Abstract
Tetrahydrofolate (vitamin B9) and its folate derivatives are essential cofactors in one-carbon (C1) transfer reactions and absolutely required for the synthesis of a variety of different compounds including methionine and purines. Most plants, microbial eukaryotes, and prokaryotes synthesize folate de novo. We have characterized an important enzyme in this pathway, the Saccharomyces cerevisiae FOL1 gene. Expression of the budding yeast gene FOL1 in Escherichia coli identified the folate biosynthetic enzyme activities dihydroneopterin aldolase (DHNA), 7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase (HPPK), and dihydropteroate synthase (DHPS). All three enzyme activities were also detected in wild-type yeast strains, whereas fol1Δ deletion strains only showed background activities, thus demonstrating that Fol1p catalyzes three sequential steps of the tetrahydrofolate biosynthetic pathway and thus is the central enzyme of this pathway, which starting from GTP consists of seven enzymatic reactions in total. Fol1p is exclusively localized to mitochondria as shown by fluorescence microscopy and immune electronmicroscopy. FOL1 is an essential gene and the nongrowth phenotype of the fol1 deletion leads to a recessive auxotrophy for folinic acid (5′-formyltetrahydrofolate). Growth of the fol1Δ deletion strain on folinic acid–supplemented rich media induced a dimorphic switch with haploid invasive and filamentous pseudohyphal growth in the presence of glucose and ammonium, which are known suppressors of filamentous and invasive growth. The invasive growth phenotype induced by the depletion of C1 carrier is dependent on the transcription factor Ste12p and the flocullin/adhesin Flo11p, whereas the filamentation phenotype is independent of Ste12p, Tec1p, Phd1p, and Flo11p, suggesting other signaling pathways as well as other adhesion proteins.
INTRODUCTION
Reduced folate supplies the cellular one-carbon pool with C1 carrier units, which act as enzyme cofactors in one-carbon transfer reactions essential for the synthesis of purines, thymidylate, glycine, methionine, pantothenic acid and N-formylmethionyl-tRNA in all organisms (Blakley and Benkovic, 1984; Appling, 1991). In eukaryotes the cytoplasmic and mitochondrial compartments each possess a parallel array of enzymes catalyzing the interconversion of folate coenzymes, which differ by the oxidation state of their one-carbon units (Appling, 1991).
Mammalian cells are unable to carry out de novo synthesis of folate. Therefore folate is considered a vitamin in these organisms, and the cells use a carrier-mediated active transport system for the uptake of folate. Folate deficiency causes massive incorporation of uracil into the DNA and chromosome breaks during the repair by uracil-DNA glycosylase and apyrimidinic endonuclease (Blount et al., 1997). Diets with low intake of folate are associated with increased risk of developing cancer (Ames et al., 1995; Duthie, 1999). In addition, it has been shown that up to 70% of neural tube defects can be prevented by folic acid supplementation in early pregnancy (Greene and Copp, 1997). Most microbial cells and plants must synthesize folates de novo because they lack the carrier-mediated active transport system of mammalian cells. Therefore early folate biosynthetic enzymes are attractive chemotherapeutic targets for the treatment of microbial diseases (Roland et al., 1979).
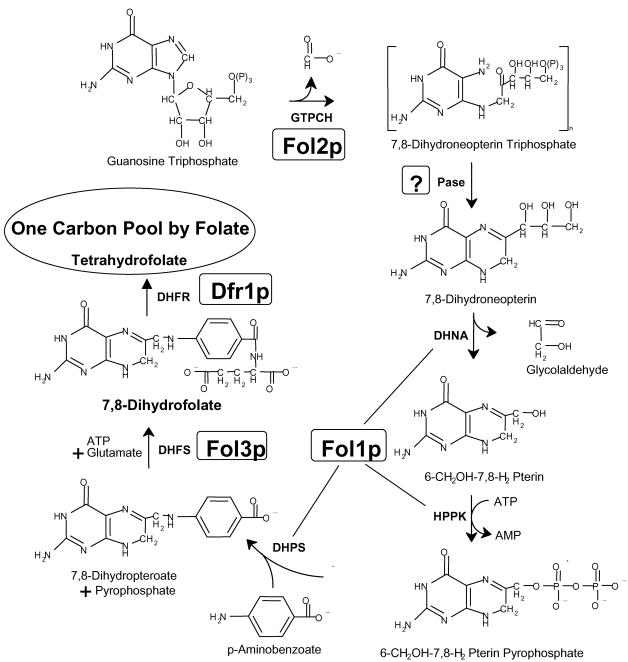
The molecular and biochemical characterization of folate biosynthetic pathways and the enzymes involved has been limited to a small number of species, in particular bacteria and plants (Talarico et al., 1992; Volpe et al., 1993; Cossins and Chen, 1997; for review see Rebeille, 1999). In most bacteria the single enzymatic activities are performed by monofunctional proteins, whereas in eukaryotes enzymatic steps are carried out by bi- or trifunctional proteins. In Saccharomyces cerevisiae the pathway leading to tetrahydrofolate (THF) has so far not been elucidated completely (Figure 1). THF synthesis starts with guanosine triphosphate, which is transformed to 7,8-dihydroneopterin triphospate by the GTP-cyclohydrolase I encoded by FOL2 (Nardese et al., 1996). The phosphatase(s) that presumably removes the phosphate residues has not been identified yet. PHO8, the only alkaline phosphatase identified in S. cerevisiae so far (Kaneko et al., 1987), is probably capable of catalyzing this reaction yielding 7,8 dihydroneopterin. We show in this work that the next three enzymes needed (dihydroneopterin aldolase [DHNA], 7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase [HPPK], and dihydropteroate synthase [DHPS]) to convert 7,8 dihydroneopterin to 7,8-dihydropteroate are all carried out by a single protein, the Fol1 protein. Subsequently, dihydrofolate synthase (DHFS) encoded by FOL3 adds the first glutamate to 7,8-dihydropteroate, yielding 7,8-dihydrofolate (DHF; Cherest et al., 2000). Finally, DHF is reduced by dihydrofolate reductase (DHFR) encoded by the DFR1 gene to yield tetrahydrofolate (THF; Barclay et al., 1988; Figure 1). Although all genes encoding enzymes of the THF biosynthetic pathway are nuclear, it was unclear in which subcellular compartment(s) THF is produced. In yeast the biochemical analysis had only revealed that dihydrofolate reductase (DHFR), which catalyzes the last step in THF synthesis is present in both the cytoplasm and mitochondria (Zelikson and Luzzati, 1977).
Figure 1.
Schematic representation of the biosynthetic pathway of tetrahydrofolate. The successive reactions shown here are catalyzed by the following enzymes: GTPCH, GTP-cyclohydrolase; DHNA, dihydroneopterin aldolase; HPPK, 7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase; DHPS, 7,8-dihydropteroate synthase; DHFS, dihydrofolate synthase (folylpolyglutamate synthase); DHFR, dihydrofolate reductase. In S. cerevisiae the GTPCH activity is encoded by FOL2 (Nardese et al., 1996). The phosphatase(s) (Pase) that presumably removes the phosphate residues of 7,8-dihydroneopterin triphosphate might be encoded by PHO8 (Kaneko et al., 1987). FOL1 described in this work encodes the next three enzymatic activities. The DHFS activity is brought about by the FOL3 gene (Cherest et al., 2000), whereas the DHFR activity is encoded by DFR1 (Lagosky et al., 1987). Tetrahydrofolate supplies the one-carbon pool with C1 carrier units, which act as enzyme cofactors in one-carbon transfer reactions involved in various biosynthetic activities (Blakley and Benkovic, 1984).
In many fungi nutrient deprivation or changes in the environment result in a distinct morphological differentiation. This dimorphic switch to filamentous growth is thought to be adaptive for nonmotile microorganisms because it facilitates foraging through the environment for scarce nutrients. This dimorphic switch is similar to morphological transitions observed for fungal pathogens such as Candida albicans and Ustilago maydis (for reviews see Lengeler et al., 2000; Sanchez-Martinez and Perez-Martin, 2001). When starved for nitrogen diploid strains of S. cerevisiae undergo a developmental transition from a single yeast cell form to filaments of elongated pseudohyphal cells (for reviews see Lengeler et al., 2000; Palecek et al., 2002). Pseudohyphal cells exhibit an altered morphology and budding pattern and can invade the growth substrate (Gimeno et al., 1992; Kron et al., 1994). In haploid S. cerevisiae cells nutrient limitation, specifically loss of fermentable sugars such as glucose causes a related process called haploid invasive growth (Roberts and Fink, 1994; Cullen and Sprague, 2000). Presence of short-chain alcohols (fusel oils; e.g., isoamyl and isobutyl alcohols) in liquid and solid media can also induce pseudohyphal-like growth in haploid cells (Dickinson, 1996), indicating that yeast may not only sense nutrient limitation but also metabolic byproducts to regulate differentiation (Lorenz et al., 2000a). Starch-degrading haploid and diploid S. cerevisiae cells are also able to switch from a yeast cell form to pseudohyphal forms in response to carbon limitation (Lambrechts et al., 1996).
Although little is known about the sensors and effectors of pseudohyphal differentiation, considerably more insight has been gained about the signal transduction pathways. The two best characterized pathways are the mating Cdc42p/Ste20p/Ste12p/Tec1p MAPK cascade, including several unique filamentation specific components, and the receptor/G protein/cAMP-PKA/Flo8p signaling pathway (for reviews see Borges-Walmsley and Walmsley, 2000; Lengeler et al., 2000; D'Souza and Heitman, 2001; Palecek et al., 2002). Other transcription factors known to regulate filamentous growth include Sok2p, Phd1p, and Ash1p and there is evidence that Phd1p could act in a pathway distinct from the cAMP and MAP kinase pathways (for review see Pan et al., 2000). Few targets of these signal transduction pathways are known, but one is the FLO11 gene encoding the most important flocculin relevant for cell-cell adhesion during filamentous growth in haploids and diploids (Rupp et al., 1999; Tamaki et al., 2000). The FLO11 gene expression is highly regulated by several transcription factors; among them Ste12p and Tec1p regulate transcription from the MAPK cascade, whereas Flo8p regulates Flo11p expression downstream of PKA (Lo and Dranginis, 1998; Madhani and Fink, 1998a; Rupp et al., 1999). New transcriptional profiling data indicate that invasive growth is not controlled solely by a dedicated invasion-specific transcriptional program and thus a variety of signals may contribute to initiate the dimporphic switch (Breitkreutz et al., 2003).
