Abstract
The intestinotrophic and cytoprotective actions of glucagon-like peptide-2 (GLP-2) are mediated by the GLP-2 receptor (GLP-2R), a member of the class II glucagon-secretin G protein-coupled receptor superfamily. Although native GLP-2 exhibits a short circulating half-life, long-acting degradation-resistant GLP-2 analogues are being evaluated for therapeutic use in human subjects. Accordingly, we examined the mechanisms regulating signaling, internalization, and trafficking of the GLP-2R to identify determinants of receptor activation and desensitization. Heterologous cells expressing the transfected rat or human GLP-2R exhibited a rapid, dose-dependent, and prolonged desensitization of the GLP-2–stimulated cAMP response and a sustained GLP-2–induced decrease in levels of cell surface receptor. Surprisingly, inhibitors of clathrin-dependent endocytosis failed to significantly decrease GLP-2R internalization, whereas cholesterol sequestration inhibited ligand-induced receptor internalization and potentiated homologous desensitization. The hGLP-2R localized to both Triton X-100–soluble and –insoluble (lipid raft) cellular fractions and colocalized transiently with the lipid raft marker caveolin-1. Although GLP-2R endocytosis was dependent on lipid raft integrity, the receptor transiently associated with green fluorescent protein tagged-early endosome antigen 1–positive vesicles and inhibitors of endosomal acidification attenuated the reappearance of the GLP-2R on the cell surface. Our data demonstrate that GLP-2R desensitization and raft-dependent trafficking represent distinct and independent cellular mechanisms and provide new evidence implicating the importance of a clathrin- and dynamin-independent, lipid raft-dependent pathway for homologous G protein-coupled receptor internalization.
INTRODUCTION
Glucagon-like peptide-2 (GLP-2) is a peptide hormone produced via posttranslational processing of proglucagon and is secreted from intestinal L-cells after nutrient intake (Drucker, 2001). GLP-2 stimulates crypt cell proliferation and inhibits apoptosis in the gut mucosal epithelium (Drucker et al., 1996; Tsai et al., 1997). The biological actions of GLP-2 are transduced by a single GLP-2 receptor (GLP-2R), a member of the class II glucagon-secretin G protein-coupled receptor (GPCR) superfamily (Munroe et al., 1999), which includes structurally related hormone receptors for peptides such as glucagon, GLP-1, and secretin (Mayo et al., 2003). The GLP-2R is expressed in human enteroendocrine cells (Yusta et al., 2000b), murine enteric neurons (Bjerknes and Cheng, 2001), and in specific regions of the central nervous system (Tang-Christensen et al., 2000; Lovshin et al., 2001). Agonist-induced activation of the GLP-2R is coupled to generation of cAMP (Yusta et al., 1999) and leads to enhanced cell survival via inhibition of the proapoptotic molecules GSK3 and Bad (Yusta et al., 2002).
GPCRs share a common regulatory pathway that governs agonist-induced desensitization and endocytosis. This pathway, originally described for many of the class I GPCR superfamily members, involves phosphorylation of receptor intracellular domains by G protein receptor kinases and binding of arrestin molecules. These actions in turn promote uncoupling of the GPCR from G proteins and effectors (desensitization) and subsequent internalization via clathrin-coated pits, where trafficking to acidic early endosomes leads to recycling of the resensitized GPCR back to the cell surface, or targets the receptor for degradation (for review, see Ferguson, 2001). However, recent studies suggest that GPCR trafficking and signaling may, in some instances, be regulated by clathrin-independent pathways (Malecz et al., 1998; Walker et al., 1999; Pao and Benovic, 2002).
Lipid raft endocytosis, initially characterized as an entry route for pathogens (Pelkmans et al., 2001), toxins (Montesano et al., 1982), extracellular ligands (Benlimame et al., 1998), and molecules such as glycosyl phosphatidylinositol-anchored proteins (Nichols et al., 2001) and sphingolipids (Puri et al., 2001), has recently been implicated as a potential pathway for GPCR endocytosis (Escriche et al., 2003; Olli-Lahdesmaki et al., 2003; Pawson et al., 2003; Venkatesan et al., 2003). Nevertheless, little is known about how GPCRs are trafficked and recycled via this mechanism or how this alternative internalization pathway influences GPCR desensitization.
Although both GLP-1 and GLP-2 are rapidly inactivated and exhibit short circulating half-lives in vivo, analogues of both GLP-1 and GLP-2 exhibiting prolonged pharmacokinetic profiles are currently being developed for the treatment of diabetes and intestinal disorders, respectively (Drucker, 2002). A recent study demonstrated that the cAMP response of cultured rat intestinal cells was diminished after pretreatment with degradation-resistant GLP-2, suggesting that GLP-2R desensitization may occur in vivo (Walsh et al., 2003). However, little is known about the mechanisms mediating class II GPCR desensitization after exposure to native or modified long-acting peptide ligands. Accordingly, we investigated the mechanisms mediating agonist-induced GLP-2R desensitization and trafficking in baby hamster kidney (BHK) fibroblasts and DLD-1 cells transfected with the rat or human GLP-2 receptors.
MATERIALS AND METHODS
Materials
Tissue culture medium was purchased from HyClone Laboratories (Logan, UT); fetal bovine serum (FBS), G418, and antibiotics were from Invitrogen (Carlsbad, CA). Forskolin, 3-isobutyl-1-methylxanthine (IBMX), forskolin, anti-FLAG (M1) antibody, anti-mouse IgG-agarose, mouse IgG, 2,2′-azino-bis(3-ethylbenthiazoline-6-sulfonic acid) reagent, protease inhibitor cocktail, phosphatase inhibitor cocktail I, filipin complex, methyl-β-cyclodextrin (MβCD), and sodium monensin were all obtained from Sigma-Aldrich (St. Louis, MO). Peptide N-glycanase F (PNGase F) and restriction enzymes were from New England Biolabs (Mississauga, Ontario, Canada). Anti-caveolin-1 (N20) antibody conjugated to fluorescein isothiocyanate (FITC) was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz Biotechnology (Santa Cruz, CA). The donkey anti-mouse-Cy3 antibody was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA), and the monoclonal anti-EEA1 antibody was obtained from BD Biosciences (San Diego, CA). The anti-GLP-2 receptor antibody has been described previously (Yusta et al., 2000b). Anti-mouse- and anti-rabbit-horseradish peroxidases (HRPs) were purchased from Amersham Biosciences (Baie d'Urfe, Quebec, Canada). The chemically synthesized human and rat glucagon-like peptide-2 (hGLP-2 or rGLP-2, respectively) were from Bachem California (Torrance, CA).
Plasmids
The expression vector pcDNA3.1 was obtained from Invitrogen (Burlington, Ontario, Canada). The pcDNA3.1-human GLP-2R (hGLP-2R) construct was a gift from NPS-Allelix (Mississauga, Ontario, Canada). A FLAG epitope-tagged human GLP-2R (FLAG-hGLP-2R) cDNA was generated using a strategy described previously (Guan et al., 1992) by ligating a synthetic oligonucleotide (ACTG, Toronto, Ontario, Canada) encoding a modified hemagglutinin (HA) signal peptide sequence upstream of the FLAG epitope (HA/FLAG) into a KpnI/BamHI-digested hGLP-2R cDNA plasmid. The authenticity of the FLAG-hGLP-2R cDNA was verified by automated sequencing (Core Molecular Biology Facility, York University, Ontario, Canada). Wild-type human dynamin (pcDNA 3.1-HA-DynI wt) was purchased from the American Type Culture Collection (Manassas, VA). The expression vector encoding the dominant-negative dynamin-I (pcDNA3.1-HA-DynK44A) was a kind gift from Dr. J.L. Benovic (Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA). The expression vector encoding the FLAG-β2-adrenergic receptor (pcDNA 3-FLAG-β2AR) was a kind gift from Dr. R. Lefkowitz (Howard Hughes Medical Institute, Duke University Medical Center, Durham, NC). The green fluorescent protein-tagged early endosome antigen 1 (GFP-EEA1) expression construct was a generous gift from Dr. S. Grinstein (University of Toronto, Toronto, Ontario, Canada).
Cell Culture
Baby hamster kidney fibroblasts (BHK-21) and human colon cancer epithelial (DLD-1) cells were obtained from American Type Culture Collection and maintained in DMEM supplemented with 10 and 5% FBS, respectively. BHK cells stably transfected with the rat GLP-2R (BHK:rGLP-2R) were propagated as described previously (Yusta et al., 1999). DLD-1 cells stably expressing the human GLP-2R were generated after transfection of the hGLP-2R construct by using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA) and selection with 1.2 mg/ml G418. A single clone (DLD-1:hGLP-2R) was used for all further experimental analysis and maintained in media supplemented with 0.5 mg/ml G418. Transient transfections of BHK or DLD-1 cells were carried out using FuGene (Roche Diagnostics, Quebec, Canada).
Homologous Desensitization Protocol and Determination of Intracellular cAMP Content
Experiments were performed using either stable cell lines or transient transfections, as indicated. To induce desensitization, cells were incubated with DMEM containing either 10 nM GLP-2 or vehicle (phosphate-buffered saline, PBS) for 20 min. After a brief wash with warm PBS, the cells were allowed to recover in DMEM containing 5% FBS for the indicated periods of time. The cells were then restimulated with media containing 0–500 nM GLP-2 and 100 μM IBMX for 10 min at 37°C. The reaction was stopped by a quick wash with PBS at 4°C followed by the addition of cAMP extraction buffer (77% ethanol in DMEM containing 100 μM IBMX). The cAMP concentration in the ethanol extracts was assessed by radioimmunoassay by using a commercially available kit (Biomedical Technologies, Stoughton, MA).
