Abstract
The optimal route for clinical delivery of oncolytic viruses is thought to be systemic intravenous injection; however, the immune system is armed with several highly efficient mechanisms to remove pathogens from the circulatory system. To overcome the challenges faced in trying to delivery oncolytic viruses specifically to tumors via the bloodstream, carrier cells have been investigated to determine their suitability as delivery vehicles for systemic administration of oncolytic viruses. Cell carriers protect viruses from neutralization, one of the most limiting aspects of oncolytic virus interaction with the immune system. Cell carriers can also possess inherent tumor tropism, thus directing the delivery of the virus more specifically to a tumor. With preclinical studies already demonstrating the success and feasibility of this approach with multiple oncolytic viruses, clinical evaluation of cell-mediated delivery of viruses is on the horizon. Meanwhile, ongoing preclinical studies are aimed at identifying new cellular vehicles for oncolytic viruses and improving current promising cell carrier platforms.
Keywords: oncolytic virus, cell carrier, systemic delivery, tumor targeting, cancer
Introduction
Oncolytic viruses infect and kill tumor cells while leaving normal tissues unharmed. Specificity toward cancer cells can be a natural feature of the virus, as is the case with reovirus, Newcastle disease virus, and mumps virus, or it can be selected for or engineered into the virus through the use of tumor-specific cell surface molecules,1 transcription factors,2 and tissue-specific microRNAs.3 Similarly, vesicular stomatitis virus, herpes simplex virus, and adenovirus have been genetically attenuated by subduing their ability to antagonize antiviral defenses, thus improving tumor specificity. This strategy leads to enhanced replication in tumor cells, which often possess defects in antiviral pathways,4 while sparing normal cells. Oncolytic viruses exert their antitumor activities through both direct and indirect mechanisms. Direct infection of tumor cells leads to virus and immune-mediated cytotoxicity, and in some cases, alerting the immune system to the previously tolerated tumor through the recruitment of natural killer (NK) cells and cluster of differentiation (CD)8+ cytotoxic T cells.5 Infection of tumor vasculature can lead to vascular collapse and compromised blood flow within the tumor, thus choking off its access to nutrients.6 To increase potency, oncolytic viruses have been engineered to express genes that augment virus replication,7 induce cytotoxicity,8 promote bystander cell killing,9 and enhance antitumor immunity.5 Of overarching concern, however, is that these numerous improvements will provide no benefit to antitumor efficacy unless the virus is successfully delivered to the tumor. Although direct intratumoral injection should deliver all virus particles directly to the tumor, there are several advantages to systemic administration. Firstly, not all tumors are amenable to direct intratumoral injection since they may consist of several small nodules spread out over a large area, or they are in an anatomic location that is inaccessible by direct injection. Systemic delivery has a greater chance of reaching disseminated metastases as well as the primary tumor. Although not thoroughly investigated, the ability of some oncolytic viruses to stimulate antitumor immune responses may be greater when administered systemically.10
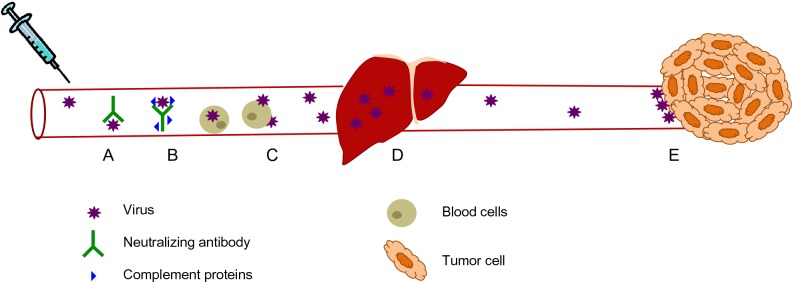
Despite the need for efficient systemic delivery of oncolytic viruses, there exist several barriers to their efficient systemic delivery (Figure 1). The immune system has evolved several mechanisms to prevent the systemic spread of microorganisms and does not discriminate between pathogens and therapeutic oncolytic viruses. Intravenous delivery exposes oncolytic viruses to circulating factors such as antibodies, which bind to and neutralize virus directly or mark them for destruction by complement and various immune cells.11 Virus is also neutralized by nonspecific binding to serum proteins and circulating cells present in the bloodstream.11 Organs such as the lung, spleen, and especially the liver, also play a significant role in clearing virus from the bloodstream because these tissues contain resident macrophages, the role of which is to scavenge the blood for circulating pathogens.12 Because these mechanisms are present in virus-naïve individuals as part of the innate immune system, the neutralization of oncolytic viruses is even greater if previous exposure to the virus has occurred. Since adaptive immunity is able to mount a significantly more specific and potent immune response compared with its innate counterpart, this represents an additional and significant hurdle to an efficacious repeat dosing regimen using oncolytic viruses. Indeed, a significant proportion of the human population has already been exposed to and thus has developed immunologic memory to many of the oncolytic viruses currently undergoing clinical development and testing, including reovirus,13 vaccinia virus,14 and measles virus.15 Apart from neutralization of virus in the bloodstream, physical barriers such as tumor extracellular matrix, as well as limited extravasation of oncolytic viruses into the tumor bed due to high interstitial fluid pressure within the tumor, can also result in fewer virions being delivered to tumor cells.16 Despite all these existing barriers to systemic delivery of oncolytic viruses, recent clinical trials17,18 suggest that intravenously administered virus can reach the tumor and replicate if administered at sufficiently high doses, presumably saturating the neutralizing mechanisms present within the human body. Although this was a milestone achievement in the field, there is still much room for improvement as several sets of preclinical19–21 and clinical13,18 data demonstrate that pre-existing, virus-specific neutralizing antibodies dramatically reduce the amount of infectious virus that can be delivered to the tumor.