In this report we show that the FOL1 gene encodes three subsequent enzymatic activities for the biosynthesis of folic acid, namely DHNA, HPPK, and DHPS. The trifunctional Fol1 protein is expressed at a very low level and is localized at mitochondrial membranes. Deletion of FOL1 leads to a nongrowth phenotype that can be suppressed by the addition of folinic acid to the media. Under these conditions haploid fol1Δ strains exhibited strong invasive growth on rich media containing glucose and ammonium compounds known to suppress invasive growth. Likewise haploid and diploid fol1Δ strains showed a switch to filamentous pseudohyphal growth on these media, which was independent of the transcription factors Ste12p, Tec1p, Phd1p, and the cell wall protein Flo11p. Thus our results indicate that loss of the C1 carrier activity mediated by folate triggers a signal for pseudohyphae development under rich nutrient supply conditions.
MATERIALS AND METHODS
Media
The YP media plus 2% glucose (YPD) or 2% galactose (YPG) and synthetic media plus 2% glucose (SD) or 2% galactose (SG) were prepared as described (Sherman, 1991). All YP media were supplemented with adenine (4 mg/l) and tryptophan (20 mg/l). G418 (geneticin, final concentration 200 μg/ml, Cal-biochem, Novabiochem GmbH, Bad Soden, Germany), phleomycin (final concentration 7.5 μg/ml, Cayla, Toulouse, France), 5′fluoro orotic acid (5-FOA, final concentration 1000 μg/ml, PCR) and folinic acid (final concentration 50–1000 μg/ml, F-7878, Sigma, Taufkirchen, Germany) were added as required.
Yeast Strains
All yeast strains used in this study are derivatives of CEN.PK2 or CGX31 (genetic background: Σ1278b) as indicated in Table 1 (Gimeno et al., 1992; Entian and Kötter, 1998). Transformation of yeast cells, sporulation, and tetrad analysis was carried out as described (Sherman and Hicks, 1991).
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| CEN.PK2a | MATa/MATα ura3-52/ura3-52 trp1-289/trp1-289 leu2-3,112/leu2-3,112 his3-Δ1/his3-Δ1 | Entian and Kötter (1998) |
| CEN.PK2-1ca | MATaura3-52 trp1-289 leu2-3,112 his3-Δ1 | Entian and Kötter (1998) |
| CEN.HE4a | MATa/MATα ura3-52/ura3-52 trp1-289/trp1-289 leu2-3,112/leu2-3,112 his3-Δ1/his3-Δ1 YNL256w/ynl256wΔ::kanMX4 | Sen-Gupta et al. (1997) |
| YGK35b | MATaura3-52 phd1Δ::loxP-ble-loxP | This study |
| YGK36b | MATaura3-52 phd1Δ::loxP-ble-loxP fol1Δ::loxP-kanMX-loxP | This study |
| YGK37b | MATaura3-52 tec1Δ::loxP-ble-loxP | This study |
| YGK38b | MATaura3-52 tec1Δ::loxP-ble-loxP fol1Δ::loxP-kanMX-loxP | This study |
| YUG1a | MATaura3-52 trp1-289 leu2-3,112 his3-Δ1 ynl256wΔ::kanMX4 | This study |
| YUG3a | MATaura3-52 trp1-289 leu2-3,112 his3-Δ1[pUG15 GALS-FOL1 CEN6 ARSH4 TRP1 Amp] | This study |
| YUG31a | MATa/MATα ura3-52/ura3-52 trp1-289/trp1-289 leu2-3,112/leu2-3,112 his3-Δ1/his3-Δ1 FOL1/FOL1-yEGFP3-loxP-kanMX-loxP | This study |
| YUG28a | MATa/MATα ura3-52/ura3-52 trp1-289/trp1-289 leu2-3,112/leu2-3,112 his3-Δ1/his3-Δ1 URA3/ura3Δ::yEGFP3-loxP-kanMX-loxP | This study |
| CGX31b | MATa/MATα ura3-52/ura3-52 | Gimeno et al. (1992) |
| YUG72b | MATa/MATα ura3-52/ura3-52 FOL1/fol1Δ::loxP FLO11/flo11Δ::loxP-kanMX-loxP | This study |
| YUG74b | MATa/MATα ura3-52/ura3-52 FOL1/fol1Δ::loxP STE12/ste12Δ::loxP-kanMX-loxP | This study |
| YUG94b | MATaura3-52 | This study |
| YUG96b | MATaura3-52 fol1Δ::loxP | This study |
| YUG98b | MATaura3-52 ste12Δ::loxP-kanMX-loxP | This study |
| YUG100b | MATaura3-52 flol1Δ::loxP-kanMX-loxP | This study |
| YUG102b | MATaura3-52 fol1Δ::loxP ste12Δ::loxP-kanMX-loxP | This study |
| YUG104b | MATaura3-52 fol1Δ::loxP flol1Δ::loxP-kanMX-loxP | This study |
| YUG105b | MATa/MATα ura3-52/ura3-52 fol1Δ::loxP/fol1Δ::loxP | This study |
Genetic background:W303
Geneticbackground: Σ1278b
Databank Searches
The NCBI's protein database was searched using BLASTP (Altschul et al., 1997). Sequence alignments were performed using CLUSTAL W (Thompson et al., 1994). Pfam domains were calculated according to Bateman et al. (2002). Isolated Pfam domains were subjected to FASTA analysis (Pearson, 1994).
Gene Deletions
ORF YNL256w (FOL1) was deleted in strain CEN.PK2 resulting in strain CEN.HE4 using the PCR-based disruption strategy. Briefly the deletion cassette was created on template plasmid pFA6-kanMX4 using oligonucleotides 278 and 279 (Table 2) and transformed into CEN.PK2 (Wach et al., 1994). The N terminal 357 nucleotides (119 amino acids) of FOL1 were not deleted. The correct deletion was verified by Southern blot analysis (unpublished data).
Table 2.
Oligonucleotides used in this study
| Oligonucleotide, no. | Sequence (5′ → 3′) | Used for |
|---|---|---|
| Plasmid constructiona,b | ||
| 556 | GTACTAGTCGACcagttatttgtacaattcatcc | pUG24 |
| 557 | CCACTAGTCGACatgtctaaaggtgaagaattattc | pUG24 |
| 1098 | CCACTAGAGCTCTCTCCACATACCAATCACTCG | pUG64 |
| 1099 | GTACTAGTCGACTGGTCTTTGCATAGTGTGCG | pUG64 |
| 327 | GCCGTAAGACTAGTATGATAAATTGCAAGGTC | pUG15 |
| 328 | GGCGCCGGGTCGACTTATTCCAAACCTTTATAAAT | pUG15 |
| 345 | CGCACGTGACGTCTTATTCCAAACCTTTATAAA | pSH50 |
| 346 | CGCATGCCATGGTAAATTGCAAGGTC | pSH50 |
| 327 | GCCGTAAGACTAGTATGATAAATTGCAAGGTC | pUG38 |
| 391 | ACTAAGAATTCTTCCAAACCTTTATAAAT | pUG38 |
| Gene disruptionb | ||
| 278 | TTTGAAATATTCTCTAAATTATGCAGTTATTTCTCGTGATcagctgaagcttcgtacgc | FOL1 |
| 279 | TACAAAACAGAATTACATATTTAATCTATATAACTAACCTgcataggccactagtggatctg | FOL1 |
| 1052 | ATGAAAGTCCAAATAACCAATAGTAGAACAGAGGAAATCTcagctgaagcttcgtacgc | STE12 |
| 1053 | ATATCAGGTTGCATCTGGAAGGTTTTTATCGGACCTTCGAgcataggccactagtggatctg | STE12 |
| 1056 | ATGCAAAGACCATTTCTACTCGCTTATTTGGTCCTTTCGCcagctgaagcttcgtacgc | FLO11 |
| 1057 | GTATCATTAGAATACAACTGGAAGAGCGAGTAGCAACCACgcataggccactagtggatctg | FLO11 |
| 591 | ACCCAACTGCACAGAACAAAAACCTGCAGGAAACGAAGATAAATCatgtctaaaggtgaagaat | URA3 |
| 592 | AATTTGTGAGTTTAGTATACATGCATTTACTTATAATACAGTTTTgcataggccactagtgga | URA3 |
| 1425 | CTGGAATTCATAATTCATTAAGCAGAAAATATGTACCATGcagctgaagcttcgtacgc | PHD1 |
| 1426 | TTGTTCATAGAGCAAAGAGTTAACGGATTATGTTATGTGCgcataggccactagtggatc | PHD1 |
| 1427 | ATAATCCACCTATTTCAACAATTCTGATACCTGTTTAACCcagctgaagcttcgtacgc | TEC1 |
| 1428 | TGCGTATTTATGTACGAGATGTATGTATGTATGTAGACATgcataggccactagtggatc | TEC1 |
| Tagging | ||
| 609 | TTTCCTCCCTTGGTTATTTTTAACGATTCTTTATTATGAAgcataggccactagtggatctg | FOL1 |
| 636 | GAGCATTAAATTAGCAGATGCTATTTATAAAGGTTTGGAAatgtctaaaggtgaagaattattcac | FOL1 |
| Verification | ||
| 363 | GGATGTATGGGCTAAATG | kanMX forw |
| 335 | CCTCGACATCATCTGCCC | kanMX rev |
| 1068 | TTTGGGATCCCTCCCGTG | YNL256w start |
| 1069 | TTGTACCATGCCAAGAAC | YNL256w stop |
| 638 | ATAGATGAAGTTTTCGTGTG | STE12 start |
| 1055 | GAAAGATGTATCGGTAGC | STE12 stop |
| 1058 | CGTTCTCTTCTGATGAGG | FLO11 start |
| 1061 | TTCATCAAAGCCTGGTCG | FLO11 stop |
| 604 | GCATCACCTTCACCTTCACC | yEGFP3 |
| S52 | GTTTCAAACACATTCAAATGG | FOL1 |
| S38 | GGAACAATCACTTATCATGC | FOL1 |
| 593 | GAGGCTACTGCGCCAATTG | URA3 |
| 604 | GCATCACCTTCACCTTCACC | URA3 |
| 1429 | GCTGGAGTTTCTCTCGATGG | TEC1 |
| 1430 | TACTCGTTCTGGCGCGTCAG | TEC1 |
| 1431 | ATATAGGAAGAACTCTACAG | PHD1 |
| 1432 | TCTAACGAATTGCGCAATAG | PHD1 |
Underlines indicate restriction enzyme recognition sites
Lower case letters indicate nucleotides homologous to sequences on plasmids used for PCR; capital letters indicate nucleotides homologous to genomic sequences in yeast used for homologous recombination
All gene deletions in the diploid yeast strain CGX31 were done using a PCR-generated deletion cassette carrying the loxP-kanMX-loxP marker created on template plasmid pUG6 (Güldener et al., 1996). Deletion of ORF YNL256w was verified by PCR using the genomic primers 1068 and 1069 in combination with the kanR-specific primers 363 and 335. Where appropriate the kanMX marker of the resulting diploid heterozygous deletion strain was rescued using the Cre recombinase as described (Güldener et al., 1996). Next in the FOL1/fol1Δ::loxP strain, the STE12 gene was deleted using a kanMX deletion cassette generated using oligonucleotides 1052 and 1053. PCR verification was done using the STE12-specific genomic primers 638 and 1055 in combination with 363 and 335. Likewise in the FOL1/fol1Δ::loxP strain the FLO11 gene was deleted using oligonucleotides 1056 and 1057. PCR verification was done using the FLO11-specific genomic primers 1058 and 1061 in combination with 363 and 335. The PHD1 and TEC1 genes were each individually deleted in the FOL1/fol1Δ::loxP-kanMX-loxP strain using a ble deletion cassette created on template plasmid pUG66 using oligonucleotides 1425 and 1426 (PHD1) and oligonucleotides 1427 and 1428 (TEC1; Gueldener et al., 2002). PCR verification was done using the PHD1-specific genomic primers 1431 and 1432 or the TEC1-specific genomic primers 1429 and 1430 in combination with 363 and 335.
