Abstract
Assembly of initiation factors on individual replication origins at onset of S phase is crucial for regulation of replication timing and repression of initiation by S-phase checkpoint control. We dissected the process of preinitiation complex formation using a point mutation in fission yeast nda4-108/mcm5 that shows tight genetic interactions with sna41+/cdc45+. The mutation does not affect loading of MCM complex onto origins, but impairs Cdc45-loading, presumably because of a defect in interaction of MCM with Cdc45. In the mcm5 mutant, however, Sld3, which is required for Cdc45-loading, proficiently associates with origins. Origin-association of Sld3 without Cdc45 is also observed in the sna41/cdc45 mutant. These results suggest that Sld3-loading is independent of Cdc45-loading, which is different from those observed in budding yeast. Interestingly, returning the arrested mcm5 cells to the permissive temperature results in immediate loading of Cdc45 to the origin and resumption of DNA replication. These results suggest that the complex containing MCM and Sld3 is an intermediate for initiation of DNA replication in fission yeast.
INTRODUCTION
Initiation of DNA replication in eukaryotic cells is regulated through an ordered assembly of replication complexes at replication origins (reviewed in Kelly and Brown, 2000; Bell and Dutta, 2002; Kearsey and Cotterill, 2003). Replication origins are recognized and bound by the origin recognition complex (ORC). In the G1 phase of the cell cycle, MCM (minichromosome maintenance) complex composed of Mcm2-7 associates with replication origins depending on ORC, Cdc6/Cdc18, and Cdt1, forming a prereplicative complex (pre-RC). At the onset of S phase, several factors, including Cdc45 and Sld3, associate with pre-RC to form a preinitiation complex (pre-IC), which is capable of origin-unwinding, and promoting assembly of replication forks at replication origin.
The MCM complex is considered to be involved in unwinding of replication origins because a heterohexameric complex composed of Mcm4, 6, and 7 has a DNA helicase activity in vitro (Ishimi, 1997; Lee and Hurwitz, 2001). However, the helicase activity has not been detected with the Mcm2-7 heterohexamer, which seems to be the intrinsic MCM complex in cells. Thus, modification of the MCM complex and/or its association with other factors might be required for activation.
Cdc45 plays a crucial role in activation of replication origins. In Xenopus egg extracts, Cdc45 is required for unwinding of template DNA (Walter and Newport, 2000). S phase cyclin-dependent kinase (S-CDK) and Cdc7-Dbf4 kinase (Dbf4-dependent kinase [DDK]) are necessary for assembly of Cdc45 with pre-RC (Mimura et al., 2000; Zou and Stillman, 2000). Because some MCM subunits are efficient substrates for CDK or DDK in vitro (Sato et al., 1997; Fujita et al., 1998), their phosphorylation might have roles in assembly of Cdc45 with pre-RC. Furthermore, several factors such as Sld3, Dpb11, Mcm10, and the GINS complex are shown to be required for chromatin loading of Cdc45 (Masumoto et al., 2000; Kamimura et al., 2001; Van Hatten et al., 2002; Wohlschlegel et al., 2002; Gregan et al., 2003; Kubota et al., 2003; Takayama et al., 2003). SLD3 was initially isolated as a synthetic lethal gene with dpb11-1 in budding yeast and it exhibits a tight genetic interaction with CDC45 (Kamimura et al., 1998). Coimmunoprecipitation of Sld3 with Cdc45 depending on cross-linking was observed throughout the cell cycle and association of Sld3 and Cdc45 with origins depended on each other (Kamimura et al., 2001). In fission yeast, however, Cdc45 was coimmunoprecipitated with Sld3 without cross-linking selectively in S phase (Nakajima and Masukata, 2002). The mechanism of Cdc45-loading in either organism has yet to be elucidated.
In budding yeast, the CDC46/MCM5 gene has been identified as an extragenic suppressor for cdc45-1 (Hennessy et al., 1991). In fission yeast, in turn, the defects of the nda4-108/mcm5 mutation are suppressed by a mutation in the sna41/cdc45 gene (Miyake and Yamashita, 1998). The genetic interactions conserved among these species suggest that Mcm5 plays an important role in the interaction of MCM with Cdc45. Furthermore, the bob1-1/mcm5 mutation in budding yeast bypasses requirement of DDK for DNA replication (Hardy et al., 1997). The bob1-1/mcm5 mutation allows loading of Cdc45 onto origin in the absence of DDK and structural changes of origin DNA in the α-factor arrested G1 cells (Geraghty et al., 2000; Sclafani et al., 2002). These findings are consistent with involvement of Mcm5 in the interactions between MCM and Cdc45.
Replication origins are likely to contain information required to promote DNA replication, because replication initiates from fixed regions in almost all eukaryotes. Structures of replication origins are well understood in budding yeast. Budding yeast origins are composed of modular elements within a 100–200-base pair region. A consensus sequence, designated as the ARS consensus sequence (ACS), is essential for origin function (Marahrens and Stillman, 1992). The ACS is the binding site for ORC complex (Bell and Stillman, 1992) and the pre-RC is formed in the vicinity of ACS (Diffley et al., 1994). On the other hand, fission yeast, similar to higher eukaryotes, requires a long chromosome region as a replication origin. Short essential consensus sequences like the budding yeast ACS have not been found. Instead, stretches of asymmetrically placed adenine/thymine residues are essential for the origin activity on both the ARS plasmid (Clyne and Kelly, 1995; Dubey et al., 1996; Okuno et al., 1999) and the chromosome (Takahashi et al., 2003). Adenine/thymine stretches are the binding sites for ORC both in vitro (Kong and DePamphilis, 2001; Lee et al., 2001; Takahashi and Masukata, 2001; Kong and DePamphilis, 2002) and in vivo (Kong and DePamphilis, 2002; Takahashi et al., 2003). The ars2004, an efficient replication origin (Okuno et al., 1997), contains three essential regions I, II, and III in about a 1-kb segment. The regions I and III, consisting of adenine stretches, are collectively required for the ORC binding, whereas the nonadenine region II stimulates loading of MCM. Interestingly, MCM in pre-RC is localized preferentially to a region near the initiation site, which is distant from ORC-binding sites (Takahashi et al., 2003). Multiple ORC binding sites and a preferential MCM binding near the initiation site seem to be general organization of fission yeast replication origins, although MCM binds to the proximal region of one of the ORC binding sites in the ars3001 (Kong and DePamphilis, 2002). Hence, the organization of fission yeast origins resembles those in multicellular organisms, such as the chorion amplification origin in Drosophila, which consists of multiple DmORC binding regions and a preferential initiation site (Austin et al., 1999). These results suggest that fission yeast replication origins are good model system for understanding the complex replication origins in higher eukaryotes. Thus, it should be important to analyze assembly of replication components such as those in pre-IC on fission yeast origins.
In the present study, we examined molecular assembly of replication factors onto the replication origins in a fission yeast nda4-108/mcm5 mutant. We show here that the mcm5 mutation does not affect pre-RC formation but greatly reduces loading of Cdc45 onto origins. In contrast, Sld3 associates with origins in the mcm5 mutant, suggesting independence from Cdc45-loading. Sld3 colocalizes with Mcm6 within the origin during the mcm5-arrest. Immediately after return to the permissive temperature from the mcm5-arrest, Cdc45 associates with origins and DNA replication initiates. Thus, the complex containing MCM and Sld3 but not Cdc45 appears to be a physiological intermediate for initiation of DNA replication.
MATERIALS AND METHODS
Strains and Media
The Schizosaccharomyces pombe haploid strains used are listed in Table 1. h- nda4-108, h- nda1-376, and h- nda3-KM311 were gifts from Dr. M. Yanagida (Kyoto University, Japan). Fission yeast strains were cultured in complete YE medium (0.5% yeast extract, 3% glucose) and minimal EMM medium (Moreno et al., 1991). Media containing 2% agar were used for plating.
Table 1.