Figure 1.
Virus neutralization during systemic delivery. (A) Circulating antibodies and (B) complement proteins bind to virus and neutralize them, as well as marking them for destruction by immune effector cells. (C) Intravenously administered virus also interacts with circulating blood cells, leading to virus sequestration. (D) Liver macrophages, which are part of the reticulo-endothelial system, filter viruses from the blood. (E) Viruses that do reach the tumor encounter extensive tumor extracellular matrix and high interstitial fluid pressure which limits their extravasation into the tumor.
Cell carriers: playing virus “hide and seek”
To overcome some of the challenges presented in delivering oncolytic viruses systemically, several investigators are exploring the use of cells as delivery vehicles for oncolytic viruses. This approach mimics what some viruses have evolved to do in order to spread systemically or gain access to various tissues within their host. For example, the human immunodeficiency virus is known to bind to circulating dendritic cells and macrophages which then naturally migrate to lymph nodes and pass on the virus to its target cell population, ie, CD4+ T cells.22 Some viruses that replicate via cell-to-cell spread are also able to evade neutralization by circulating antibodies,23 a feature that would clearly be of benefit for the systemic delivery of oncolytic viruses. The utility of cells as delivery vehicles for oncolytic viruses has therefore been investigated for several oncolytic viruses using a variety of cell carrier types and different tumor models in numerous preclinical studies. Notably, in recent clinical trials,17,18 intravenously administered virus was found to bind several types of circulating cells, and in the case of reovirus, the cell-bound virus was still infectious and could deliver virus to tumor cells in vitro despite the presence of neutralizing antibodies in patients.18
Based on the preclinical studies completed so far, most of the cell types that have been studied as delivery vehicles for oncolytic viruses fall under one of the following three categories: transformed cells; immune cells; and progenitor cells. Each of these cell types has its unique advantages and potential disadvantages. In theory, the ideal cell carrier would not only protect its viral cargo from neutralization and direct it specifically to the location of the tumor, thus limiting toxicity to normal tissues, but the cell carrier would also have antitumor activity of its own. Additional properties, such as a favorable safety profile, ease of isolation, and/or manufacturing, are important features to consider when deciding what carrier cell type is most suitable for clinical application. In this review, we aim to summarize the promising research that has been accomplished thus far using carrier cells for efficient delivery of viral therapeutics and what lies ahead in terms of bringing this approach to a clinical setting for evaluation in patients.
Virus protection
There are several preclinical studies demonstrating that carrier cells can deliver a variety of oncolytic viruses to tumors. Indeed, successful delivery in vivo has been achieved with several oncolytic virus platforms using a wide range of carrier cell types (Table 1). Clearly this approach is not limited to a single virus platform or to a single carrier cell type. In choosing a suitable cell carrier for the delivery of oncolytic viruses, one must consider the susceptibility of the cell to the virus, the kinetics of viral replication and release, as well as the kinetics of trafficking of the cell carrier from the site of injection to the tumor location. All of these parameters must be optimized to ensure that virus remains unseen by the immune system and therefore protected from neutralization. After a virus enters a cell, there is a period of time before progeny virions are released from the infected cell. Ideally, the cell carrier should reach the tumor during this stage of virus replication. For some rapidly replicating viruses, such as vesicular stomatitis virus, this eclipse phase can be as short as an hour,24 whereas for slower replicating viruses, such as vaccinia, this period of time is much longer.25 Therefore, with slower replicating viruses, there is more flexibility in optimizing the timing of infection and delivery of the carrier cell. For example, with vesicular stomatitis virus, we found that successful delivery could be achieved in the presence of virus-specific neutralizing antibodies using tumor cell carriers if the cells were injected after being infected for only 1–2 hours.19 On the other hand, studies performed by Thorne et al demonstrated that cytokine-induced killer (CIK) cells took 72 hours to traffic to tumors and this was compatible with vaccinia virus replication kinetics in CIK cells in which peak virus release also occurred at 72 hours post-infection.26 From these studies and several others, it is clear that proper characterization of virus replication in the cell carrier of choice and subsequent optimization is important in achieving successful delivery to tumors.
Table 1.
Cell carriers used to deliver virus vectors
| Transformed cells |
| Adenovirus83 |
| Vesicular stomatitis virus19 |
| Measles virus21 |
| Herpes simplex virus38 |
| Vaccinia84 |
| Parvovirus85 |
| Newcastle disease virus34 |
| T cells/cytokine-induced killer cells |
| Reovirus20 |
| Retrovirus35 |
| Measles virus44,86 |
| Vesicular stomatitis virus32 |
| Herpes simplex virus33 |
| Vaccinia26 |
| Newcastle disease virus34 |
| Dendritic cells |
| Reovirus20 |
| Measles virus87 |
| Macrophages |
| Adenovirus46 |
| Measles virus45 |
| Newcastle disease virus34 |
| Peripheral blood mononuclear cells |
| Reovirus88 |
| Myeloid-derived suppressor cells |
| Vesicular stomatitis virus47 |
| Mesenchymal stem cells |
| Adenovirus89 |
| Measles virus90 |
| Myxoma91 |
| Neural progenitor cells |
| Adenovirus59 |
| Herpes simplex virus57 |
| Endothelial progenitor cells |
| Retrovirus62 |
It is important to note that even before progeny virions are released from an infected cell, viral proteins can accumulate at the surface of the infected cell either as membrane-bound proteins, as is the case with vesicular stomatitis virus G protein, or in complexes with host cell surface proteins such as major histocompatibility complex molecules which present intracellular peptides to immune cells. This raises the possibility that extracellular viral antigens present on the cell carrier can make it a target for immune-mediated destruction, thus negatively impacting successful delivery. Because of the premature clearing of infected cell carriers as a result of viral antigens being exposed on the cell surface, some investigators have attempted to regulate virus replication in the carrier cell such that virus replication and release is initiated once the carrier cells localize to the tumor. One such study by Muthana et al27 used an adenovirus/macrophage cell carrier system to selectively target prostate cancer. Macrophages which have a natural propensity to migrate to hypoxic areas of tumor tissue, were infected with a replication-deficient adenovirus carrying a therapeutic transgene controlled by prostate-specific promoters, and then transfected with a hypoxia-driven E1A/B plasmid. Upon migration of these cotransduced macrophages into hypoxic areas of prostate tumors, hypoxic response element-driven expression of E1A/B initiated viral replication and release, thus allowing the adenovirus to infect prostate cancer cells and express the therapeutic transgene. A similar approach used human mesenchymal stem cells (MSCs) to deliver conditionally replicating adenovirus under the control of osteocalcin.28 This delivery system relied on the observation that MSCs normally express low levels of osteocalcin but that its expression can be highly induced by vitamin D3. Thus, it is possible to deliver the carrier cell and wait until it reaches the tumor, at which point virus replication can be induced by intraperitoneal injection of vitamin D3. In both of these approaches, the end result was that virus release occurred only once the carrier cell reached the tumor, thus potentially minimizing neutralization of the virus in the bloodstream and limiting off-target toxicities.