Tetrad dissection of the resulting diploid heterozygous deletion strains was done on YPD + 1 mg/ml folinic acid, and the mating type was determined via PCR analysis (Huxley et al., 1990). To generate a diploid homozygous fol1Δ/fol1Δ deletion strain the haploid fol1Δ deletion strain YUG1 was transformed with the HO plasmid Ycp50-HO-12 (gift from C. Hollenberg). Several uracil-prototrophic transformants were streaked out and diploids were identified by mating type-specific PCR. The HO plasmid was recovered by 5-FOA counterselection (Boeke et al., 1984).
Quantification of Fol1 Protein
The amount of Fol1 protein was quantified by fluorescence-activated cell analysis of a Fol1-GFP fusion protein. The genomic fusion of yEGFP to the C-terminus of FOL1 in yeast strain CEN.PK2 was done by homologous integration of a yEGFP3-loxP-kanMX-loxP integration cassette, which was created by PCR on template plasmid pUG24 using oligonucleotides 609 and 636. In the resulting strain YUG31 the yEGFP3 gene was integrated in frame at the C-terminal end of FOL1, replacing the FOL1 stop codon and the following 60 base pairs downstream. The correct integration was verified by two PCRs using 1) the yEGFP3 specific primer 604 and the FOL1 specific primer S52, and 2) the kanMX module-specific primer 335 and a primer in the FOL1 terminator region (S38). Plasmid pUG24 was obtained by cloning a PCR-generated yEGFP3 SalI fragment (template plasmid: pYGFP3; Cormack et al., 1997; oligonucleotides 556 and 557) into SalI cut pUG6 (Güldener et al., 1996).
Wild-type and yEGFP3 integration strains were grown overnight at 30°C in the appropriate liquid media as indicated. From these cultures cells were freshly inoculated in the same media (starting OD600 0,1) and grown to an OD600 of 0.3–0.5. Cells were diluted to an OD600 of 0.03 in 10 mM Tris, pH 7.5, and analyzed directly. Flow cytometry was carried out using a FACSort system (Becton Dickinson, Heidelberg, Germany; Niedenthal et al., 1996, 1999). Ten thousand particles (living cells) were analyzed per sample. The autofluorescence obtained for the wild-type (wt) strain was set electronically to channel 200 and the deletion strains were then analyzed using the same parameters. For each data point five independent cultures were measured.
Quantification of the FLO11 Promoter Activity
GFP/FACS Analysis For quantification of the FLO11 promoter activity a 3-kb-long FLO11 promoter fragment was cloned in front of the yEGFP gene located on CEN plasmid. The FLO11 promoter fragment was obtained by PCR on genomic DNA of strain CGX31 using oligonucleotides 1098 and 1099. The resulting PCR product was digested with SacI/SalI and cloned into SacI/SalI cut pUG35 (a pUG23 derivative carrying yEGFP3, URA3, and CEN/ARSH4), thus replacing the MET25 promoter in front of the yEGFP3 gene. The resulting plasmid pUG64 and the control plasmid p416MET25 (Mumberg et al., 1995) were expressed in Σ1278b-based strains and analyzed by FACS as described before (Niedenthal et al., 1999). Wild-type and yEGFP3 integration strains were grown overnight at 30°C in liquid synthetic minimal glucose media. From these cultures cells were freshly inoculated in the same media (starting OD600 0.1) and grown to an OD600 of 0.3–0.5 and analyzed by FACS as described above.
LacZ/Overlay Assay Yeast cells transformed with pFLO11-2/1, pFLO11-4/3, pFLO11-6/5, pFLO11-8/7, pFLO11-9/10, pFLO11-12/11, pFLO11-14/13, pFLO11::LacZ and the empty vector (Rupp et al., 1999) were pregrown in SC-ura medium containing 200 μg/ml folinic acid at 30°C O/N. Approximately 1 × 106 cells (wt) and 28 × 106 cells (fol1Δ) were spotted on SD plates without amino acids containing 50 μg/ml folinic acid and incubated at 30°C for 25 h. On average spots carried 4 × 107 cells. The overlay assay was performed as previously described (Suckow and Hollenberg, 1998). Briefly spots were covered with 5 ml of 70°C hot agarose (0.5% agarose, 0.5 M sodium phosphate buffer, pH 7.0, 0.2% SDS, and 0.4 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and incubated for 24 h at 37°C.
Quantification of Fol1p Enzymatic Activities
FOL1 Overexpression in Yeast A 2.6-kb-long FOL1 fragment was obtained by PCR on an EcoRI subclone of cosmid 14-4 (obtained from P. Philippsen, Basel) using oligonucleotides 327 and 328. The SpeI/SalI-cleaved PCR product was cloned into SpeI/SalI cut p414GALS (Mumberg et al., 1994) yielding pUG15 (selection marker: TRP1). pUG15 was transformed into strain CEN.HE4 (relevant genotype: FOL1/fol1Δ::KanMX). Subsequent tetrad analysis and replica platting identified G418-resistant and Trp+ haploid segregants (YUG3). The GAL1/2 μ FOL1 overexpression vector pUG14 was constructed by subcloning the SpeI/SalI fragment of pUG15 into SpeI/SalI-cut p424GAL1 (Mumberg et al., 1994).
FOL1 Expression in E. coli The FOL1 gene was cloned behind the tac promoter on plasmid pHK255-I (Duesterhoeft, 1990). To this end the FOL1 gene was first PCR-amplified from an EcoRI subclone of cosmid 14-4 using oligonucleotides 345 and 346, cleaved with EcoRV, and cloned into pUG7 (pBluescript derivative; Güldener et al., unpublished results) resulting in plasmid pSH50. pSH50 was cleaved by NcoI and SacI and the DNA fragment carrying the complete FOL1 gene was cloned into NcoI/SacI cut pHK255-I resulting in plasmid pSH51, which was transformed into E. coli strain XL1 blue for expression of the Fol1 protein.
Enzymatic Assays
The following materials were purchased from B. Schircks (Jona, Switzerland): 6-hydroxymethylpterin, 6-hydroxymethylpterin pyrophosphate, and 7,8-dihydro-d-neopterin. Pterines were hydrated with Pd-Charcoal (4 h, RT, atmospheric pressure) under the formation of 7,8-dihydropterins.
Assay of 7,8-Dihydroneopterin Aldolase (Haussmann et al., 1998) Assay mixtures contained 100 mM Tris/HCl, pH 8.0, 5 mM β-mercaptoethanol, 100 μM 7,8-dihydro-d-neopterin and protein. The mixture was incubated at 37°C for 20 min. The reaction was terminated by the addition of 30 μl 1 M HCl containing 1% I2 and 2% KI (wt/vol). The samples were incubated at room temperature (RT) in the dark for 5 min. Excess iodine was reduced by the addition of 20 μl 2% ascorbinic acid (wt/vol). Samples were analyzed by reversed-phase HPLC using a column of Nucleosil RP18 (4 × 250 mm). The eluent contained 30 mM HCOOH and 7% MeOH. The flow rate was 2 ml/min. 6-hydroxymethylpterin was detected fluorometrically (Ex: 365 nm; Em: 446 nm). The retention volume of the reaction product was 6.5 ml.
Assay of 6-Hydroxymethyldihydropterin Pyrophosphokinase Assay mixtures contained 100 mM Tris/HCl, pH 8.0, 10 mM MgCl2, 5 mM β-mercaptoethanol, 50 μM 6-hydroxymethyl-7,8-dihydropterin, and protein. After incubation for 20 min the reaction was stopped as described above. The samples were then anaylzed for 6-hydroxymethylpterin pyrophosphate as described above. The retention volume of 6-hydroxymethylpterin pyrophosphate was 2.5 ml.
