Abstract
Background and Objectives:
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is an effective method for tissue diagnosis of gastrointestinal submucosal tumors (SMTs) that are difficult to diagnose by standard endoscopic biopsy. However, the learning curve, especially for gastrointestinal SMT, has not been sufficiently established. The aim of our study was to assess the skill acquisition and diagnostic accuracy of EUS-FNA for gastrointestinal SMT in trainee endoscopists in order to elucidate the optimal starting standards of EUS-FNA.
Materials and Methods:
We prospectively evaluated 51 EUS-FNA procedures for gastrointestinal SMT between May 2010 and March 2014. The procedure was performed by two trainee endoscopists and two expert endoscopists. We investigated the diagnostic yield of EUS-FNA and the factors associated with the accuracy between the trainee endoscopists and expert endoscopists.
Results:
The rate of adequate EUS-FNA materials for histological examination was 86.3%. Although infections occurred in two cases (3.9%), which were managed conservatively, no severe complications were identified. Comparing the trainee endoscopists with expert endoscopists, there was no significant difference in the rate of gaining adequate specimen (76.5% vs. 82.3%, P = 0.4626). However, the mean number of passes of the trainees tended to be more than that of the expert endoscopists (2.1 pass vs. 1.7 pass, P = 0.0511), and lesions located in the middle third of the stomach were the predictive factors for nondiagnostic tumors by the trainee endoscopists (P = 0.0075).
Conclusion:
EUS-FNA for gastrointestinal SMT by trainee endoscopists can be safely performed under the supervision of EUS-FNA expert endoscopists.
Keywords: Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), gastrointestinal submucosal tumors (GI SMTs), skill acquisition
INTRODUCTION
Recently, with the improvement of endoscopic skills, the detection of gastrointestinal (GI) submucosal tumors (SMTs) such as gastrointestinal stromal tumors (GISTs) is increasing worldwide.[1] GISTs are now considered as potentially malignant. Thus, all GISTs may need to be resected even when they appear as small lesions,[2] and differentiating these lesions from benign submucosal lesions such as leiomyoma or schwannoma is crucial.[3] However, standard endoscopic biopsy specimens are usually nondiagnostic because the overlying mucosa is thick enough to prevent retrieving the tissue from the lesion.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has therefore, become a well-established diagnostic technique for assessing lesions of the GI tract and adjacent organs. The indications have expanded to the mediastinal lesions, liver, spleen, adrenal gland, ascites, pleural effusion, and intraabdominal lymph nodes.[4] In the centers where the volume of EUS procedures are high, EUS-FNA has a sensitivity and specificity of at least 80% and 100%, respectively.[4,5,6,7,8,9] However, EUS-FNA for GI SMT is limited to relatively small series, and is more difficult for this than for others such as pancreatic lesions.[10,11,12,13,14] The diagnosis of GIST should be based on immunohistochemical analysis.[15,16] Therefore, it is necessary to obtain enough tissue for the immunohistochemical analysis in the case of GI SMTs.
To the best of our knowledge, there have been few educational guidelines or training programs especially for GI SMT, and the optimal number of procedures that should be performed to become proficient at EUS-FNA for SMT is unknown. In this study, we prospectively assessed the skill acquisition of EUS-FNA for GI SMT for two trainee endoscopists to elucidate the optimal starting standards of EUS-FNA, and the technical steps associated with increased diagnostic accuracy.
MATERIALS AND METHODS
Between May 2010 and March 2014, 51 consecutive patients who had a GI SMT with submucosal hypoechoic tumors originating in the third to fourth sonographic layers of the gastrointestinal wall suspected as GIST by standard EUS underwent EUS-FNA for histopathological diagnosis at the University of Tokyo Hospital. All patients were informed of the risks and benefits of EUS-FNA, and written informed consent was obtained from them to perform EUS-FNA. Patients who refused to participate in the study were excluded. This study was conducted only at our institute, and was approved by our ethics committee.
