centrosome staining). (B and B′) Control mitotic cell expressing β-galactosidase. (C and C′) Cell expressing pericentrin 1340–1920 and spindles with reduced astrals and pole-to-pole distance (C′, compare with B and B′). Insets at bottom right of B′, C′ show protein expression. (D) β-Galactosidase–expressing control cell. (E) Cell expressing pericentrin 1680–1810 shows reduced γ tubulin at spindle poles (compare with D). D and E, γ tubulin (red), DNA (blue), insets show 5051 staining. (F) β-Galactosidase–expressing cell. (G) Cell expressing 1680–1810 (inset, bottom right) shows reduced astral microtubules and decreased pole-to-pole distance compared with F. DNA, insets, left. Overexpressed protein, insets, right. (H) Monopolar spindle in cell expressing 1680–1810 showing centrosomes (yellow, 5051) and microtubules (H) or DNA (H′). (I) Spindle from cell expressing 1680–1810 with one tiny spindle pole (arrowhead, 5051) and unfocused microtubules at this pole (I′, merge, I″). (J) Telophase cell expressing 1680–1810 undergoing tripolar division. One nascent daughter cell lacks a centrosome (bottom). Images at right show protein expression (top), microtubules (middle) and centrosomes (bottom). (A–J) Immunofluorescence images of SAOS cells. All images except H and H′ are shown with deconvolution. Paired images were stained in parallel and collected on the same day, without modification to the laser or acquisition settings between images. (K–K″) Graphs showing percentage of transfected mitotic SAOS cells with total mitotic defects (K), monopolar spindles (K′), and reduced or absent astral microtubules (K″) 1–3, 20 h posttransfection; 4–6, 40 h posttransfection. 1, nontransfected mitotics, n = 368; 2, βgal, n = 82; 3, 1618–1810, n = 31; 4, Peri B 1826–2117; n = 95. 5, βgal, n = 39. 6; 1618–1810, n = 14. p values comparing βgal- and 1618–1810–expressing cells were calculated using the Student's t test for both time points. K, p < 0.0001 at 22 h; p < 0.0001 at 44 h. K′, p = 0.049 at 22 h; p < 0.0001 at 44 h. (L) Soluble pericentrin is more abundant in mitotic cells. Western blot of HeLa whole cell lysates from asynchronous cells (lane 1), from cells treated 4 h with nocodazole (lane 2), and from mitotic cells after shake-off (lane 3). Total protein loads were normalized for each lane using the Bio-Rad protein assay.
Overexpression of the GCP2/3 Binding Domain of Pericentrin and Reduction in Pericentrin Levels Both Induce G2/Antephase Delay and Apoptosis
During the course of these studies, we observed a marked reduction in cell density in cultures transfected with the GCP2/3 binding domain (Figure 7, A and A′). Typically, half the cells detached from their substrate by 44 h, whereas there was little change in cell density before protein expression (Figure 7A′, 20 h). To investigate this further, we examined cells for apoptosis and found that a significant fraction of the cells stained with an apoptosis-specific marker that detects a caspase 3 product of cytokeratin 18 produced early in apoptosis (Figure 7A″, M30); control cells showed low levels of M30 staining.
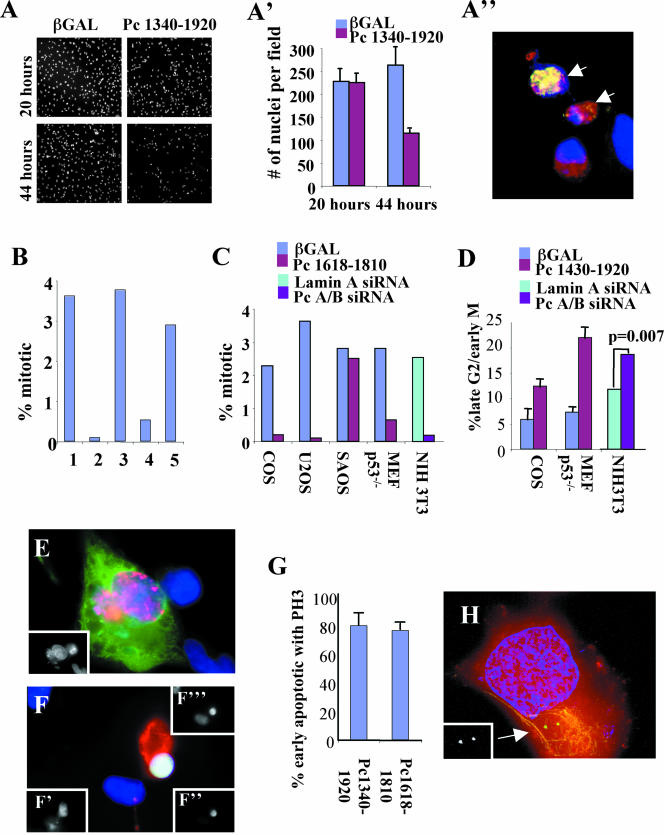
Figure 7.