Assay of Dihydropteroate Synthase Assay mixtures contained 0.1 M Tris hydrochloride, pH 8.0, 10 mM MgCl2, 5 mM β-mercaptoethanol, 20 μM 6-hydroxymethyl-7,8-dihydropterin pyrophosphate, 0.04 nM [carboxyl-14C]4-aminobenzoate, and protein in a total volume of 6 μl. The mixture was incubated at 37°C for 30 min. The reaction was terminated by immersion into a boiling water bath. Aliquots (3 μl) were applied to Whatman 3MM paper that was subjected to descending paper chromatography using 0.1 M potassium phosphate as solvent. The enzyme product remained at the start of the chromatogram. Radioactivity of paper segments was determined by scintillation counting.
For the DHNA and HPPK activity measurements two independent cultures were measured in triplicate and the median and the SD was calculated.
Yeast Protein Analysis
Wild-type and fol1Δ strains (strain background: Σ1278b) were grown to an OD600 of 0.6–0.8. Then cells were incubated with 0.15 μM α factor for 15 min. Cells from 20-ml cultures were pelleted, and crude protein extracts were produced according to a protocol provided by Miltenyi Biotec GmbH (Bergisch-Gladbach, Germany). Protein samples were immediately boiled with SDS loading buffer, aliquoted, and frozen at -20°C.
Protein samples were electrophoresed by SDS-PAGE on 12% polyacrylamide gels (acrylamide:bis-acrylamide at a ratio of 37.5:1) using the Hoefer Mighty Small gele system (Amersham Biosciences, Freiburg, Germany). After protein transfer the membranes were blocked in TBST buffer as described by the ECL Advance Western Blotting Detection Kit (Amersham Biosciences). Membranes were incubated with primary antibody (antiphospho p44/42 MAPK; Cell Signaling Technology, Inc., Beverly, MA; 9101) in TBST-2% block overnight at 4 C° followed by three washings with TBST at RT. Immunoblots were incubated with secondary antibody (rabbit Ig, HRP-linked Whole antibody; Amersham Biosciences) in TBST-2% block for 7 h at 4°C and then washed three times with TBST at RT and developed using the Amersham ECL kit.
Microscopy
The FOL1 gene was PCR amplified using oligonucleotides 327 and 391 and plasmid pUG16 as template. The SpeI/EcoRI 2.6-kb PCR product was cloned into SpeI/EcoRI cut yEGFP3 vector pUG23 (Güldener et al., unpublished results) resulting in the CEN-vector pUG38.
For Fol1p localization experiments yeast strain CEN.PK2 was transformed with plasmid pUG38. For staining of mitochondria and nuclei in living cells, exponential growth phase cells were incubated in YPD media supplemented with 4′6-diamino-2-phenylindoledihydrochloride (DAPI; stock:1 μg/μl, final concentration: 1 μg/ml) for 30 min. Cells were fixed by addition of formaldehyde (500 μl of 37% formaldehyde were added to 5 ml yeast culture) and incubation for 30 min at 30°C. Cells were washed once with PBS and then adsorbed onto a polylysine-coated coverslip. Cells were viewed using a Zeiss Axioskop (Oberkochen, Germany) microscope.
Assessment of Budding Pattern
Haploid cells with the indicated genotype grown on YPD plates containing 50 μg/ml folinic acid were taken from plates, resuspended in PBS buffer, and stained with the chitin-specific dye calcofluor white (concentration 4 μg/ml). Cells were spotted onto polylysine-coated microscope slides and viewed using a Zeiss Axioskop microscope. Cells with two or more bud scars were classified as follows: unipolar/axial (scars only at one end), bipolar (scars at both ends), or random (scars not localized to the ends; number of cells ≥100).
Immunocytochemistry and Electron Microscopy
For electron microscopy a gfp-FOL1 fusion was constructed by cloning a SpeI/SalI FOL1 fragment (out of pUG15) into plasmid pGFP-N-FUS (Niedenthal et al., 1996) resulting in pUG16. Yeast strain CEN.PK2 was transformed with plasmid pUG16. Cells were grown in selective minimal media, harvested, and fixed with a mixture of 0.5% glutaraldehyde and 4% freshly depolymerized paraformaldehyde in 0.1 M sodium citrate buffer, pH 4.7, for 1.5 h at 30°C (Zimmer et al., 1995). Subsequent treatment with 1% sodium metaperiodate (Sigma) for 1 h at 4°C was performed to support penetration of the cell wall by the cryoprotectant sucrose. Cryoprotection of the cells was achieved by infusion with a mixture containing 25% polyvinylpyrrolidone (PVP, MW 10,000, Sigma) and 1.6 M sucrose for 3 h at 30°C (Tokuyasu, 1989). Cells were mounted on specimen holders and immediately frozen in liquid nitrogen. Ultrathin cryosections were prepared at -100°C and deposited on formvar/carbon-coated copper grids after thawing. Immunolabeling with polyclonal anti-GFP antibody (Clontech, Palo Alto, CA) or polyclonal anti-Fol1p antibody (D. Becher, unpublished results) and secondary colloidal gold (10 nm) goat anti-rabbit IgG conjugate (British BioCell, Cardiff, Wales) was performed according to Griffiths et al., (1984) with minor modifications. As controls, the sections were incubated directly with gold IgG conjugate, omitting the primary antibody step, or cells without GFP-fusion protein were used. Finally, the frozen-thawed sections were stabilized and stained with a mixture of 0.3% uranyl acetate and 2% methylcellulose (25 cp; Sigma). Specimens were examined with a Zeiss 906 electron microscope at 60 kV acceleration voltage.
RESULTS
The FOL1 Gene Product Has Been Conserved in Evolution
Data base searches using the 864-amino acid-long protein sequence derived from ORF YNL256w on the left arm of chromosome XIV revealed significant homologies to proteins of prokaryotic and eukaryotic microorganisms known to be involved in the biosynthesis of folate (Sen-Gupta et al., 1997). Therefore we named ORF YNL256w FOL1 according to the standard yeast genetic nomenclature. Furthermore a folate auxotroph mutant named fol1 had been mapped previously to this region (Little and Haynes, 1979).
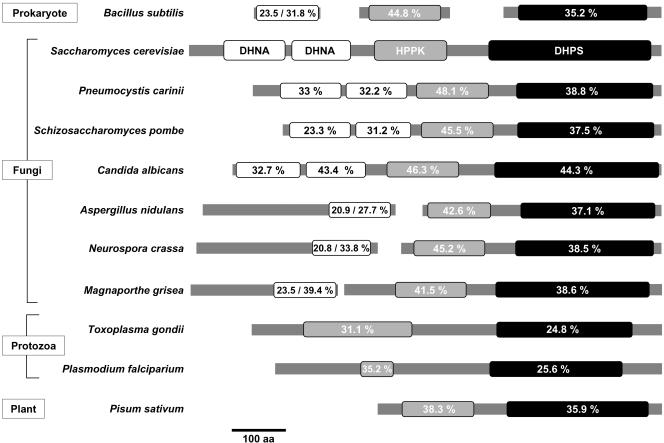
Detailed analysis of the Fol1p protein sequence indicated the presence of three putative enzymatic activities: DHNA, HPPK, and DHPS (Figure 2). These three enzymatic activities perform three subsequent steps in the biosynthesis of folate (Figure 1). In bacteria these three enzymatic activities are usually encoded by three separate ORFs (e.g., Bacillus subtilis; Slock et al., 1990). In contrast in filamentous fungi and probably in other higher eukaryotes only the HPPK and DHPS domains are fused to one protein, e.g., Toxoplasma gondii and in pea (Triglia and Cowman, 1994; Rebeille et al., 1997; Figure 2). However, in the fungi Schizosaccharomyces pombe and C. albicans and in Pneumocystis carinii (zygomycete), all three enzyme activities have been found to be located on a single polypeptide (Volpe et al., 1993; Figure 2). Detailed FASTA alignments of Fol1p indicated that its N-terminus carries two domains with 24 and 32% identity, respectively, to ORF1 from B. subtilis encoding DHNA. This organization has also been found in the P. carinii fas protein, where the two domains FasA and FasB are both required for DHNA function (Volpe et al., 1995; Figure 2). The S. cerevisiae DHNA domains show the lowest identity (21%) to the corresponding domain from Neurospora crassa and the highest identity (43%) to the domain from C. albicans. The central Fol1p domain exhibits identities between 31 and 48%, respectively, to the HPPK carrying domains from T. gondii and P. carinii. The C-terminal domain of Fol1p shares between 25% identity with the corresponding domain from T. gondii and 44% identity with the C-terminal domain from C. albicans (Figure 2). Taken together these data indicate that Fol1p shares its trifunctional nature with the corresponding proteins from a limited number of fungi, whereas in most other eukaryotes this protein is bifunctional.
Figure 2.
The Fol1 protein is conserved in evolution. Comparison of Pfam domains of the Fol1 protein from S. cerevisiae with the corresponding proteins from other species (B. subtilis P28823, S66109, P28822; P. carinii P29251; S. pombe SPBC1734.03; C. albicans CAC08858; A. nidulans an4979.1, an6032.1; N. crassa ncu02798.1, b21j21_140; M. grisea mg01629.1, mg01474.1; T. gondii AAB93492.1; P. falciparium CAA83456.1; P. sativum CAA69903.1; numbers correspond to GenBank entries). For the protozoa and for P. sativum only the bifunctional HPPK- and DHPS-carrying proteins are presented. The percentage identity to the corresponding S. cerevisiae Pfam domain was calculated using FASTA.
FOL1 Codes for the Enzyme Activities DHNA, HPPK, and DHPS
To confirm that the three enzymatic activities inferred from homology were indeed encoded by FOL1, we performed enzymatic tests for all three enzyme activities. First the FOL1 gene was expressed in E. coli via the inducible tac promoter. As expected, control E. coli cells exhibited all three endogenous enzyme activities (Table 3; XL1 Blue). However in E. coli cells expressing the S. cerevisiae FOL1 gene all three enzyme activities increased significantly. The DHNA and HPPK enzyme levels were increased fivefold compared with the enzyme levels in protein extracts from control cells, whereas the DHPS enzyme level increased about ninefold (Table 3; ptac:FOL1). These data demonstrate that FOL1 encodes all three enzyme activities.
Table 3.
