Abstract
Background and Objectives:
Standard endoscopic ultrasound-fine-needle aspiration (EUS-FNA) needles are in widespread use. Meaningful differences between the available needles have been difficult to identify. Recently, a new EUS needle (Shark Core®, Covidien, Dublin, Leinster, Ireland), has been introduced in an attempt to improve diagnostic accuracy, tissue yield, and to potentially obtain a core tissue sample. We performed a pilot study prospectively to evaluate this new needle when compared to a standard EUS-FNA needle.
Materials and Methods:
Analysis of the first 15 patients undergoing EUS-FNA with the Shark Core needle was performed and it was compared to EUS-FNA in 15 patients who underwent EUS-FNA with a standard needle.
Results:
The Shark Core needle required fewer needle passes to obtain diagnostic adequacy than the standard needle [(χ2(1) = 11.3, P < 0.001]. The Shark Core needle required 1.5 passes to reach adequacy, whereas the standard needle required three passes. For cases with cell blocks, the Shark Core needle produced diagnostic material in 85% of cases [95% confidence interval (CI): 54–98], whereas the standard needle produced diagnostic material in 38% of the cases (95% CI: 9-76). The Shark Core needle produced actual tissue cores 82% of the time (95% CI: 48–98) and the standard needle produced no tissue cores (95% CI: 0-71) (P = 0.03).
Conclusion:
This pilot study found that the Shark Core needle had a high rate of producing adequate cytologic material for the diagnosis of pancreatic and peri-pancreatic lesions sampled by EUS with fewer passes required to obtain a definitive diagnosis and with a high rate of tissue cores being obtained when compared to a standard FNA needle.
Keywords: Core tissue samples, endoscopic ultrasound-fine-needle aspiration (EUS-FNA) needle, EUS needle, Shark Core
INTRODUCTION
Over the past 20 years, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has become the most widely utilized technique to sample a variety of lesions including solid and cystic pancreatic masses, mediastinal and gastric lymph nodes, gastrointestinal submucosal lesions, and peri-rectal lesions. EUS-FNA is estimated to have a sensitivity of 75%-92% and a specificity of 82%-100%.[1]
Many different EUS-FNA needles are available, the vast majority of which are designed to obtain cytologic specimens. Standard EUS needles are in widespread use and work with a high level of clinical efficacy. Meaningful differences between the available needle types and sizes have been difficult to identify. A recent meta-analysis showed that there was little clinical difference between different EUS-FNA needle sizes when 19-gauge, 22-gauge, and 25-gauge needles were compared, and other studies have shown a similar lack of meaningful difference between needle sizes.[2,3,4] Differences in FNA technique (i.e., the use or lack of use of a stylet) have also been shown to have limited value on the final result.[5,6] Still, EUS-FNA is a complex process that is not uniformly standardized.[7]
EUS-FNA needles with novel tip shapes have been available for some time. These devices have been designed to attempt to obtain a core biopsy and not just cytology. One of these, Trucut® biopsy needle, Wilson Cook, Winston Salem, NC, USA has proven to be effective but does not have widespread use, largely due to issues regarding cost and technical ease of use, and is largely used as an adjunctive tool and/or in special situations.[8,9,10,11] Another device intended to produce a core sample (Procore®, Wilson Cook, Winston Salem, NC, USA) was found to be of a similar value to standard EUS-FNA needles, and did not reliably produce a core of tissue or have additional advantages over standard EUS-FNA needles.[2,12]
Recently, a new EUS needle (Shark Core®, Covidien, Dublin, Leinster, Ireland) has been introduced, which has a novel needle tip shape in an attempt to improve diagnostic accuracy, tissue yield, and to potentially obtain a core tissue sample via EUS. The needle tip design incorporates two sharp points of different lengths and a multifaceted bevel in an attempt to capture additional tissue, preferably in a core of tissue [Figure 1]. No clinical data are available on this needle at the time of this writing.
Figure 1.