Overexpression of GCP2/3 binding domain or silencing of Pc A/B induces cell cycle arrest and apoptosis at the G2/M phase of the cell cycle. (A) Cells expressing the GCP2/3 binding domain are lost through apoptosis. Low-magnification image of COS cells stained with DAPI showing cell loss when 1340–1920 is expressed for 44 h compared with nonexpressing cells (20 h) or β-galactosidase (βgal)–expressing cells. (A′) Quantification of cells in A (mean and SD, 10 fields). (A″) Image of COS cells from A stained for DNA (blue), 1340–1920 (red) and M30 cytodeath (green). Arrows, cells with both 1340–1920 and M30. (B) Mitotic index of U2OS cells expressing: 1, βgal; 2, 1618–1810 p < 0.001; 3, Pc B1826–2117 p = 0.437; 5, 1572–1816 p = 0.011; 6, 1572–1816m p = 0.226 (n = 1000 cells/bar at 40–44 h posttransfection). p values were calculated using the t-test relative to βgal controls). (C) Mitotic index in indicated cell types overexpression the indicated constructs or treated with siRNA. p values calculated as described above: COS, p < 0.001; U2OS, p < 0.001; SAOS, p = 0.479; Mefp53-/-, p = 0.001; NIH3T3 (siRNA), p = 0.0004. (D) Cells expressing GCP2/3 binding domain constructs or treated with Pc A/B siRNA have a greater proportion of late G2 cells. Shown are mean and SD of three experiments or p value based upon scoring from 1000 treated cells. Cells immunostained for overexpressed protein (green), phosphorylate histone H3 (PH3, red), and DAPI (blue). (E) Antephase cell overexpressing 1340–1920 (stains for phosphorylated histone H3 and does not show condensed chromatin). Inset, DAPI. (F) Apoptotic antephase cells expressing 1340–1920. M30 (red), phosphorylated histone H3 (green; inset, bottom right), DNA (blue; inset, above), overexpressed protein (inset, bottom left). (G) Graph showing that most early apoptotic cells expressing GCP2/3 binding domains stain for phosphorylated histone H3. Shown are mean, SD, n = 3 experiments. (H) Early apoptotic cell expressing 1340–1920 stained for centrosomes (5051, green), DNA (blue), HA pericentrin (red), M30, yellow. Inset 5051. Imaged as in Figure 5.
Apoptosis required that cells be actively cycling, because we did not detect apoptosis when cells were plated at high density to induce G1/G0 arrest during the period of protein expression. In cycling cells of several different origins, we observed a low mitotic index (Figure 7C) suggesting that cells were delayed at some point in the cell cycle. We found that cells accumulated in a premitotic stage based on their ability to stain for a form of histone H3 that is phosphorylated by aurora B in early mitotic cells (Swedlow and Hirano, 2003; Hans and Dimitrov, 2001); control cell staining was significantly lower (Figure 7, D and F). The cell cycle period between late G2 and mitosis (before chromosome condensation occurs) is termed antephase (Pines and Rieder, 2001). Antephase arrest was linked to apoptosis because most early mitotic cells (phospho-H3-positive) were also early apoptotic (Figure 7G, M30-positive). Moreover, most centrosomes in apoptotic cells seemed duplicated and separated (Figure 7H, two γ tubulin spots), consistent with cells in late G2 or early prophase.
To confirm the link between cell cycle arrest and cell death, we microinjected cDNA into nuclei of COS cells arrested in S phase by thymidine block. Approximately 8 h after release from the block cells entered mitosis. At this time, a significant proportion of cells expressing the GCP2/3 binding domain of pericentrin expressed the M30 antigen or detached from the substrate whereas control cells remained attached and often increased in number (Figure 8, A, B, and D). Cell loss was cell cycle specific because premitotic cycling cells or cells kept under S phase arrest remained viable and adherent (Figure 8C). These results suggested that uncoupling the pericentrin–γ TuRC interaction and disruption of astral microtubules induced apoptosis at the G2/M transition. (Figure 8C).
Figure 8.
COS cells expressing Pc1618–1810 undergo apoptosis during the G2/M transition. (A) Cells expressing 1618–1810 undergo apoptosis at mitosis. Microinjected cells released from a double thymidine block were stained for DNA (DAPI) and overexpressed protein (250 cells/bar). 1618–1810–expressing cells were apoptotic or detached 8–10 h after injection, whereas control cells (Pc B1826–2117) increased in number. At 14 h, p value comparing the two treatments, p < 0.0001 (B) Mitotic index of COS cells after release from double thymidine block as in A (peak, 9 h). (C) 1618–1810–expressing cells arrested in S phase do not undergo apoptosis. Microinjected cells retained in thymidine for the times indicated. No loss of cells was observed 8–14 h later. 10*, microinjected cells arrested in S phase for 6 h and then released from the block for 4 h (10 h total, p = 0.25). (D) Immunofluorescence image of an apoptotic cell expressing 1618–1810 at 10 h postinjection as in A. D (overlay), DNA (blue and upper inset), M30 (red), overexpressed protein (lower inset).