Schizosaccharomyces pombe strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| HM144 | h- nda1-376 | Our stock |
| HM5 | h- leu1-32 nda1-376 | M. Yanagida |
| YYY72 | h- nda1-376 Δcig2::ura4+ | This work |
| YYY51 | h- nda4-108 | Our stock |
| HM9 | h- leu1-32 nda4-108 | M. Yanagida |
| YYY84 | h- nda4-108 Δcig2::ura4+ | This work |
| YYY36 | h- cdc19-P1 | S. L. Forsburg |
| YYY74 | h- cdc19-P1 Δcig2::ura4+ | This work |
| HM130 | h- cdc21-M68 | S. E. Kearsey |
| YYY75 | h- cdc21-M68 Δcig2::ura4+ | This work |
| OGM1 | h- mis5-268 | M. Yanagida |
| YYY86 | h+ mis5-268 Δcig2::ura4+ | This work |
| YYY164 | h+ orp1-4 | P. Nurse |
| YYY163 | h+ orp1-4 Δcig2::ura4+ | This work |
| OGC17 | h- cdc18-K46 | H. Nishitani |
| YYY159 | h- cdc18-K46 Δcig2::ura4+ | This work |
| YYY63 | h- sna41-928 | Our stock |
| YYY67 | h- nda4-108 sna41-928 | Our stock |
| HM65 | h- leu1-32 sna41-928 | Our stock |
| HM59 | h- cdc25-22 | Our stock |
| YYY32 | h- nda4-108 cdc25-22 | This work |
| YYY97 | h- FLAG-cdc45 | T. Uchida |
| YYY100 | h- cdc25-22 flag-cdc45 | This work |
| YYY104 | h- nda4-108 cdc25-22 flag-cdc45 | This work |
| YYY136 | h+ ura4-D18 cdc25-22 5flag-sld3(ura4+) | R. Nakajima |
| YYY137 | h- ura4-D18 nda4-108 cdc25-22 5flag-sld3(ura4+) | This work |
| HM128 | h- leu1-32 nda3-KM311 | M. Yanagida |
| YYY173 | h- ura4-D18 nda3-KM311 5flag-sld3(ura4+) | This work |
| YYY156 | h- ura4-D18 sna41-928 nda3-KM311 5flag-sld3(ura4+) | This work |
The following are names of genes relevant to this study in S. pombe and in Saccharomyces cerevisiae: S. pombe: nda1+/cdc19+, cdc21+, nda4+, mis5+, sna41+; S. cerevisiae: MCM2, MCM4, MCM5, MCM6, CDC45.
Cell Cycle Synchronization
To arrest the cell cycle in G1 phase, cells were incubated at 30°C for 32 h in EMM medium lacking nitrogen source but supplemented with 250 μg/ml leucine, and then the cell cycle was resumed by addition of a nitrogen source. For synchronization from G2/M boundary, cdc25-22 and cdc25-22 mcm5 cells were grown at 28°C to log phase and then incubated at 36°C for 3 h. The cell cycle was restarted at 20°C, the restrictive temperature for mcm5. The nda3-KM311 and sna41-928/cdc45 nda3-KM311 cells grown at 28°C were incubated at 20°C for 4 h to block the cell cycle at M phase and then released at 37.5°C, the restrictive temperature for sna41-928/cdc45.
Epitope Tagging of cdc45+
To express FLAG-Cdc45 protein, the recombinant cdc45 gene carrying a FLAG epitope sequence between the first and second codons was made as described below. The N-terminal region of cdc45+ was amplified by PCR from pSNA41 containing a 3072-base pair Sau3AI genomic fragment of the cdc45+ (Nakajima and Masukata, 2002) using primers 5′-AACATATGGACTACAAGGACGACGATGACAAGTTCATCAAGAGATCCG-3′, containing an NdeI site and a FLAG epitope sequence, and 5′-AAGGATCCTCATTACTGGAATCGG-3′, and the product digested with NdeI and BamHI was cloned into pUCTL1. The upstream region of the cdc45+ gene was PCR-amplified from pSNA41 using primers 5′-GGAATTCCATATGTAATAAATAAACGTTACT-3′ and the M13 reverse primer. The PstI-NdeI fragment containing the upstream of cdc45+ and NdeI-SphI fragment containing the FLAG-tagged N-terminal region of cdc45 were cloned into PstI-SphI site of pBS-SNA41, resulting in an integration plasmid pFL-cdc45. A diploid strain ade6-M210/ade6-M216 ura4-D18/ura4-D18 leu1-32/leu1-32 cdc45+/cdc45::ura4+ was transformed with the BstBI-XbaI fragment of pFL-cdc45 and 5-fluoroorotic acid (5-FOA)-resistant transformants were selected. Integration of the flag-cdc45 gene at the cdc45+ locus was confirmed by PCR and a haploid cdc45+::flag-cdc45 strain was obtained by tetrad analysis. YYY97 h- cdc45+::flag-cdc45 without auxotrophy was used to construct YYY100 h- cdc25-22 flag-cdc45 and YYY104 h- nda4-108 cdc25-22 flag-cdc45 by mating with appropriate strains.
Immunoprecipitation and Immunoblotting
For immunoprecipitation of Mcm6, S. pombe haploid cells (5 × 108 cells) were sequentially washed with cold water and HNG buffer (10 mM HEPES-NaOH [pH 7.5], 50 mM sodium acetate, 10% glycerol, 1 mM dithiothreitol, 1 mM PMSF, 2 μg/ml leupeptin, 50 μg/ml TLCK, 2 μg/ml aprotinin) and then suspended in 0.4 ml of HNG buffer. Cells were disrupted with acid-washed glass beads by a bead beater (45 s, 6 times with 2-min intervals). Total cell extracts were obtained by centrifugation at 15,000 rpm for 10 min. For immunoprecipitation, the extracts were incubated at 4°C for 1.5 h with 20 μl of 10% (wt/vol) protein A-Sepharose (Sigma-Aldrich, St. Louis, MO) preincubated with rabbit anti-Mcm6 antibody. The Sepharose beads were washed five times with cold HNG buffer and then suspended in an equal volume of SDS-sample buffer (120 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 10% β-mercaptoethanol).
For immunoprecipitation of Cdc45, haploid cells (3 × 108 cells) were washed with cold water and EB buffer (25 mM HEPES-NaOH [pH 7.5], 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 2 mM EGTA, 2 mM dithiothreitol, 0.25% Triton X-100, 1 mM PMSF, 2 μg/ml leupeptin, 50 μg/ml TLCK, 2 μg/ml aprotinin) and then suspended in 0.5 ml of EB buffer. After cells were disrupted with glass beads, the extracts were incubated with 70 U of DNaseI (TAKARA) at 4°C for 30 min and then centrifuged at 15,000 rpm for 30 min to obtain the total extracts. A portion of the extracts was incubated at 4°C for 1.5 h with 50 μl of anti-rabbit IgG-conjugated magnetic beads (Dynal Biotech.) preincubated with rabbit anti-Cdc45 antibody. The magnetic beads were washed five times with cold EB buffer and suspended in SDS-sample buffer. Immunoblotting was performed as described previously (Takahashi and Masukata, 2001), with rabbit polyclonal antibodies against Mcm2, Mcm3 (Sherman and Forsburg, 1998), Mcm4 (Sherman et al., 1998), Mcm5, Mcm6, Mcm7 or Cdc45 at 1:200, 1:3000, 1:2000, 1:200, 1:2000, 1:500 or 1:1000 dilutions, respectively. Rabbit anti-Mcm3 and Mcm4 antibodies were gifts from Dr. S. Forsburg.
Chromatin Immunoprecipitation (ChIP) Assay and ChIP Scanning
ChIP assays were performed as described previously (Ogawa et al., 1999). Three sets of primers were used to amplify a segment in the ars2004 (239 base pairs) and adjacent nonorigin regions non-ARSa (295 base pairs) and non-ARSb (195 base pairs) on the chromosome II. Another three sets of primers to amplify ars3002 segment (246 base pairs) and nonorigin regions non-ARSc (195 base pairs) and non-ARSd (289 base pairs) on the chromosome III. The primers that amplify ars3002, non-ARSa and non-ARSb, are identical to those in the previous work (Ogawa et al., 1999). The nucleotide sequences of the remaining primers are ars2004F, 5′-CTTTTGGGTAGTTTTCGGATCC-3′; ars2004R, 5′-ATGAGTACTTGTCACGAATTC-3′; non-ARScF, 5′-GAATCTTCAGACCTTGCAGC-3′; non-ARScR, 5′-ACGGACTCCAACAACCATTTG-3′; non-ARSdF, 5′-TTGCTGGCAGGGGTGAGTTG-3′; and non-ARSdR, 5′-TATGCTCTTTTCCCTCCACC-3′. ChIP scanning analyses were carried out as described previously (Takahashi et al., 2003). The nucleotide sequences of the primers for amplification of segments 8A and non-ARS were ars2004–8AF, 5′-GAGTTAAGAGTGAAATAAGATTCATGGC-3′; ars2004–8AR, 5′-CCTTACCCTTTCCTACCTGCC-3′; non-ARS-F, 5′-TCGAAGATCCTACCGCTTTC-3′; and non-ARS-R, 5′-CTTGCGCTGAAGCTTTAGTAAAAG-3′.