Enzymatic activities encoded by FOL1
| DHNA (nmol/mg h) | HPPK (nmol/mg h) | DHPS (nmol/mg h) | |
|---|---|---|---|
| S. cerevisiae strain | |||
| FOL1 | h6.0 ± 1 | 1.0 ± 0.5 | 16 |
| fol1Δ | <1.0a | <0.3a | 5 |
| FOL1 + pGALS:FOL1 | 61.7 ± 20 | 43.8 ± 15 | 60 |
| E. coli strain | |||
| XL1Blue | 73.8 ± 10 | 61.4 ± 15 | 185 |
| XL1Blue + ptac:FOL1 | 364.0 ± 25 | 310.7 ± 25 | 1714 |
The specific enzyme activity assays were performed using protein extracts from E. coli or yeast. E. coli XL1Blue was assayed without plasmid (control) and was transformed with pSH51 carrying the yeast FOL1 gene under control of the tac promoter (ptac-FOL1). Yeast strains that were derived from the same genetic back ground were grown in media complemented with 1 mg/ml folinic acid. Haploid CEN.PK2-1C carries a FOL1+ wt gene (FOL1), whereas YUG1 is deleted for FOL1 (fol1Δ). Strain YUG3 expresses FOL1 via the GALS promoter on a plasmid (FOL1 + pGALS-FOL1; Mumberg et al., 1994).
The levels were below the detection level of the assay
We next characterized the FOL1 enzyme activities in yeast. Protein extracts prepared from a haploid wt strain exhibited low but significant levels for all three enzymatic reactions (Table 3; FOL1). Analysis of an isogenic FOL1 deletion strain (kept alive by the presence of folinic acid in the media; see later) revealed severely reduced activities for all three enzyme reactions (Table 3; fol1Δ). In contrast moderate overexpression of FOL1 from the GALS promoter in a wt strain resulted in a strong increase of all three enzymatic activities (Table 3; FOL1 + pGALS:FOL1).
These data demonstrate that Fol1p carries three enzymatic activities for the biosynthesis of folic acid, namely DHNA, HPPK, and DHPS (Figure 1).
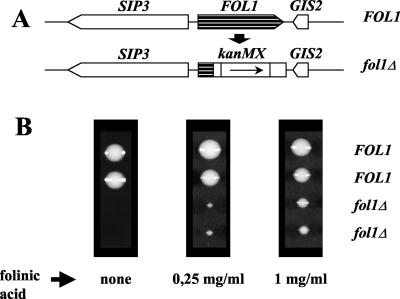
The FOL1 Gene Is Required for Growth
Deletion of one allele of FOL1 in a diploid yeast strain (Figure 3A) and subsequent tetrad analysis revealed that FOL1 is an essential gene, as spores carrying the FOL1 deletion were unable to form visible colonies on YPD (Figure 3B, left panel). The terminal phenotype of the fol1 deletion on rich media (YPD) was a microcolony of uneven shape containing 50–100 slightly elongated cells. The nongrowth phenotype of the fol1Δ strain could be rescued by addition of 5-formyl tetrahydrofolate (folinic acid, FA) as an alternative one-carbon source into the media (Figure 3B, middle and right panels). Folinic acid can only be metabolized by 5,10-methenyl-THF synthetase yielding 5,10-methenyl-THF, a folate coenzyme that can be converted into the other folate coenzymes (Holmes and Appling, 2002). However although the fol1Δ strain could be rescued by addition of folinic acid, the colonies never reached wt size (Figure 3B; 1 mg/ml FA), probably because folinic acid is known to be an inhibitor of several folate-dependent enzymes (Girgis et al., 1997).
Figure 3.
FOL1 is an essential gene.(A) Schematic representation of a FOL1 deletion using the kanMX PCR-generated gene deletion cassette. (B) Tetrad analysis of the heterozygous diploid fol1 deletion strain CEN.HE4 on YPD supplemented with folinic acid as indicated. The genotype of the spores is shown. Plates were incubated at 30°C for 72 h. Spores carrying the fol1Δ deletion germinated on media without folinic acid and went through 4–6 cell divisions before stopping to divide. This nongrowth phenotype could be rescued by addition of folinic acid.
Additionally, although the fol1 deletion strains could grow on media supplemented with folinic acid, they were unable to grow on the nonfermentable carbon sources ethanol, glycerol, or acetate (supplemented with folinic acid), indicating that Fol1p and thus 1C carrier are also required for mitochondrial function in yeast and that addition of exogenous folinic acid cannot fill up the mitochondrial one-carbon carrier pools adequately (unpublished data).
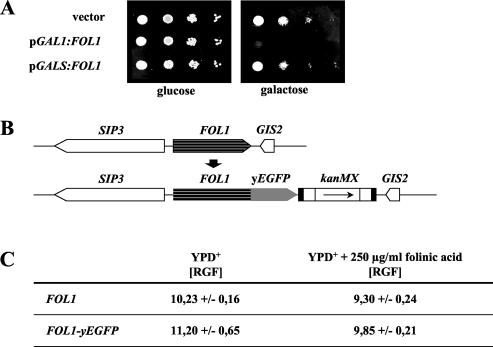
Moderate But Not High FOL1 Expression Rescues the Nongrowth Phenotype of a fol1Δ Strain
Interestingly, strong overexpression of FOL1 by expression from the GAL1 promoter on a 2-μ–based plasmid did not complement the nongrowth phenotype of the fol1Δ strain. Likewise strong overexpression of FOL1 in a diploid wt strain was toxic and resulted in a significant increase of cells with a 2C DNA content (Figure 4A; unpublished data). However FOL1 expression from the moderate GALS promoter on a CEN-based plasmid exhibited almost wt-like growth (Figure 4A). These data suggest that high levels of THF-synthesizing enzymes and/or of C1 carrier units are detrimental to the yeast cell. Thus it can be assumed that the Fol1p level in the cell is low, and indeed our own data indicate that the Fol1 promoter activity is low (unpublished data).
Figure 4.
High levels of Fol1p are detrimental to yeast. (A) Serial dilution patch tests of cells expressing Fol1p from the GAL1 promoter from a 2-μ plasmid or from the GALS promoter from a CEN/ARS plasmid in the diploid strain CEN.PK2 grown on rich media containing glucose or galactose. Dilutions shown were 10-fold starting with 104 cells. (vector = empty vector p424GAL1 without FOL1; Mumberg et al., 1994); (B) Diagrammatic representation of a chromosomal FOL1-yEGFP fusion. The yEGFP3 gene was fused in frame to the 3′ end of one of the 2 FOL1 alleles in strain CEN-PK2 by integration of a PCR-mediated yEGFP3-loxP-kanMX-loxP replacement cassette. (C) The diploid strain CEN.PK2 and the heterozygous FOL1/FOL1-yEGFP3 strainYUG31 were grown in YPD+ or in YPD+ supplemented with 250 μg/ml folinic acid, and the amount of green fluorescence was quantified by flow cytometry. For each strain five independent cultures were measured and the median and SD is given. RGF, relative green fluorescence.
Next we wanted to know whether the amount of Fol1 protein is regulated under conditions where the intracellular C1 carrier concentration changes (here: addition of folinic acid). A genomic FOL1-yEGFP fusion construct replaced one of the two chromosomal wt FOL1 alleles in a diploid strain (Figure 4B). Quantification of the background fluorescence of the parental wt strain (FOL1) by flow cytometry revealed a relative green fluorescence (RGF) of 10.23 U, while the Fol1-yEGFP fusion strain exhibited 11.2 U, which represents a small increase of 0.97 RGF units (see MATERIALS AND METHODS for details; Figure 4C). Addition of folinic acid to the media led to a reduction in background fluorescence for the wt control strain (RGF 9.30) of ∼0.93 RGF units; however, the FOL1-yEGFP fusion strain showed a stronger reduction (RGF 9.85) of 1.35 RGF units, suggesting that the FOL1 promoter might be autoregulated (Figure 4C).
Together these data indicate that excess amounts of Fol1p in yeast hampers growth and that cells therefore may have evolved sensing and regulating mechanisms to adjust the Fol1p levels adequately.
Fol1p Is Localized at the Mitochondrial Membrane
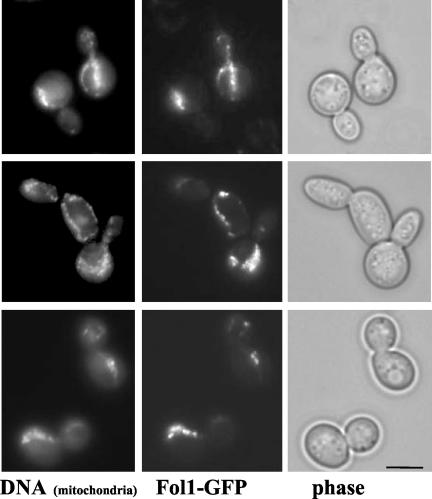
To study the subcellular localization of the Fol1 protein, we expressed a C-terminal FOL1-yEGFP3 fusion protein from a CEN plasmid under control of the regulatable MET25 promoter. The fusion protein was functional as it was able to rescue the nongrowth phenotype of the fol1 deletion strain (unpublished data). The Fol1p-yEGFPp fusion protein showed a punctate staining and colocalized with DAPI-stained mitochondria (Figure 5).
Figure 5.
Localization of Fol1-yEGFP3 fusion protein. Cells expressing a plasmid-borne version of FOL1-yEGFP were grown in YPD+ to midlog followed by the addition of 1 μg/ml DAPI and incubation for 30 min at 30°C. Under these conditions DAPI stains mainly the mitochondrial DNA. Subsequently cells were adsorbed onto polylysine-coated coverslips, and photomicrographs were taken. Bar, 5 μm.