Measurement of Cell Surface and Total GLP-2 Receptor Levels
To assess levels of cell surface receptor, cells transiently transfected with either FLAG-hGLP-2R or FLAG-β2AR were fixed with 4% paraformaldehyde in PBS for 10 min at 4°C. Fixed cells were incubated at room temperature with FLAG (M1) antibody (1:2000) diluted in DMEM containing 10% FBS and 1 mM CaCl2 for 1 h and then with anti-mouse-HRP (1:1000) in DMEM (10% FBS/1 mM CaCl2) for 1 h. Cell-associated horseradish peroxidase activity was quantified by incubation at room temperature with 2,2′-azino-bis(3-ethylbenthiazoline-6-sulfonic acid) (1 mg/ml) in 0.1 M Na citrate, 0.1 M Na2HPO4, and 1 μl/ml H2O2 at pH 4.0. The reaction was stopped with 0.5% SDS/0.1% sodium azide, and the absorbance of the HRP reaction product measured at 405 nm.
To assess receptor internalization, transfected cells were incubated with agonist for the indicated periods of time before fixation. To assess receptor recycling, cells were incubated with agonist for 20 min at 37°C, washed briefly with warm PBS, and allowed to recover in DMEM supplemented with serum for the 30–180 min before fixation. To measure levels of total cellular FLAG-hGLP-2R, cells were permeabilized with 0.2% Triton X-100 in PBS for 10 min at room temperature after fixation and before incubation with the anti-FLAG (M1) antibody. For inhibitor studies, cells were pretreated with 4 μg/ml filipin, 10 mM MβCD, 0.5 M sucrose, or 25 μM monensin diluted in DMEM with serum 30 min before and during treatment with agonist. Cells were cotransfected with FLAG-hGLP-2R and either wild-type (wt) or mutant (K44A) dynamin in a 1:3 ratio 24 h before treatment. Wild-type dynamin-I was used as a negative control for all experiments involving Dyn K44A.
Immunofluorescence Microscopy
BHK cells grown in chamber slides (Nalge Nunc, Naperville, IL) and transiently transfected with FLAG-GLP-2R were incubated at 4°C with anti-FLAG (M1) antibody (1:350) for 1h in 25 mM HEPES, 1.2 mM MgSO4, 118 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4 buffer at pH 7.4 supplemented with 10% FBS and 0.2% glucose. After washing with PBS + 1 mM CaCl2, cells were incubated at 37°C for the indicated period of time in the absence (control) or presence of 10 nM hGLP-2 diluted in DMEM containing 10% FBS and 1 mM CaCl2, to induce receptor endocytosis, and immediately fixed with 4% paraformaldehyde + 1 mM CaCl2 at 4°C. After permeabilization with 0.2% Triton X-100/1 mM CaCl2 and blocking with 10% horse serum, 1% bovine serum albumin (BSA), 1 mM CaCl2, and 0.02% azide in Tris-buffered saline (TBS) for 1 h at room temperature, the cells were incubated overnight at 4°C with anti-caveolin-1-FITC (1:100) antibody diluted in 5% BSA/1 mM CaCl2 in TBS. Cells were washed with Tris-buffered saline (TBS)/1 mM CaCl2 and incubated with donkey anti-mouse-Cy3 diluted in 5% BSA in TBS/1 mM CaCl2. The slides were mounted using antifade mounting media (DakoCytomation Canada, Ontario, Canada) and visualized using an LSM 510 confocal microscope with a 63× oil immersion objective (Carl Zeiss, Thornwood, NY). For colocalization of FLAG-hGLP-2R and EEA1, cells were transiently cotransfected with FLAG-hGLP-2R and GFP-EEA1 before treatment and staining. For inhibitor studies, inhibitor was present in media 30 min before and during incubation with the M1 antibody, and during stimulation with agonist as described above.
Cell Fractionation and Western Blot Analysis
Lipid raft-enriched fractions were prepared from cells as described previously (Ma et al., 2003). Briefly, 48 h after transfection with either FLAG-hGLP-2R or expression vector alone (pcDNA3.1), cells were lysed on ice in 20 mM HEPES pH 7.2, 100 mM NaCl, containing 1% Triton X-100, 10% glycerol, protease inhibitor cocktail, phosphatase inhibitor cocktail I, 500 μM sodium orthovanadate, and 5 mM NaF. The lysate was spun at 16,000 × g for 10 min at 4°C, and the supernatant (Triton X-100–soluble fraction) was saved. The pellet was washed once with PBS at 4°C; resuspended in lysis buffer also containing 0.5% SDS; passed through a 30-gauge syringe; and sonicated (Triton X-100–insoluble fraction). Protein concentration was determined using the BCA protein assay kit (Pierce Chemical, Rockford, IL). Thirty micrograms of protein from both the Triton X-100–soluble and –insoluble fractions was deglycosylated with PNGase F (New England Biolabs), resolved by discontinuous SDS-PAGE, and electrotransferred to Hybond-ECL nitrocellulose membrane (Amersham Biosciences). Blots were incubated overnight at 4°C with either anti-EEA1 (1:2500), anti-GLP-2R (1:500), or anti-caveolin-FITC (1:1000) antibodies diluted in 5% milk in PBS containing 0.2% Tween 20. Antigen–antibody complexes were visualized with a secondary antibody conjugated to horseradish peroxidase and an enhanced chemiluminescence kit (Amersham Biosciences).
Immunoprecipitation of FLAG-hGLP-2R
Cells transfected with either FLAG-hGLP-2R, wild-type hGLP-2R, or pcDNA 3.1 constructs were lysed in PBS containing 1% Triton X-100, 1 mM CaCl2, and protease inhibitor cocktail. Five hundred micrograms of each protein lysate was precleared by incubation at 4°C for 1 h with anti-mouse IgG-agarose beads linked to 1 μg of mouse IgG in lysis buffer. Lysates were then incubated overnight at 4°C with anti-mouse IgG-agarose beads linked to 2 μg of anti-FLAG antibody. Beads were washed on ice with lysis buffer and immunoprecipitates were deglycosylated, resolved by SDS-PAGE, and immunoblotted as described above.
Statistical Analysis
Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by the Bonferroni multiple comparison post hoc test by using GraphPad Prism 3.03 (GraphPad Software, San Diego, CA).
RESULTS
Rapid, Dose- and Time-dependent Homologous Desensitization of the hGLP-2 Receptor
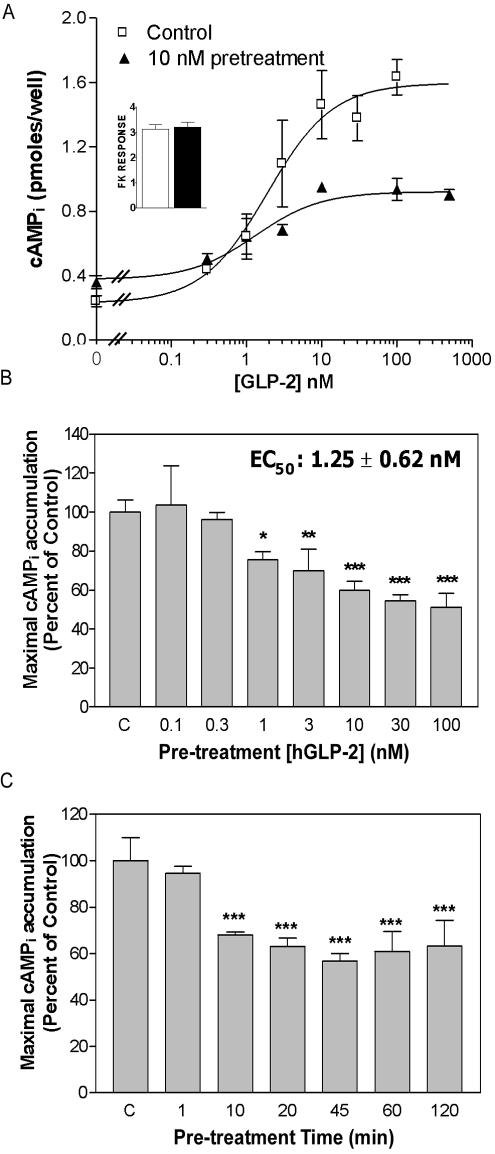
The GLP-2R has been localized to human enteroendocrine cells and enteric neurons; however, cell lines that express the endogenous GLP-2R have not yet been identified. Accordingly, as GLP-2 acts predominantly to stimulate cell growth in the mucosal epithelium, we chose to study receptor signaling and trafficking in a human epithelial colon cancer (DLD-1) cell line, and in BHK fibroblasts, an established cellular model for analysis of GLP-2 receptor signaling (Yusta et al., 1999; Yusta et al., 2000a; Yusta et al., 2002). Pretreatment of stably transfected DLD-1:hGLP-2R cells with GLP-2 caused a dose-dependent decrease in maximal intracellular cAMP (cAMPi) accumulation in response to subsequent hGLP-2 rechallenge (Figure 1, A and B), with no apparent shift in EC50 relative to nonpretreated cells (2.2 ± 0.7 nM vs. 1.8 ± 0.3 nM, n = 5 independent experiments). In contrast, hGLP-2 pretreatment did not affect forskolin-induced cAMP production (Figure 1A, inset). Maximal receptor desensitization, which corresponded to a 30–40% decrease in maximal agonist-induced cAMPi accumulation, was achieved with a 10 nM hGLP-2 pretreatment for 10 min (Figure 1, B and C, EC50 for desensitization was 1.25 ± 0.62 nM).
Figure 1.
Rapid, dose- and time-dependent homologous desensitization of the hGLP-2R in DLD-1 cells. (A) DLD-1 cells stably transfected with the hGLP-2R (DLD-1:hGLP-2R) were pretreated with vehicle (control, C) or 10 nM hGLP-2 for 20 min, allowed to recover for 30 min, and then rechallenged with 0–500 nM hGLP-2 before intracellular cAMPi measurement. The pretreatment agonist concentrations (B) or times (C) were varied, as shown, and the cells were rechallenged with 100 nM GLP-2 after a 30-min recovery to determine maximal agonist-induced cAMPi accumulation. cAMP concentrations were determined by radioimmunoassay as indicated in MATERIALS AND METHODS. Data are means ± SD from triplicate values and are representative of three to four independent experiments. In B and C, data were normalized to the control (C) values. The control value represents hGLP-2-induced cAMPi accumulation in cells pretreated with vehicle before rechallenge. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control values.