RESULTS
CDK Modulation Suppresses Mutations in pre-RC Components except for nda4/mcm5
To identify factors that have functional interaction with MCM complex, we screened for fission yeast genes on a multicopy plasmid, which suppressed the cold sensitivity of the nda1-376/mcm2. We thereby obtained the rum1+ gene, encoding an inhibitor of cyclin-dependent kinase (CDK; Figure 1A). Because Rum1 is required for maintenance of the G1 phase by inhibiting the CDK kinase activity (Benito et al., 1998), we speculate that the suppression was due to transient inhibition of CDK. Consistent with this idea, deletion of the cig2+ gene (cig2Δ), encoding a B-type cyclin that controls entry into S phase (Mondesert et al., 1996), suppressed the growth defect of the mcm2 mutant at restrictive temperatures (Figure 1B). It has been shown that deletion of cig2 suppresses the growth defect of cdc19-P1/mcm2, allelic to nda1-376/mcm2, cdc18-K46 and cdc21-M68/mcm4 (Jallepalli et al., 1997; Lopez-Girona et al., 1998; Grallert et al., 2000). In addition, we here found that mis5-268/mcm6 and orp1-4/orc1 were suppressed by cig2Δ or by the multicopy rum1+ (unpublished data). A possible explanation for such wide-spectrum suppression is that extended G1 phase by transient inhibition of CDK provides time to form pre-RC in the mutants that have defects in pre-RC formation. Indeed, the nda1-376/mcm2 and orp1-4/orc1 mutants are defective in chromatin loading of Mcm6 (T. Asahara and H. Masukata, unpublished results). An exception that was not suppressed by the multicopy rum1+ or cig2Δ was the nda4-108/mcm5 mutation (Figure 1, A and B). Therefore, we hypothesized that the mcm5 mutation may have a defect rather in a later step than the pre-RC formation.
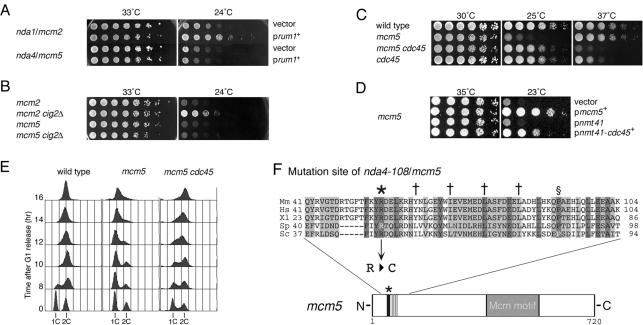
Figure 1.
Genetic interactions between mcm5+ and cdc45+. (A) Suppression of mcm2 and mcm5 by multicopy rum1+. Five-fold serial dilutions of nda1-376/mcm2 leu1 and nda4-108/mcm5 leu1 cells carrying a vector or rum1+ plasmid were spotted onto EMM plates and incubated at permissive (33°C) and restrictive (24°C) temperatures. (B) Effects of cig2Δ on the mcm2 and the mcm5 mutations. Serial dilutions of cig2+ and cig2Δ derivatives of mcm2 and mcm5 cells were spotted onto YE plates as in A and their growth was examined. (C) Suppression of mcm5 by the cdc45 mutation. Growth of wild-type (972), mcm5, sna41-928/cdc45, and mcm5 cdc45 cells was examined as in B. (D) Effects of overexpression of Cdc45 on mcm5. Growth of mcm5 leu1 derivatives carrying vector (ars2004-LEU2), pmcm5+, pnmt41-vector and pnmt41-cdc45+ plasmids was examined as in A. (E) Effects of the cdc45 mutation on DNA replication in the mcm5 mutant. Wild-type (972), mcm5, and mcm5 cdc45 cells arrested in G1 phase by culturing at 30°C in EMM medium lacking a nitrogen source were released at 20°C by adding a nitrogen source, and their DNA contents were analyzed by flow cytometry. Positions of 1C and 2C DNA are indicated. Minor peaks at 2C DNA content at 0 h correspond to cells arrested in G2 phase under conditions used. (F) Site of the nda4-108/mcm5 mutation. Amino acid sequences of N-terminal regions of Mcm5 of mouse (Mm), human (Hs), Xenopus laevis (Xl), S. pombe (Sp), and Saccharomyces cerevisiae (Sc), respectively, are aligned for maximum homology. Identical amino acids are shaded and similar amino acids are boxed in gray. The asterisk indicates the position of the amino acid altered by the nda4-108 mutation. Leucine residues in a putative leucine-zipper motif and the mutation site for the S. cerevisiae bob1-1/mcm5 are indicated by † and §, respectively (Hardy et al., 1997).
DNA Replication in the mcm5 Mutant Is Rescued by a Mutation in the cdc45
It has been shown that the defects of nda4/mcm5 mutation are suppressed by an extragenic mutation in the sna41/cdc45 gene, and the second mutation itself endows the temperature-sensitive growth (Miyake and Yamashita, 1998). In an independent screening, we obtained another allele of sna41/cdc45, identical to the sna41-928/cdc45 mutation (S. Miyake, personal communication). The sna41-928/cdc45 mutation suppressed the growth defect of mcm5 mutant at 25°C and, in turn, the temperature sensitivity of the sna41-928/cdc45 mutation was enhanced by the mcm5 mutation (Figure 1C). In addition, overexpression of cdc45+ from the inducible promoter (nmt41) partially suppressed the cold sensitivity of the mcm5 mutant (Figure 1D). These genetic observations suggest that the mcm5 mutant has a defect in a step where Cdc45 functions.
To test whether the cdc45 mutation rescued DNA replication of the mcm5 mutant, DNA contents of mcm5 and mcm5 cdc45 cells were examined after synchronization of the cell cycle from G1 phase by depleting nitrogen source (see MATERIALS AND METHODS). Wild-type, mcm5, and mcm5 cdc45 cells arrested in G1 phase were released at the restrictive temperature for the mcm5 by adding a nitrogen source. The results of flow cytometry analysis showed that wild-type cells with 1C DNA content shifted to 2C DNA cells by 10 h after release, whereas proportion of mcm5 cells with 1C DNA did not decrease significantly even at 16 h (Figure 1E), showing a defect in an early step of DNA replication as described previously (Miyake et al., 1993). In contrast to the results of mcm5 cells, 1C DNA peak of mcm5 cdc45 cells gradually shifted to 2C at 16 h, although the increment of DNA content was slower than that in wild-type cells. These results show that DNA replication in the mcm5 mutant is rescued in some extent by the cdc45 mutation.
Determination of the nucleotide sequence of the nda4-108/mcm5 mutant gene revealed that substitution of cysteine for a conserved arginine residue at position 50 from the N-terminus of Mcm5 (R50C; Figure 1F). The mutation is located proximal to the putative leucine zipper motif. Because leucine-zippers are considered to be involved in protein-protein interactions, the mcm5 mutation may affect possible interactions between the N-terminus of Mcm5 and other factors for initiation of DNA replication.
The mcm5 Mutation Does not Affect MCM Complex Formation
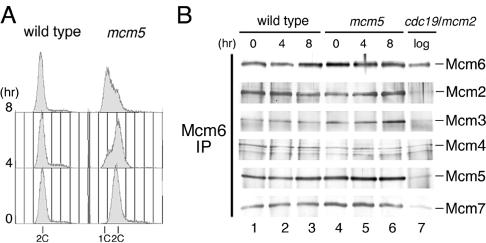
To determine the step affected by the mcm5 mutation at a molecular level, we first examined MCM complex formation. It has been reported that MCM complex is unstable in the cdc19-P1/mcm2 mutant (Sherman et al., 1998). Exponentially growing wild-type and mcm5 cells were cultured at 20°C for 4 and 8 h. The results of flow cytometry analysis showed that mcm5 cells with 1C DNA content accumulated at 8 h at 20°C, showing that cells were arrested with unreplicated DNA (Figure 2A). To examine whether MCM complexes were maintained, Mcm6 was immunoprecipitated with polyclonal antibody and analyzed by Western blotting with antibodies against various MCM subunits. The results in Figure 2B showed that all other components of MCM complex, Mcm2, 3, 4, 5, and 7, were coprecipitated with Mcm6 from the mcm5 extracts at similar efficiencies with those from wild-type extracts. As reported previously, amounts of Mcm2, Mcm3, 5, and 7 coprecipitated with Mcm6 from the cdc19-P1/mcm2 extracts were greatly reduced, even when cultured at a permissive temperature (Sherman et al., 1998; Figure 2B, lane 7). These results show that MCM complexes containing the mutant Mcm5 are stably maintained under restrictive conditions.
Figure 2.
Stable MCM complexes are maintained in the mcm5 mutant. (A) Wild-type (972) and mcm5 cells grown at 33°C were incubated at 20°C, and DNA contents were analyzed by flow cytometry. (B) Immunoprecipitates with anti-Mcm6 antibody from the extracts of wild-type and mcm5 cells cultured as in A were analyzed by Western blotting with antibodies against Mcm2, Mcm3, Mcm4, Mcm5, Mcm6, and Mcm7. An extract prepared from exponentially growing cdc19-P1/mcm2 cells was also analyzed in lane 7.