The use of a cell carrier that supports viral replication is an attractive approach because it has the added advantage of en route dose amplification as the cell carrier produces virus and makes its way to the tumor. Given that for some viruses, the current doses used in clinical trials are at their maximum due to limits in production of the clinical grade virus stocks, a cell carrier with the capacity to produce hundreds of virus particles per cell can dramatically increase the total amount of therapeutic virus that is delivered to the tumor and increase the likeliness of overcoming neutralizing mechanisms. Because oncolytic viruses are selected or engineered to replicate poorly in normal cells, transformed cell carriers are more likely to be permissive to virus replication and produce more virus particles per cell.29 While the use of transformed cells as carriers does not come without safety concerns, it has been demonstrated that irradiation of the cell before administration can halt its ability to grow, and thus its tumorigenic potential, while still maintaining virus infectivity.30,31 Finally, when using a replicating virus in a permissive carrier cell, one must consider the impact of virus replication on carrier cell viability, trafficking, and effector functions because these may be altered as a consequence of infection.
Although considered an advantage, amplification of the virus by the carrier cell is not absolutely necessary for successful protection and delivery to tumors, as exemplified by tumor antigen-specific T cell carriers. It was demonstrated that vesicular stomatitis virus,32 reovirus,20 herpes simplex virus,33 Newcastle disease virus,34 and retrovirus35 particles could “hitchhike” on the surface of T cells and could be delivered to tumor cells through various mechanisms, either passively or involving cellular synapses between the carrier cell and the tumor cell. With retrovirus particles, it was found that the virus was handed off either passively or via intracellular perforin-containing cytotoxic granules released from activated T cells when they engaged their target cells.36 Importantly, surface-adsorbed virus was protected from neutralizing antibodies. With vesicular stomatitis virus-loading of T cells,32 it was observed that at a high multiplicity of infection, the T cells could not deliver virus to tumors in passively immunized mice but that this could be achieved if loaded at a low multiplicity of infection. This suggests that at high multiplicities of infection, surface-bound virus was still accessible to neutralizing antibodies, thus emphasizing the importance of optimizing infection conditions when preparing cell carriers.
T cells are not the only cell type capable of protecting and delivering surface-adhered virus to tumor cells. A study by Ilett et al20 compared delivery of reovirus using either murine T cells, immature dendritic cells, or mature dendritic cells, and found that mature dendritic cells were superior at delivering virus to tumors in immunized mice, a finding that can perhaps be explained in part by the observation that mature dendritic cells trafficked to tumors in greater numbers than T cells. In contrast, immature dendritic cells were not able to deliver reovirus to tumors in immunized mice, leading the authors to speculate that the high level of reovirus replication in this cell type marked it for immune destruction. In support of this hypothesis, in a follow-up study37 performed in vitro with human T cells and dendritic cells, both immature and mature dendritic cells were able to protect reovirus from immune serum whereas T cells could not. Moreover, in these studies, both mature and immature dendritic cells supported low levels of reovirus replication and thus were not neutralized by immune serum, whereas the T cells failed to internalize the virus, leaving the authors to hypothesize that the virus was exposed and thus vulnerable to neutralization. In summary, future studies aimed at better understanding of the mechanisms by which viruses bind to and enter cells will most certainly shed some light on how we can take advantage of these complex interactions between viruses and carrier cells to further enhance the systemic delivery of oncolytic viruses.
Tumor-specific delivery
Virus protection, as discussed above, is not the only benefit of using carrier cells for the delivery of oncolytic viruses. Indeed, a protected virus is not useful unless it is delivered specifically to the site of the tumor. For this reason, there has been a lot of effort invested in identifying cell types that not only protect viruses during systemic delivery, but also have the ability to traffic to tumors. This section briefly reviews the current knowledge regarding promising tumor-targeted cellular vehicles for oncolytic virus delivery.