The indicated lesions for EUS-FNA in our hospital were as follows: SMT with suspicion of GIST (>2 cm or if <2 cm, increasing in size); and SMT for which we could not obtain tissue using conventional endoscopic biopsy. EUS-FNA procedures were operated by four endoscopists. Two endoscopists were trainees (GO and KN) and two were experienced (KK and YN) in performing EUS-FNA. In our hospital, there were no definite rules to start EUS-FNA for GI SMTs but an essential condition was to perform EUS for more than 40 GI tumors before beginning the EUS-FNA procedures. The two trainee endoscopists had sufficient experience of conducting more than 8,000 regular esophagogastroduodenoscopies (EGDs), 2,500 colonoscopies, and 50 EUS procedures before starting EUS-FNA. They had also attended 20 EUS-FNA procedures performed by EUS-FNA experts as assistants, and learned about EUS-FNA techniques sufficiently through a review of the literature, videos, and attendance at seminars. The two expert endoscopists had performed regular EGD, colonoscopy, and EUS procedures for over 8 years. They were extremely familiar with EUS-FNA and had performed more than 50 EUS-FNA procedures before the beginning of this study.
We prospectively evaluated the patients' age, sex, location and size of the long axis of the lesions, the number of punctures, procedure-related complications, the diagnostic yield of gaining a sufficient material for immunohistological diagnosis, and accuracy by comparison with the final diagnosis. We defined a case of gaining sufficient material for immunohistological diagnosis as diagnostic and calculated the diagnostic yield. The standard criteria for final diagnosis were as follows:
Initial EUS-FNA diagnoses are consistent with subsequent histopathological examination of a surgical resection material of the same lesions,
The clinical follow-up course during at least 6 months without surgical resection correspond to that of initial EUS-FNA diagnoses.
Furthermore, the procedure results categorized for the overall diagnostic yield were as follows:
Diagnostic group, if sufficient samples were obtained, such that a specific diagnosis could be established;
Nondiagnostic group, if the samples were primarily insufficient, and/or if the results were discordant with the standard criteria.
We assessed the factors associated with the diagnostic yield of EUS-FNA from the trainee endoscopists and the expert endoscopists. The results of EUS-FNA and final diagnosis were categorized into two groups:
Malignant or potentially malignant group including GIST, carcinoma, carcinoid, and mesothelial tumor;
Benign group including leiomyomas, schwannomas, and aberrant pancreas.
Sensitivity, specificity, positive and negative predictive values, and diagnostic accuracy of EUS-FNA were determined by comparison with the final diagnoses of the lesions according to surgical pathology or clinical follow-up.
EUS-FNA PROCEDURE
The EUS-FNA procedure was performed as previously reported elsewhere.[7,13,17] Standard EUS was principally performed under moderate sedation with diazepam (10 mg) and pentazosine (15 mg), using an oblique forward-viewing electronic linear scanning video echoendoscope (GF-UCT240-AL5 or GF-UCT260; Olympus Medical Systems Co., Tokyo, Japan), which was connected to a processor featuring color doppler function (EU-ME1; Olympus Medical Systems Co., Tokyo, Japan). The EUS-FNA was mainly performed using a 22-gauge needle (Expect; Boston Scientific, Natick, MA, USA) inserted through the working channel of the echoendoscope. According to the situation, a 19-gauge or 25-gauge needle was used in combination with a 22-gauge needle at the discretion of the endoscopists. After localization of the target lesion, color flow doppler sonography was performed to exclude intervening vascular structures and to select a vessel-free needle track to avoid puncturing vessels. Once the tip of the catheter was visualized, the needle was advanced from the catheter sheath through the wall of the gastrointestinal tract and into the target lesion under EUS guidance. The EUS-FNA was performed using either of the two methods described below: The suction technique or the slow-pull technique.[17] The EUS-FNA was performed for one lesion first by the trainee and then by the expert, and at least one pass was performed by each endoscopist for the target lesion. The aspirate was entirely placed in a petri dish. The material was then carefully inspected and the visible specimen, if any, was lifted from the slide and placed in formalin in a bottle for histopathological examination. The remaining specimen was sent for cytological examination. A cytology technician was not present to smear the aspirated material for immediate review. The procedure was terminated when adequate cellular specimens were achieved. The procedure time was measured from the scope insertion to the scope removal. After the completion of EUS-FNA, the patient stayed in bed for 3 hours, and if the patient's symptoms, the laboratory data, and abdominal X-rays revealed no significant abnormality, a soft diet was started. Prophylactic antibiotic with cefmetazole sodium, 1 g twice daily, was administered on the day of the procedure and the following day. The patients were discharged from the ward 2 days after EUS-FNA.