We reasoned that if apoptosis resulted from a cellular defect common to both overexpression and reduction of pericentrin, we should observe cell cycle arrest and apoptosis after pericentrin silencing. Significant cell death was in several cell types knocked down for pericentrin A and B at 48–72 h posttreatment (our unpublished data). Pericentrin A/B silencing also induced a significant increase in antephase, and a decrease in mitotic index 48–72 h after protein silencing (Figure 7, C and D). These provide further support for the idea that antephase arrest and apoptosis may be caused by disruption of the pericentrin–γ tubulin interaction.
DISCUSSION
Our previous results demonstrated that pericentrin and γ tubulin interacted in Xenopus extracts and that the proteins were in proximity at centrosomes in vertebrate cells, suggesting that they interacted at this site as well (Dictenberg et al., 1998). The additional data provided in this study show that pericentrin interacts with the γ TuRC via domains that bind GCP2 and GCP3 and that this interaction is important for microtubule organization in mitotic cells. The results of this study are consistent with our previous work showing that pericentrin overexpression induces severe spindle defects (Purohit et al., 1999). We propose a model in which pericentrin acts as a scaffold for anchoring γ TuRCs at mitotic centrosomes/spindle poles. This interaction seems to be required not only for astral microtubule organization but also for maintaining spindle bipolarity and for mitotic entry. The monopolar spindles and “minispindles” induced by disruption of the pericentrin-GCP2/3 interaction, indicate that pericentrin anchoring of γ TuRCs also may play a role in organizing microtubules of the central spindle.
Centrosomal Anchoring of γ TuRCs by Pericentrin Is Required for Mitotic Microtubule Aster Organization in Xenopus Extracts and Somatic Cells
Our results indicate that pericentrin anchoring of γ TuRCs at centrosomes is required for mitotic aster organization. If anchoring is disrupted, γ tubulin is dramatically depleted at mitotic centrosomes in Xenopus extracts and reduced at spindle poles in somatic cells. The more dramatic loss of centrosomal γ tubulin from Xenopus asters suggests that pericentrin plays a more dominant role in the organization of γ TuRCs at centrosomes in this system and perhaps in embryonic systems in general. We have not investigated the fate of γ TuRCs once dissociated from centrosomes, although one possibility is that they remain attached to the minus ends of microtubules where they could cap microtubule growth (Wiese and Zheng, 2000). In somatic cells, a fraction of γ tubulin remains at centrosomes/spindle poles under conditions that disrupt the GCP2/3-pericentrin interaction. This fraction could be anchored by other proteins that have been shown to bind γ TuRC components such as AKAP450, pericentrin B (Takahashi et al., 2002) Nlp (Casenghi et al., 2003), and centrosomin (Terada et al., 2003)
In this study, we map the GCP2/3 binding site of pericentrin to the C terminus of the protein, a region that shows no apparent homology to AKAP450, Spc110, Spc72, or CP309 (Kawaguchi and Zheng, 2003; Takahashi et al., 2002), although it is conserved between mouse, human, and rat (66–75% identical, 78–84% similarity). Whereas the amino terminus of pericentrin B binds GCP2 (Takahashi et al., 2002) (W. Zimmerman and S. Doxsey, unpublished observations), a similar region in the smaller pericentrin isoform does not, perhaps because it lacks exons found in pericentrin B. More information on the GCP2/3 interacting domain will require mapping these sites in all the GCP2 binding proteins.
The phenotype observed with the GCP2/3-pericentrin disrupting polypeptides and after pericentrin silencing is similar in many respects to that seen after functional abrogation of γ tubulin and other proteins of the γ TuRC. Under these conditions, centrosomes in Caenorhabditis elegans and Drosophila embryos were compromised in their ability to form mitotic asters (Hannak et al., 2002; Strome et al., 2001), separate from one another (Barbosa et al., 2003; Sampaio et al., 2001), and organize meiotic and mitotic spindles (Sunkel et al., 1995; Barbosa et al., 2000, 2003). It is of interest that mitotic asters in some of these systems formed in the absence of γ tubulin or other γ tubulin ring complex proteins (Strome et al., 2001; Hannak et al., 2002; Barbosa et al., 2003). This is in contrast to our results in Xenopus extracts where microtubule asters did not form in the presence of the pericentrin interacting domain of GCP2/3 even after extended periods (30 min). Moreover, preformed mitotic asters were rapidly disassembled after addition of this polypeptide. Future studies will be required to determine whether pericentrin and γ tubulin are more critical for mitotic aster formation in Xenopus extracts than in the other systems, or whether uncoupling γ tubulin from pericentrin prevents both γ tubulin-mediated microtubule nucleation and nucleation by a proposed γ tubulin-independent pathway (Hannak et al., 2002).