Persistent Desensitization of the GLP-2R
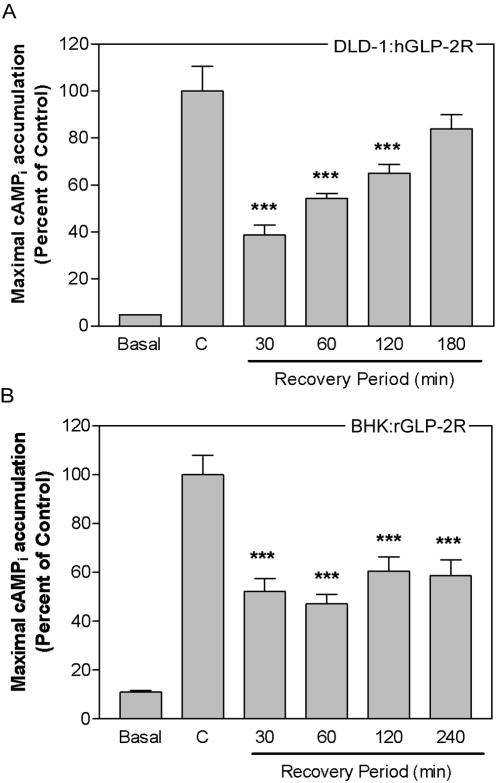
To assess the ability of cells to regain responsiveness to agonist after homologous desensitization, desensitization and resensitization kinetics were studied using DLD-1 and BHK fibroblasts, to ensure that the results obtained were not cell line specific. Recovery of the hGLP-2-induced cAMP response of the human (Figure 2A) or rat (our unpublished data) GLP-2R in DLD-1 cells required more than 2 h, a recovery time significantly longer than that observed for the related GLP-1 receptor (Gromada et al., 1996). Similarly, no resensitization to agonist was detectable up to 4 h after GLP-2 pretreatment in BHK cells expressing the rat (Figure 2B) or human (our unpublished data) GLP-2R.
Figure 2.
Persistent desensitization of the GLP-2 receptor is not species or cell type specific. DLD-1:hGLP-2R cells (A) or BHK cells stably transfected with the rGLP-2R (BHK:rGLP-2R) (B) were incubated with either 10 nM human (A) GLP-2, 10 nM rat (B) GLP-2, or vehicle (control, C) for 20 min; allowed to recover for the indicated periods of time; and then rechallenged with 100 nM GLP-2 or media alone (basal) for 10 min to determine maximal agonist-induced cAMPi accumulation. Data (means ± SD from triplicate values) were normalized and compared with control (C) (*p < 0.05, **p < 0.01, ***p < 0.001) as described in Figure 1 and are representative of two to four independent experiments.
Rapid Agonist-induced Internalization Followed by Slow Cell Surface Reappearance of the GLP-2R
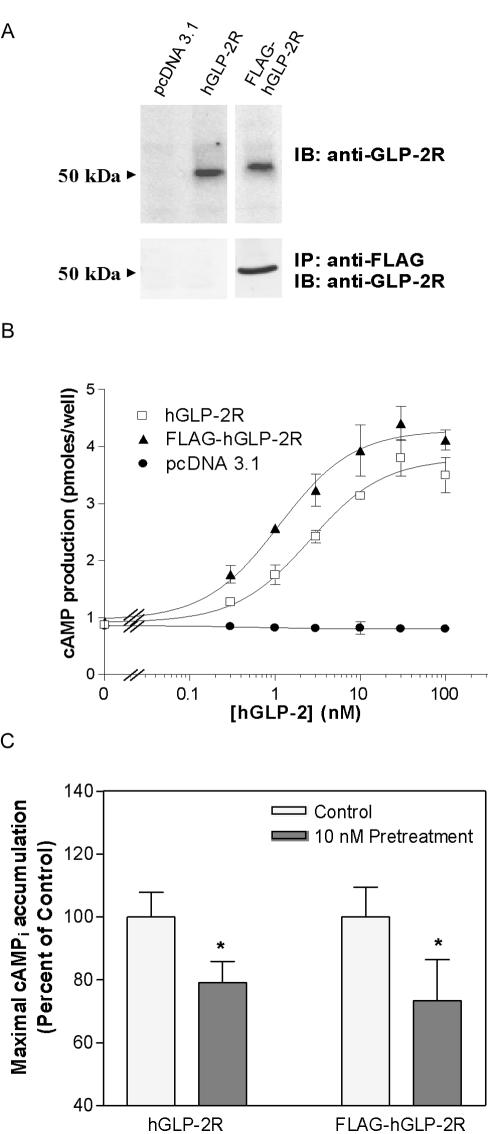
To address whether ligand-induced endocytosis and/or receptor degradation was responsible for the persistent loss of receptor responsiveness, we monitored the trafficking of a FLAG-tagged hGLP-2R in BHK and DLD-1 cells. The FLAG epitope-tagged receptor (FLAG-hGLP-2R) was expressed at comparable levels to the wild-type hGLP-2R in BHK cells (Figure 3A). Furthermore, the activity of the FLAG-hGLP-2R mirrored the wild-type receptor, with comparable profiles of both GLP-2–stimulated cAMP accumulation and homologous desensitization demonstrated in BHK and DLD-1 cells, respectively (Figure 3, B and C).
Figure 3.
An N-terminal FLAG-epitope does not alter hGLP-2-induced cAMP accumulation or desensitization of the hGLP-2R. (A) BHK cells were transiently transfected with either vector alone (pcDNA 3.1), untagged wild-type human GLP-2R (hGLP-2R), or FLAG-epitope–tagged human GLP-2R (FLAG-hGLP-2R). PNGase F-deglycosylated total cell extracts from BHK cells transiently transfected with the indicated constructs were analyzed by immunoblotting (IB) for the GLP-2R as described in MATERIALS AND METHODS. Alternatively, anti-FLAG antibody immunoprecipitates (IP) from the transfected cell extracts were subjected to deglycosylation before Western blot analysis to detect the GLP-2R. (B) Receptor activation was assessed by monitoring cellular cAMP production in response to hGLP-2 in transiently transfected BHK cells. Data were fit to a sigmoidal dose-response curve by using GraphPad Prism software. (C) DLD-1 cells transiently transfected with either the hGLP-2R or the FLAG-hGLP-2R constructs, were pretreated with vehicle or 10 nM hGLP-2, allowed to recover for 30 min, and then rechallenged with 100 nM hGLP-2 to determine maximal agonist-induced cAMPi accumulation. Data (means ± SD from triplicate values) were normalized and compared with control (*p < 0.05) as described in Figure 1.
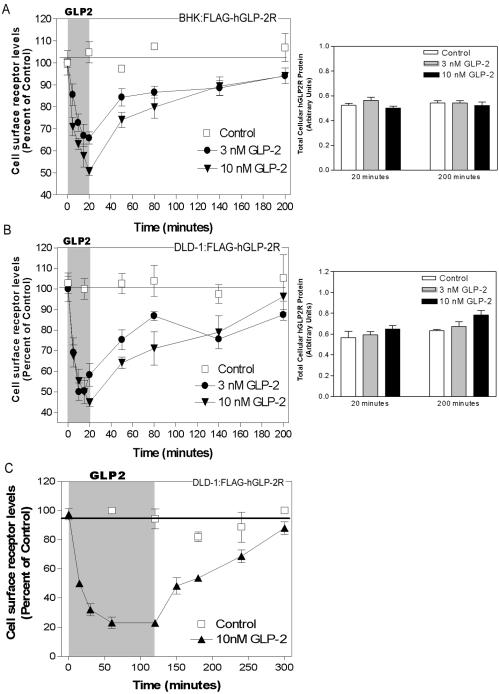
Exposure of BHK or DLD-1 cells to GLP-2 for 5–20 min produced a rapid, dose- and time-dependent disappearance of hGLP-2R from the cell surface (Figure 4, A and B). Maximal receptor internalization (∼75%) was evident ∼60 min after exposure to 10 nM GLP-2 in DLD-1 cells (Figure 4C). Surprisingly, despite the prolonged desensitization kinetics demonstrated for the GLP-2R in BHK cells (Figure 2B), 80–90% of the internalized hGLP-2R reappeared on the cell surface of both BHK (Figure 4A) and DLD-1 (Figure 4B) cells within 3 h. Hence, trafficking of the hGLP-2R back to the cell surface after internalization is not sufficient for receptor resensitization. Furthermore, receptor degradation does not contribute to acute GLP-2R desensitization, because no change in the total amount of receptor protein was detected in BHK or DLD-1 cells either immediately after the 20-min treatment with hGLP-2 or 3 h after removal of the peptide (Figure 4, A and B). Moreover, exposure of DLD-1 cells to hGLP-2 for up to 2 h did not impair subsequent recovery of cell surface receptor (Figure 4C) nor decrease total cellular receptor content (our unpublished data).
Figure 4.