Transformed cells
Transformed cells were among the first cell carriers tested for oncolytic virus delivery to tumors.31,38 They are relatively easy to grow to sufficiently large numbers and are more readily infected with oncolytic viruses than normal cells, and are thus capable of delivering large payloads of virus to tumors. However, their ability to home to specific body and tumor locations is rather limited. In fact, we have observed that cancer cells that form solid tumors (HeLa cervical carcinoma, A549 lung carcinoma, MCF-7 breast carcinoma, CT26 colorectal carcinoma, and SF268 glioblastoma) tend to arrest in the small capillary beds of the lungs and fail to recirculate when injected intravenously into mice19 (and unpublished data, Bell, 2007). This is most likely due to their large size and the fact that organs such as the lung and liver act as filters. Although these findings suggest that solid tumor cells may be ideal carriers for targeting oncolytic viruses to lung tumors, as demonstrated by Power et al,19 these studies led us to explore the use of transformed cells of hematologic origin, such as leukemia cells, with the rationale that they would circulate much better than solid tumor cells and be able to bypass the lungs for delivery to tumors in anatomic locations other than the lung. Although the L2120 murine leukemia cells used in these studies did transiently accumulate in the lungs, as determined by bioluminescent imaging, they eventually managed to recirculate and thus were able to deliver virus to subcutaneous tumors.19 In this model, delivery to the subcutaneous tumors was most likely by nonspecific accumulation of infected cells within the tumor as opposed to specific homing of the cell carriers to the tumor location. Tumor cells can, however, have the propensity to traffic, or home, to specific organs, as is seen with metastatic disease. The ability of tumor cells to migrate to specific tissues can be exploited to achieve targeted delivery to metastatic tumor beds. For example, myeloma cells, which have been used as cell carriers for delivery of oncolytic measles virus,39 express high levels of CXC chemokine receptor 4 (CXCR4) and thus often metastasize to the bone marrow.40 Therefore, it has been proposed that myeloma cells themselves can be used to achieve delivery of measles virus to metastatic tumor beds in the bone marrow.30,39 Although an “off the shelf” cell carrier is possible when using transformed cells, use of a patient’s own tumor cells as a carrier for oncolytic virus delivery is also attractive from an immunologic standpoint, since infected cell vaccines have been demonstrated to enhance antitumor immune responses.10,41,42 Finally, even though transformed cells have been used in clinical trials,43 proper safety measures must be considered when using any transformed cell line as a cell carrier.
Immune cells
Several immune cell types, including T cells,20,32,34,35 CIK cells,26,44 monocytes/macrophages,45,46 and myeloid-derived suppressor cells47 have been investigated as carrier cells because they can circulate systemically, have the ability to specifically recognize tumors or tumor-associated features, and possess antitumor activity on their own, therefore delivering a one-two punch to the tumor. Adoptive transfer of tumor antigen-specific T cells has been extensively investigated as a cancer immunotherapy in both preclinical and clinical settings. Since this cell type has direct anticancer effector functions and can also protect and deliver oncolytic viruses, it seems like an ideal combination of cancer therapeutics. In preclinical studies using OT-1 T cells specific for ovalbumin,32 it was found that 5%–14% of adoptively transferred T cells migrated to B16-OVA tumors, which is quite remarkable. However, in this tumor model, all tumor cells, and only tumor cells, express the highly immunogenic ovalbumin antigen and this does not mirror what is seen in human patients, where truly tumor-specific and highly immunogenic tumor antigens are rare.
Adoptively transferred T cells that do not migrate to the tumor are often found in the spleen and lymph nodes, thus having the potential to target any metastatic disease in these locations or conversely leading to off-target toxicities. One advantage of combining adoptively transferred T cells loaded with oncolytic viruses is that the highly proinflammatory nature of a virus infection in the tumor milieu can help to prevent T cell silencing and inactivation as a consequence of the immune suppressive microenvironment of the tumor, which is often seen with adoptive T cell therapy.48 Despite the attractiveness of this approach, its clinical application is challenging and expensive, so the overall feasibility of this approach is limited.
CIK cells, which are a population of CD3+, CD56+ NK-like T cells obtained from human peripheral blood or mouse splenocytes after ex vivo expansion with interferon gamma, CD3-specific antibody, and interleukin-2, are another type of cell carrier possessing direct anticancer functions.49,50 However, unlike tumor antigen-specific T cells, they are not restricted to one single antigen. Rather, they recognize NKG2D ligands, which are often upregulated on tumor cells as a result of stress,51 thus increasing the chances of targeting a larger percentage of tumor cells within the tumor mass. Additionally, it has been demonstrated that expression of the NKG2D ligands, MICA (MHC class I polypeptide-related sequence A) and MICB, within tumors can be upregulated by treatment with histone deacetylase inhibitors, thus increasing the trafficking of CIK cells to tumors.52 These cells are also easier to obtain from patients and expand ex vivo as compared with antigen-specific T cells, so clinical application of this strategy may be a simpler alternative to tumor antigen-specific T cells. However, vaccinia virus26 and measles virus44 are the only oncolytic viruses that have been successfully delivered to tumors by CIK cells, so it remains unknown if this cell carrier will be useful for delivery of other oncolytic virus platforms.
Macrophages are another class of immune cells that have been associated with tumors and tumor stroma, and have the ability to either enhance or inhibit tumor growth depending on their cytokine expression profile. Tumor cells often secrete monocyte chemotactic protein-1, macrophage colony-stimulating factor, and vascular endothelial growth factor, which promote migration of monocytes to the tumor, where they differentiate into tumor-associated macrophages and localize to hypoxic regions within the tumor.53 Because of the natural homing of macrophages to tumors and the ease of clinical translation of this approach, the delivery of oncolytic measles45 and adenovirus46 has been explored in preclinical studies, with promising results. Interestingly, using a similar approach, a relatively recent study has investigated the use of myeloid-derived suppressor cells as cell carriers for delivery of vesicular stomatitis virus.47 Myeloid-derived suppressor cells are a heterogeneous population of immature myeloid cells critical to the development of tumor-induced immune tolerance.54 However, it was found that infection of this cell population with vesicular stomatitis virus promoted their conversion from an M2 immune suppressive phenotype to an M1-like tumor killing phenotype.47 In summary, with their ability to circulate throughout the body, to specifically recognize and migrate to tumors or tumor stroma, and to have natural antitumor activities, future studies using immune cells as carriers may hopefully lead to clinical testing.