Pericentrin Is Not Essential for Assembly and Anchoring of γ TuRCs at Interphase Centrosomes
The GCP2/3-interacting pericentrin domains described in this study had no detectable effect on assembly of asters in interphase extracts prepared from Xenopus or in interphase somatic cells. Moreover, silencing of both isoforms also had no apparent effect on localization of γ tubulin at the centrosome or microtubule organization in interphase cells. This suggests that the protein does not play a major role in γ tubulin assembly or anchoring at interphase centrosomes but rather that the asterorganizing function of pericentrin is mitosis specific. Because both proteins are normally present at the centrosome throughout the cell cycle, we cannot conclude that they do not interact during interphase. Only that this specific interaction is not necessary for γ tubulin localization. It has been shown that γ tubulin and associated proteins are crucial for microtubule nucleation from interphase centrosomes (Joshi et al., 1992; Hannak et al., 2002). It is thus likely that proteins other than pericentrin provide microtubule-anchoring sites at centrosomes in interphase cells.
Other Proteins Involved in Centrosomal γ TuRC Anchoring and Microtubule Organization
Several other proteins play a role in centrosome organization and microtubule nucleation. However, their ability to directly anchor components of the γ TuRC and thus serve as molecular scaffolds for tethering these complexes to centrosomes has not been demonstrated. These include the centrosome proteins Asp (do Carmo Avides and Glover, 1999), NuMA (Merdes et al., 1996), TPX-2 (Wittmann et al., 2000; Garrett et al., 2002), SPD-5 (Hamill et al., 2002), PCM-1 (Dammermann and Merdes, 2002), Sas-4 (Kirkham et al., 2003) centrosomin (Megraw et al., 1999; Terada et al., 2003), and several regulatory molecules, including Aurora A (Hannak et al., 2001; Giet et al., 2002), Polo (Lane and Nigg, 1996; Barbosa et al., 2000), PP1 (Katayama et al., 2001), and PP4 (Sumiyoshi et al., 2002).
Some of these proteins play a critical role in a centrosome-independent spindle assembly pathway mediated by the Ran GTPase (see Dasso, 2002) including NuMA (Nachury et al., 2001; Wiese et al., 2001) and TPX-2 (Gruss et al., 2001). This is in contrast with pericentrin, which seems to be critical for assembly of mitotic asters but not Ran-mediated asters. In this regard, the proposed function of pericentrin in aster formation also differs from that of epsilon tubulin, which seems to be required for centrosome-independent but not centrosome-dependent microtubule aster formation (Chang et al., 2003). From this discussion, it seems that different molecules are required to organize asters in centrosome-dependent and -independent pathways as well as at different stages of the cell cycle.
Regulation of the Pericentrin–GCP2/3 Interaction
Pericentrin, γ tubulin, and γ tubulin-associated proteins are localized to centrosomes throughout the cell cycle (Stearns et al., 1991; Zheng et al., 1991; Dictenberg et al., 1998). However, the pericentrin–GCP2/3 interaction seems to be involved in γ TuRC anchoring only during mitosis. This suggests that the interaction of pericentrin and γ TuRCs is regulated. The mechanism and regulation of cell cycle-specific binding between these centrosome components is unknown. One model is that γ TuRCs are anchored to different centrosome scaffold proteins at different cell cycle stages and that these interactions are regulated in a cell cycle-dependent manner. For example, the γ TuRC binding activity of pericentrin could be regulated by phosphorylation by mitotic kinases. γ TuRC binding also could be regulated at least in part, through differential patterns of protein expression. Consistent with this idea is the observation that pericentrin, which is expressed primarily in mitosis and in tissues that are highly proliferative (Doxsey et al., 1994; Figure 6N), has a mitotic phenotype. Future experiments will be required to determine the contribution of these and other centrosome proteins in the anchoring of γ TuRCs to centrosomes at different cell cycle stages.
G2/Antephase Delay and Apoptosis
G2 accumulation of cells expressing the GCP2/3 binding domain of pericentrin or after silencing of pericentrin A/B suggests that disruption of the pericentrin γ TuRC interaction in vivo elicits a checkpoint response at this time in the cell cycle. Recent studies have implicated γ tubulin as well as the Spc110p homologue Pcp1p in regulation of the metaphase to anaphase transition (Prigozhina et al., 2004; Rajagopalan et al., 2004), but this is the first study suggesting a role for these or related molecules in regulation of mitotic entry. We do not yet know what this checkpoint may be monitoring. We favor a model in which the checkpoint senses spindle pole assembly/centrosome maturation because disruption of the γ tubulin–pericentrin interaction disrupts spindle pole assembly and possibly centrosome maturation, which increases in size fourfold between G2 and early prophase (Piehl et al., 2004), concurrent with the onset of γ tubulin mislocalization and antephase arrest that we observe.