Rapid agonist-induced hGLP-2R internalization is followed by slow cell surface receptor reappearance. BHK (A) or DLD-1 (B and C) cells transiently transfected with FLAG-hGLP-2R were treated with vehicle (control), 3 nM, or 10 nM hGLP-2 for either up to 20 min (A and B) or 2 h (C) to assess hGLP-2R internalization. After the hGLP-2 treatment, cells were washed briefly to remove the agonist and incubated for the indicated times in media with serum to assess hGLP-2R reappearance on the cell surface (left). Cell surface receptor levels were measured using an enzyme-linked immunoassay as described in MATERIALS AND METHODS. Data (means ± SD of triplicate values) are expressed as percentage of the cell surface receptor level at time 0. Similar results were obtained in two independent experiments for each cell type. Total cellular levels of FLAG-hGLP-2R in BHK cells (A, right) and DLD-1 cells (B, right) at the end of the internalization (20 min), and washout (200-min) periods were measured as indicated above, after permeabilization of the cells with 0.2% Triton X-100. Data are means ± SD of triplicate values (n = 2 independent experiments for each cell line).
hGLP-2R Internalizes via a Clathrin-independent Mechanism
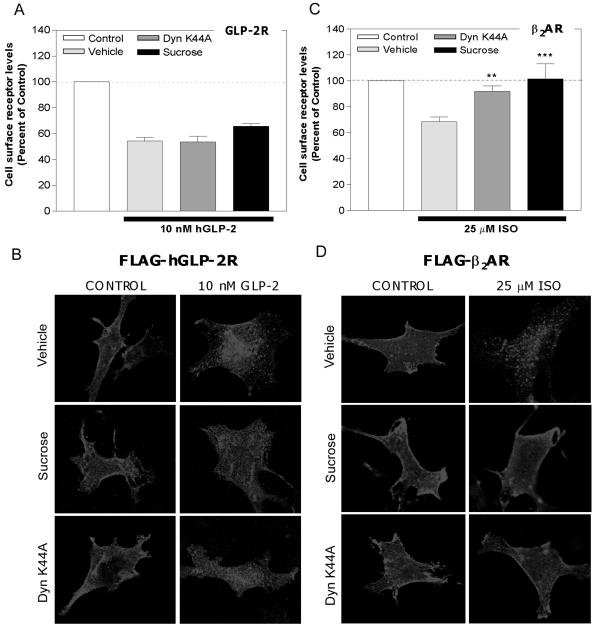
GPCR endocytosis commonly occurs via clathrin-coated pits after ligand activation (Ferguson et al., 1998). Surprisingly, hGLP-2R endocytosis was not significantly inhibited after pretreatment of DLD-1 cells with hypertonic (0.5 M) sucrose, or after cotransfection of a dominant-negative dynamin-1 cDNA (DynK44A), two commonly used inhibitors of clathrin-dependent endocytosis (Figure 5, A and B). However, under similar conditions, these inhibitors abrogated internalization of the β2AR, a GPCR known to internalize via coated pits (Figure 5, C and D). Confocal microscopy of immunofluorescently stained FLAG-tagged receptors in BHK cells indicated that although the β2AR rapidly internalized into small, distinct vesicles, GLP-2R internalization was characterized by a loss of receptor density in the plasma membrane and a gradual localized perinuclear accumulation (Figures 5, A and B, and 6B). BHK cells were chosen to visualize receptor trafficking because they consistently exhibited high transfection efficiency and grew well on glass slides. Wild-type dynamin-I was used to control for Dyn K44A overexpression and had no effect on hGLP-2R internalization (our unpublished data).
Figure 5.
hGLP-2R internalization is not prevented by inhibition of clathrin-dependent endocytosis. DLD-1(A and C) or BHK (B and D) cells transiently transfected with either the FLAG-hGLP-2R (A and B) or FLAG-β2AR (C and D) were pretreated with either 0.5 M sucrose or vehicle for 30 min before and during a 20-min incubation with media alone (control), 10 nM hGLP-2 (A and B) or 25 μM isoproterenol (ISO) (C and D). The wild-type or dominant-negative dynamin (Dyn K44A) cDNAs were cotransfected with the corresponding receptor cDNAs 24 h before agonist treatment. Cell surface receptor levels were quantified in A and C by enzyme-linked immunoassay as described in MATERIALS AND METHODS. Data (means ± SEM of two to three independent experiments each performed in triplicate or quadruplicate) are expressed relative to control and compared with vehicle-treated cells (**p < 0.01, ***p < 0.001). The normalized control value represents cell surface receptor levels in cells exposed to each of the indicated inhibitors or vehicle, but not to agonist. (B and D) After a 30-min inhibitor treatment at 37°C, transiently transfected BHK cells were incubated with anti-FLAG antibody on ice for 1 h in the presence of vehicle or sucrose, as described in MATERIALS AND METHODS. After a wash with PBS, cells were treated and either media alone (control), 10 nM hGLP-2, or 25 μM ISO at 37°C for 20 min in the absence (vehicle) or presence of inhibitors. After fixation and permeabilization, the receptor/anti-FLAG antibody complexes were visualized by confocal microscopy by using a Cy3-conjugated secondary antibody.
Figure 6.
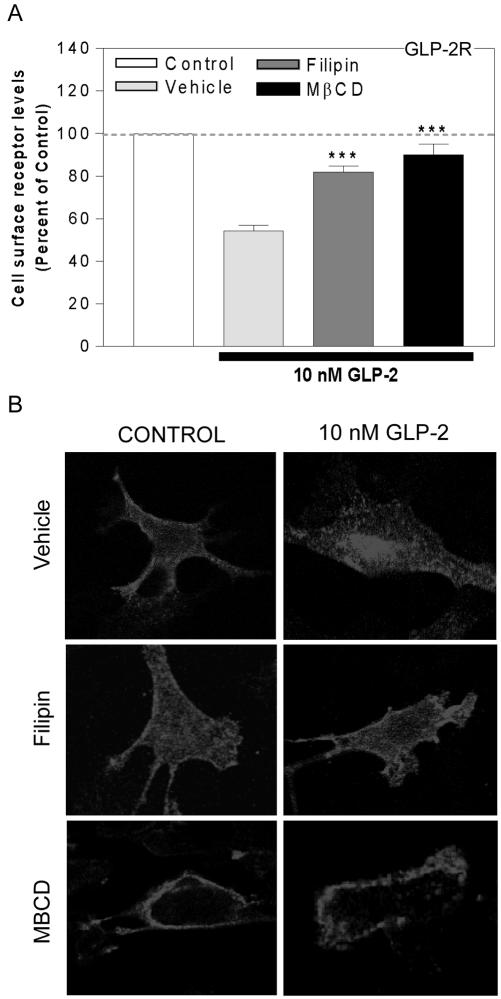
hGLP-2R endocytosis is inhibited by disruption of lipid raft integrity. DLD-1(A) or BHK (B) cells transiently transfected with the FLAG-hGLP-2R were pretreated with either 4 μg/ml filipin, 10 mM MβCD, or vehicle for 30 min before and during a 20-min incubation with media alone (control) or 10 nM hGLP-2. Cell surface receptor levels were quantified by enzyme-linked immunoassay as described in MATERIALS AND METHODS. Data (means ± SEM of 3 independent experiments each performed in triplicate or quadruplicate) are expressed relative to control and compared with vehicle-treated cells (***p < 0.001). The normalized control value represents cell surface receptor levels in cells exposed to each of the indicated inhibitors or vehicle, but not to agonist. (B) After a 30-min inhibitor treatment at 37°C, transfected BHK cells were incubated with anti-FLAG antibody on ice for 1 h in the presence of vehicle, filipin, or MβCD, as described in MATERIALS AND METHODS. After a wash with PBS, cells were treated with media alone (control) or 10 nM hGLP-2 at 37°C for 20 min in the absence (vehicle) or presence of inhibitors. After fixation and permeabilization, the receptor/anti-FLAG antibody complexes were visualized by confocal microscopy using a Cy3-conjugated secondary antibody.
hGLP-2R Internalization Is Dependent on Lipid Raft Integrity
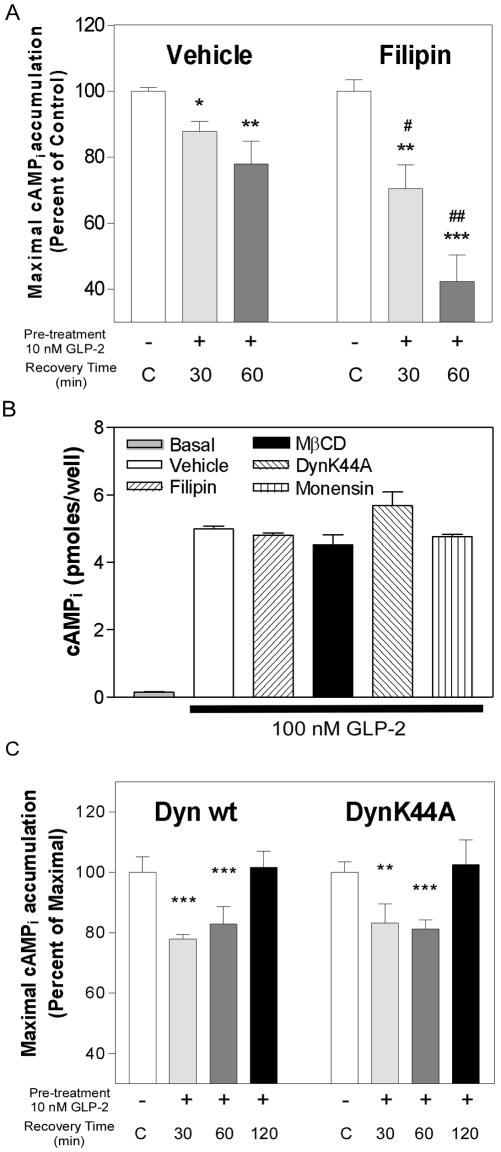
Cholesterol sequestration with filipin or cholesterol depletion with MβCD significantly inhibited agonist-induced hGLP-2R internalization, suggesting that hGLP-2R endocytosis proceeds via a lipid raft-mediated pathway in BHK and DLD-1 cells (Figure 6, A and B). Neither treatment with the clathrin-coated pit inhibitors nor cholesterol disruption affected hGLP-2-induced cAMP accumulation (Figure 8B; see below), indicating that this downstream signaling pathway was not perturbed by inhibitor treatment and that GLP-2-stimulated adenylyl cyclase activation occurs independently of GLP-2R internalization.
Figure 8.