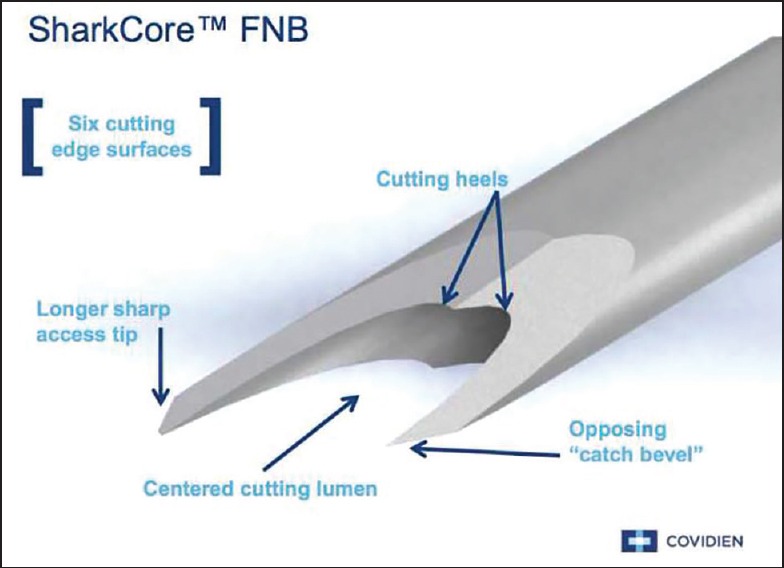
Image of tip of Shark Core needle (used with the permission of Covidien)
We performed a retrospective pilot study to evaluate the clinical value and efficacy of this needle when compared to a standard EUS-FNA needle.
MATERIALS AND METHODS
An analysis of the first 15 patients (between October 2014 and January 2015) to undergo EUS FNA with the Shark Core® needle at the University of Utah School of Medicine/Huntsman Cancer Center was performed. For comparison, 15 patients who underwent EUS-FNA performed by the same endoscopist using a standard needle (EchoTip®, Wilson Cook, Winston Salem, NC, USA) were included.
EUS-FNA with both needle types was performed in the standard manner using linear echoendoscopes. All EUS-FNA procedures were performed by a single endosonographer (DGA) with over 12 years of experience. The technique for both the needles was to insert the needle, remove the stylette, and take several slow passes (three to five). Rapid on-site evaluation (ROSE) by a cytopathologist was performed on all of the 30 included EUS-FNA cases. Slides were prepared for on-site evaluation using the rapid DiffQuik® stain; in some cases, alcohol-fixed slides were also submitted for Papanicolau staining. For standard needle specimens, FNA direct-smear slides were created at the time of the exam and excess needle rinse, if present, was submitted into a saline tube for cell block preparation. For Shark Core® specimens, touch or squash preparation slides were created and the core, if intact, was submitted for formalin fixation for cell block preparation. The technique for touch preparations was to either move the core fragment around the majority of the surface of a slide with a needle cap or to gently press the sample between two slides, and then place the core fragment into formalin or saline. The squash preparation entailed compressing the core fragment between two slides and using shear force (in the direction of the long axis of the slides) to help disperse the cells for immediate cytologic evaluation. This method usually disrupted the core fragment to the extent that no remaining intact fragment was available to be placed into formalin or saline. In some cases, the Shark Core® needle was rinsed in sterile saline and cell blocks were prepared from the rinse.
Each case was independently reviewed by three board certified cytopathologists (BLW, BEC, and JMW) for the following cytologic parameters on the aspirate smears or touch/squash preparations: overall cellularity [1 (low) to 3 (high)] and percentage of obtained cells that were lesional/representative (<25%, 26%-50%, and >50%), relative ease of interpretation [1 (difficult) to 3 (easy)]. Pathologic material and reporting records were also reviewed for each case to confirm the number of needle passes to achieve diagnostic adequacy, the presence or absence of diagnostic material on an hematoxylin and eosin (H and E) slide (from cell block, if prepared), whether a definitive diagnosis was able to be rendered, and the presence or absence of a true core of tissue (within the cell block, if prepared). Contingency tables for cellularity, production of diagnostic material in cell blocks, and production of cores were evaluated using Fisher's exact test. The number of needle passes was evaluated using the Kruskal-Wallis test. Statistical calculations were performed using Stat 13 (StatCorp LP, College Station, TX, USA).
This study was approved by our Institutional Review Board (IRB) at the University of Utah/ARUP Laboratories. It is to be noted that no consent was obtained due to the retrospective nature of the study, and no patients were contacted during the completion of this study.