Our results showing that pericentrin A/B silencing has no significant affect on interphase microtubule arrays confirms previous work (Dammermann and Merdes, 2002). In this earlier study, the authors did not address mitotic defects most likely because a G2 checkpoint is activated, apoptosis follows, and mitotic cells are rarely observed, a phenomenon that we have encountered in two of the cell lines used by these authors; U2OS and HeLa (Figure 7C; data not shown). In this study, we overcame this problem by using a cell line that that apparently lacks this checkpoint and fails to undergo apoptosis.
Apoptosis is commonly observed after checkpoint activation if a cellular imbalance cannot be repaired. We have not determined which molecular pathway is involved in the G2/antephase arrest identified in this study. DNA damage induces two molecularly distinct pathways involved in G2 arrest, one ATM dependent, the other ATM independent (Xu et al., 2002). Cellular insults other than DNA damage also can induce late G2 arrest, including microtubule disruption, hypothermia, fluoride treatment, and viral protein expression (Pines and Rieder, 2001; Tyler et al., 2001; Elder et al., 2002; Mikhailov and Rieder, 2002).
Antephase delay and subsequent apoptosis also can be activated through pathways that include p53 and Rb. Our data demonstrate that that p53 is not involved because primary MEFs lacking p53 retain the checkpoint response. SAOS cells, which do not arrest and apoptose have been reported to lack Rb (Scolnick and Halazonetis, 2000). We are currently testing the role of Rb in the checkpoint response. We also are investigating other mechanisms for inducing apoptosis. For example, apoptosis can be triggered by mislocalization of antiapoptotic signals from centrosomes (Li, 1998). γ Tubulin has recently been shown to associate with DAP-like kinase, which is implicated in apoptosis (Preuss et al., 2003). However, the role of γ tubulin mislocalization from centrosomes and induction of apoptosis through DAP-like kinase has not been explored. Mislocalization of survivin and other antiapoptotic proteins from centrosomes can induce apoptosis (Reed and Reed, 1999; Piekorz et al., 2002; Sandal et al., 2003). Additional studies will be required to identify the proteins and pathways involved in the apoptotic response observed after disruption of the pericentrin–GCP2/3 interaction.
Acknowledgments
We thank T. Stearns and S. Murphy for antibodies to GCP2 and 3 and for plasmids encoding GCP2, GCP3, and γ tubulin. We thank T.N. Davis for pericentrin B/kendrin-specific antibody. We also thank C. Wiese for constitutively active RanL43E This work was supported by National Institutes of Health GM-51994 (to S.J.D.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-11-0796. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-11-0796.
References
- Barbosa, V., Gatt, M., Rebollo, E., Gonzalez, C., and Glover, D.M. (2003). Drosophila dd4 mutants reveal that gammaTuRC is required to maintain juxtaposed half spindles in spermatocytes. J. Cell Sci. 116, 929-941. [DOI] [PubMed] [Google Scholar]
- Barbosa, V., Yamamoto, R.R., Henderson, D.S., and Glover, D.M. (2000). Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 14, 3126-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagden, S.P., and Glover, D.M. (2003). Polar expeditions–provisioning the centrosome for mitosis. Nat. Cell Biol. 5, 505-511. [DOI] [PubMed] [Google Scholar]
- Bobinnec, Y., Khodjakov, A., Mir, L.M., Rieder, C.L., Edde, C.L., and Bornens, M. (1998). Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 143, 1575-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casenghi, M., Meraldi, P., Weinhart, U., Duncan, P.I., Korner, R., and Nigg, E.A. (2003). Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev. Cell. 5, 113-125. [DOI] [PubMed] [Google Scholar]
- Chang, P., Giddings, Jr., T.H., Winey, M., and Stearns, T. (2003). Epsilontubulin is required for centriole duplication and microtubule organization. Nat. Cell Biol. 5, 71-76. [DOI] [PubMed] [Google Scholar]
- Dammermann, A., and Merdes, A. (2002). Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159, 255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso, M. (2002). The Ran GTPase: theme and variations. Curr. Biol. 12, R502-508. [DOI] [PubMed] [Google Scholar]
- Dictenberg, J., Zimmerman, W., Sparks, C., Young, A., Vidair, C., Zheng, Y., Carrington, W., Fay, F., and Doxsey, S.J. (1998). Pericentrin and gamma tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141, 163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo Avides, M., and D. M. Glover. (1999). Abnormal spindle protein, ASP, and the integrity of mitotic centrosomal microtubule organizing centers. Science 283, 1733-1735. [DOI] [PubMed] [Google Scholar]