Disruption of lipid raft integrity potentiates homologous desensitization of the hGLP-2R. (A) DLD-1:hGLP-2R cells were incubated with either vehicle or 4 μg/ml filipin for 30 min before and during a 20-min incubation in the absence (-) or presence of 10 nM hGLP-2 (+). This was followed by recovery in media alone for the indicated periods of time and assessment of maximal agonist-induced cAMPi accumulation. The controls (C, white bars) represent normalized data for cells pretreated with media alone and allowed to recover for each of the times shown in the presence or absence of inhibitor. (B) To assess the effect of the inhibitors on agonist-induced cAMP production, transfected DLD-1 cells were incubated with either vehicle or the indicated inhibitor for 80 min (30 min for MβCD) before and during a 10-min incubation in the absence (basal) or presence of 100 nM hGLP-2. (C) Desensitization/recovery experiments, as described in A, were performed on DLD-1 cells 24 h after cotransfection with FLAG-hGLP-2R and either wild-type dynamin-I (Dyn wt) or the dominant-negative mutant (Dyn K44A). Data (means ± SD of triplicate values) are representative of three independent experiments and are expressed relative to their corresponding control. Statistical comparisons between recovery time and corresponding control values are indicated with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Statistical significance between recovery time points in the presence or absence of inhibitors is represented by #p < 0.05, ##p < 0.01.
The hGLP-2R Transiently Colocalizes with the Lipid Raft Marker Caveolin-1
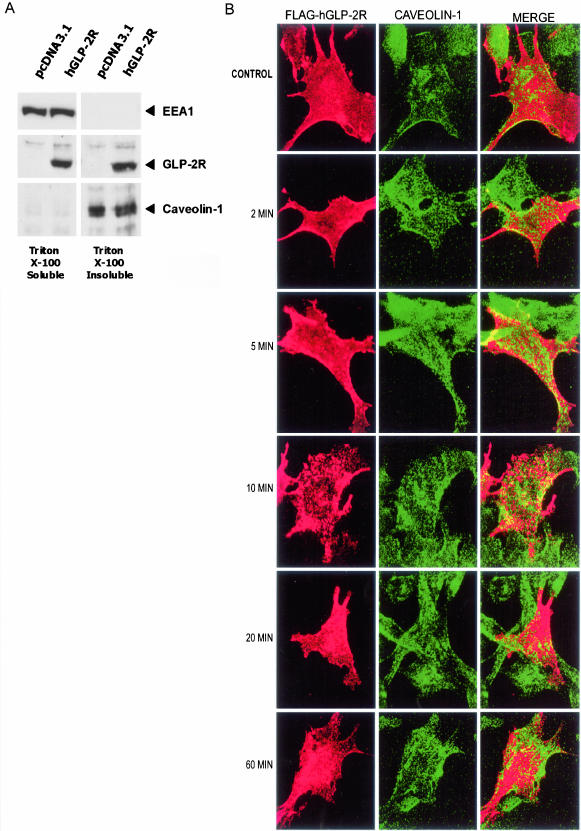
We next assessed whether the hGLP-2R associates with markers of the lipid raft endocytotic pathway. Because lipid rafts and lipid raft-associated proteins are insoluble in Triton X-100 at 4°C (Brown and Rose, 1992; Hooper, 1999), we isolated Triton-soluble and -insoluble protein fractions of transfected DLD-1 (Figure 7A) or BHK cells (our unpublished data). The FLAG-hGLP-2R protein was detected in both Triton X-100–soluble and –insoluble fractions by Western blot analysis (Figure 7A). Antibodies against caveolin-1, a palmitoylated integral membrane protein specific for the lipid raft cellular fraction (Nabi and Le, 2003), and EEA1 were used to verify the quality of the differential fractions isolated using detergent extraction (Simons and Toomre, 2000).
Figure 7.
A pool of the hGLP-2R is associated with the cellular detergent insoluble fraction and transiently colocalizes with caveolin-1 in transfected cells. (A) DLD-1 cells transiently transfected with FLAG-hGLP-2R or pcDNA3.1 were lysed in a 1% Triton X-100 containing buffer as described in MATERIALS AND METHODS. After centrifugation, the supernatant (Triton X-100–soluble fraction) was recovered, and the pellet was solubilized in the same buffer also containing 0.5% SDS (Triton X-100–insoluble fraction). Aliquots of the Triton X-100–soluble and –insoluble fractions were deglycosylated, and analyzed by immunoblotting for EEA1, hGLP-2R, and caveolin-1. Antigen/antibody complexes were visualized by enhanced chemiluminescence after incubation with a horseradish peroxidase-conjugated secondary antibody. (B) BHK cells transiently expressing the FLAG-hGLP-2R were prelabeled on ice with anti-FLAG antibody and then incubated with media alone (control) or 10 nM hGLP-2R at 37°C for 0–60 min as described in MATERIALS AND METHODS. After fixation and permeabilization, cells were incubated with anti-caveolin-1-FITC. A Cy3-conjugated secondary antibody was used to visualize hGLP-2R/anti-FLAG antibody complexes. Confocal microscopy images are representative of two to three independent experiments.
Fluorescent costaining of FLAG-hGLP-2R and caveolin-1 in BHK cells revealed that, in the absence of hGLP-2, only plasma membrane located hGLP-2R costained with caveolin-1, confirming that the receptor colocalized with lipid raft domains on the cell surface (Figure 7B). A time course of receptor internalization revealed that hGLP-2R colocalization with caveolin-1 increased transiently after a 5- to 10-min stimulation with hGLP-2. GLP-2 increased plasma membrane colocalization of caveolin-1 and the GLP-2R, and vesicles containing both proteins were detected in the cytosol. However, 20 min after addition of ligand, GLP-2 receptor and caveolin-1 colocalization diminished, with each protein being found predominantly in separate cellular compartments after 60 min (Figure 7B).
Sequestration of Cellular Cholesterol Potentiates hGLP-2R Homologous Desensitization
Ligand-induced decreases in the levels of cell surface receptor, due to internalization and/or degradation, might explain the decrease in maximal cAMP accumulation observed after hGLP-2 pretreatment. However, for receptors that undergo clathrin-mediated endocytosis, desensitization and internalization are not obligatorily linked processes, with endocytosis and trafficking of GPCRs through acidic compartments representing a mechanism for receptor resensitization (Ferguson, 2001). Because the hGLP-2R was not significantly degraded after hGLP-2 treatment (Figure 4), we analyzed receptor desensitization after inhibition of hGLP-2R endocytosis with filipin. Consistent with the belief that internalization serves to resensitize GPCRs to ligand, filipin potentiated homologous desensitization in DLD-1 cells (Figure 8A), without affecting the ability of the receptor to activate downstream signaling to adenylyl cyclase (Figure 8B). Recovery from homologous hGLP-2R desensitization could not be assessed at later time points, because the cells did not tolerate longer treatments with filipin. To further demonstrate that hGLP-2R desensitization and resensitization occurred independently of dynamin-I, we assessed hGLP-2R resensitization in cells cotransfected with FLAG-hGLP-2R and either the wild-type or dominant-negative dynamin-I (K44A) constructs (Figure 8C). Consistent with the data that dynamin-I does not regulate hGLP-2R endocytosis (Figure 5, A and B), hGLP-2R homologous desensitization and subsequent resensitization proceeded similarly in cells transfected with the wild-type or mutant dynamin constructs (Figure 8C).
Inhibition of Endosomal Acidification Prevents hGLP-2R Recycling
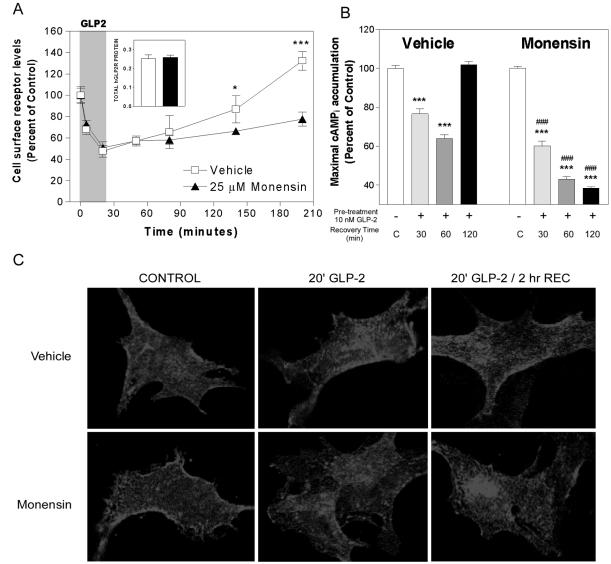
To determine the cellular pathways important for hGLP-2R postendocytotic trafficking, DLD-1 cells were treated with monensin, an ionophore that prevents receptor recycling from early endosomes by neutralizing intralumenal pH (Basu et al., 1981). Monensin did not alter the kinetics of hGLP-2R endocytosis or change the total cellular level of hGLP-2R protein; however, it prevented reappearance of the receptor on the cell surface of DLD-1 (Figure 9A) and BHK cells (our unpublished data). These data suggest that hGLP-2R trafficking involves a pH-sensitive intracellular compartment.
Figure 9.
Inhibition of endosomal acidification prevents receptor recycling and resensitization. (A) DLD-1 cells transiently transfected with FLAG-hGLP-2R were pretreated with vehicle or 25 μM monensin for 30 min before and during assessment of hGLP-2R internalization and cell surface reappearance as described in Figure 4. Data (means ± SD of triplicate values) are expressed as percentage of the cell surface receptor level at time 0. *p < 0.05, ***p < 0.001, vehicle vs. monensin treated. Inset, total cellular receptor protein following the hGLP-2 washout period (200 min) in vehicle- (open bar) and monensin (filled bar)-treated cells. (B) hGLP-2R resensitization, as described in Figure 8, was performed in the absence (vehicle) and presence of monensin. Data (means ± SD of triplicate values) are representative of three independent experiments and are expressed relative to their corresponding control. Statistical comparisons between recovery time and corresponding control values are indicated with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Statistical significance between recovery time points in the presence or absence of inhibitors is represented by ### p < 0.001. (C) After a 30-min treatment with vehicle or monensin at 37°C, transfected BHK cells were incubated with anti-FLAG antibody on ice for 1 h media containing vehicle or monensin, as described in MATERIALS AND METHODS. Cells were treated with either media alone (control) or 10 nM hGLP-2 at 37°C for 20 min, washed, and allowed to recover for 0 or 2 h at 37°C in the absence (vehicle, top) or presence of monensin (bottom). After fixation and permeabilization, the receptor/anti-FLAG antibody complexes were visualized by confocal microscopy using a Cy3-conjugated secondary antibody.