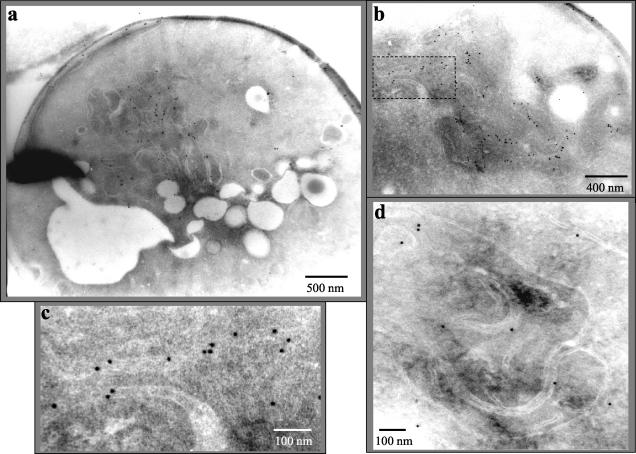
This localization was confirmed by electron microscopy of yeast cells expressing a N-terminal GFP-Fol1 fusion protein using anti-GFP antibodies (Figure 6, a–c). Expression of this fusion protein again complemented the nongrowth phenotype of the fol1 deletion strain. However sometimes clumped mitochondria were observed in this strain. In cells expressing the GFP-Fol1 fusion protein the gold-particles labeled mainly the mitochondria (Figure 6a). A detailed inspection at higher resolution revealed that the gold particles were found associated mainly with both mitochondrial membranes and rarely with the mitochondrial matrix (Figure 6, b and c). To exclude that the GFP part of the fusion protein influenced the localization we also localized the Fol1 protein in a diploid wt strain using peptide antibodies against an antigenic epitope of Fol1p itself. Although the Fol1 protein level is very low in yeast and the antibody exhibited only limited specificity, most of the gold particles were again located at the mitochondrial membrane (Figure 6d). Taken together the data indicate that the Fol1 protein is located at the mitochondrial membrane system.
Figure 6.
Electron microscopy of Fol1 protein. Cells from the diploid CEN.PK2 strain expressing plasmid-borne GFP-Fol1p were processed and the localization of the fusion protein was determined using gold-labeled anti-GFP antibody (a–c). In panel c an enlargement of a mitochondrial membrane shown in panel b is presented (indicated by boxed area). (d) The analysis was also carried out using anti-Fol1p peptide antibodies. Size markers are indicated.
Absence of FOL1 Induces Filamentous and Adhesive Growth
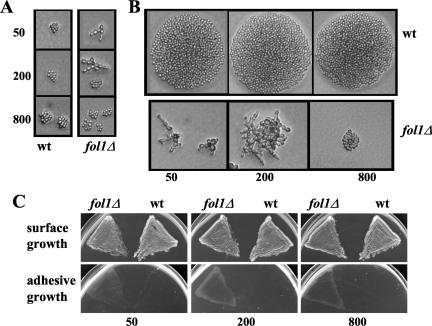
Rescue of the nongrowth phenotype of fol1Δ cells was seen on media containing folinic acid. The degree of rescue observed was dependent on the concentration of folinic acid (Figure 3B). Microscopic inspection of haploid fol1Δ cells grown for only 8 h on rich YPD media in the presence of folinic acid revealed elongated connected cells forming branching filamentous asters (Figure 7A). The extent of filamentation was dependent on the amount of folinic acid present: on media with 50 μg/ml all cells formed filaments consisting of elongated and bigger cells (Figure 7A, top panel), on media with 200 μg/ml 50–80% of the cells formed filaments (Figure 7A, middle panel, both types shown), whereas on media with 800 μg/ml, most cells showed an almost regular yeast-like morphology (Figure 7A, bottom panel). After growth for 24 h filamentous growth of fol1Δ cells grown on media with 50 and 200 μg/ml folinic acid had continued, whereas cells on media with 800 μg/ml folinic acid were yeast-like but had formed uneven microcolonies (Figure 7B). These data indicated that deletion of the FOL1 gene induces a filamentous growth phenotype and prompted us to test if fol1Δ cells showed adhesive growth. Haploid fol1Δ cells grown for 48 h on YPD media supplemented with varying amounts of folinic acid had invaded the agar, whereas isogenic wt cells did not show agar invasion (Figure 7C).
Figure 7.
Pseudohyphal and adhesive growth of a fol1Δ strain. (A and B) CEN.PK2 derived wild-type (wt) and fol1Δ strains were pregrown on YPD medium containing different amounts of folinic acid (50, 200, or 800 μg/ml) for 24 h at 30°C. Subsequently cells were resuspended in water and aliquots plated for colonies derived from single cells on YPD medium containing 50, 200, or 800 μg/ml folinic acid. Single colonies were photographed after an 8- (A) or 24-h (B) incubation at 30°C. (C) The same strains as described in A were pregrown on YPD medium containing folinic acid, restreaked on YPD containing different amounts of folinic acid (50, 200, or 800 μg/ml), and incubated at 30°C for 48 h. Plates were photographed (surface growth) and then washed extensively with water and rephotographed (adhesive growth). Background of all strains is CEN.PK2/W303.
These growth phenotypes are reminiscent of the filamentous growth phenotype observed for diploid cells under nitrogen limitation, or for haploid cells exhibiting invasive growth when grown on rich media for an extended period of time or when starved for glucose (Cullen and Sprague, 2000; Lengeler et al., 2000; Palecek et al., 2002).
The wt and fol1Δ strains used were derived from CEN.PK2 and have a W303 background. We next assayed the consequences of a FOL1 deletion in a Σ1278b/CGX31 strain background which is commonly used for filamentation and invasion studies (Gimeno et al., 1992; Palecek et al., 2002). Haploid strains of this strain background show invasive growth on rich YPD media but only after prolonged incubation for 4–6 days (Roberts and Fink, 1994; Rupp et al., 1999; Braus et al., 2003). As expected, microcolonies of the Σ1278b-based haploid wt strain showed yeast-like growth forming round colonies on rich YPD media supplemented with folinic acid (Figure 8, a and b) and showed the typical substrate invasiveness after growth for 5 days (Figure 9, c and d, wt), whereas after growth for 24 h no agar invasion could be observed (Figure 9, a and b, wt). In contrast, deletion of the FOL1 gene in this strain background gave rise to slightly larger and often elongated cells with filamentous structures and uneven microcolony morphologies (Figure 8, g and h). Moreover, in contrast to wt cells the fol1Δ strain showed strong adhesive growth after 24 h of growth on media supplemented with folinic acid (Figure 9, a and b, fol1Δ). After prolonged growth for 5 days the fol1Δ strain despite its significantly reduced overall growth behavior showed adhesive growth similar to that observed for the wt strain (Figure 9, c and d, fol1Δ).
Figure 8.
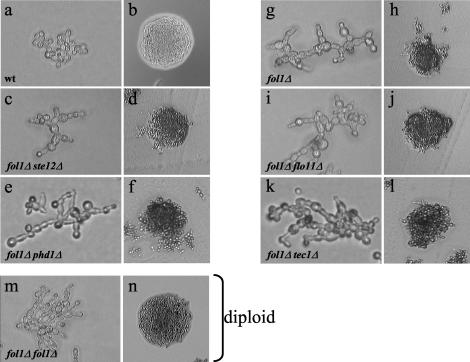
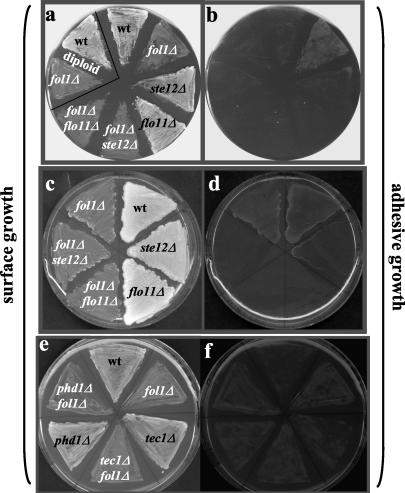
The fol1Δ deletion strain shows filamentous growth, which is independent of STE12, TEC1, PHD1, and FLO11. Cells of the haploid wt strain YUG94 (wt), the fol1Δ single deletion (fol1Δ), the fol1Δ ste12Δ (fol1Δ/ste12Δ), the fol1Δ tec1Δ (fol1Δ/tec1Δ), the fol1Δ phd1Δ (fol1Δ/phd1Δ), and the fol1Δ flo11Δ (fol1Δ/flo11Δ) double deletion strains, and of the homozygous diploid fol1Δ/fol1Δ strain (fol1Δ/fol1Δ) were incubated for 6 h (a), 24 h (b, c, e, g, i, k, and m), and 72 h (d, f, h, j, l, and n) at 30°C on YPD + 50 μg/ml folinic acid, respectively. All fol1Δ strains showed filamentous growth after 24 h and formed uneven colonies. Background of all strains is Σ1278b.
Figure 9.
Adhesive growth of fol1Δ strains is dependent on Ste12p and Flo11p, but independent of Tec1p and Phd1p. (a, b, e, and f) Single and double deletion strains were streaked on synthetic media containing 50 μg/ml folinic acid and grown at 30°C for 24 h. Strains shown are haploid except the strains in the white triangle, which are homozygous FOL1 (wt) or fol1Δ (fol1Δ) diploid strains. (c and d) Single and double deletion strains were streaked on solid 2× YPD containing 125 μg/ml folinic acid and incubated for 5 days at 30°C. All plates were photographed (surface growth) and then washed extensively with water and rephotographed (adhesive growth).
In diploid strains, starvation for nitrogen also induces pseudohyphal growth. Therefore we tested the consequences of the deletion of both alleles of the FOL1 gene in a diploid Σ1278b-based strain. Growth of this strain for 24 h on rich YPD media supplemented with folinic acid resulted in filamentous growth and after growth for 72 h an uneven colony was formed (Figure 8m and n, diploid). However, after growth for 24 h the adhesive growth properties of the fol1Δ fol1Δ strain were similar to those of the diploid wt strain (Figure 9, a and b, diploid).
The haploid fol1Δ cells exhibited an altered budding pattern in comparison to wt cells. Haploid wt cells typically exhibit an axial budding pattern in which they choose the location of the new bud adjacent to the sites of the previous ones (for review see Madden and Snyder, 1998; Chant, 1999). The haploid fol1Δ strain showed mainly bipolar distal bud site selection (91%), where most of the buds emerged at the distal pole opposite to the birth end, whereas only 47% of the wt cells exhibited bipolar budding (Table 4).
Table 4.