RESULTS
Fifteen patients underwent either transgastric or transduodenal EUS-FNA with the Shark Core® needle. Five patients underwent EUS-FNA with a 25-gauge Shark Core® needle while 10 patients underwent EUS-FNA with a 22-gauge Shark Core® needle. Twelve patients underwent EUS-FNA for the evaluation of solid pancreatic lesions and three patients underwent EUS-FNA for the evaluation of peripancreatic adenopathy. The comparison cohort comprised 15 patients who underwent either trasgastric or transduodenal EUS-FNA with the EchoTip® (22-gauge) needle for evaluation of solid pancreatic lesions. A distribution of the final cytologic diagnosis for each needle type is given in Figure 2. Representative tissue samples from each needle are shown in Figures 3 and 4.
Figure 2.
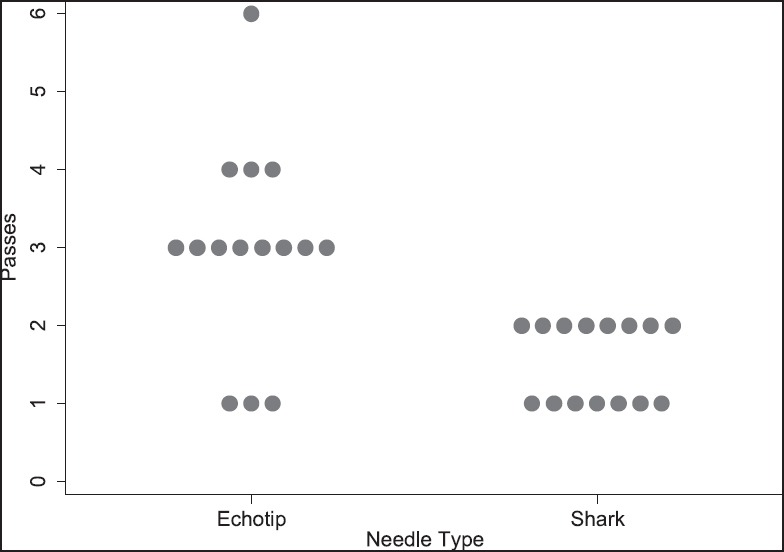
Comparison of needle passes by needle type
Figure 3.
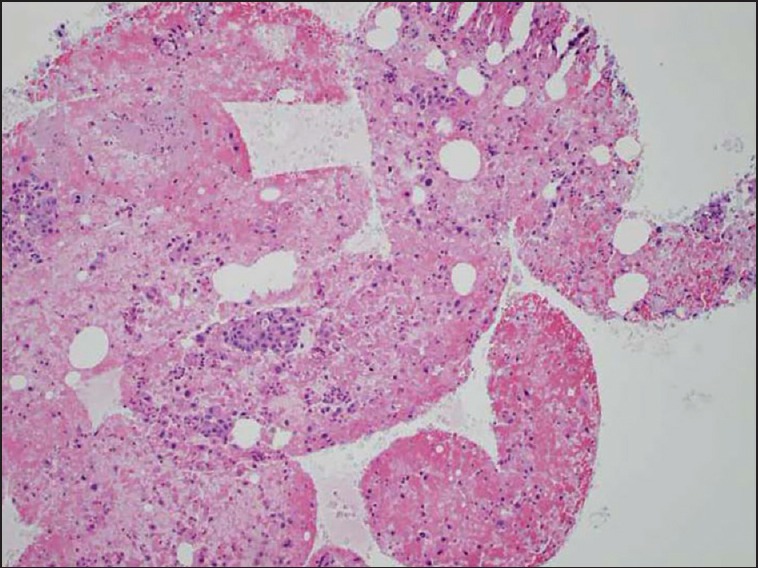
Cell block with a tissue core of diagnostic material. Hematoxylin and eosin (10×) stain of a cell block preparation from a case using the Shark Core® needle. The photomicrograph shows mucin producing malignant cells in groups and single cell distribution, diagnostic of adenocarcinoma
Figure 4.