Histopathological evaluations
Specimens were sent to the Department of Pathology for histopathological assessment. Pathological diagnosis was based on a combination of cytological diagnosis and histopathological diagnosis. Immunoperoxidase stains were subsequently performed using commercially available antibodies against c-Kit (CD117), CD34, S-100, and smooth-muscle actin. The histopathological analysis was performed on a per pass basis. Diagnosis of GIST was performed when pathologic examination showed spindle or epithelioid cells that stained positive for c-Kit. In case of histological diagnosis of a potential malignant tumor such as GIST, the patients were generally sent to a surgeon for resection.
Statistical analysis
Continuous variables were expressed as mean and standard deviation (SD). Categorical variables were expressed as number and percentages. Statistical analysis for categorical variables was conducted using an χ2 test and for continuous variables using the Student's t-test. A P value of less than 0.05 was considered significant. Differences within the outcome of EUS-FNA by operators were evaluated with the MacNemar test. All statistical analyses were performed with JMP version 10.0 software (SAS Institute, Cary, NC, USA).
RESULTS
The clinicopathological features and outcomes of EUS-FNA for the 51 GI SMTs are shown in Tables 1 and 2. A total of 51 patients who had undergone EUS-FNA of GI SMTs were identified. Of the total 51 cases, 44 (86.3%) had adequate EUS-FNA materials for cytological and histological examinations, and seven cases (13.7%) were judged as inadequate. Final diagnoses after EUS-FNA were GIST (n = 26), leiomyoma (n = 6), schwannoma (n = 3), aberrant pancreas (n = 2), carcinoid (n = 1), ectopic liver (n = 1), carcinoma (n = 2), lymph node (n = 1), duplication cyst (n = 1), and mesothelial tumor (n = 1). Surgical resection was performed for 29 of the 51 patients; 25 patients were diagnosed as GIST and 4 were nondiagnostic by EUS-FNA. Four nondiagnostic lesions by EUS-FNA were diagnosed by surgical resection as GIST (n = 2), schwannoma (n = 1), and aberrant pancreas (n = 1). In comparing the histopathological findings in the tissues by EUS-FNA with the specimens obtained by surgical resection, the EUS-FNA results were concordant with the final diagnosis in 25 of the 25 lesions (accuracy rate 100%). Final diagnoses after surgical resection were GIST (n = 25), schwannoma (n = 1), aberrant pancreas (n = 1), carcinoid (n = 1), and carcinoma (n = 1). In differentiating benign lesions from potentially malignant lesions, EUS-FNA had a sensitivity of 90.5% [95% confidence interval (CI) 77.6%-96.2%], a specificity of 93.3% (95% CI 84.3%-97.4%), a positive predictive value of 90.5% (95% CI 77.6%-96.2%), a negative predictive value of 93.3% (95% CI 84.3%-97.4%), and an accuracy rate of 92.2% (95% CI 81.6%-96.9%).
Table 1.
Clinicopathological features of EUS-FNA for 51 gastrointestinal submucosal tumors

Table 2.
Outcomes of EUS-FNA for 51 gastrointestinal submucosal tumors
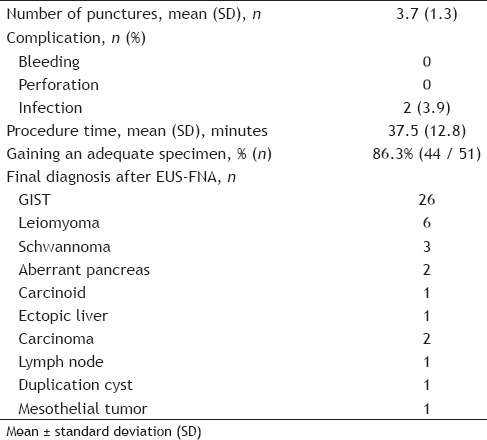
Infection after EUS-FNA occurred in two patients. One was esophageal SMT diagnosed as a duplication cyst by EUS-FNA[18] and the other was gastric SMT diagnosed as GIST by EUS-FNA. The first lesion was identified as a well-defined, homogenous, hypoechoic mass adjacent to the esophagus by EUS and a 3-cm unenhanced mass to the left of the abdominal esophagus by computed tomography (CT). An esophageal submucosal tumor was suspected; however, the cystic nature of the lesion was not diagnosed due to the solid-tissue appearance of the lesion presumably because of the mucinous nature of the fluid, and the typical wall-layers were not appreciated. Both patients were discharged without complications on the third day after EUS-FNA. They were admitted to the hospital on the fourth day and eighth day after the EUS-FNA and were managed conservatively with antibiotic therapy and with antibiotics and percutaneous transhepatic abscess drainage, respectively. Technical complications, such as intestinal perforation or hemorrhage, were not identified during the follow-up periods.