Increased bipolar budding pattern in haploid fol1 deletion strains
| Strain | Unipolar (%) | Random (%) | Bipolar (%) |
|---|---|---|---|
| wt | 30 | 23 | 47 |
| fol1Δ | 5 | 4 | 91 |
| fol1Δ ste12Δ | 6 | 21 | 73 |
| fol1Δ fol1Δ | 6 | 7 | 87 |
| fol1Δ phd1Δ | 4 | 8 | 88 |
| fol1Δ tec1Δ | 11 | 5 | 84 |
Yeast cells were grown for 24 hrs on YPD plates supplemented with 50 μg/ml folinic acid, then analyzed. For the determination of budding pattern of cells having two or more bud scars, the following criteria applied: unipolar: cells with all bud scars at one pole; bipolar: cells with bud scars at both poles; random: cells with at least one bud scar not localized at either pole (Freedman, et al.; 2000). Values are shown as percentages (n ≥ 100 cells).
The actin cytoskeleton plays a central role in fungal dimorphism (Kron et al., 1994). Therefore we also analyzed the actin cytoskeleton in a fol1Δ strain grown on YPD media supplemented with 50 μg/ml folinic acid. Cells of the fol1Δ strain showed an enhanced polarity of actin patches toward the distal tip of daughter cells throughout bud emergence relative to the distribution of actin patches in wt cells. Furthermore the actin cables were more pronounced in fol1Δ cells when compared with wt cells (unpublished data). These findings indicate that in a fol1Δ strain the actin cytoskeletal structures have adopted characteristics of pseudohyphal growth (Kron et al., 1994; Cali et al., 1998).
Filamentous Growth of fol1Δ Strains Is Independent of Ste12p, Tec1p, Pdh1p, and Flo11p
The Ste12p and Tec1p transcription factors are critical for the induction of filamentous and invasive growth stimulated by the MAPK cascade and strains lacking these genes are deficient in both filamentous and invasive growth (Liu et al., 1993; Gavrias et al., 1996; Madhani et al., 1997; Palecek et al., 2002). To test whether the folate starvation signal is mediated by the Ste12 and/or Tec1p proteins fol1Δ ste12Δ and fol1Δ tec1Δ double mutant strains were analyzed for their ability to show pseudohyphal and adhesive growth. Surprisingly the haploid fol1Δ ste12Δ as well as the fol1Δ tec1Δ double mutant strain still showed increased bipolar budding and filamentous growth as well as uneven colony morphologies (Figure 8, c and d, k and l; Table 4). However, adhesive growth could no longer be observed for the fol1Δ ste12Δ double mutant strain after 24 h of growth (Figure 9, a and b). Likewise, no invasive growth could be observed for the fol1Δ ste12Δ strain after growth for 5 days, whereas the single ste12Δ mutant strain showed residual substrate invasiveness (Figure 9, c and d). In contrast the fol1Δ tec1Δ double mutant strain showed adhesive growth like the single fol1Δ mutant strain, whereas the single tec1Δ mutant strain showed some residual invasiveness (Figure 9, e and f). Thus our data suggest that the filamentation process induced by loss of the Fol1p activity and presence of folinic acid in the media does not require the Ste12p and Tec1p transcription factors, whereas invasiveness is dependent on the presence of the Ste12 protein.
Next we tested the role of the transcription factor Phd1p, which also is thought to be involved in pseudohyphal growth by acting in a pathway distinct from the cAMP and MAP kinase pathways (Gimeno and Fink, 1994; Palecek et al., 2002). The haploid fol1Δ phd1Δ double mutant strain exhibited filamentous growth and increased bipolar budding pattern and an uneven colony shape to the same extent as the fol1Δ single mutant strain (Figure 8, e and f; Table 4). Furthermore in adhesion assays the fol1Δ phd1Δ double mutant strain after 24 h of growth exhibited invasiveness similar to that observed for the phd1Δ single mutant strain despite its significantly reduced overall growth behavior (Figure 9, e and f). These results suggest that Phd1p is not required for pseudohyphal growth and for agar invasive growth of a fol1Δ mutant strain.
One of the few known Ste12p target genes leading to and required for the induction of pseudohyphal growth and agar invasion is the FLO11 gene encoding a cell surface flocculin protein (Lo and Dranginis, 1998). We therefore tested whether the Flo11 protein is required for the filamentous and invasive growth behavior of the fol1Δ strain. The haploid fol1Δ flo11Δ double mutant strain exhibited filamentous growth and increased bipolar budding and an uneven colony shape to the same extent as the fol1Δ single mutant strain (Figure 8, i and j; Table 4). However, it showed no adhesive growth after 24 h and 5 days of growth (Figure 9, a–d). These data suggest that Flo11p similar to Ste12p is not required for the fol1Δ induced filamentous growth but required for agar invasion.
The Phosphorylation Level of the Fus3p and Kss1p MAP Kinases Is Not Increased in a fol1Δ Strain
Filamentous growth depends in part on the mitogen-activated protein kinase (MAPK) cascade, which results in activation of Ste12p (for review see Madhani and Fink, 1998b). It has been shown that activation of the final MAP kinase Kss1p by phosphorylation increases filamentation, whereas the kinase activity of Fus3p appears to suppress filamentous growth (Madhani and Fink, 1997; Sabbagh et al., 2001). Stimulation of the mating pheromone response pathway also leads to phophorylation and thus activation of both Kss1p and Fus3p (Sabbagh et al., 2001). We tested whether deletion of the FOL1 gene results in an increased phosphorylation level of Kss1p and/or Fus3p. The fol1Δ single mutant strain showed a Kss1p and Fus3p phosphorylation level very similar to that of the corresponding wt strain (Figure 10, wt and Δ). The level of Kss1p and Fus3p phosphorylation found in the fol1Δ strain did not change when cells were grown in media containing different amounts of folinic acid. However in control experiments Kss1 and Fus3p phosphorylation could be induced in both strains by addition of α factor mating pheromone, indicating that the MAPK pathway can be activated (Figure 10). This experiment indicates that deletion of FOL1 does not result in an increased level of Kss1p and Fus3p phosphorylation.
Figure 10.
Kss1p and Fus3p phosphorylation levels are not altered in fol1Δ strains. Cells of haploid wild-type (wt) and fol1Δ (Δ) strains were incubated for 24 h at 30°C in liquid YPD media containing different amounts of folinic acid (FA; 50, 200, or 800 μg/ml). Midlog phase cultures of both strains were treated (+) or not treated (-) for 15 min with 0.15 μM α-factor mating pheromone (α-factor). MAPK phosphorylation levels were determined by immunoblotting using a phosphorylation-state specific antibody (αpTEpY; Sabbagh et al., 2001). Total protein amounts loaded were determined by Coomassie staining (loading control).
Deletion of FOL1 Results in Increased Flo11p Expression Levels
Our data suggested that the fol1Δ induced invasive growth was dependent on Ste12p and Flo11p. This would imply that Flo11p transcription is activated in a fol1Δ strain. The FLO11 promoter, the largest known promoter in S. cerevisiae, is regulated by several transcription factors including Ste12p and Tec1p (Lo and Dranginis, 1998; Rupp et al., 1999; Palecek et al., 2002). We thus assayed the FLO11 promoter activity in a haploid fol1Δ background. We placed a 3-kb-long DNA fragment containing the FLO11 promoter in front of the yEGFP encoding gene on a CEN plasmid and quantified the FLO11 promoter-dependent green fluorescence by flow cytometry in haploid strains grown in liquid synthetic glucose media supplemented with 250 μg/ml folinic acid. Cells were analyzed in early log phase when they were not depleted for glucose or ammonium. In the wt strain the FLO11 promoter showed ∼22 relative green fluorescence (RGF) units, whereas in a fol1Δ deletion strain the promoter was found to be strongly activated, exhibiting 104 (RGF) units (Table 5). A ste12Δ strain showed a FLO11 promoter activity similar to that of a wt strain, indicating that the basal FLO11 promoter activity is not dependent on Ste12p. The fol1Δ ste12Δ double deletion strain exhibited a promoter activity of 24 ± 6 RGF, indicating that the elevated FLO11 promoter activity observed in the flo11Δ strain is indeed dependent on Ste12p (Table 5). Thus these data indicate that depletion of the cellular folate pool triggers the activation of FLO11 gene expression in a Ste12p-dependent manner.
Table 5.
FLO11 promoter activity in different strains
| Strain | RGF |
|---|---|
| wt | 21.91 ± 3.36 |
| ste12Δ | 17.15 ± 5.04 |
| fol1Δ | 104.87 ± 18.68 |
| ste12Δfol1Δ | 24.11 ± 6.16 |
Yeast strains expressing yEGFP from the FLO11 promoter present on a plasmid were grown in liquid synthetic glucose media supplemented with 250 μg/ml folinic acid to an OD600 of 0.3–0.5. The amount of yEGFP protein was quantified by flow cytometry. For each data point, five independent cultures were measured. Median ± SD are given. RGF, relative green fluorescence.
To assay which FLO11 promoter regions were affected in a fol1Δ strain, we used a set of seven plasmid-born reporter constructs containing individual nonoverlapping 400-base pair FLO11 promoter regions covering the first 2800 base pairs of the FLO11 promoter cloned in front of a CYC1-lacZ reporter unit (Rupp et al., 1999). Wild-type or fol1Δ cells carrying these plasmids were plated onto synthetic glucose media containing 50 μg/ml folinic acid, incubated for 24 h, and analyzed by a β-gal overlay assay. The full-length FLO11 promoter showed a small but significant increase in β-galactosidase activity in the fol1Δ strain when compared with wt confirming our GFP measurements (Figure 11, full-length prom.) Furthermore the FLO11 promoter segments 6/5 and 12/11 exhibited a strong increase in β-galactosidase activity in the fol1Δ deletion strain compared with wild type, indicating that these regions become activated in the absence of Fol1p (Figure 11).
Figure 11.