Progenitor cells
Progenitor cells that have been utilized to deliver oncolytic viruses include MSCs, neural stem cells, and vascular progenitor cells, with MSCs being the most extensively studied progenitor cell type. MSCs are multipotent stromal cells that can differentiate into a variety of cell types, including adipocytes, chondrocytes, and osteoblasts.55 They are readily obtainable from various tissues including bone marrow, adipose tissue, the umbilical cord, and peripheral blood, and proliferate fairly rapidly in culture, making it possible to expand to sufficiently large numbers for clinical application.55 MSCs naturally home to areas of inflammation, stress, and tissue injury, and thus the tumor microenvironment, which is often viewed as a wound that never heals, also provides the necessary signals to direct MSCs to traffic to their location. Apart from their ability to migrate to tumors, factors released by MSCs are known to have antitumor properties capable of reducing the proliferation of glioma, melanoma, lung cancer, and breast cancer cells.56 Neural stem cells are another progenitor cell type that has been investigated specifically for intracranial delivery of oncolytic viruses to brain tumors.57–59 These cells are characterized by their ability to differentiate into cells of the nervous system (neurons, astrocytes, or oligodendrocytes) and by their capacity to self-renew.60 Their ability to migrate to tumors resembles that of MSCs; however, it is quite difficult to obtain sufficient numbers of these cells for clinical studies. Advancements in the isolation, propagation, or generation of these cells will immensely benefit future studies. Finally, it has been suggested that endothelial progenitor cells, with their ability to contribute to neovascularization of growing tumors, would also allow for tumor-specific delivery of oncolytic viruses.61 These cells are readily obtainable from peripheral blood and can be expanded in vitro, and a study performed by Jevremovic et al demonstrated that these cells could transfer retroviruses to tumors in mice upon systemic administration.62 Therefore, future studies aimed at determining if endothelial progenitor cells can deliver other oncolytic viruses would further support the use of these cells as carriers for systemic delivery of viruses.
Clinical trials with carrier cells: are we there yet?
Based on the above summary of what has been accomplished by the use of carrier cells for delivery of oncolytic viruses, it seems that there is no shortage of potentially useful cell carriers for protecting and delivering oncolytic viruses specifically to tumors. The challenge now is to apply what has been learned in these preclinical studies and evaluate the safety, feasibility, and efficacy of this approach in human patients. But how do we decide what is the best cell type that will allow for successful delivery of virus to tumor beds? It is likely that different tumor types may require different cell carriers in order to achieve tumor specific delivery and thus preclinical testing of these strategies remains an important and informative exercise. Apart from the observation that the only infectious virus recovered from the blood of patients treated with reovirus was cell-associated,18 suggesting that intravenous delivery of the virus might naturally occur via a cell carrier, no clinical trial has been conducted looking specifically at carrier cell-mediated delivery of oncolytic viruses. However, in a translational study looking at the viability of this approach, Mader et al assessed the feasibility of obtaining MSCs from adipose tissue of patients with ovarian cancer for the delivery of measles virus to ovarian tumors.63 In this study, it was found that MSCs could be obtained and expanded to sufficient numbers for a clinical dose within 14 days. Furthermore, the MSCs supported measles virus infection and were able to migrate to human ovarian cancers both in vitro and in vivo. Interestingly, the MSCs could be infected and subsequently frozen, and upon thawing, still displayed active viral replication and antitumor activity comparable with that of freshly infected cells. Infection of the MSCs with measles expressing a sodium iodide symporter gene allowed for SPECT-CT (single-photon emission computed tomography/computed tomography) imaging of the cells trafficking to tumors.63 Imaging of the cell carriers is invaluable because it will be informative in understanding the exact trafficking kinetics and ultimate fate of the cell carriers in patients and may guide the optimization of clinical protocols for future studies. Importantly, the MSCs did not form tumors in immune compromised mice nor did they increase the growth of ovarian cancer xenografts. From this study, a clinical protocol is proposed whereby certified MSCs will be thawed, mixed with clinical grade virus on the day of treatment, followed by a 5-minute low speed centrifugation to increase loading efficiency, then incubated for 2 hours at 37°C, and finally infused into the patient by a catheter in the peritoneal cavity. Hopefully, the findings from this trial will not only validate the carrier cell approach for protecting and delivering oncolytic viruses, but will pave the way for future trials involving different cell carrier and oncolytic virus combinations. Equally important, careful analysis of the interactions between oncolytic viruses and cells naturally found in the bloodstream may lead to the discovery of new cell carriers for systemic delivery.
Future of cell carriers: from Trojan horses to stealth fighters
As pointed out by Willmon et al, even with tumor-targeted cell carriers, only 10%, at best, of the administered cell carriers actually find their way to the target tissue.64 Although this is a big improvement from having only 0.001% of systemically administered virions reach the tumor, as estimated for intravenous delivery of “naked” vesicular stomatitis virus,65 there is still the potential for improvement. Given the vast number of biological tools available and our growing understanding in the areas of tumor biology and cell migration, it is possible that cell trafficking can be increased through biological engineering of the carrier cell and/or oncolytic virus. In fact, some investigators are already devising strategies that can perhaps be translated to cell carriers. One strategy is to conjugate or express molecules that bind to exposed tumor antigens or to tumor vasculature. Expression of certain adhesion molecules such as integrins and selectins is known to be upregulated on tumor vasculature as a consequence of the inflammatory environment within tumors.66 Tumor vasculature is also an attractive aspect of tumor biology to target since it is a common feature of most cancer types and some oncolytic viruses are already known to infect the tumor vasculature,6,67,68 thus leading to reduced blood flow to the tumor as a result of vascular collapse.65 There are several published reports of investigators who have exploited this phenomenon to target viruses to the tumor vasculature,69–72 and so perhaps similar targeting strategies could be applied to cell carriers. Indeed, chemical conjugation73 and polymer coating74 of sialyl Lewis X to the surface of MSCs improves their ability to bind to P-selectin in vitro. Similarly, enzymatic conversion of carbohydrate groups on the glycoprotein CD44, which is expressed by MSCs and subsets of T cells, to the sialyl Lewis X epitope enabled CD44 binding to E-selectin.75 Whether these modifications would result in preferential binding of circulating cell carriers to the tumor vasculature remains to be seen.