Cell Cycle–specific Interaction between Cdc45 and MCM Is Impaired by the mcm5 Mutation
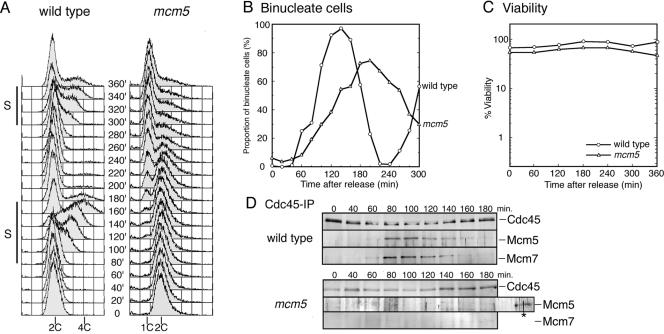
On the basis of tight genetic interactions between mcm5+ and cdc45+, we tested the possibility that physical interaction between Mcm5 and Cdc45 was affected by the mcm5 mutation. Because MCM-Cdc45 interaction was observed only in G1 and S phases in fission yeast (Uchiyama et al., 2001b), the cell cycle was synchronized using the cdc25-22 mutation, which causes cell cycle arrest at the G2/M boundary (Russell and Nurse, 1986). The cdc25 (wild-type for mcm5) and mcm5 cdc25 cells grown at 28°C, a permissive temperature for both mutations, were first incubated at 36°C for 3 h for arrest and then released into 20°C. Progression of the cell cycle was monitored by assessing the proportion of cells with divided nuclei (Figure 3B). In fission yeast, nuclear division is not immediately followed by cytokinesis, which occurs at a similar time with S phase of the next cell cycle. Thus, binucleate cells correspond to those in G1-S phases. For wild-type cells, the proportion of binucleate cells increased from 60 min with a peak at 140 min, and the second increase was observed from 260 min (Figure 3B). In the mcm5 mutant, the proportion of binucleate cells increased from 80 min and peaked at 200 min with a slight delay for unknown reasons (Figure 3B). Flow cytometry analysis of wild-type showed that cells 2C-4C DNA content appeared during 100–160 min and 300–360 min, indicative of S phases (Figure 3A, left). In contrast, for the mcm5 mutant 1C DNA cells increased from 180 min and remained even after 360 min (Figure 3A, right). Increase of 1C DNA cells well correlates with decrease in proportion of binucleate cells (Figure 3, A and B). These results show that the mcm5 cells arrest without significant DNA synthesis. It should be noted that mcm5 cells maintained their viability at the restrictive temperature even at 360 min (Figure 3C), suggesting that the mutation does not cause an irreversible defect.
Figure 3.
Coimmunoprecipitation of MCM subunits with Cdc45 is reduced in the mcm5 mutant. Wild-type (cdc25-22) and mcm5 (mcm5 cdc25-22) cells grown in EMM medium at 28°C were incubated at 36°C for 3 h to arrest at the G2/M boundary. Cells were then shifted to 20°C, which is the restrictive temperature for mcm5. (A) DNA contents analyzed by flow cytometry are shown. Approximate periods of the first and the second S phases are indicated along with positions of 1C, 2C, and 4C DNA. (B) Percentages of binucleate wild-type and mcm5 cells are shown by circles and triangles, respectively. (C) Viability of wild-type (○) and mcm5 (▵) cells was determined as percentage of number of colonies formed at the permissive temperature among cell numbers measured by Sysmex F-520. (D) The immunoprecipitates with anti-Cdc45 antibody were analyzed by Western blotting with antibodies against Cdc45, Mcm5, and Mcm7. The immunoprecipitates from wild-type cells at 80 min was included as a positive control in a lane marked by an asterisk.
Extracts prepared at indicated time points were treated with DNaseI to eliminate interactions mediated by chromosome DNA, and then Cdc45 was immunoprecipitated with anti-Cdc45 polyclonal antibody. The results of Western blotting showed that Mcm5 and Mcm7 subunits were coimmunoprecipitated with Cdc45 in wild-type extracts specifically at 80–140 min (Figure 3D), consistent with previous observations (Uchiyama et al., 2001b). In contrast, neither Mcm5 nor Mcm7 was pulled down with Cdc45 from the mcm5 extracts at any time point (Figure 3D). These results show that interaction between MCM complex and Cdc45 was greatly reduced in the mcm5 mutant.
Cdc45-loading onto Replication Origins Is Diminished in the mcm5 Mutant
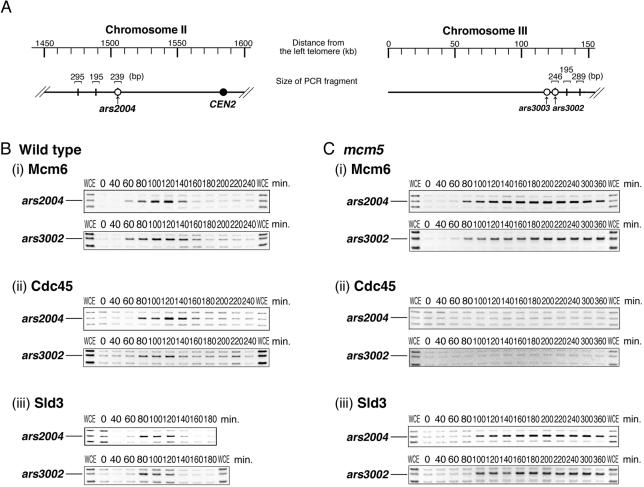
To examine the effect of the mcm5 mutation on origin-association of MCM and Cdc45, we used a chromatin-immunoprecipitation (ChIP) assay. The cell cycle was synchronized as described above using the cdc25 mutation. DNA immunoprecipitated with Mcm6 or FLAG-Cdc45 was used for PCR to determine the enrichment of the ars2004 or ars3002, efficient replication origins on chromosomes II and III, respectively (Zhu et al., 1994; Okuno et al., 1997). We used three sets of primers to amplify ars2004 or ars3002, and two adjacent nonorigin regions as controls (Figure 4A). PCR amplification with these primers from the total cellular DNA prepared without immunoprecipitation yielded similar amounts of products (Figure 4, B and C, lanes WCE). On the other hand, the ars2004 and the ars3002 fragments were preferentially amplified from Mcm6-IP DNA of wild-type during 60–140 min and then diminished (Figure 4Bi), whereas surrounding bands were not significantly amplified at any time point, showing that Mcm6 associated preferentially with the origins during G1-S phase, as described previously (Ogawa et al., 1999). In contrast, when Mcm6-IP DNA from mcm5 cells was used as a template, the ars2004 and the ars3002 fragments were amplified at 80 min and did not decrease even at 360 min (Figure 4Ci). These results indicate that Mcm6 persistently associates with the origins in mcm5 cells. DNA immunoprecipitated with the anti-Mcm2 antibody yielded similar results as shown for Mcm6-IP (unpublished data). Taken together, the MCM complex persistently associates with replication origins in the mcm5 mutant. The findings demonstrate that the mcm5 mutant does not affect pre-RC formation but has a defect in a later step.
Figure 4.
Sld3 efficiently associates with origins, whereas Cdc45 does not in the mcm5 mutant. Wild-type and mcm5 cells were arrested at G2/M boundary and released at 20°C as described for Figure 3. (A) Positions of segments examined by ChIP analysis are shown along with the distance from the left telomere. PCR reactions were carried out using three sets of primers that amplify ars2004 or ars3002 origin and adjacent nonorigin regions on same chromosome. Synchronized wild-type (B) and mcm5 (C) cells taken at indicated time points were cross-linked and DNA fragments immunoprecipitated with antibodies were analyzed by PCR with three sets of primers. PCR products amplified from total cellular DNA without immunoprecipitation are shown in lane WCE. Positions of ars2004 (239 base pairs) and ars3002 (246 base pairs) bands are indicated. DNA was immunoprecipitated with anti-Mcm6 antibody (i) and with anti-FLAG antibody (ii) from cell extracts of YYY100 (cdc25 flag-cdc45) and YYY104 (mcm5 cdc25 flag-cdc45). Sld3-associated DNA (iii) was immunoprecipitated with anti-FLAG antibody from YYY136 (cdc25 5flag-sld3) and YYY137 (mcm5 cdc25 5flag-sld3).
To examine association of Cdc45 with origins in mcm5 cells, we used strains in which the cdc45+ gene was replaced with FLAG-tagged cdc45. As shown in Figure 4Bii, the ars2004 and the ars3002 fragments were selectively amplified from the FLAG-Cdc45-IP DNA in wild-type cells (cdc25 flag-cdc45) at 80–140 min. In contrast, in mcm5 cells, the ars2004 and the ars3002 fragments were not amplified significantly from FLAG-Cdc45-IP DNA at any time point (Figure 4Cii). These results show that cell cycle–specific association of Cdc45 with the origins is greatly reduced in mcm5 cells.