To characterize the role of postendocytotic trafficking in hGLP-2R resensitization, we next inhibited hGLP-2R recycling from pH-sensitive compartments with monensin and monitored maximal cAMPi accumulation after homologous desensitization. Although treatment with monensin alone did not affect ligand-induced cAMPi accumulation (Figure 8B), monensin potentiated homologous desensitization and prevented recovery of the maximal cAMPi response after pretreatment with hGLP-2 (Figure 9B). Taken together with the data indicating that the kinetics of hGLP-2R internalization and trafficking do not correspond to the kinetics of hGLP-2R resensitization (Figures 2 and 4), hGLP-2R endocytosis, followed by reappearance of the receptor on the cell surface, seems to be necessary, but not sufficient, for resensitization of the hGLP-2R.
Immunofluorescent staining of the FLAG-hGLP-2R in BHK cells revealed that intracellular accumulation of receptor was seen after a 20-min stimulation with 10 nM hGLP-2; however, when cells were washed and allowed to recover in media alone for 2 h after stimulation, plasma membrane localization of theGLP-2 receptor returned (Figure 9C). Because only cell surface hGLP-2Rs were labeled with FLAG antibody before stimulation and fixation, the labeled receptor observed in the membrane after recovery is most consistent with recycled hGLP-2R, and not newly synthesized receptor protein. This provides further evidence that agonist stimulation does not target the hGLP-2R for degradation after endocytosis. In the presence of monensin, agonist-induced intracellular hGLP-2R accumulation was similar to that observed in vehicle-treated cells (Figure 9C); in contrast however, monensin treatment prevented return of the hGLP-2R to the cell membrane and resulted in accumulation of the receptor in a large, perinuclear compartment (Figure 9C). These findings suggest that trafficking of the GLP-2 receptor from the intracellular pool to the cell membrane is dependent on the maintenance of intracellular pH gradients.
The hGLP-2R Transiently Colocalizes with Early Endosomal Antigen-1 after Ligand Stimulation
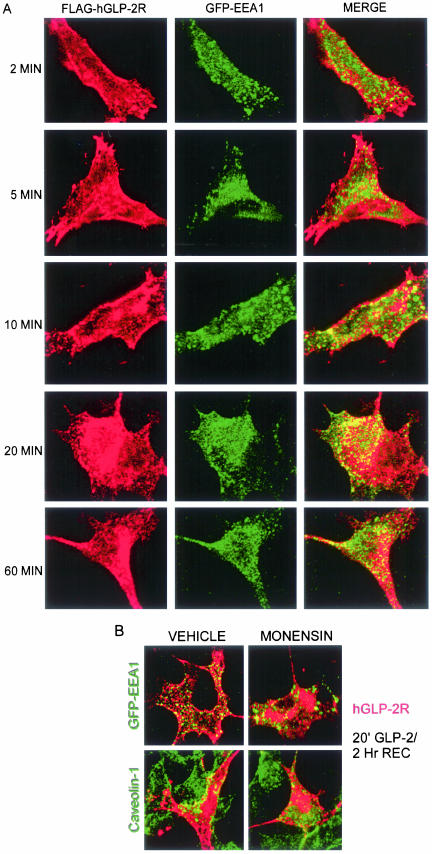
To further address the fate of the internalized hGLP-2R after agonist-induced internalization, we monitored whether the receptor could be detected in vesicles containing markers of the recycling pathway. After coexpression of a GFP-tagged early-endosome antigen-1(Lawe et al., 2002), the FLAG-hGLP-2R was primarily localized to the plasma membrane in cells treated with agonist for 0–2 min (Figures 7B and 10A), whereas GFP-EEA1 was located exclusively in the cytosol (Figure 10A). After 5–10 min of hGLP-2 treatment, the hGLP-2R was detected in the cytosol, and we observed vesicles containing both hGLP-2R and GFP-EEA1 immunofluorescence. However, the greatest extent of colocalization was detected after 20 min of agonist treatment, whereas GLP-2R-EEA1 colocalization was diminished after 60 min of GLP-2 stimulation. Because maximal GLP-2R colocalization with caveolin-1 occurred transiently after a 5–10-min stimulation (Figure 7B), these findings suggest that the hGLP-2R may be rapidly trafficking from caveolin-1–positive vesicles to early endosomes after stimulation with cognate ligand.
Figure 10.
hGLP-2R transiently colocalizes with early endosomes after agonist-induced internalization. (A) BHK cells cotransfected with FLAG-hGLP-2R and GFP-EEA1 were prelabeled on ice with anti-FLAG antibody and then treated with 10 nM hGLP-2 for 2–60 min at 37°C, as described in MATERIALS AND METHODS. (B) Transfected BHK cells were pretreated with vehicle or monensin for 30 min at 37°C, labeled with FLAG-antibody as in A, treated with 10 nM hGLP-2 for 20 min, washed, and allowed to recover in media alone for in the absence (vehicle) or presence of monensin for 2 h. After fixation and permeabilization, cells were stained with anti-caveolin-1-FITC and a Cy3-conjugated secondary antibody was used to visualize hGLP-2R/anti-FLAG antibody complexes. GFP-EEA1 was visualized directly. Confocal microscopy images are representative of two independent experiments.
To more precisely localize the specific intracellular compartments traversed by the GLP-2R after ligand-dependent receptor activation, we reexamined GLP-2R trafficking after an initial 20-min exposure to ligand followed by a 2-h recovery period. hGLP-2R trafficking into and out of early endosomes proceeded with similar kinetics in the presence or absence of monensin (our unpublished data). In the absence of monensin, the majority of the internalized hGLP-2R had returned to the plasma membrane 2 h after a 20-min agonist treatment and did not extensively colocalize with GFP-EEA1 (Figure 10B). In addition, the large intracellular pool of internalized receptor that accumulated in the presence of monensin was not caveolin-1 or GFP-EEA1–positive, providing further evidence that the hGLP-2R interaction with caveolin-1–positive and EEA1-positive endosomes is transient and that trafficking through these endosomes is not dependent on intracellular endosomal acidification.
DISCUSSION
In this study, we show that the human GLP-2 receptor undergoes rapid, sustained, agonist-induced homologous desensitization accompanied by a significant, but transient, reduction in levels of cell surface receptor in transfected BHK or DLD-1 cells. However, the sustained loss of receptor responsiveness and slow receptor reappearance on the plasma membrane were not attributable to ligand-induced hGLP-2R degradation. hGLP-2Rs were localized to lipid raft microdomains and underwent rapid agonist-induced endocytosis via a cholesterol-dependent, clathrin- and dynamin-I–independent mechanism. Internalized hGLP-2R associated transiently with cytosolic vesicles containing caveolin-1, and then EEA1, and then slowly recycled back to the cell surface. Furthermore, cholesterol sequestration potentiated receptor desensitization and inhibition of early endosome acidification prevented receptor recycling and recovery of receptor responsiveness.
In contrast to the generally accepted paradigm governing GPCR recycling and endocytosis, our data clearly demonstrate that hGLP-2R internalization is inhibited by cholesterol depletion or sequestration and not by classical inhibitors of clathrin-mediated endocytosis. Furthermore, the GLP-2R associates with the Triton X-100–insoluble protein fraction along with caveolin-1, a marker of lipid rafts (Nabi and Le, 2003). Immunofluorescence studies localized internalized hGLP-2R to large cytosolic aggregates, which may represent caveosomes, neutral endosomes formed by the budding of a subset of cell surface lipid rafts termed caveolae (Pelkmans et al., 2001). Moreover, some GLP-2R can be found transiently colocalizing with caveolin-1, a raft-associated protein, before and after agonist treatment in transfected BHK cells. Nevertheless, the partial colocalization with caveolin does not necessarily invoke an essential functional role for caveolae or caveosomes in GLP-2 receptor trafficking.
Our data demonstrate that rapid agonist-induced GLP-2R endocytosis seems to proceed by a clathrin- and dynamin-I independent pathway involving lipid rafts. Although there is some controversy as to the involvement of dynamin in lipid raft/caveolae–mediated endocytosis, there is a growing amount of evidence suggesting there may be distinct dynamin-dependent and -independent endocytotic mechanisms for lipid raft-associated proteins (Vickery and von Zastrow, 1999; Schlador et al., 2000; Lamb et al., 2001; Guha et al., 2003; Jayanthi et al., 2004).
Internalization of transforming growth factor-β receptors into lipid raft-derived endosomes seems to target them for ubiquitination and degradation (Di Guglielmo et al., 2003); however, we did not detect a decrease in GLP-2 receptor protein after agonist stimulation. Moreover, the transient colocalization of the hGLP-2R with EEA1, and the inhibition of receptor recycling by monensin, suggests that this newly emerging endocytotic pathway converges with the endosomal recycling machinery. Although the early/recycling endosomes are believed to be a sorting compartment for receptors internalized via coated pits, lipid raft-dependent internalization of the interleukin-2 receptor or other glycosyl phosphatidylinositol -anchored proteins targets these proteins to early/recycling endosomes (Lamaze et al., 2001; Sabharanjak et al., 2002; Sharma et al., 2003). Similarly, the A1 adenosine receptor is visualized in endosome-like vesicles after lipid raft-mediated endocytosis (Escriche et al., 2003). Little is known about how trafficking between lipid raft-derived endosomes and early endosomes occurs; however, epidermal growth factor can stimulate redistribution of caveolin-1 from plasma membrane to early/recycling endosomal fractions in purified rat hepatocyte membrane fractions (Pol et al., 2000).