Magnetic targeting is another attractive approach that can be applicable to several tumor types, as it does not rely on expression of certain molecules by the carrier cell or the tumor. Although it is more commonly utilized to target smaller agents such as drugs76 or liposomes77 to tumors, it has been demonstrated to be feasible to target a wide range of cell types to several tissues for various applications.78–80 Another potential approach to enhance the trafficking of carrier cells to tumors is to modify the tumor microenvironment itself. As pointed out earlier, treatment of ovarian tumors with histone deacetylase inhibitors or radiation led to an increase in the number of tumor-infiltrating CIK cells due to increased NKG2D ligand expression.44,52 Induction of inflammation within the tumor, either via drug treatment or radiation, can lead to increased expression of adhesion molecules, promote leakiness within the tumor vasculature, and induce the release of cytokines, which may enhance carrier cell homing or extravasation from blood vessels and into the tumor.
In addition to increasing the trafficking of cells to tumors, cell carriers and their viral cargoes can be manipulated in order to increase various aspects of cell-mediated delivery of oncolytic viruses, such as loading capacity, virus production, and delivery to tumor cells. It is possible that the oncolytic virus of choice may not enter or replicate in a useful cell carrier that migrates to tumors. If the cell carrier does not express the entry receptor for the virus, viruses can be engineered or pseudotyped to bind to a different receptor that is expressed by the carrier cell. There are several drugs that have been reported to enhance virus replication81,82 and thus can be used to increase virus output by the carrier cell. In doing so, it will be important to ensure the drugs do not alter the trafficking patterns of the carrier cell. Cell carriers could also be engineered to express immune modulatory molecules, to either suppress the immune response, therefore priming the tumor for virus infection, or to call on the immune system for a secondary wave of attack on the tumor. As our understanding of cell trafficking, tumor biology, and virology increases, next-generation cell carriers have the potential of being transformed from simple delivery tools into sophisticated, mobile biological factories capable of delivering a coordinated multipronged attack on tumors.
Summary
There is a clear need for improving specificity and enhancing systemic delivery of oncolytic viruses for clinical regimens. Cell carriers are a promising delivery vehicle for the protection and tumor-specific delivery of oncolytic virus and could address both these concerns. As the field waits for the first clinical testing of this approach, the search for better cell carriers continues. Future studies aimed at improving the trafficking of cells to tumors and increasing virus production by cell carriers will only add to the therapeutic gains provided by this approach.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6(7):529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61(7–8):554–571. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Kelly EJ, Russell SJ. MicroRNAs and the regulation of vector tropism. Mol Ther. 2009;17(3):409–416. doi: 10.1038/mt.2008.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9(9):1163–1176. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 5.Melcher A, Parato K, Rooney CM, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther. 2011;19(6):1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitbach CJ, De Silva NS, Falls TJ, et al. Targeting tumor vasculature with an oncolytic virus. Mol Ther. 2011;19(5):886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Boeuf F, Batenchuk C, Vaha-Koskela M, et al. Model-based rational design of an oncolytic virus with improved therapeutic potential. Nat Commun. 2013;4:1974. doi: 10.1038/ncomms2974. [DOI] [PubMed] [Google Scholar]
- 8.Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WS. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74(13):6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9(9):1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 10.Bridle BW, Stephenson KB, Boudreau JE, et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther. 2010;18(8):1430–1439. doi: 10.1038/mt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012:805629. doi: 10.1155/2012/805629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher K. Striking out at disseminated metastases: the systemic delivery of oncolytic viruses. Curr Opin Mol Ther. 2006;8(4):301–313. [PubMed] [Google Scholar]
- 13.White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15(12):911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 14.Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19(3):329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galanis E, Hartmann LC, Cliby WA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70(3):875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojton J, Kaur B. Impact of tumor microenvironment on oncolytic viral therapy. Cytokine Growth Factor Rev. 2010;21(2–3):127–134. doi: 10.1016/j.cytogfr.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 18.Adair RA, Roulstone V, Scott KJ, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4(138):138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Power AT, Wang J, Falls TJ, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15(1):123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 20.Ilett EJ, Prestwich RJ, Kottke T, et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16(5):689–699. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iankov ID, Blechacz B, Liu C, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15(1):114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 22.Martin N, Sattentau Q. Cell-to-cell HIV-1 spread and its implications for immune evasion. Curr Opin HIV AIDS. 2009;4(2):143–149. doi: 10.1097/COH.0b013e328322f94a. [DOI] [PubMed] [Google Scholar]