Sld3 Binds to Replication Origins without Cdc45 in the mcm5 Mutant
Sld3 is a factor that has tight genetic interactions with Cdc45 and is required for origin-loading of Cdc45 in both fission yeast and budding yeast (Kamimura et al., 2001; Nakajima and Masukata, 2002). In budding yeast loading of Sld3 onto the origin depends on Cdc45. Thus, we tested whether loading of Sld3 onto origins was also impaired in the mcm5 mutant, using strains with FLAG-tagged Sld3. FLAG-Sld3-ChIP analyses were carried out after synchronization as described above. In wild-type cells, the ars2004 and the ars3002 fragments were preferentially amplified at 80–120 min, showing Sld3-association with the origin during S phase (Figure 4Biii). In contrast, Sld3 in mcm5 cells started to associate with the origins at 100 min and remained even at 360 min (Figure 4Ciii). These results show that Sld3 efficiently binds to the origins without origin-association of Cdc45.
Loading of Cdc45 and DNA Replication upon Return to Permissive Condition
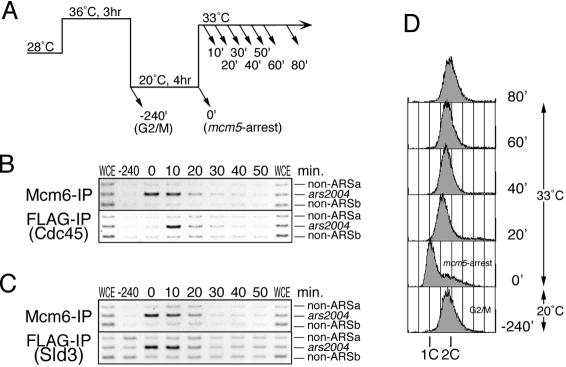
Because the mcm5 mutant retains the viability at the restrictive temperature, the process of initiation of DNA replication might be arrested with a physiological intermediate in the mcm5 mutant. To assess this possibility, we examined loading of Cdc45 and subsequent dissociation of Mcm6 and Sld3 from the origin, when the arrested mcm5 cells were returned to the permissive temperature (Experimental scheme in Figure 5A). The ChIP analysis with anti-Mcm6 antibody and anti-FLAG(Sld3) antibody showed that the ars2004 fragment, which was amplified efficiently at the arrested condition, clearly diminished at 20 min, showing efficient translocation or dissociation of Mcm6 and Sld3 from the origin (Figure 5, B, top panel, and C). In contrast, by FLAG-Cdc45-IP, the ars2004 fragment, which was not amplified in mcm5 arrested cells (0 min), was preferentially amplified at 10 min after return to the permissive temperature, and then diminished (Figure 5B, bottom panel). These results indicate that Cdc45 binds to the origin immediately after Mcm5 function is restored. Consistent with these results, the DNA content of the mcm5 cells increased from 1C to 2C within 40 min after return to the permissive temperature (Figure 5D), suggesting that replication of chromosome DNA is resumed in a short period. Taken together, it is likely that the complex formed in the mcm5 mutant can readily resume the normal initiation.
Figure 5.
Quick restoration of initiation reaction from mcm5-arrest. (A) Experimental scheme. mcm5 (mcm5 cdc25) cells were first arrested at G2/M boundary by incubation at 36°C for 3 h and then incubated at 20°C for 4 h to arrest at Mcm5-execution point. Cells were then released from the mcm5-arrest at 33°C and samples were collected at indicated time points. (B) Synchronized mcm5 (mcm5 cdc25 flag-cdc45) cells as in A were subjected to immunoprecipitation with anti-Mcm6 and anti-FLAG antibodies. (C) Samples were same as in B except that FLAG-Sld3 was immunoprecipitated with anti-FLAG antibody. (D) DNA contents were analyzed by flow cytometry for samples collected in B.
Proficient Origin Association of Sld3 without Cdc45 in the cdc45 Mutant
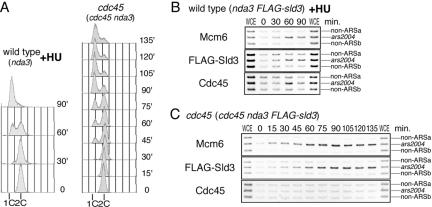
If the complex containing MCM and Sld3 without Cdc45 observed in the mcm5 mutant is a physiological one, it could be observed in the other conditions. To test this possibility, we examined association of initiation factors in the temperature-sensitive cdc45 mutant, which also maintains the viability at the restrictive temperature (R. Nakajima and H. Masukata, unpublished results). For synchronization, we used a cold-sensitive nda3 mutation, which is defective in polymerization of β-tubulin (Hiraoka et al., 1984). The sna41-928/cdc45 nda3 flag-sld3 and nda3 flag-sld3 (wild-type for cdc45) cells grown at 28°C were incubated at 20°C for 4 h for M phase arrest, and then released at 37.5°C, which is the restrictive temperature for the cdc45. The nda3 flag-sld3 (as a control) cells were released in the presence of hydroxyurea (HU), which depletes the nucleotide pool and perturbs replication fork progression in the early S phase. Flow cytometry analysis showed that 1C DNA cells accumulated in both HU-arrested wild-type and cdc45 cells (Figure 6A). At the indicated time points, DNA recovered by immunoprecipitation with anti-Mcm6, anti-FLAG and anti-Cdc45 antibodies, respectively, were analyzed by PCR. At 0 min, both wild-type and cdc45 cells being arrested at metaphase, no significant amplification of ars2004 fragment was observed from Mcm6-IP or FLAG-Sld3-IP or Cdc45-IP (Figure 6, B and C). In HU-arrested wild-type cells, ars2004 fragment was preferentially amplified at 60–90 min from Mcm6-IP, FLAG-Sld3-IP and Cdc45-IP, showing association of these proteins with the replication origin at S phase (Figure 6B). In contrast, the ars2004 fragment was not preferentially recovered at any time point by Cdc45-IP from the cdc45 mutant, whereas the recovery of the fragment by Mcm6-IP or Sld3-IP increased during 15–60 min and remained stable (Figure 6C). These results indicate that the cdc45 mutant has a defect in Cdc45-loading but allows proficient association of Sld3 with the origin, confirming independent loading of Sld3 from Cdc45.
Figure 6.
Sld3 associates with the origin in the cdc45 mutant. Wild-type (nda3 5flag-sld3) cells grown at 28°C were incubated at 20°C for 4 h to arrest in M phase and incubated at 37.5°C in the presence of 12 mM HU, whereas cdc45 (cdc45 nda3 5flag-sld3) cells synchronized similarly were incubated at 37.5°C without HU. (A) DNA contents were analyzed by flow cytometry. Cell extracts of wild-type (B) and cdc45 (C) were divided into three and subjected to immunoprecipitation with anti-Mcm6, anti-FLAG (Sld3), and anti-Cdc45 antibodies, respectively. DNA recovered by immunoprecipitation was analyzed by PCR as described for Figure 4.
Colocalization of Sld3 with Mcm6 in the Origin during mcm5 Arrest
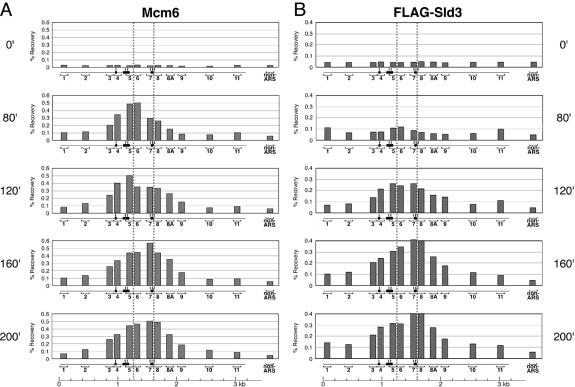
We have previously shown that MCM complex is located in a narrow region distant from ORC-binding sites within ars2004 in G1-arrested cells (Takahashi et al., 2003). Here, we addressed whether Sld3 colocalizes with MCM in mcm5-arrested cells, which allow us to map the location of Sld3. We adopted the ChIP-scanning method using 12-primer sets amplifying within ars2004 as well as non-ARS control primer sets (Figure 7) as described previously (Takahashi et al., 2003). Recoveries of DNA fragments by Mcm6-IP and Sld3-IP from synchronized mcm5 cdc25 flag-sld3 cells were determined by quantitative PCR analyses. When cells were arrested at the G2/M boundary (0 min), no region was significantly recovered by Mcm6-IP or Sld3-IP (Figure 7, A and B, top panels). At 80 min after release, origin region was recovered by Mcm6-IP several times more efficiently than non-ARS control fragment (Figure 7A). An apparent peak of the recovery resides at segments 5 and 6, showing binding of Mcm6 at a narrow region. These results are very similar to those described previously for pre-RC in G1 arrested cells (Takahashi et al., 2003). At this time point, Sld3-IP did not enrich any specific fragment (Figure 7B), showing the absence of Sld3 in pre-RC. At 120 min, however, recovery of regions of ars2004 by Sld3-IP increased, as distribution of Mcm6 became slightly broader than that at 80 min. At 160 and 200 min, recoveries of segments 7–9 by Mcm6-IP and Sld3-IP became higher than other regions and the profiles of Sld3 and Mcm6 localization were almost identical (Figure 7, A and B). These results show that Sld3 colocalizes with Mcm6 within the origin in mcm5-arrested cells. It should be noted that the peak of Mcm6 localization shifted by ∼300 base pairs from segments 5–6 to 7–8 (indicated by two broken lines in Figure 7, A and B). This may indicate that a significant fraction of Mcm6 changes its localization within the origin as Sld3 associates.