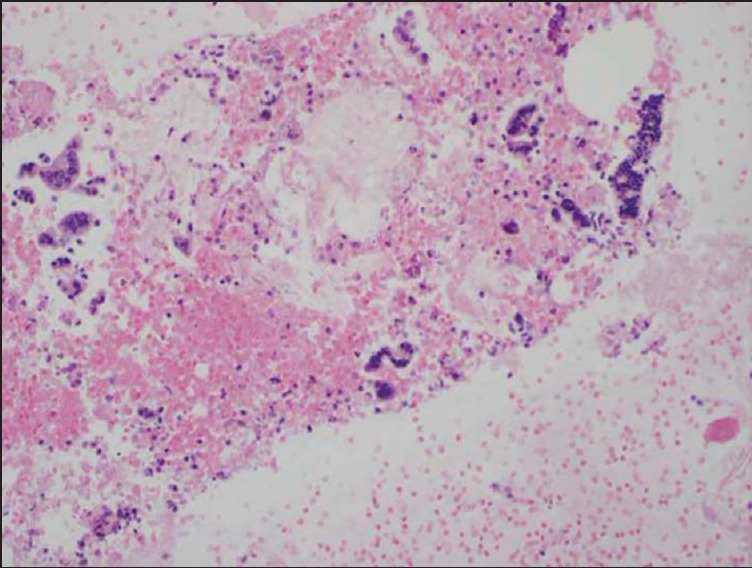
Cell block with diagnostic material without a tissue core. Hematoxylin and eosin (20×) stain of a cell block preparation from a case using the EchoTip® needle. The photomicrograph shows malignant-appearing strips of columnar cells consistent with adenocarcinoma. It is to be noted that the cell clusters are not present in a true tissue core and are instead dispersed within a red blood cell background
The Shark Core® needle required fewer needle passes to obtain diagnostic adequacy than the EchoTip® needle [(χ2(1) = 11.3, P < 0.001]. On an average, the Shark Core® needle required 1.5 passes to reach adequacy whereas the EchoTip® needle required three passes [Table 1]. Overall, 21 out of 30 cases had cell blocks prepared including 13/15 cases using the Shark Core® and 8/15 cases using the EchoTip® needle. Where cell blocks were prepared, the Shark Core® needle showed a trend toward increased production of diagnostic material within the blocks (P = 0.06). For cases with cell blocks, the Shark Core® needle produced diagnostic material in 85% of the cases [95% confidence interval (CI): 54-98], whereas the EchoTip needle produced diagnostic material in 38% of the cases (95% CI: 9-76) [Table 2]. When diagnostic material was present in the cell block, the Shark Core® needle produced actual tissue cores 82% of the time (95% CI: 48-98) and the EchoTip® needle produced no tissue cores (95% CI: 0-71) [Table 3]. Figures 2 and 3 show representative photomicrographs of diagnostic cell blocks with tissue cores and without tissue cores, respectively. The difference in the production of tissue cores was statistically significant (P = 0.03).
Table 1.
Comparison of needle passes for Shark Core® needle and EchoTip® needle
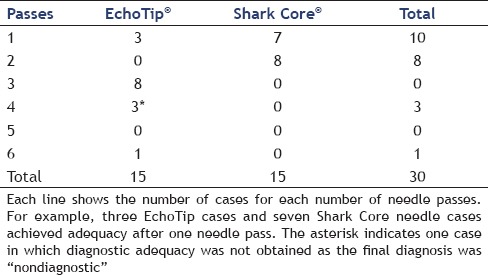
Table 2.
Production of diagnostic material in cell blocks
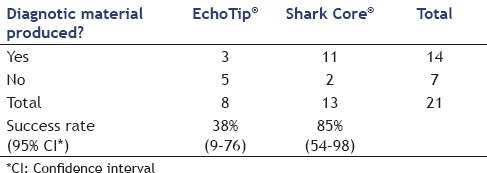
Table 3.
Presence of cores in cell block (given the presence of diagnostic tissue in blocks)
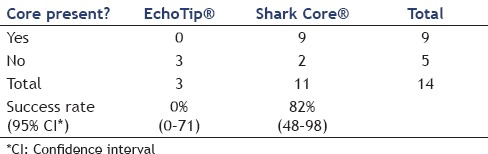
With respect to the retrospectively assessed cytologic parameters on the aspirate smears and touch/crush preparations, there was no significant difference between specimen cellularity, percentage of lesional material, or ease of interpretation between the two needle types (based on the combined blinded review by the three cytopathologist observers).
DISCUSSION
Our pilot investigation utilized the novel Shark Core® (Beacon Endoscopic) needle (22-gauge and 25-gauge) with a 4 cutting edge structure, which is designed to optimize tissue yield and coring potential during EUS-guided FNA procedures. Our preparations with the Shark Core® needle allowed simultaneous cytology material in all cases and diagnostic core biopsy material in a significant subset of cases where cell blocks were prepared [9 out of 13 (69%) cases in which blocks were made].