- Doxsey, S.J. (2001). Re-evaluating centrosome function. Nat. Rev. Mol. Biol. 2, 688-699. [DOI] [PubMed] [Google Scholar]
- Doxsey, S.J., Stein, P., Evans, L., Calarco, P., and Kirschner, M. (1994). Pericentrin, a highly conserved protein of centrosomes involved in microtubule organization. Cell 76, 639-650. [DOI] [PubMed] [Google Scholar]
- Elder, R.T., Benko, Z., and Zhao, Y. (2002). HIV-1 VPR Modulates cell cycle G2/M transition through an alternative cellular mechanism other than the classic mitotic checkpoints. Front. Biosci. 7, d349-357. [DOI] [PubMed] [Google Scholar]
- Felix, M.-A., Antony, C., Wright, M., and Maro, B. (1994). Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol. 124, 19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory, M.R., and Davis, T.N. (2003). The centrosomal proteins pericentrin and kendrin are encoded by alternatively spliced products of one gene. Genomics 82, 401-405. [DOI] [PubMed] [Google Scholar]
- Flory, M.R., Morphew, M., Joseph, J.D., Means, A.R., and Davis, T.N. (2002). Pcp1p, an Spc110p-related calmodulin target at the centrosome of the fission yeast Schizosaccharomyces pombe. Cell Growth Differ. 13, 47-58. [PubMed] [Google Scholar]
- Flory, M.R., Moser, M.J., Monnat, Jr., R.J., and Davis, T.N. (2000). Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc. Natl. Acad. Sci. USA 97, 5919-5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, S., Auer, K., Compton, D.A., and Kapoor, T.M. (2002). hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr. Biol. 12, 2055-2059. [DOI] [PubMed] [Google Scholar]
- Giet, R., McLean, D., Descamps, S., Lee, M.J., Raff, J.W., Prigent, C., and Glover, D.M. (2002). Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 156, 437-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham, A.K., and Munro, S. (2000). The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 1, 524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley, A., Jurczyk, A., Sillibourne, J., Halilovic, E., Mogensen, M., Groisman, I., Blomberg, M., and Doxsey, S. (2003). A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 161, 535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss, O.J., Carazo-Salas, R.E., Schatz, C.A., Guarguaglini, G., Kast, J., Wilm, M., Le Bot, N., Vernos, I., Karsenti, E., and Mattaj, I.W. (2001). Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104, 83-93. [DOI] [PubMed] [Google Scholar]
- Hamill, D.R., Severson, A.F., Carter, J.C., and Bowerman, B. (2002). Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev. Cell 3, 673-684. [DOI] [PubMed] [Google Scholar]
- Hannak, E., Kirkham, M., Hyman, A.A., and Oegema, K. (2001). Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155, 1109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak, E., Oegema, K., Kirkham, M., Gonczy, P., Habermann, B., and Hyman, A.A. (2002). The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is gamma-tubulin dependent. J. Cell Biol. 157, 591-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Hans, F., and Dimitrov, S. (2001). Histone H3 phosphorylation and cell division. Oncogene 20, 3021-3027. [DOI] [PubMed] [Google Scholar]
- Job, D., Valiron, O., and Oakley, B. (2003). Microtubule nucleation. Curr. Opin. Cell Biol. 15, 111-117. [DOI] [PubMed] [Google Scholar]
- Joshi, H.C., Palacios, M.J., McNamara, L., and Cleveland, D.W. (1992). Gamma-tubulin is a centrosome protein required for cell cycle-dependent microtubule nucleation. Nature 356, 80-83. [DOI] [PubMed] [Google Scholar]
- Katayama, H., Zhou, H., Li, Q., Tatsuka, M., and Sen, S. (2001). Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle. J. Biol. Chem. 276, 46219-46224. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, S.-I., and Zheng, Y. (2003). Characterization of a Drosophila centrosome protein CP309 that shares homology with Kendrin and CG-NAP. Mol. Biol. Cell 15, 37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer, G., Di Fiore, B., Celati, C., Lechtreck, K.F., Mogensen, M., Delouvee, A., Lavia, P., Bornens, M., and Tassin, A.-M. (2003). Part of Ran is associated with AKAP450 at the centrosome: involvement in microtubule-organizing activity. Mol. Biol. Cell 14, 4260-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., Rieder, C.L., Sluder, G., Cassels, G., Sibon, O., and Wang, C.L. (2002). De novo formation of centrosomes in vertebrate cells arrested during S phase. J. Cell Biol. 158, 1171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham, M., Muller-Reichert, T., Oegema, K., Grill, S., and Hyman, A.A. (2003). SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell 112, 575-587. [DOI] [PubMed] [Google Scholar]