Figure 7.
Colocalization of Mcm6 and Sld3 within the ars2004. The mcm5 cdc25 5flag-sld3 cells arrested at G2/M boundary as described for Figure 3 were released at 20°C. Total cellular DNA and DNA immunoprecipitated with anti-Mcm6 and anti-FLAG (Sld3) were prepared at the indicated time points, serially diluted and used as the templates for PCR amplification with 12 primer sets within the ars2004 and a primer set in the non-ARS region. PCR products were separated by 2% agarose gel electrophoresis and the corresponding DNA bands stained with ethidium bromide were quantitated with LAS1000-plus Image analyzer (Fuji Film). Recoveries of segments by immunoprecipitation with Mcm6 (A) and with FLAG-Sld3 (B) determined as the ratio of immunoprecipitated DNA against total DNA are presented by histograms above the corresponding regions. Locations of three essential regions for ARS activity and 12 fragments amplified by PCR are indicated below the panels. Peak positions of Mcm6-localization in G1 and mcm5-arrested cells are shown by vertical broken lines between fragments 5–6 and 7–8, respectively. A representative result from three independent experiments is shown.
DISCUSSION
At the onset of S phase, the pre-RC (prereplicative complex) formed on replication origin is converted into pre-IC (preinitiation complex), which is capable of origin DNA unwinding and of assembly of DNA polymerases to initiate DNA replication. DNA helicase activity of Mcm2-7 complex should be activated during pre-IC formation. Several factors such as Cdc45, Sld3, Dpb11, Mcm10, and GINS complex as well as DDK and S-CDK kinases are involved in this step. However, because origin-loading of these factors appears to be mutually dependent, little is known about the precise process of pre-IC formation and MCM activation. In this report, taking advantage of a point mutation that caused a defect in a specific function of the gene product, we were able to identify a novel step in pre-IC assembly in fission yeast. In the nda4/mcm5 mutant, which does not affect MCM complex stability or pre-RC formation, loading of Cdc45 is impaired, but Sld3 can proficiently associate with the origin. Furthermore, the sna41/cdc45 mutation reduces loading of Cdc45 onto the origin without affecting Sld3-loading. These results strongly suggest that origin-association of Sld3 is a step independent from Cdc45-loading. ChIP-scanning experiments revealed that Sld3 colocalizes with Mcm6 in the origin in the mcm5 mutant. The complex containing MCM and Sld3 but not Cdc45 might be a physiological intermediate for the initiation of DNA replication, because Cdc45-loading and DNA replication are resumed immediately after return to permissive conditions.
Involvement of the N-terminal Region of Mcm5 in Interactions with Cdc45
It has been previously shown that defects of the nda4-108/mcm5 mutation are suppressed by a mutation in the sna41+/cdc45+ gene (Miyake and Yamashita, 1998). The results of suppression of mcm5 mutation by overexpression of Cdc45 in addition to synthetic enhancement of temperature sensitivity of cdc45 by mcm5 presented in this work provide strong evidence for a tight genetic interaction between mcm5+ and cdc45+ (Figure 1, C and D). This genetic interaction suggests that physical interaction between Mcm5 and Cdc45 is affected by the mcm5 or cdc45 mutation. Indeed, we demonstrated that coprecipitation of Mcm5 and Mcm7 with Cdc45 was greatly reduced in the mcm5 mutant (Figure 3D). This could be the cause for failure in origin-loading of Cdc45 (Figure 4C). Consistent with the results of genetics, the cdc45 mutation that rescues the replication defect of mcm5, in turn, causes a defect in Cdc45-loading at its restrictive temperature (Figure 6C). These results suggest that the interaction between Mcm5 and Cdc45 is affected by these mutations.
Based on the results of previous studies and those presented here, conditional lethal mcm mutations are classified into three groups due to the defects in MCM functions. First group includes mutations, such as cdc19-P1/mcm2 (Sherman et al., 1998), that affect stability of the MCM complex. The mutations in the second group, such as nda1-376/mcm2, have a defect in loading of MCM complex, which is stable, onto the origin (Y. Yamada, unpublished results). The third group affects neither MCM complex stability nor MCM-loading, but is defective in a later stage. The nda4-108/mcm5 belongs to this group. In budding yeast, all the mutations reported, including cdc46-1 that has genetic interaction with CDC45, appear to belong to the first group (Hennessy et al., 1990). Thus, the effects of nda4-108/mcm5 mutation on the initiation process might be allele specific, although no other mcm5 mutation is available in fission yeast to test this possibility.
The mcm5 mutation alters a conserved amino acid residue in the N-terminal region of Mcm5 (Figure 1F). A leucine zipper motif, which may be involved in protein-protein and DNA-protein interactions, is predicted near the mcm5 mutation. Furthermore, the budding yeast mcm5-bob1 mutation has been mapped proximal downstream of the leucine zipper motif (Hardy et al., 1997; Figure 1F). The mcm5-bob1 bypasses the requirement of DDK for initiation of DNA replication and stimulates association of Cdc45 with origins in G1 phase (Sclafani et al., 2002). Studies of Archaea MCM structure suggest that the mcm5-bob1 mutation appears to increase structural flexibility in the N-terminus of Mcm5 (Fletcher et al., 2003). Taken these results together with those obtained with the nda4/mcm5 mutation, possible structural change caused either by DDK-phosphorylation of MCM subunits or by the mcm5-bob1 mutation may allow the N-terminal region of Mcm5 to interact with Cdc45.
Sequential Assembly of pre-IC Components
We have previously shown that binding of Cdc45 to the chromatin depends on the functional Sld3 (Nakajima and Masukata, 2002). On the other hand, we show here that Sld3 can efficiently bind to replication origins without detectable association of Cdc45 in the mcm5 and cdc45 mutants (Figures 4 and 6). Furthermore, temperature-sensitive mutations of psf3, a component of fission yeast GINS complex, inhibit loading of Cdc45 but not Sld3 onto the origin (H. Yabuuchi and H. Masukata, unpublished results). Although the results obtained by mutations are attributed to specific alleles, these results strongly suggest that Sld3-loading is independent of Cdc45-loading in fission yeast.
One cannot exclude the possibility that a step detected in a certain mutant could be an aberrant dead-end product. However, the following observations suggest that this is not the case. The mcm5 mutant retains the viability at the restrictive temperature (Figure 3C). Furthermore, Cdc45 is loaded onto the origin immediately after return to the permissive temperature, and replication of the bulk chromosome is efficiently resumed (Figure 5, B and D). These results suggest that the complex containing MCM and Sld3 but not Cdc45 is a functional intermediate in initiation of DNA replication.
Loading of Cdc45 and Sld3 is interdependent in budding yeast (Kamimura et al., 2001; Takayama et al., 2003). Origin association of Sld3 is decreased in cdc45-1 mutant. The finding in this study that loading of Sld3 does not depend on Cdc45 in fission yeast is somewhat different from the observations in budding yeast. Although the possibility that the difference could be due to allele specificity is not excluded, there are two other possible explanations, not exclusive each other, for this difference. Interactions of Sld3 with other factors may be more stable in fission yeast than those in budding yeast. For example, fission yeast Sld3 is coprecipitated with Mcm6 and Cdc45 without cross-linking, whereas coprecipitation of Sld3 with Cdc45 depends on cross-linking in budding yeast (Kamimura et al., 2001; Nakajima and Masukata, 2002). For the second explanation, the process of pre-IC assembly could be diverse in some extents among eukaryotes. In Xenopus egg extracts, Cut5, a possible counterpart of Dpb11, associates with the chromatin without GINS (Kubota et al., 2003), whereas loading of these components are mutually dependent in budding yeast (Kamimura et al., 2001; Takayama et al., 2003). More careful investigation in various replication systems should be required to elucidate the principle scheme of pre-IC formation.