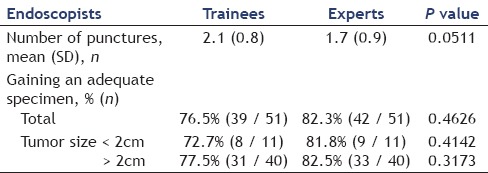
We compared the mean number of punctures and the rate of gaining an adequate specimen between the trainee endoscopists and expert endoscopists [Table 3]. The mean number of punctures by the trainee endoscopists and expert endoscopists were 2.1 passes and 1.7 passes, respectively, and the rate of gaining adequate specimen was 76.5% (39/51) and 82.3% (42/51), respectively. On comparing the trainee endoscopists with the expert endoscopists, there were no significant differences in the mean number of punctures (difference 0.4, P = 0.0511) and the rate of gaining adequate specimen (difference 5.8%, P = 0.4626), even when taking into consideration of the tumor size (tumor size <2 cm: difference 9.1%, P = 0.4142, tumor size >2 cm: difference 5%, P = 0.3173). However, the mean number of passes of the trainee endoscopists tended to be more than those of the expert endoscopists.
Table 3.
Comparison of the trainee with the expert endoscopists of EUS-FNA outcomes
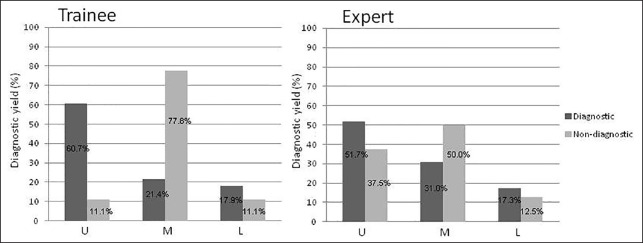
We also evaluated the outcomes of the EUS-FNA in the trainee and expert endoscopists compared with diagnostic and nondiagnostic factors [Table 4]. Although there were no significant differences between the diagnostic and nondiagnostic lesions from both the trainee endoscopists and expert endoscopists, lesions located in the middle third of the stomach were significantly lower with regard to diagnostic factors with the trainee endoscopists (P = 0.0075) [Figure 1].
Table 4.
Factors associated with the diagnostic yield of EUS-FNA in the trainees and the expert endoscopists
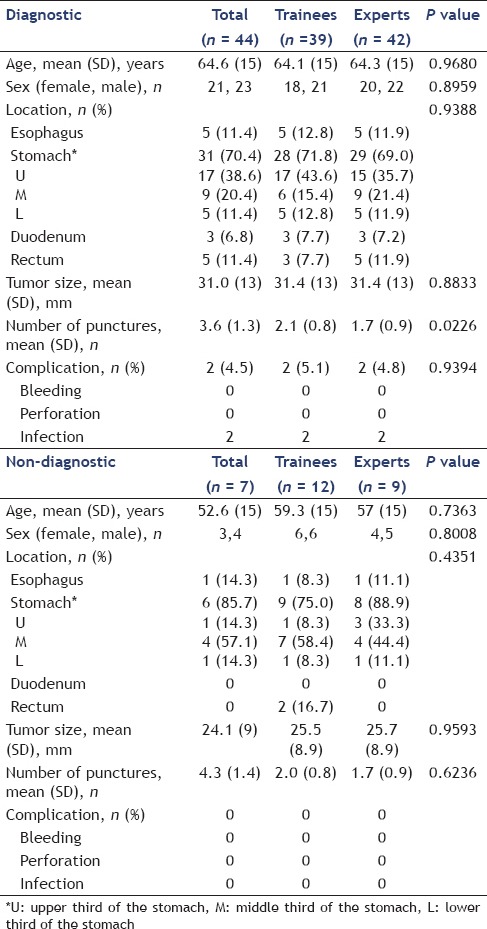
Figure 1.