- Knop, M., and Schiebel, E. (1997). Spc98p and Spc97p of the yeast gammatubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 16, 6985-6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., and Schiebel, E. (1998). Receptors determine the cellular localization of a gamma-tubulin complex and thereby the site of microtubule formation. EMBO J. 17, 3952-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, H.A., and Nigg, E.A. (1996). Antibody microinjection reveals an essential role for human polo-like kinase (Plk1) in the functional maturation of centrosomes. J. Cell Biol. 135, 1701-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., Ambrosini, G., Chu, E.Y., Plescia, J., Tognin, S., Marchisio, P.C., and Altiere, D.C. (1998). Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 396: 580-584. [DOI] [PubMed] [Google Scholar]
- Li, Q., Hansen, D., Killilea, A., Joshi, H.C., Palazzo, R.E., and Balczon, R. (2001). Kendrin/pericentrin-B, a centrosome protein with homology to pericentrin that complexes with PCM-1. J. Cell Sci. 114, 797-809. [DOI] [PubMed] [Google Scholar]
- Marshall, W.F., Vucica, Y., and Rosenbaum, J.L. (2001). Kinetics and regulation of de novo centriole assembly. Implications for the mechanism of centriole duplication. Curr. Biol. 11, 308-317. [DOI] [PubMed] [Google Scholar]
- Martin, O.C., Gunawardane, R.N., Iwamatsu, A., and Zheng, Y. (1998). Xgrip 109, a gamma tubulin-associated protein with an essential role in gamma tubulin ring complex (gammaTuRC) assembly and centrosome function. J. Cell Biol. 141, 675-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw, T.L., Li, K., Kao, L.R., and Kaufman, T.C. (1999). The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development 126, 2829-2839. [DOI] [PubMed] [Google Scholar]
- Merdes, A., Ramyar, K., Vechio, J.D., and Cleveland, D.W. (1996). A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87, 447-458. [DOI] [PubMed] [Google Scholar]
- Mikhailov, A., and Rieder, C.L. (2002). Cell cycle: stressed out of mitosis. Curr. Biol. 12, R331-R333. [DOI] [PubMed] [Google Scholar]
- Moritz, M., Braunfeld, M.B., Sedat, J.W., Alberts, B., and Agard, D.A. (1995a). Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature 378, 638-640. [DOI] [PubMed] [Google Scholar]
- Moritz, M., Zheng, Y., Alberts, B.M., and Oegema, K. (1998). Recruitment of the g tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 142, 775-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S.M., Preble, A.M., Patel, U.K., O'Connell, K.L., Dias, D.P., Moritz, M., Agard, D., Stults, J.T., and Stearns, T. (2001). GCP5 and GCP 6, two new members of the human gamma-tubulin complex. Mol. Biol. Cell 12, 3340-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S.M., Urbani, L., and Stearns, T. (1998). The mammalian gammatubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J. Cell Biol. 141, 663-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, A.W. (1991). Cell cycle extracts. Methods Cell Biol. 36, 581-605. [PubMed] [Google Scholar]
- Nachury, M.V., Maresca, T.J., Salmon, W.C., Waterman-Storer, C.M., Heald, R., and Weis, K. (2001). Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104, 95-106. [DOI] [PubMed] [Google Scholar]
- Nguyen, T., Vinh, D.B., Crawford, D.K., and Davis, T.N. (1998). A genetic analysis of interactions with Spc110p reveals distinct functions of Spc97p and Spc98p, components of the yeast gamma-tubulin complex. Mol. Biol. Cell 9, 2201-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema, K., Wiese, C., Martin, O.C., Milligan, R.A., Iwamatsu, A., Mitchison, T.J., and Zheng, Y. (1999). Characterization of two related Drosophila gammatubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144, 721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl, M., Tulu, U.S., Wadsworth, P., and Cassimeris, L. (2004). Centrosome maturation: measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1. Proc Natl Acad Sci USA 101, 1584-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekorz, R.P., Hoffmeyer, A., Duntsch, C.D., McKay, C., Nakajima, H., Sexl, V., Snyder, L., Rehg, J., and Ihle, J.N. (2002). The centrosomal protein TACC3 is essential for hematopoietic stem cell function and genetically interfaces with p53 regulated apoptosis. EMBO Journal 21, 653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines, J., and Rieder, C.L. (2001). Re-staging mitosis: a contemporary view of mitotic progression. Nat. Cell Biol. 3, E3-E6. [DOI] [PubMed] [Google Scholar]
- Preuss, U., Bierbaum, H., Buchenau, P., and Scheidtmann, K.H. (2003). DAP-like kinase, a member of the death-associated protein kinase family, associates with centrosomes, centromeres, and the contractile ring during mitosis. Eur. J. Cell Biol. 82, 447-459. [DOI] [PubMed] [Google Scholar]