Distinct Roles of Sld3 and Cdc45 in Initiation of Replication
What are the roles of Cdc45 and Sld3 in pre-IC assembly and MCM helicase activation? It has been shown, by yeast two-hybrid analyses, that budding yeast Sld3 interacts with Psf1, a component of GINS complex, and with Dpb11 (Takayama et al., 2003). Interaction of Dpb11 with Sld2 depends on phosphorylation of Sld2 by S-CDK and is required for loading of DNA Pol ε (Masumoto et al., 2002; Noguchi et al., 2002). We detected a reduced but significant level of origin association of Dpb2, the second largest subunit of DNA Pol ε, by ChIP assay in the mcm5-arrested condition (unpublished data). Hence, Sld3 may play an important role in assembly of initiation factors including DNA Pol ε onto replication origins. It has been suggested that Cdc45 is involved in chromatin-loading of DNA Pol α (Mimura and Takisawa, 1998; Aparicio et al., 1999), and genetic interaction between Cdc45 and DNA Pol α has been suggested (Uchiyama et al., 2001a). Thus, Cdc45 may function in recruiting Pol α onto origins. These results suggest that Cdc45 and Sld3 are involved in assembly of distinct factors.
In the mcm5 mutant arrested at the restrictive temperature, we could not detect single-stranded DNA in the origin either by ChIP assay with anti-Ssb2 (RPA) antibody or by genomic foot-printing with potassium permanganate modification (unpublished data). These results suggest that MCM helicase is not activated. It has been reported that complexes containing MCM and Cdc45 from Xenopus egg extracts exhibit DNA helicase activity (Masuda et al., 2003). Considering genetic and physical interactions of Cdc45 and MCM, Cdc45 may play a direct role in activation of MCM helicase. We show that localization of Sld3 and Mcm6 is changed from initial MCM binding site in ars2004 (Figure 7). This result could be interpreted by the possibility that Sld3-associated MCM may move along the DNA without unwinding. The interaction between MCM and DNA might be altered by association of Sld3. A possibility that additional association of MCM and subsequent Sld3-loading occur at the secondary preferred site in later periods is not excluded.
Initiation of replication is regulated by two independent kinase pathways, DDK and CDK. Chromatin loading of Cdc45 requires activation of both DDK and CDK (Bousset and Diffley, 1998; Donaldson et al., 1998; Zou and Stillman, 2000). However, the apparent dependency of Cdc45-loading on DDK should be, at least in part, mediated by Sld3-loading in fission yeast, because DDK-dependent origin-loading of Sld3 does not depend on Cdc45 (Nakajima and Masukata, 2002). Thus, Sld3-loading is a step critical for temporal regulation of origin firing and for repression of late origin firing by replication checkpoint. On the other hand, one of the steps regulated by CDK is demonstrated to be complex formation between Dpb11/Cut5 and Sld2/Drc1 (Masumoto et al., 2002; Noguchi et al., 2002). Taken these results together, DDK and CDK have distinct execution points, namely Sld3 loading and assembly of Dpb11/Cut5-Sld2/Drc1 complex, respectively, although some other step could be regulated by these kinases. Hence, loading of Sld3 before Cdc45 might be important for step-wise activation of initiation complex to ensure that origin is unwound only after assembly of replication factors.
Although many replication factors are conserved from yeast to human, a possible homolog of Sld3 has not been identified in higher eukaryotes. Considering essential role of Sld3 in assembly of pre-IC, its functional homolog should be present in higher eukaryotes, although its amino acid sequence might be diversified. Future biochemical studies are necessary to understand the roles of replication factors in initiation of replication.
Acknowledgments
We thank T. Tsurimoto and T. Takahashi for critical reading of the manuscript and helpful discussions; M. Yanagida for generous gifts of strains; S. Forsburg, D. Sherman, and S. Pasion for providing anti-Mcm3 and anti-Mcm4 antibodies; and T. Uchida for preparation of anti-Cdc45 antibody. This study was supported in part by a grant-in-aid from the Ministry of Education, Science, Technology, Sports, and Culture, Japan, to H.M.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-04-0292. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-04-0292.
References
- Aparicio, O.M., Stout, A.M., and Bell, S.P. (1999). Differential assembly of Cdc45p and DNA polymerases at early and late origins of DNA replication. Proc. Natl. Acad. Sci. USA 96, 9130-9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin, R.J., Orr-Weaver, T.L., and Bell, S.P. (1999). Drosophila ORC specifically binds to ACE3, an origin of DNA replication control element. Genes Dev. 13, 2639-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, S.P., and Dutta, A. (2002). DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71, 333-374. [DOI] [PubMed] [Google Scholar]
- Bell, S.P., and Stillman, B. (1992). ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357, 128-134. [DOI] [PubMed] [Google Scholar]
- Benito, J., Martin-Castellanos, C., and Moreno, S. (1998). Regulation of the G1 phase of the cell cycle by periodic stabilization and degradation of the p25rum1 CDK inhibitor. EMBO J. 17, 482-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousset, K., and Diffley, J.F. (1998). The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 12, 480-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne, R.K., and Kelly, T.J. (1995). Genetic analysis of an ARS element from the fission yeast Schizosaccharomyces pombe. EMBO J. 14, 6348-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley, J.F., Cocker, J.H., Dowell, S.J., and Rowley, A. (1994). Two steps in the assembly of complexes at yeast replication origins in vivo. Cell 78, 303-316. [DOI] [PubMed] [Google Scholar]
- Donaldson, A.D., Fangman, W.L., and Brewer, B.J. (1998). Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev. 12, 491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, D.D., Kim, S.M., Todorov, I.T., and Huberman, J.A. (1996). Large, complex modular structure of a fission yeast DNA replication origin. Curr. Biol. 6, 467-473. [DOI] [PubMed] [Google Scholar]
- Fletcher, R.J., Bishop, B.E., Leon, R.P., Sclafani, R.A., Ogata, C.M., and Chen, X.S. (2003). The structure and function of MCM from archaeal M. Thermoautotrophicum. Nat. Struct. Biol. 10, 160-167. [DOI] [PubMed] [Google Scholar]
- Fujita, M., Yamada, C., Tsurumi, T., Hanaoka, F., Matsuzawa, K., and Inagaki, M. (1998). Cell cycle- and chromatin binding state-dependent phosphorylation of human MCM heterohexameric complexes. J. Biol. Chem. 273, 17095-17101. [DOI] [PubMed] [Google Scholar]
- Geraghty, D.S., Ding, M., Heintz, N.H., and Pederson, D.S. (2000). Premature structural changes at replication origins in a yeast minichromosome maintenance (MCM) mutant. J. Biol. Chem. 275, 18011-18021. [DOI] [PubMed] [Google Scholar]
- Grallert, B., Kearsey, S.E., Lenhard, M., Carlson, C.R., Nurse, P., Boye, E., and Labib, K. (2000). A fission yeast general translation factor reveals links between protein synthesis and cell cycle controls. J. Cell Sci. 113, 1447-1458. [DOI] [PubMed] [Google Scholar]
- Gregan, J., Lindner, K., Brimage, L., Franklin, R., Namdar, M., Hart, E.A., Aves, S.J., and Kearsey, S.E. (2003). Fission yeast Cdc23/Mcm10 functions after pre-replicative complex formation to promote Cdc45 chromatin binding. Mol. Biol. Cell 14, 3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, C.F., Dryga, O., Seematter, S., Pahl, P.M., and Sclafani, R.A. (1997). mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc. Natl. Acad. Sci. USA 94, 3151-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy, K.M., Clark, C.D., and Botstein, D. (1990). Subcellular localization of yeast CDC46 varies with the cell cycle. Genes Dev. 4, 2252-2263. [DOI] [PubMed] [Google Scholar]
- Hennessy, K.M., Lee, A., Chen, E., and Botstein, D. (1991). A group of interacting yeast DNA replication genes. Genes Dev. 5, 958-969. [DOI] [PubMed] [Google Scholar]
- Hiraoka, Y., Toda, T., and Yanagida, M. (1984). The NDA3 gene of fission yeast encodes β-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell 39, 349-358. [DOI] [PubMed] [Google Scholar]
- Ishimi, Y. (1997). A DNA helicase activity is associated with an MCM4, -6, and -7 protein complex. J. Biol. Chem. 272, 24508-24513. [DOI] [PubMed] [Google Scholar]