Accumulation of the hGLP-2R in a perinuclear region after long-term agonist stimulation, or in the presence of monensin, suggests that hGLP-2 recycling may be mediated via the previously described perinuclear recycling compartment (PNRC), an organelle that serves as an intermediate recycling depot for other membrane-bound receptors (Innamorati et al., 2001). Trafficking via this compartment, described as the “long cycle,” is consistent with the slow recycling kinetics of the hGLP-2R. Furthermore, rapid hGLP-2R trafficking into and out of early endosomes is not dependent on endosomal acidification. However, maintenance of the pH gradient seems to be required for recycling via the PNRC, suggesting that postendocytotic processing of the hGLP-2R receptor, including ligand dissociation, dephosphorylation, or segregation from interacting signaling/scaffold proteins, may occur in this compartment and not in early endosomes, as was described for the rapidly recycled B2-adrenergic receptor (Seachrist et al., 2000).
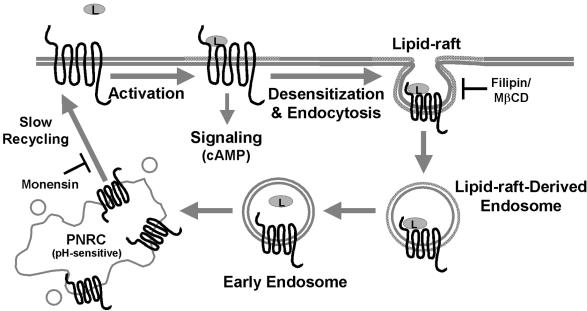
Although a limited number of class I (rhodopsin-like) GPCRs internalize in part via lipid raft endocytosis, this pathway may be used as an alternative internalization mechanism after inhibition of coated-pit endocytosis (Gines et al., 2001; Di Guglielmo et al., 2003; Escriche et al., 2003; Rapacciuolo et al., 2003; Venkatesan et al., 2003). The secretin receptor, another member of the class II GPCR family, internalizes via a nonclathrin, dynamin-independent manner (Walker et al., 1999) and also exhibits delayed resensitization kinetics (Mundell and Kelly, 1998). Little is known about the desensitization and trafficking mechanisms regulating the related members of the class II GPCR family. Together with our data on trafficking of the GLP-2R, the evidence suggests that internalization via lipid rafts may be a more general characteristic of the class II GPCR family than previously recognized. To our knowledge, the GLP-2R represents the first GPCR that is internalized and trafficked primarily via a lipid raft- and recycling endosome-dependent, dynamin-independent mechanism (Figure 11).
Figure 11.
Proposed mechanism for human GLP-2 receptor agonist-induced internalization and postendocytotic trafficking. The GLP-2R is localized in lipid raft domains on the cell surface in the basal state. After ligand-induced GLP-2R activation, the receptor undergoes desensitization and rapid internalization into neutral lipid raft-derived endosomes, a process that can be blocked by cholesterol sequestration with filipin or cholesterol depletion with methyl-β-cyclodextrin. The receptor is then rapidly and transiently shuttled to early endosomes and then slowly recycled to the cell surface most likely via the pH-sensitive PNRC. Prevention of endosomal acidification with monensin inhibits the recycling of the GLP-2R from the PNRC after ligand-induced endocytosis. L, receptor ligand, GLP-2.
Receptor recycling is believed to be a mechanism by which GPCRs regain responsiveness to ligand after desensitization (Ferguson, 2001). Our data demonstrate that although agonist-induced internalization and subsequent reappearance of cell surface receptor is required for recovery from desensitization, receptor trafficking alone cannot account for resensitization of receptor responsivity. Although the wild-type receptor internalized and trafficked with identical kinetics in both DLD-1 and BHK cells, receptor desensitization in BHK cells was prolonged relative to more rapid resensitization kinetics observed in DLD-1 cells. Furthermore, mutant GLP-2 receptors exhibiting altered recycling kinetics retain the ability to resensitize similarly to wild-type receptors (manuscript in preparation). Nonetheless, inhibition of internalization potentiated homologous hGLP-2R desensitization, and resensitization was prevented when recycling of the hGLP-2R was abrogated, suggesting that, in addition to hGLP-2R trafficking, other mechanisms also are involved in receptor resensitization. These findings provide further evidence that the GLP-2R is regulated via pathways distinct from those classically characterized for the majority of class I receptors.
Whereas GPCR desensitization may contribute to dampening of receptor signal transduction, endocytosis has been implicated as a regulatory mechanism for differential control of downstream signaling. Receptor internalization via coated pits and lipid rafts functions as a prerequisite for activation of signal transduction pathways, including MAPK (Daaka et al., 1998; Maudsley et al., 2000; Ma et al., 2003), and is necessary for survival and differentiation of cultured neurons (Tansey et al., 2000). Caveolin-1 has been shown to act as a scaffold for lipid-modified signaling molecules, including G proteins, Src-like kinases, H-Ras, and endothelial nitric-oxide synthase, and may suppress their activity (Li et al., 1996; Couet et al., 1997; Garcia-Cardena et al., 1997), a function similar to that described for arrestin molecules involved in clathrin-mediated endocytosis. Because caveolin-1 may suppress molecular signaling, agonist-induced binding to this scaffold suggests a possible novel mechanism for GLP-2R desensitization. Some receptors also have been shown to internalize via both clathrin- and lipid raft-associated pathways. Colocalization of receptors with alternative signaling machinery (Simons and Toomre, 2000; Di Guglielmo et al., 2003; Rapacciuolo et al., 2003) is consistent with the possibility that selective membrane localization differentially couples receptors to distinct intracellular signaling pathways (Felberbaum-Corti et al., 2003). Whether internalization via lipid rafts targets the hGLP-2R to distinct microdomains associated with regulation of downstream signaling has yet to be determined.
In conclusion, our data outline a new mechanism regulating human GLP-2 receptor signaling and provides further evidence that GPCRs undergo desensitization, endocytosis, and resensitization via increasingly diverse pathways. Because long-acting analogues of GLP-2 are currently being evaluated in clinical trials for the treatment of intestinal failure (Jeppesen et al., 2001), elucidating mechanisms underlying GLP-2R desensitization may have potential therapeutic relevance. Further analysis of the molecular events regulating GLP-2R signaling may provide a useful model for characterization of an incompletely understood regulatory pathway for control of GPCR-dependent cellular signaling.
Acknowledgments
We thank William Harper for generation of the DLD-1 cell line stably expressing the wild-type human GLP-2 receptor; Shannon McCue for assistance with the confocal immunofluorescence microscopy; and Drs. Jeffrey Benovic, Robert Lefkowitz, and Sergio Grinstein for providing the expression constructs. The research was supported in part by operating grants from the Canadian Institutes of Health Research, the National Cancer Institute of Canada, and the Ontario Research and Development Challenge Fund. D.J.D. is a Senior Scientist of the Canadian Institutes of Health Research, and J.E. is supported by grants from the National Science and Engineering Research Council and the Banting and Best Diabetes Centre/Novo-Nordisk.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-11-0825. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-11-0825.
References
- Basu, S.K., Goldstein, J.L., Anderson, R.G., and Brown, M.S. (1981). Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell 24, 493-502. [DOI] [PubMed] [Google Scholar]
- Benlimame, N., Le, P.U., and Nabi, I.R. (1998). Localization of autocrine motility factor receptor to caveolae and clathrin-independent internalization of its ligand to smooth endoplasmic reticulum. Mol. Biol. Cell 9, 1773-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes, M., and Cheng, H. (2001). Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc. Natl. Acad. Sci. USA 98, 12497-12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D.A., and Rose, J.K. (1992). Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68, 533-544. [DOI] [PubMed] [Google Scholar]
- Couet, J., Li, S., Okamoto, T., Ikezu, T., and Lisanti, M.P. (1997). Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 272, 6525-6533. [DOI] [PubMed] [Google Scholar]
- Daaka, Y., Luttrell, L.M., Ahn, S., Della Rocca, G.J., Ferguson, S.S., Caron, M.G., and Lefkowitz, R.J. (1998). Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J. Biol. Chem. 273, 685-688. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo, G.M., Le Roy, C., Goodfellow, A.F., and Wrana, J.L. (2003). Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5, 410-421. [DOI] [PubMed] [Google Scholar]
- Drucker, D.J. (2001). Minireview: the glucagon-like peptides. Endocrinology 142, 521-527. [DOI] [PubMed] [Google Scholar]
- Drucker, D.J. (2002). Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology 122, 531-544. [DOI] [PubMed] [Google Scholar]
- Drucker, D.J., Erlich, P., Asa, S.L., and Brubaker, P.L. (1996). Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA 93, 7911-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriche, M., Burgueno, J., Ciruela, F., Canela, E.I., Mallol, J., Enrich, C., Lluis, C., and Franco, R. (2003). Ligand-induced caveolae-mediated internalization of A1 adenosine receptors: morphological evidence of endosomal sorting and receptor recycling. Exp. Cell Res. 285, 72-90. [DOI] [PubMed] [Google Scholar]
- Felberbaum-Corti, M., Van Der Goot, F.G., and Gruenberg, J. (2003). Sliding doors: clathrin-coated pits or caveolae? Nat. Cell Biol. 5, 382-384. [DOI] [PubMed] [Google Scholar]
- Ferguson, S.S. (2001). Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53, 1-24. [PubMed] [Google Scholar]
- Ferguson, S.S., Zhang, J., Barak, L.S., and Caron, M.G. (1998). Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci. 62, 1561-1565. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena, G., Martasek, P., Masters, B.S., Skidd, P.M., Couet, J., Li, S., Lisanti, M.P., and Sessa, W.C. (1997). Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J. Biol. Chem. 272, 25437-25440. [DOI] [PubMed] [Google Scholar]