- 23.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6(11):815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 24.Pittman D, St John RC, Shechmeister IL. Latent period of vesicular stomatitis virus in chick embryo cells. Nature. 1965;206(990):1228–1231. doi: 10.1038/2061228a0. [DOI] [PubMed] [Google Scholar]
- 25.Furness G, Youngner JS. One-step growth curves for vaccinia virus in cultures of monkey kidney cells. Virology. 1959;9:386–395. doi: 10.1016/0042-6822(59)90130-8. [DOI] [PubMed] [Google Scholar]
- 26.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311(5768):1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 27.Muthana M, Giannoudis A, Scott SD, et al. Use of macrophages to target therapeutic adenovirus to human prostate tumors. Cancer Res. 2011;71(5):1805–1815. doi: 10.1158/0008-5472.CAN-10-2349. [DOI] [PubMed] [Google Scholar]
- 28.Hsiao WC, Sung SY, Liao CH, Wu HC, Hsieh CL. Vitamin D3- inducible mesenchymal stem cell-based delivery of conditionally replicating adenoviruses effectively targets renal cell carcinoma and inhibits tumor growth. Mol Pharm. 2012;9(5):1396–1408. doi: 10.1021/mp200649g. [DOI] [PubMed] [Google Scholar]
- 29.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anticancer agents. Cancer Cell. 2003;4(4):263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 30.Munguia A, Ota T, Miest T, Russell SJ. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 2008;15(10):797–806. doi: 10.1038/gt.2008.45. [DOI] [PubMed] [Google Scholar]
- 31.Coukos G, Makrigiannakis A, Kang EH, et al. Use of carrier cells to deliver a replication-selective herpes simplex virus-1 mutant for the intraperitoneal therapy of epithelial ovarian cancer. Clin Cancer Res. 1999;5(6):1523–1537. [PubMed] [Google Scholar]
- 32.Qiao J, Wang H, Kottke T, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15(8):604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanzaki A, Kasuya H, Yamamura K, et al. Antitumor efficacy of oncolytic herpes simplex virus adsorbed onto antigen-specific lymphocytes. Cancer Gene Ther. 2012;19(4):292–298. doi: 10.1038/cgt.2011.91. [DOI] [PubMed] [Google Scholar]
- 34.Pfirschke C, Schirrmacher V. Cross-infection of tumor cells by contact with T lymphocytes loaded with newcastle disease virus. Int J Oncol. 2009;34(4):951–962. doi: 10.3892/ijo_00000221. [DOI] [PubMed] [Google Scholar]
- 35.Cole C, Qiao J, Kottke T, et al. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat Med. 2005;11(10):1073–1081. doi: 10.1038/nm1297. [DOI] [PubMed] [Google Scholar]
- 36.Kottke T, Qiao J, Diaz RM, et al. The perforin-dependent immunological synapse allows T-cell activation-dependent tumor targeting by MLV vector particles. Gene Ther. 2006;13(15):1166–1177. doi: 10.1038/sj.gt.3302722. [DOI] [PubMed] [Google Scholar]
- 37.Ilett EJ, Barcena M, Errington-Mais F, et al. Internalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralization. Clin Cancer Res. 2011;17(9):2767–2776. doi: 10.1158/1078-0432.CCR-10-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambright ES, Caparrelli DJ, Abbas AE, et al. Oncolytic therapy using a mutant type-1 herpes simplex virus and the role of the immune system. Ann Thorac Surg. 1999;68(5):1756–1760. doi: 10.1016/s0003-4975(99)00852-8. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Russell SJ, Peng KW. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol Ther. 2010;18(6):1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moller C, Stromberg T, Juremalm M, Nilsson K, Nilsson G. Expression and function of chemokine receptors in human multiple myeloma. Leukemia. 2003;17(1):203–210. doi: 10.1038/sj.leu.2402717. [DOI] [PubMed] [Google Scholar]
- 41.Conrad DP, Tsang J, Maclean M, et al. Leukemia cell-rhabdovirus vaccine: personalized immunotherapy for acute lymphoblastic leukemia. Clin Cancer Res. 2013;19(14):3832–3843. doi: 10.1158/1078-0432.CCR-12-3199. [DOI] [PubMed] [Google Scholar]
- 42.Lemay CG, Rintoul JL, Kus A, et al. Harnessing oncolytic virus-mediated antitumor immunity in an infected cell vaccine. Mol Ther. 2012;20(9):1791–1799. doi: 10.1038/mt.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59(20):5160–5168. [PubMed] [Google Scholar]
- 44.Liu C, Suksanpaisan L, Chen YW, Russell SJ, Peng KW. Enhancing cytokine-induced killer cell therapy of multiple myeloma. Exp Hematol. 2013;41(6):508–517. doi: 10.1016/j.exphem.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng KW, Dogan A, Vrana J, et al. Tumor-associated macrophages infiltrate plasmacytomas and can serve as cell carriers for oncolytic measles virotherapy of disseminated myeloma. Am J Hematol. 2009;84(7):401–407. doi: 10.1002/ajh.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muthana M, Rodrigues S, Chen YY, et al. Macrophage delivery of an oncolytic virus abolishes tumor regrowth and metastasis after chemotherapy or irradiation. Cancer Res. 2013;73(2):490–495. doi: 10.1158/0008-5472.CAN-12-3056. [DOI] [PubMed] [Google Scholar]
- 47.Eisenstein S, Coakley BA, Briley-Saebo K, et al. Myeloid derived suppressor cells as a vehicle for tumor-specific oncolytic viral therapy. Cancer Res. 2013;73(16):5003–5015. doi: 10.1158/0008-5472.CAN-12-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97(10):2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 50.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153(4):1687–1696. [PubMed] [Google Scholar]
- 51.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103(8):3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 52.Huang B, Sikorski R, Sampath P, Thorne SH. Modulation of NKG2D-ligand cell surface expression enhances immune cell therapy of cancer. J Immunother. 2011;34(3):289–296. doi: 10.1097/CJI.0b013e31820e1b0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 54.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oreffo RO, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1(2):169–178. doi: 10.1385/SCR:1:2:169. [DOI] [PubMed] [Google Scholar]