- Jallepalli, P.V., Brown, G.W., Muzi-Falconi, M., Tien, D., and Kelly, T.J. (1997). Regulation of the replication initiator protein p65cdc18 by CDK phosphorylation. Genes Dev. 11, 2767-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura, Y., Masumoto, H., Sugino, A., and Araki, H. (1998). Sld2, which interacts with Dpb11 in Saccharomyces cerevisiae, is required for chromosomal DNA replication. Mol. Cell. Biol. 18, 6102-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura, Y., Tak, Y.S., Sugino, A., and Araki, H. (2001). Sld3, which interacts with Cdc45 (Sld4), functions for chromosomal DNA replication in Saccharomyces cerevisiae. EMBO J. 20, 2097-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearsey, S.E., and Cotterill, S. (2003). Enigmatic variations: divergent modes of regulating eukaryotic DNA replication. Mol. Cell 12, 1067-1075. [DOI] [PubMed] [Google Scholar]
- Kelly, T.J., and Brown, G.W. (2000). Regulation of chromosome replication. Annu. Rev. Biochem. 69, 829-880. [DOI] [PubMed] [Google Scholar]
- Kong, D., and DePamphilis, M.L. (2001). Site-specific DNA binding of the Schizosaccharomyces pombe origin recognition complex is determined by the Orc4 subunit. Mol. Cell. Biol. 21, 8095-8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, D., and DePamphilis, M.L. (2002). Site-specific ORC binding, prereplication complex assembly and DNA synthesis at Schizosaccharomyces pombe replication origins. EMBO J. 21, 5567-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota, Y., Takase, Y., Komori, Y., Hashimoto, Y., Arata, T., Kamimura, Y., Araki, H., and Takisawa, H. (2003). A novel ring-like complex of Xenopus proteins essential for the initiation of DNA replication. Genes Dev. 17, 1141-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.K., and Hurwitz, J. (2001). Processive DNA helicase activity of the minichromosome maintenance proteins 4, 6, and 7 complex requires forked DNA structures. Proc. Natl. Acad. Sci. USA 98, 54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.K., Moon, K.Y., Jiang, Y., and Hurwitz, J. (2001). The Schizosaccharomyces pombe origin recognition complex interacts with multiple AT-rich regions of the replication origin DNA by means of the AT-hook domains of the spOrc4 protein. Proc. Natl. Acad. Sci. USA 98, 13589-13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona, A., Mondesert, O., Leatherwood, J., and Russell, P. (1998). Negative regulation of Cdc18 DNA replication protein by Cdc2. Mol. Biol. Cell 9, 63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marahrens, Y., and Stillman, B. (1992). A yeast chromosomal origin of DNA replication defined by multiple functional elements. Science 255, 817-823. [DOI] [PubMed] [Google Scholar]
- Masuda, T., Mimura, S., and Takisawa, H. (2003). CDK- and Cdc45-dependent priming of the MCM complex on chromatin during S-phase in Xenopus egg extracts: possible activation of MCM helicase by association with Cdc45. Genes Cells 8, 145-161. [DOI] [PubMed] [Google Scholar]
- Masumoto, H., Muramatsu, S., Kamimura, Y., and Araki, H. (2002). S-Cdk-dependent phosphorylation of Sld2 essential for chromosomal DNA replication in budding yeast. Nature 415, 651-655. [DOI] [PubMed] [Google Scholar]
- Masumoto, H., Sugino, A., and Araki, H. (2000). Dpb11 controls the association between DNA polymerases α and ε and the autonomously replicating sequence region of budding yeast. Mol. Cell. Biol. 20, 2809-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura, S., Masuda, T., Matsui, T., and Takisawa, H. (2000). Central role for Cdc45 in establishing an initiation complex of DNA replication in Xenopus egg extracts. Genes Cells 5, 439-452. [DOI] [PubMed] [Google Scholar]
- Mimura, S., and Takisawa, H. (1998). Xenopus Cdc45-dependent loading of DNA polymerase α onto chromatin under the control of S-phase Cdk. EMBO J. 17, 5699-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, S., Okishio, N., Samejima, I., Hiraoka, Y., Toda, T., Saitoh, I., and Yanagida, M. (1993). Fission yeast genes nda1+ and nda4+, mutations of which lead to S-phase block, chromatin alteration and Ca2+ suppression, are members of the CDC46/MCM2 family. Mol. Biol. Cell 4, 1003-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, S., and Yamashita, S. (1998). Identification of sna41 gene, which is the suppressor of nda4 mutation and is involved in DNA replication in Schizosaccharomyces pombe. Genes Cells 3, 157-166. [DOI] [PubMed] [Google Scholar]
- Mondesert, O., McGowan, C.H., and Russell, P. (1996). Cig2, a B-type cyclin, promotes the onset of S in Schizosaccharomyces pombe. Mol. Cell. Biol. 16, 1527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795-823. [DOI] [PubMed] [Google Scholar]
- Nakajima, R., and Masukata, H. (2002). SpSld3 is required for loading and maintenance of SpCdc45 on chromatin in DNA replication in fission yeast. Mol. Biol. Cell 13, 1462-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, E., Shanahan, P., Noguchi, C., and Russell, P. (2002). CDK phosphorylation of Drc1 regulates DNA replication in fission yeast. Curr. Biol. 12, 599-605. [DOI] [PubMed] [Google Scholar]
- Ogawa, Y., Takahashi, T., and Masukata, H. (1999). Association of fission yeast Orp1 and Mcm6 proteins with chromosomal replication origins. Mol. Cell. Biol. 19, 7228-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno, Y., Okazaki, T., and Masukata, H. (1997). Identification of a predominant replication origin in fission yeast. Nucleic Acids Res. 25, 530-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno, Y., Satoh, H., Sekiguchi, M., and Masukata, H. (1999). Clustered adenine/thymine stretches are essential for function of a fission yeast replication origin. Mol. Cell. Biol. 19, 6699-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, P., and Nurse, P. (1986). cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 45, 145-153. [DOI] [PubMed] [Google Scholar]
- Sato, N., Arai, K., and Masai, H. (1997). Human and Xenopus cDNAs encoding budding yeast Cdc7-related kinases: in vitro phosphorylation of MCM subunits by a putative human homologue of Cdc7. EMBO J. 16, 4340-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani, R.A., Tecklenburg, M., and Pierce, A. (2002). The mcm5-bob1 bypass of Cdc7p/Dbf4p in DNA replication depends on both Cdk1-independent and Cdk1-dependent steps in Saccharomyces cerevisiae. Genetics 161, 47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, D.A., and Forsburg, S.L. (1998). Schizosaccharomyces pombe Mcm3p, an essential nuclear protein, associates tightly with Nda4p (Mcm5p). Nucleic Acids Res. 26, 3955-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, D.A., Pasion, S.G., and Forsburg, S.L. (1998). Multiple domains of fission yeast Cdc19p (MCM2) are required for its association with the core MCM complex. Mol. Biol. Cell 9, 1833-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, T., and Masukata, H. (2001). Interaction of fission yeast ORC with essential adenine/thymine stretches in replication origins. Genes Cells 6, 837-849. [DOI] [PubMed] [Google Scholar]
- Takahashi, T., Ohara, E., Nishitani, H., and Masukata, H. (2003). Multiple ORC-binding sites are required for efficient MCM loading and origin firing in fission yeast. EMBO J. 22, 964-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama, Y., Kamimura, Y., Okawa, M., Muramatsu, S., Sugino, A., and Araki, H. (2003). GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev. 17, 1153-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama, M., Arai, K., and Masai, H. (2001a). sna41goa1, a novel mutation causing G1/S arrest in fission yeast, is defective in a CDC45 homolog and interacts genetically with polα. Mol. Genet. Genomics 265, 1039-1049. [DOI] [PubMed] [Google Scholar]
- Uchiyama, M., Griffiths, D., Arai, K., and Masai, H. (2001b). Essential role of Sna41/Cdc45 in loading of DNA polymerase α onto minichromosome maintenance proteins in fission yeast. J. Biol. Chem. 276, 26189-26196. [DOI] [PubMed] [Google Scholar]
- Van Hatten, R.A., Tutter, A.V., Holway, A.H., Khederian, A.M., Walter, J.C., and Michael, W.M. (2002). The Xenopus Xmus101 protein is required for the recruitment of Cdc45 to origins of DNA replication. J. Cell Biol. 159, 541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, J., and Newport, J. (2000). Initiation of eukaryotic DNA replication: origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase α. Mol. Cell 5, 617-627. [DOI] [PubMed] [Google Scholar]
- Wohlschlegel, J.A., Dhar, S.K., Prokhorova, T.A., Dutta, A., and Walter, J.C. (2002). Xenopus Mcm10 binds to origins of DNA replication after Mcm2-7 and stimulates origin binding of Cdc45. Mol. Cell 9, 233-240. [DOI] [PubMed] [Google Scholar]
- Zhu, J., Carlson, D.L., Dubey, D.D., Sharma, K., and Huberman, J.A. (1994). Comparison of the two major ARS elements of the ura4 replication origin region with other ARS elements in the fission yeast, Schizosaccharomyces pombe. Chromosoma 103, 414-422. [DOI] [PubMed] [Google Scholar]
- Zou, L., and Stillman, B. (2000). Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase. Mol. Cell. Biol. 20, 3086-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]