- Gines, S., Ciruela, F., Burgueno, J., Casado, V., Canela, E.I., Mallol, J., Lluis, C., and Franco, R. (2001). Involvement of caveolin in ligand-induced recruitment and internalization of A(1) adenosine receptor and adenosine deaminase in an epithelial cell line. Mol. Pharmacol. 59, 1314-1323. [PubMed] [Google Scholar]
- Gromada, J., Dissing, S., and Rorsman, P. (1996). Desensitization of glucagon-like peptide 1 receptors in insulin-secreting beta TC3 cells: role of PKA-independent mechanisms. Br. J. Pharmacol. 118, 769-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, X.M., Kobilka, T.S., and Kobilka, B.K. (1992). Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J. Biol. Chem. 267, 21995-21998. [PubMed] [Google Scholar]
- Guha, A., Sriram, V., Krishnan, K.S., and Mayor, S. (2003). Shibire mutations reveal distinct dynamin-independent and -dependent endocytic pathways in primary cultures of Drosophila hemocytes. J. Cell Sci. 116, 3373-3386. [DOI] [PubMed] [Google Scholar]
- Hooper, N.M. (1999). Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review). Mol. Membr. Biol. 16, 145-156. [DOI] [PubMed] [Google Scholar]
- Innamorati, G., Le Gouill, C., Balamotis, M., and Birnbaumer, M. (2001). The long and the short cycle. Alternative intracellular routes for trafficking of G-protein-coupled receptors. J. Biol. Chem. 276, 13096-13103. [DOI] [PubMed] [Google Scholar]
- Jayanthi, L.D., Samuvel, D.J., and Ramamoorthy, S. (2004). Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters: evidence for localization in lipid rafts and lipid raft mediated internalization. J. Biol. Chem. 279, 19315-19326. [DOI] [PubMed] [Google Scholar]
- Jeppesen, P.B., et al. (2001). Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120, 806-815. [DOI] [PubMed] [Google Scholar]
- Lamaze, C., Dujeancourt, A., Baba, T., Lo, C.G., Benmerah, A., and Dautry-Varsat, A. (2001). Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 7, 661-671. [DOI] [PubMed] [Google Scholar]
- Lamb, M.E., De Weerd, W.F., and Leeb-Lundberg, L.M. (2001). Agonist-promoted trafficking of human bradykinin receptors: arrestin- and dynamin-independent sequestration of the B2 receptor and bradykinin in HEK293 cells. Biochem. J. 355, 741-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawe, D.C., Chawla, A., Merithew, E., Dumas, J., Carrington, W., Fogarty, K., Lifshitz, L., Tuft, R., Lambright, D., and Corvera, S. (2002). Sequential roles for phosphatidylinositol 3-phosphate and Rab5 in tethering and fusion of early endosomes via their interaction with EEA1. J. Biol. Chem. 277, 8611-8617. [DOI] [PubMed] [Google Scholar]
- Li, S., Couet, J., and Lisanti, M.P. (1996). Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J. Biol. Chem. 271, 29182-29190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovshin, J., Estall, J., Yusta, B., Brown, T.J., and Drucker, D.J. (2001). Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J. Biol. Chem. 276, 21489-21499. [DOI] [PubMed] [Google Scholar]
- Ma, L., et al. (2003). Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J. Neurosci. 23, 3164-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecz, N., Bambino, T., Bencsik, M., and Nissenson, R.A. (1998). Identification of phosphorylation sites in the G protein-coupled receptor for parathyroid hormone. Receptor phosphorylation is not required for agonist-induced internalization. Mol. Endocrinol. 12, 1846-1856. [DOI] [PubMed] [Google Scholar]
- Maudsley, S., Pierce, K.L., Zamah, A.M., Miller, W.E., Ahn, S., Daaka, Y., Lefkowitz, R.J., and Luttrell, L.M. (2000). The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J. Biol. Chem. 275, 9572-9580. [DOI] [PubMed] [Google Scholar]
- Mayo, K.E., Miller, L.J., Bataille, D., Dalle, S., Goke, B., Thorens, B., and Drucker, D.J. (2003). International Union of Pharmacology. XXXV. The Glucagon Receptor Family. Pharmacol. Rev. 55, 167-194. [DOI] [PubMed] [Google Scholar]
- Montesano, R., Roth, J., Robert, A., and Orci, L. (1982). Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature 296, 651-653. [DOI] [PubMed] [Google Scholar]
- Mundell, S.J., and Kelly, E. (1998). The effect of inhibitors of receptor internalization on the desensitization and resensitization of three Gs-coupled receptor responses. Br. J. Pharmacol. 125, 1594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe, D.G., et al. (1999). Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA 96, 1569-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi, I.R., and Le, P.U. (2003). Caveolae/raft-dependent endocytosis. J. Cell Biol. 161, 673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B.J., Kenworthy, A.K., Polishchuk, R.S., Lodge, R., Roberts, T.H., Hirschberg, K., Phair, R.D., and Lippincott-Schwartz, J. (2001). Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olli-Lahdesmaki, T., Scheinin, M., Pohjanoksa, K., and Kallio, J. (2003). Agonist-dependent trafficking of alpha2-adrenoceptor subtypes: dependence on receptor subtype and employed agonist. Eur. J. Cell Biol. 82, 231-239. [DOI] [PubMed] [Google Scholar]
- Pao, C.S., and Benovic, J.L. (2002). Phosphorylation-independent desensitization of G protein-coupled receptors? Sci. STKE 2002, PE42. [DOI] [PubMed] [Google Scholar]
- Pawson, A.J., Maudsley, S.R., Lopes, J., Katz, A.A., Sun, Y.M., Davidson, J.S., and Millar, R.P. (2003). Multiple determinants for rapid agonist-induced internalization of a nonmammalian gonadotropin-releasing hormone receptor: a putative palmitoylation site and threonine doublet within the carboxyl-terminal tail are critical. Endocrinology 144, 3860-3871. [DOI] [PubMed] [Google Scholar]
- Pelkmans, L., Kartenbeck, J., and Helenius, A. (2001). Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3, 473-483. [DOI] [PubMed] [Google Scholar]
- Pol, A., Calvo, M., Lu, A., and Enrich, C. (2000). EGF triggers caveolin redistribution from the plasma membrane to the early/sorting endocytic compartment of hepatocytes. Cell Signal 12, 537-540. [DOI] [PubMed] [Google Scholar]
- Puri, V., Watanabe, R., Singh, R.D., Dominguez, M., Brown, J.C., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2001). Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154, 535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapacciuolo, A., Suvarna, S., Barki-Harrington, L., Luttrell, L.M., Cong, M., Lefkowitz, R.J., and Rockman, H.A. (2003). Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J. Biol. Chem. 278, 35403-35411. [DOI] [PubMed] [Google Scholar]
- Sabharanjak, S., Sharma, P., Parton, R.G., and Mayor, S. (2002). GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2, 411-423. [DOI] [PubMed] [Google Scholar]
- Schlador, M.L., Grubbs, R.D., and Nathanson, N.M. (2000). Multiple topological domains mediate subtype-specific internalization of the M2 muscarinic acetylcholine receptor. J. Biol. Chem. 275, 23295-23302. [DOI] [PubMed] [Google Scholar]
- Seachrist, J.L., Anborgh, P.H., and Ferguson, S.S. (2000). beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J. Biol. Chem. 275, 27221-27228. [DOI] [PubMed] [Google Scholar]
- Sharma, D.K., Choudhury, A., Singh, R.D., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2003). Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278, 7564-7572. [DOI] [PubMed] [Google Scholar]
- Simons, K., and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1, 31-39. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen, M., Larsen, P.J., Thulesen, J., Romer, J., and Vrang, N. (2000). The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med 6, 802-807. [DOI] [PubMed] [Google Scholar]
- Tansey, M.G., Baloh, R.H., Milbrandt, J., and Johnson, E.M., Jr. (2000). GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron 25, 611-623. [DOI] [PubMed] [Google Scholar]
- Tsai, C.H., Hill, M., Asa, S.L., Brubaker, P.L., and Drucker, D.J. (1997). Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am. J. Physiol. 273, E77-E84. [DOI] [PubMed] [Google Scholar]
- Venkatesan, S., Rose, J.J., Lodge, R., Murphy, P.M., and Foley, J.F. (2003). Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4. Mol. Biol. Cell 14, 3305-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery, R.G., and von Zastrow, M. (1999). Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J. Cell Biol. 144, 31-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, J.K., Premont, R.T., Barak, L.S., Caron, M.G., and Shetzline, M.A. (1999). Properties of secretin receptor internalization differ from those of the beta(2)-adrenergic receptor. J. Biol. Chem. 274, 31515-31523. [DOI] [PubMed] [Google Scholar]
- Walsh, N.A., Yusta, B., DaCambra, M.P., Anini, Y., Drucker, D.J., and Brubaker, P.L. (2003). Glucagon-like peptide-2 receptor activation in the rat intestinal mucosa. Endocrinology 144, 4385-4392. [DOI] [PubMed] [Google Scholar]
- Yusta, B., Estall, J., and Drucker, D.J. (2002). Glucagon-like peptide-2 receptor activation engages bad and glycogen synthase kinase-3 in a protein kinase A-dependent manner and prevents apoptosis following inhibition of phosphatidylinositol 3-kinase. J. Biol. Chem. 277, 24896-24906. [DOI] [PubMed] [Google Scholar]
- Yusta, B., Boushey, R.P., and Drucker, D.J. (2000a). The glucagon-like peptide-2 receptor mediates direct inhibition of cellular apoptosis via a cAMP-dependent protein kinase-independent pathway. J Biol Chem 275, 35345-35352. [DOI] [PubMed] [Google Scholar]
- Yusta, B., Huang, L., Munroe, D., Wolff, G., Fantaske, R., Sharma, S., Demchyshyn, L., Asa, S.L., and Drucker, D.J. (2000b). Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119, 744-755. [DOI] [PubMed] [Google Scholar]
- Yusta, B., Somwar, R., Wang, F., Munroe, D., Grinstein, S., Klip, A., and Drucker, D.J. (1999). Identification of glucagon-like peptide-2 (GLP-2)-activated signaling pathways in baby hamster kidney fibroblasts expressing the rat GLP-2 receptor. J. Biol. Chem. 274, 30459-30467. [DOI] [PubMed] [Google Scholar]