Fourteen out of 15 (93%) EUS-FNA cases of either solid pancreatic lesions or peri-pancreatic lymph nodes had definitive diagnoses rendered using the Shark Core® needle. By comparison, our control population of patients receiving EUS-FNA with a standard needle (EchoTip®, 22-gauge) had fewer overall cell blocks that were attempted with only three out of eight (38%) harboring diagnostic material and none demonstrating actual tissue cores. Cases performed with the standard needle yielded a similar, albeit slightly lesser rate [12 out of 15 (80%)] of definitively diagnostic samples.
Perhaps more important is the result that the cytologic material afforded on site by the Shark Core® needle allowed a significantly reduced number of needle passes to reach specimen adequacy (P < 0.001). The reduction in the number of endoscopist passes to adequacy was twofold less with the Shark Core® needle (average 1.5 passes/case) versus the standard needle (3.0 passes/case). Finally, the touch/squash preparations made from Shark Core® needle samples were at least equivalent in terms of overall cellularity, estimated amount of lesional cellularity, and ease of interpretation compared to the aspirate smears from the standard needle. Additional available tissue from each pass could be used for special stains, a cell block, or other testing as needed. Prior data show that the addition of a cell block to FNA samples may increase the sensitivity and negative predictive value for diagnosing pancreatic malignancy in patients with pancreatic masses who undergo EUS-FNA.[13]
The most common lesion to undergo EUS-FNA at our institution is a solid pancreatic mass. From a purely diagnostic standpoint, tissue cores with diagnostic lesional material are generally not necessary for the diagnosis of pancreatic adenocarcinoma, as the cytologic morphology for this diagnosis has very good sensitivity and specificity.[14] However, some of the well-differentiated pancreatic adenocarcinomas are not straightforward from a cytomorphologic standpoint, and increased lesional tissue in a cell block could allow the application of immunohistochemical markers (such as SMAD4) or show perineural invasion that could support a malignant diagnosis.[15] In addition, some primary pancreatic neoplasms such as pancreatic neuroendocrine tumors, acinar cell carcinomas, and solid pseudopapillary neoplasms have extensive cytomorphologic overlap; in these settings, a more robust cell block with diagnostic cores could provide a better likelihood of achieving a definitive diagnosis. Lastly, as we continue to move forward to the era of molecular diagnostics, it is possible that pancreatic malignancies will be assessed for biomarkers to aid in chemotherapeutic management. In general, from a pathologic/cytologic point of view, it is always advantageous to obtain more tissue and higher quality tissue. It is also always best if tissue samples are adequate enough for reliable ancillary tests to be performed, i.e., special stains.
EUS needles continue to be an area of development. The desire for a needle that could produce a core sample easily, reliably, and inexpensively has been longstanding. Current needles available to obtain a core sample are either costly or less then universally reliable.[2,9,10,11,16] It should be noted that the Shark Core® needle also did not obtain a core sample in all cases. When diagnostic material was present in the cell block, the Shark Core® needle produced actual tissue cores 82% of the time. Cost is also likely to be an issue as historically, needles that can potentially obtain a core sample are more expensive than standard EUS-FNA needles.
Our pilot study was based on a relatively small sample; however, the sample was sufficient to show a statistically significant difference between needles. Questions regarding sample size generally arise with negative results (i.e., did the study fail to detect a difference because the sample size was too low or because there truly no difference?). We obtained a positive result and it is very unlikely (P < 0.001) that a difference of this magnitude could have been obtained by chance. Thus, the sample size is not a limitation.
Our study has several limitations. First, it was conducted at a single site. Studies at additional sites will be required to establish the generalizability of our results. Our study was not randomized or blinded. Unfortunately, it is not possible to blind the endoscopist to the needle type and we believe it is unlikely that knowledge of the needle type would cause performance bias. Although the needle type was not randomized, there is no indication that there was a significant difference in the distribution of cases. To date, there is no published literature on the Shark Core® needle, and this study represents the first evaluation of its clinical and pathologic roles.