- 56.Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010;21(11):1513–1526. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrlinger U, Woiciechowski C, Sena-Esteves M, et al. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol Ther. 2000;1(4):347–357. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- 58.Tyler MA, Ulasov IV, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16(2):262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed AU, Thaci B, Alexiades NG, et al. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther. 2011;19(9):1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 61.Deng W, Jia J. Endothelial progenitor cells as cellular vehicles to deliver oncolytic virus therapies to metastatic tumors: the “Trojan horse” approach. Med Hypotheses. 2008;70(4):842–844. doi: 10.1016/j.mehy.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 62.Jevremovic D, Gulati R, Hennig I, et al. Use of blood outgrowth endothelial cells as virus-producing vectors for gene delivery to tumors. Am J Physiol Heart Circ Physiol. 2004;287(2):H494–H500. doi: 10.1152/ajpheart.00064.2004. [DOI] [PubMed] [Google Scholar]
- 63.Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med. 2013;11:20. doi: 10.1186/1479-5876-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willmon C, Harrington K, Kottke T, Prestwich R, Melcher A, Vile R. Cell carriers for oncolytic viruses: fed ex for cancer therapy. Mol Ther. 2009;17(10):1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breitbach CJ, Paterson JM, Lemay CG, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15(9):1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 66.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 67.Breitbach CJ, Arulanandam R, De Silva N, et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73(4):1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 68.Benencia F, Courreges MC, Conejo-Garcia JR, et al. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther. 2005;16(6):765–778. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 69.Ong HT, Trejo TR, Pham LD, Oberg AL, Russell SJ, Peng KW. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol Ther. 2009;17(6):1012–1021. doi: 10.1038/mt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jing Y, Tong C, Zhang J, et al. Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 2009;69(4):1459–1468. doi: 10.1158/0008-5472.CAN-08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hallak LK, Merchan JR, Storgard CM, Loftus JC, Russell SJ. Targeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regression. Cancer Res. 2005;65(12):5292–5300. doi: 10.1158/0008-5472.CAN-04-2879. [DOI] [PubMed] [Google Scholar]
- 72.Bachtarzi H, Stevenson M, Subr V, Ulbrich K, Seymour LW, Fisher KD. Targeting adenovirus gene delivery to activated tumour-associated vasculature via endothelial selectins. J Control Release. 2011;150(2):196–203. doi: 10.1016/j.jconrel.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarkar D, Vemula PK, Teo GS, et al. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug Chem. 2008;19(11):2105–2109. doi: 10.1021/bc800345q. [DOI] [PubMed] [Google Scholar]
- 74.Sarkar D, Spencer JA, Phillips JA, et al. Engineered cell homing. Blood. 2011;118(25):e184–e191. doi: 10.1182/blood-2010-10-311464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14(2):181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 76.Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fattahi H, Laurent S, Liu F, Arsalani N, Vander Elst L, Muller RN. Magnetoliposomes as multimodal contrast agents for molecular imaging and cancer nanotheragnostics. Nanomedicine (Lond) 2011;6(3):529–544. doi: 10.2217/nnm.11.14. [DOI] [PubMed] [Google Scholar]
- 78.Muthana M, Scott SD, Farrow N, et al. A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther. 2008;15(12):902–910. doi: 10.1038/gt.2008.57. [DOI] [PubMed] [Google Scholar]
- 79.Polyak B, Fishbein I, Chorny M, et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci U S A. 2008;105(2):698–703. doi: 10.1073/pnas.0708338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arbab AS, Jordan EK, Wilson LB, Yocum GT, Lewis BK, Frank JA. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum Gene Ther. 2004;15(4):351–360. doi: 10.1089/104303404322959506. [DOI] [PubMed] [Google Scholar]
- 81.Diallo JS, Le Boeuf F, Lai F, et al. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol Ther. 2010;18(6):1123–1129. doi: 10.1038/mt.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacTavish H, Diallo JS, Huang B, et al. Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PLoS One. 2010;5(12):e14462. doi: 10.1371/journal.pone.0014462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamada K, Zhang T, Desaki J, et al. Carrier cell-mediated cell lysis of squamous cell carcinoma cells by squamous cell carcinoma antigen 1 promoter-driven oncolytic adenovirus. J Gene Med. 2010;12(6):545–554. doi: 10.1002/jgm.1467. [DOI] [PubMed] [Google Scholar]
- 84.Guo ZS, Parimi V, O’Malley ME, et al. The combination of immunosuppression and carrier cells significantly enhances the efficacy of oncolytic poxvirus in the pre-immunized host. Gene Ther. 2010;17(12):1465–1475. doi: 10.1038/gt.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raykov Z, Balboni G, Aprahamian M, Rommelaere J. Carrier cell-mediated delivery of oncolytic parvoviruses for targeting metastases. Int J Cancer. 2004;109(5):742–749. doi: 10.1002/ijc.20013. [DOI] [PubMed] [Google Scholar]
- 86.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14(4):324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 87.Iankov ID, Msaouel P, Allen C, et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat. 2010;122(3):745–754. doi: 10.1007/s10549-009-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adair RA, Scott KJ, Fraser S, et al. Cytotoxic and immune-mediated killing of human colorectal cancer by reovirus-loaded blood and liver mononuclear cells. Int J Cancer. 2013;132(10):2327–2338. doi: 10.1002/ijc.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5(3):755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 90.Mader EK, Maeyama Y, Lin Y, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15(23):7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Josiah DT, Zhu D, Dreher F, Olson J, McFadden G, Caldas H. Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol Ther. 2010;18(2):377–385. doi: 10.1038/mt.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]