- Prigozhina, N.L., Oakley, C.E., Lewis, A.M., Nayaka, T., Osmani, S.A., and Oakley, B.R. (2004). γ-Tubulin plays an essential role in the coordination of mitotic events. Mol. Biol. Cell 15, 1374-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit, A., Pihan, G., and Doxsey, S. (2001). Methods for the study of pericentrin in centrosome assembly and function. Methods Cell Biol. 2001, 53-69. [DOI] [PubMed] [Google Scholar]
- Purohit, A., Tynan, S.H., Vallee, R., and Doxsey, S.J. (1999). Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 147, 481-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan, S., Bimbo, A., Balasubramanian, M.K., and Oliferenko, S. (2004). A potential tension-sensing mechanism that ensures timely antephase onset upon metaphase spindle orientation. Curr. Biol. 14, 69-74. [DOI] [PubMed] [Google Scholar]
- Reed, J.C., and Reed, S.I. (1999). Survivin'cell-separation anxiety. Nat. Cell Biology 1, E199-E200. [DOI] [PubMed] [Google Scholar]
- Sampaio, P., Rebollo, E., Varmark, H., Sunkel, C.E., and Gonzalez, C. (2001). Organized microtubule arrays in gamma-tubulin-depleted Drosophila spermatocytes. Curr. Biol. 11, 1788-1793. [DOI] [PubMed] [Google Scholar]
- Sandal, T., Aumo, L., Hedin, L., Gjertsen, B.T., and Doskeland, S.O. (2003). Irod/Ian 5, an inhibitor of γ-radiation- and okadaic acid-induced apoptosis. Mol. Biol. Cell 14, 3292-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick, D.M., and Halazonetis, T.D. (2000). Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature 406, 430-435. [DOI] [PubMed] [Google Scholar]
- Stearns, T., Evans, L., and Kirschner, M. (1991). Gamma tubulin is a highly conserved component of the centrosome. Cell 65, 825-836. [DOI] [PubMed] [Google Scholar]
- Stearns, T., and Kirschner, M. (1994). Reconstitution of centrosome assembly, role of gamma tubulin. Cell 76, 623-637. [DOI] [PubMed] [Google Scholar]
- Strome, S., Powers, J., Dunn, M., Reese, K., Malone, C.J., White, J., Seydoux, G., and Saxton, W. (2001). Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell 12, 1751-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi, E., Sugimoto, A., and Yamamoto, M. (2002). Protein phosphatase 4 is required for centrosome maturation in mitosis and sperm meiosis in C. elegans. J. Cell Sci. 115, 1403-1410. [DOI] [PubMed] [Google Scholar]
- Sunkel, C.E., Gomes, R., Sampaio, P., Perdigao, J., and Gonzalez, C. (1995). Gamma-tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO J. 14, 28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedlow, J.R., and Hirano, T. (2003). The making of the mitotic chromosome: modern insights into classical questions. Mol. Cell 11, 557-569. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Yamagiwa, A., Nishimura, T., Mukai, H., and Ono, Y. (2002). Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell 13, 3235-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin, A.M., Celati, C., Paintrand, M., and Bornens, M. (1997). Identification of an Spc110p-related protein in vertebrates. J. Cell Sci. 110, 2533-2545. [DOI] [PubMed] [Google Scholar]
- Terada, Y., Uetake, Y., and Kuriyama, R. (2003). Interaction of Aurora-A and centrosomin at the microtubule-nucleating site in Drosophila and mammalian cells. J. Cell Biol. 162, 757-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, K.L., Clarke, P., DeBiasi, R.L., Kominsky, D., and Poggioli, G.J. (2001). Reoviruses and the host cell. Trends Microbiol. 9, 560-564. [DOI] [PubMed] [Google Scholar]
- Tynan, S.H., Purohit, A., Doxsey, S.J., and Vallee, R.B. (2000). Light intermediate chain 1 defines a functional subfraction of cytoplasmic dynein which binds to pericentrin. J. Biol. Chem. 275, 32763-32768. [DOI] [PubMed] [Google Scholar]
- Wiese, C., Wilde, A., Moore, M.S., Adam, S.A., Merdes, A., and Zheng, Y. (2001). Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science 291, 653-656. [DOI] [PubMed] [Google Scholar]
- Wiese, C., and Zheng, Y. (2000). A new function for the gamma-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2, 358-364. [DOI] [PubMed] [Google Scholar]
- Wilde, A., and Zheng, Y. (1999). Stimulation of microtubule aster formation and spindle assembly by the small GTPase ran. Science 284, 1359-1362. [DOI] [PubMed] [Google Scholar]
- Wittmann, T., Wilm, M., Karsenti, E., and Vernos, I. (2000). TPX2, A novel Xenopus MAP involved in spindle pole organization. J. Cell Biol. 149, 1405-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, B., Kim, S., Lim, D., and Kastan, M.B. (2002). Two molecularly distinct G2/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 22, 1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., Jung, M.K., and Oakley, B.R. (1991). γ-Tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell 65, 817-823. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Wong, M.L., Alberts, B., and Mitchison, T. (1995). Nucleation of microtubule assembly by a gamma tubulin-containing ring complex. Nature 378, 578-583. [DOI] [PubMed] [Google Scholar]