Diagnostic yield of EUS-FNA for the stomach in the trainee endoscopists and expert endoscopists
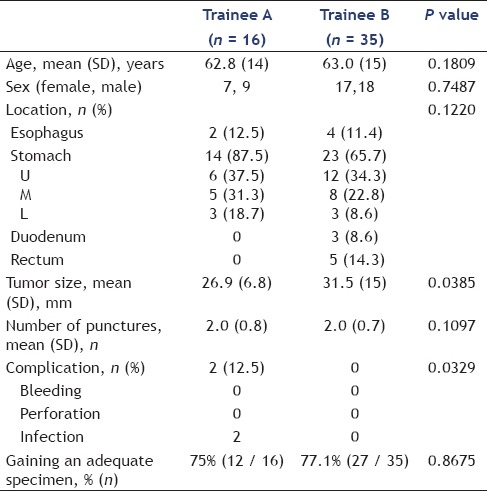
Table 5 summarizes the clinicopathological features and diagnostic outcomes of the GI SMTs performed using EUS-FNA by two trainee endoscopists (trainees A and B). From the beginning of the EUS-FNA until March 2014, trainee A performed EUS-FNA for 16 lesions and trainee B performed it for 35 lesions. The rates of gaining adequate specimens were 75% (12/16) in trainee A and 77.1% (27/35) in trainee B. The mean tumor size from trainee A was smaller than from trainee B (P = 0.0385) and the complication rate from trainee A was higher than from trainee B (P = 0.0329), with significant differences. However, there were no significant differences in the location of the tumors, the rate of gaining adequate specimen, and the mean number of punctures between trainee A and trainee B.
Table 5.
The outcomes of EUS-FNA comparison of the two trainee endoscopits
DISCUSSION
This study demonstrated that the method for performing EUS-FNA for GI SMTs was safely and efficiently taught to trainee endoscopists under the supervision of suitable experts. Although the number of punctures tended to be fewer and the rate of gaining an adequate specimen was higher among expert endoscopists than among trainee endoscopists, the outcome of EUS-FNA for the trainee endoscopists after experiencing EUS procedures for more than 40 GI tumors was sufficiently acceptable.
GISTs are the most commonly identified SMTs in the GI tract. Recently, minimally invasive surgery by the collaboration of endoscopy and laparoscopy have gradually gained acceptance in Japan. Laparoscopic and endoscopic cooperative surgery (LECS) and nonexposed endoscopic wall-inversion surgery (NEWS) are thought to be effective treatments for gastric SMT as a minimally-invasive and minimally-sized endoluminal surgery.[19,20] Early diagnosis as GIST can lead to minimally invasive surgery. Thus, accurate diagnosis is necessary even for small SMT.
EUS-FNA is considered to be a reliable and accurate method for the pathological estimation of submucosal lesions in the GI tract although substantial procedural inadequacies are reported. Operator experience is a major factor with respect to diagnostic yield, whereas tumor size and location are less important. The procedure time also tended to decrease with experience. However, the steps to achieve expertise have not been evaluated systematically. We found that there was no statistically significant difference in the diagnostic yield between the trainee endoscopists and expert endoscopists although the mean number of punctures tended to be less and the rate of gaining an adequate specimen tended to be higher among the expert endoscopists than among the trainee endoscopists. It can thus be concluded that EUS-FNA for gastrointestinal SMT by trainee endoscopists upon acquiring enough EUS skill can be safely performed under the supervision of EUS-FNA expert endoscopists. However, compared with diagnostic and nondiagnostic lesions only for the stomach, the diagnostic yield in the middle third of the stomach was significantly lower among the trainee endoscopists. This was probably because the scope handling and stability at the middle third of the stomach, with a wide open space, was more difficult than at other locations. Therefore, it might be recommended that trainee endoscopists start EUS-FNA for lesions located in the middle third of the stomach.