Overall, this pilot study found that the Shark Core needle had a high rate of producing adequate cytologic material for the diagnosis of pancreatic and peri-pancreatic lesions sampled by EUS with fewer passes required to obtain a definitive diagnosis and with a high rate of tissue cores being obtained when compared to a standard FNA needle. Larger studies, multicenter studies, and studies comparing the device to other EUS-FNA needles and other EUS needles designed to obtain a core sample are warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fabbri C, Polifemo AM, Luigiano C, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: A prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647–52. doi: 10.1016/j.dld.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Witt BL, Adler DG, Hilden K, et al. A comparative needle study: EUS FNA procedures using the HD ProCore(™) and EchoTip(®) 22-gauge needle types. Diagn Cytopathol. 2013;41:1069–74. doi: 10.1002/dc.22971. [DOI] [PubMed] [Google Scholar]
- 3.Songür N, Songür Y, Bırcan S, et al. Comparison of 19- and 22-gauge needles in EUS-guided fine needle aspiration in patients with mediastinal masses and lymph nodes. Turk J Gastroenterol. 2011;22:472–8. doi: 10.4318/tjg.2011.0322. [DOI] [PubMed] [Google Scholar]
- 4.Imazu H, Uchiyama Y, Kakutani H, et al. A prospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol Res Pract 2009. 2009:546390. doi: 10.1155/2009/546390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimeno-García AZ, Paquin SC, Gariépy G, et al. Comparison of endoscopic ultrasonography-guided fine-needle aspiration cytology results with and without the stylet in 3364 cases. Dig Endosc. 2013;25:303–7. doi: 10.1111/j.1443-1661.2012.01374.x. [DOI] [PubMed] [Google Scholar]
- 6.Wani S, Gupta N, Gaddam S, et al. A comparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Dig Dis Sci. 2011;56:2409–14. doi: 10.1007/s10620-011-1608-z. [DOI] [PubMed] [Google Scholar]
- 7.Tadic M, Stoos-Veic T, Kusec R. Endoscopic ultrasound guided fine needle aspiration and useful ancillary methods. World J Gastroenterol. 2014;20:14292–300. doi: 10.3748/wjg.v20.i39.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahnschaffe U, Ullrich R, Mayerle J, et al. EUS-guided trucut needle biopsies as first-line diagnostic method for patients with intestinal or extraintestinal mass lesions. Surg Endosc. 2009;23:2351–5. doi: 10.1007/s00464-009-0345-2. [DOI] [PubMed] [Google Scholar]
- 9.Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: A prospective study. Endoscopy. 2009;41:329–34. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 10.Kipp BR, Pereira TC, Souza PC, et al. Comparison of EUS-guided FNA and trucut biopsy for diagnosing and staging abdominal and mediastinal neoplasms. Diagn Cytopathol. 2009;37:549–56. doi: 10.1002/dc.21042. [DOI] [PubMed] [Google Scholar]
- 11.Storch I, Shah M, Thurer R, et al. Endoscopic ultrasound-guided fine-needle aspiration and Trucut biopsy in thoracic lesions: When tissue is the issue. Surg Endosc. 2008;22:86–90. doi: 10.1007/s00464-007-9374-x. [DOI] [PubMed] [Google Scholar]
- 12.Strand DS, Jeffus SK, Sauer BG, et al. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn Cytopathol. 2014;42:751–8. doi: 10.1002/dc.23116. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Lee SJ, Moon SH, et al. Incremental value of cell block preparations over conventional smears alone in the evaluation of EUS-FNA for pancreatic masses. Hepatogastroenterology. 2014;61:2117–22. [PubMed] [Google Scholar]
- 14.Cohen MB, Egerter DP, Holly EA, et al. Pancreatic adenocarcinoma: Regression analysis to identify improved cytologic criteria. Diagn Cytopathol. 1991;7:341–5. doi: 10.1002/dc.2840070404. [DOI] [PubMed] [Google Scholar]
- 15.Layfield LJ, Ehya H, Filie AC, et al. Utilization of ancillary studies in the cytologic diagnosis of biliary and pancreatic lesions: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 2014;11(Suppl 1):4. doi: 10.4103/1742-6413.133352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhutani MS, Koduru P, Lanke G, et al. The emerging role of endoscopic ultrasound-guided core biopsy for the evaluation of solid pancreaticmasses. Minerva Gastroenterol Dietol. 2015;61:51–9. [PubMed] [Google Scholar]