Basically, acquiring the necessary skills for performing EUS-FNA depends on a fundamental understanding of normal and abnormal EUS anatomies to avoid inadvertent sampling of structures that should not be biopsied.[21] The American Society for Gastrointestinal Endoscopy (ASGE) has developed guidelines for the formal training of individuals to achieve competence in performing EUS examinations, which should also permit adequate exposure to learn EUS-FNA.[22] The guidelines also represent a minimum number of procedures necessary to gauge competency and may serve as a resource for practitioners interested in acquiring these skills.[23] In this study, the two trainees started to perform EUS-FNA after experiencing EUS for more than 40 GI tumors. The rate of gaining an adequate specimen was around 75%, and there was no significant difference in the diagnostic yield between the trainee endoscopists and the expert endoscopists. Therefore, from our study, we found that at least 40 cases of EUS procedures would be appropriate. This result appears to confirm that the ASGE guidelines for training in EUS-FNA are acceptable. Endoscopists should obtain the requisite experience so as to acquire safe and stable techniques by performing a sufficient number of EUS procedures before starting GI EUS-FNA.
After EUS observation technique is mastered to some extent, training should begin with relatively easily accessible lesions followed by more difficult lesions. The next step is puncturing the pancreatic body lesions and mediastinal lymph nodes followed by puncturing pancreatic head lesions and bile duct mass. In general, transesophageal and transgastric EUS-FNAs are technically easier and transduodenal EUS-FNAs are more challenging.[24] The guidelines for training in pancreatic EUS-FNA suggests that the trainee should be competent to perform diagnostic pancreaticobiliary EUS and have done at least 25 supervised EUS-FNA of pancreatic lesions.[25] The majority of reports on EUS-FNA have focused on pancreatic lesions and lymphadenopathy. Meanwhile, experience with EUS-FNA for the diagnosis of SMT is limited to relatively small series.[26] In this study, the two trainees started EUS-FNA with GI SMT and not with pancreatic lesions. The outcome of EUS-FNA in trainee endoscopists was just as good as in expert endoscopists. We speculate that it is most important for us to keep the scope stable and in a good position during the EUS-FNA procedure. It might not be required to start EUS-FNA from pancreatic lesion in terms of the location.
As for safety, complications such as endoscope-induced perforation, febrile episodes following aspiration of cystic lesions, hemorrhage, and seeding, were reported, all of which were nonfatal.[5,8] A learning curve exists for EUS-FNA, which may have a bearing on the likelihood of complications, whereas several studies have found low complications rates by EUS-FNA.[27,28,29] Our study showed that two infections occurred. We presume that it is not due to technical issues but because of the nature of the lesions. EUS-FNA of a duplication cyst resulted in procedure-induced infection even with antibiotics.[5,27] We should always take into consideration that EUS-FNA for a duplication cyst might cause infection as in this case.
This study is the first report showing the skill acquisition for EUS-FNA of GI SMT in trainee endoscopists. The limitations of this study are that it is a single-center analysis, with a small sample of only two trainee endoscopists. EUS-FNA started with GI tumors was introduced in our hospital for the first time and therefore, only two trainees were studied. The skill of endoscopy may be different with each person, and the trainee's skill affects the result greatly. Referring back to their experience, the essential skill of EUS of the two trainees was almost equal before beginning the EUS-FNA procedures in this study. We are not sure whether the result can be generalized for all endoscopists but this study will help the beginner in EUS-FNA to perform EUS-FNA for the GI tract in accordance with the guidelines. Further investigations with a larger number of EUS-FNA procedures and participating endoscopists will be necessary to confirm our results.
CONCLUSION
In conclusion, EUS-FNA of GI SMT by trainee endoscopists can be safely performed under the supervision of EUS-FNA experts. By acquiring enough EUS skill, trainee endoscopists could start EUS-FNA safely and effectively from GI SMTs. From the viewpoint of the early diagnosis and rapid cure of GI SMTs, we hope that our study concerning skill acquisition will help trainees to start EUS-FNA of GI SMT.
AUTHOR'S CONTRIBUTIONS
Keiko Niimi conceived and designed the study. Keiko Niimi drafted the manuscript. Keiko Niimi, Osamu Goto, Kazumichi Kawakubo, Yousuke Nakai, Chihiro Minatsuki, Itsuko Asada-Hirayama, and Satoshi Mochizuki collected and assembled the data. Keiko Niimi analyzed and interpreted the data. Osamu Goto, Kazumichi Kawakubo, and Yousuke Nakai offered critical revision of the manuscript for important intellectual content. All authors discussed the results and commented on the manuscript. Kazuhiko Koike gave his final approval of the article.
Financial support and sponsorship
Nil.
Conflicts of interest
None of the authors have any relevant financial interests to disclose.
REFERENCES
- 1.Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–45. doi: 10.1055/s-2005-861422. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 3.Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gress FG, Hawes RH, Savides TJ, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc. 1997;45:243–50. doi: 10.1016/s0016-5107(97)70266-9. [DOI] [PubMed] [Google Scholar]
- 5.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 6.Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: Results in 141 patients. Endoscopy. 1995;27:171–7. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 7.Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–82. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut. 1999;44:720–6. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin LF, Tung JN. Experience of endoscopic ultrasound-guided fine needle aspiration in a regional teaching hospital. Indian J Gastroenterol. 2008;27:156–8. [PubMed] [Google Scholar]
- 10.Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: A randomized crossover study. Endoscopy. 2010;42:292–9. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 11.Watson RR, Binmoeller KF, Hamerski CM, et al. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56:1757–62. doi: 10.1007/s10620-011-1646-6. [DOI] [PubMed] [Google Scholar]
- 12.Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218–23. doi: 10.1016/j.gie.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T, Arai M, Matsumura T, et al. Factors associated with inadequate tissue yield in EUS-FNA for gastric SMT. ISRN Gastroenterol 2011. 2011:619128. doi: 10.5402/2011/619128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okubo K, Yamao K, Nakamura T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004;39:747–53. doi: 10.1007/s00535-004-1383-0. [DOI] [PubMed] [Google Scholar]
- 15.Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 16.Elliott DD, Fanning CV, Caraway NP. The utility of fine-needle aspiration in the diagnosis of gastrointestinal stromal tumors: A cytomorphologic and immunohistochemical analysis with emphasis on malignant tumors. Cancer. 2006;108:49–55. doi: 10.1002/cncr.21376. [DOI] [PubMed] [Google Scholar]
- 17.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 18.Goto O, Isayama H, Fujishiro M, et al. Education and imaging. Gastrointestinal: EUS-guided FNA for a mimicking esophageal submucosal tumor in an immunocompromised patient. J Gastroenterol Hepatol. 2011;26:1215. doi: 10.1111/j.1440-1746.2011.06675.x. [DOI] [PubMed] [Google Scholar]
- 19.Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729–35. doi: 10.1007/s00464-007-9696-8. [DOI] [PubMed] [Google Scholar]
- 20.Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594–9. doi: 10.1007/s10120-013-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goto O, Kambe H, Niimi K, et al. Discrepancy in diagnosis of gastric submucosal tumor among esophagogastroduodenoscopy, CT, and endoscopic ultrasonography: A retrospective analysis of 93 consecutive cases. Abdom Imaging. 2012;37:1074–8. doi: 10.1007/s00261-012-9928-9. [DOI] [PubMed] [Google Scholar]
- 22.Van Dam J, Brady PG, Freeman M, et al. Guidelines for training in electronic ultrasound: Guidelines for clinical application. From the ASGE. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1999;49:829–33. doi: 10.1016/s0016-5107(99)70312-3. [DOI] [PubMed] [Google Scholar]
- 23.Eisen GM, Dominitz JA, Faigel DO, et al. American Society for Gastrointestinal Endoscopy. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811–4. doi: 10.1016/s0016-5107(01)70082-x. [DOI] [PubMed] [Google Scholar]
- 24.Varadarajulu S, Fockens P, Hawes RH. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2012;10:697–703. doi: 10.1016/j.cgh.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–7. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 26.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: A learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–8. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 27.O'Toole D, Palazzo L, Arotçarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–4. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 28.Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: A prospective evaluation. Gastrointest Endosc. 2006;63:622–9. doi: 10.1016/j.gie.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Hamada T, Yasunaga H, Nakai Y, et al. Rarity of severe bleeding and perforation in endoscopic ultrasound-guided fine needle aspiration for submucosal tumors. Dig Dis Sci. 2013;58:2634–8. doi: 10.1007/s10620-013-2717-7. [DOI] [PubMed] [Google Scholar]