The FLO11 promoter is activated in fol1Δ strains. Wild-type (wt) and fol1Δ (fol1Δ) cells were transformed with CYC::lacZ reporter constructs carrying individual 400-base pair-long FLO11 promoter elements (2/1–14/13), the full-length promoter (wt p.) and no promoter (no insert; Rupp et al., 1999). Boundaries of promoter fragments are given relative to the FLO11 translational start site + 1 (2/1: -421 to -1; 3/4: -820 to -381; 6/5: -1220 to -779; 8/7: -1620–1182; 10/9: -2020 to -1573; 12/11: -2420 to-1981; 14/13: -2820 to -2381). Strains were pregrown in synthetic medium without uracil plus 200 μg/ml folinic acid at 30°C o/n. Cells were spotted onto SD plates without amino acids containing 50 μg/ml folinic acid and incubated for 25 h at 30°C. For the overlay assay X-gal–containing agarose was added on top of the spots and incubated at 37°C for 24 h. Background of the strains is Σ1278b.
DISCUSSION
One-carbon transfer reactions mediated by folate cofactors play a essential role in a variety of cellular reactions. Similar to bacteria and plants S. cerevisiae produces folate de novo from pterin, p-aminobenzoate, and glutamate moieties. The yeast folate-synthesis pathway is not completely understood. In this work we have shown that the S. cerevisiae FOL1 gene encodes the essential enzymatic activities DHNA, HPPK, and DHPS, which perform three subsequent steps in the biosynthesis of folate. Our data suggest that the biosynthesis of C1 carrier appears to be carried out at the mitochondria because Fol1p is localized to the mitochondrial membrane system. Interestingly, deletion of FOL1 gives rise to induction of filamentation and adhesive growth on media supplemented with folinic acid in the presence of excess amounts of glucose and ammonium, which are known suppressors of the dimorphic switch. Invasive growth induced in fol1Δ strains is dependent on the MAP kinase transcription factor Ste12p as well as on the major adhesin Flo11p. Indeed Flo11p expression levels are increased in a Ste12p-dependent way in strains where FOL1 is absent. Interestingly, however, filamentous growth observed in fol1Δ strains is not dependent on the transcription factors Ste12p, Tec1p, and Phd1p and also not on Flo11p, suggesting that in fol1Δ strains yet unknown mechanisms/pathways/adhesins are required for pseudohyphal growth.
The Trifunctional Nature of Fol1p Is Conserved in Evolution
The three different enzymatic activities performed by Fol1p have been found in most procaryotes, microbial eukaryotes, and plants but are absent in mammals (Figure 2). In most bacteria the three enzyme activities are encoded by three distinct ORFs (Slock et al., 1990; Dallas et al., 1992; Talarico et al., 1992; Jardine et al., 2002). However in some prokaryotes the enzymatic activities HPPK and DHNA are linked as a bifunctional protein, whereas DHPS is encoded by a separate ORF (Lopez and Lacks, 1993). In bacteria the genes encoding consecutive enzymes of a biosynthetic pathway are often adjacent or close to each other on the chromosome, suggesting that they are coregulated in some way (Jardine et al., 2002).
In eukaryotes, biochemical analysis of the folate biosynthetic genes characterized so far revealed that the corresponding proteins are always multifunctional, containing fused HPPK and DHPS activities (Allegra et al., 1990; Triglia and Cowman, 1994; Rebeille et al., 1997) and fused DHNA, HPPK, and DHPS activities (Volpe et al., 1992, 1995; this work). Moreover in the yeasts S. pombe and C. albicans ORFs exist that could code for a protein with homology to all three enzyme activities (Figure 2). However, in other fungi like N. crassa or in the plant pathogenic fungus Magnaporthe grisea, the bifunctional HPPK/DHPS protein seems to be separated from the DHNA activity. The multifunctional nature of this protein appears to be common in fungal and plant species; however, only a subset of fungi contain the trifunctional structure. It is unclear whether the other fungi and the plants have relocated the third gene because the initial fusion events had occurred or whether the trifunctional fusion occurred later in evolution in only a subset of fungal species. Whether enzymatic activities come from one or several genes affects both the regulation of gene expression and the localization of the enzymes (Figure 2).
Very recently folic acid biosynthetic enzymes have been used for rooting the eukaryotic tree. Stechmann and Cavalier-Smith (2002) used a derived gene fusion between dihydrofolate reductase and thymidylate synthase in order to locate the root of the eukaryotic tree between bikonts and opisthokonts. However the position of the Amoebozoa needs to be clarified before the root can be precisely pin-pointed. Thus analysis of folic acid biosynthesis and in particular the DHNA, HPPK, and DHPS activities in Amoebozoa might initiate further studies with regard to the identification of early-branching eukaryotic lineages.
Fol1p Is Essential for Growth
Our data show that the FOL1 gene is essential for the S. cerevisiae vegetative life cycle, and fol1Δ strains can only be kept alive by the addition of folinic acid. This has also been shown for a partial deletion of just the C-terminal part of the FOL1 gene encoding the DHPS activity (Bayly et al., 2001). Similar results have been obtained for FOL2 (encoding GTP-cyclohydrolase I) and FOL3 (encoding dihydrofolate synthetase; Nardese et al., 1996; Cherest et al., 2000). Folinic acid is a folate derivative, which supplements the C1 carrier pool at a site distinct from DHF (Holmes and Appling, 2002). We have not tested whether supplementation of fol1Δ strains with folic acid also restores growth as reported for other folate-minus strains (Cherest et al., 2000; Bayly et al., 2001; Bayly and Macreadie, 2002). That the intracellular folate pool was indeed limiting for growth was shown by the fact that the growth rate of the fol1Δ strain was dependent on the amount of extracellular folinic acid added although wt-like growth could never be observed (Figure 3; unpublished data). Moreover the fact that high amounts of folinic acid are needed indicates that the yeast cell probably does not possess an active uptake transport system for folic acid derivatives.
The terminal phenotype of a FOL1 deletion is a microcolony of ∼50 slightly elongated cells indicating that the folate pool in the spore derived from a heterozygous diploid deletion strain is sufficient for the sporulation process and the subsequent first 5–6 cell divisions. This very low demand for folate can be explained by the fact that one carbon carrier are reused and not consumed. Because there is no consumption of folate derivatives, it can be assumed that FOL1 gene expression is low. Indeed quantification of the FOL1 transcript as well as our own FOL1 promoter studies (unpublished data) revealed a very low mRNA level (Planta et al., 1999). Moreover Fol1p expression is regulated as in wt strains grown in the presence of extracellular folinic acid the FOL1 promoter is repressed (see Figure 4C). In addition glucose activates Fol1p expression, whereas the stationary phase results in repression of Fol1p expression (Planta et al., 1999). These data indicate a complex regulation of FOL1 gene expression, suggesting that the Fol1 protein level is adjusted adequately to the nutrient situation of the yeast cell.
Interestingly strong overexpression of FOL1 is toxic to yeast (Figure 4A) and bacteria (unpublished data). Recently an inverse relationship has been reported between FOL1 expression levels and the growth rate of the corresponding yeast strains (Iliades et al., 2003). Increased FOL1 expression could result in production of excess amounts of 7,8-dihydropteroate depletes the GTP pool, which is also needed for other biosynthetic pathways or because overproduction of these folate intermediates inhibits folate-dependent enzymes similar to the action of folinic acid (Girgis et al., 1997). Alternatively, growth of FOL1 overexpressing strains could be hampered because increased levels of 7,8-dihydropteroate are toxic to yeast cells (Bayly and Macreadie, 2002).
Fol1p Is Localized at the Mitochondrial Membrane System
In vivo localization of a Fol1 fusion protein showed a similar pattern as observed for mitochondria (Figure 5). Electron microcopy confirmed the mitochondrial localization of a GFP-Fol1 fusion protein, because the mitochondrial membranes were labeled nearly exclusively by the gold particles. However, because the overexpression of GFP-Fol1p gave rise to clumped mitochondria, we were unable to make a distinction between inner and outer membrane localization. The localization of Fol1p was also analyzed by EM studies using anti-Fol1p peptide antibodies and again predominant staining at the mitochondrial membranes was observed (Figure 6d).
What are the implications of the mitochondrial membrane-associated localization of Fol1p as the central multifunctional component of the folate biosynthetic machinery for the production of folate in S. cerevisiae? In yeast cytosol and mitochondria both contain folate coenzymes and both compartments posses a parallel array of enzymes catalyzing the interconversion of folate units in different oxidation states (Appling, 1991; McNeil et al., 1996). Based on our data, it could now be speculated that in yeast biosynthesis of folate is associated with mitochondria and that 7,8-dihydropteroate (no γ-glutamate residue) and/or DHF/THF (1 γ-glutamate residue; see Figure 1) are transported into the mitochondrial matrix and into the cytosol where their conversion to the physiologically relevant oligo-γ-glutamyl derivatives could occur. Recently it has been reported that the cytosolic as well as the mitochondrial folylpolyglutamate synthetase activity is encoded by MET7 (Cherest et al., 2000; DeSouza et al., 2000).
In addition to FOL1 being essential for folate biosynthesis, we found that loss of FOL1 impairs growth on nonfermentable carbon sources (unpublished data). Similarly, it has been found that loss of dihydrofolate reductase (DHFR) activity also results in the inability of the yeast mutant to grow on glycerol (Huang et al., 1992). These data suggest that C1 carrier directly or indirectly are essential for mitochondrial function.
Depletion of Folate Coezymes Induces Pseudohyphal Growth and Agar Invasion
The data presented here demonstrate that growth of haploid fol1Δ cells on media supplemented with folinic acid results in filamentous growth and the ability to adhere to the agar. The slow-growth phenotype of a fol1Δ mutant strain even in the presence of high amounts of folinic acid suggests that the starvation of the cells for C1 carrier units induces the dimorphic switch. Interestingly both filamentous growth and the ability of the fol1Δ cells to adhere to the agar substrate is induced in the presence of high glucose and ammonium. Thus our data indicate that starvation for C1 carrier is a further nutritional signal that activates filamentous and adhesive growth.
To test if the haploid invasive and pseudohyphal growth phenotype was dependent on the MAP kinase pathway and on the major adhesin Flo11p, we constructed fol1Δ ste12Δ, fol1Δ tec1Δ, and fol1Δ flo11Δ double deletion strains. Ste12p is the transcription factor at the end of the MAP kinase cascade, which together with Tec1p binds to so called FREs (filamentation and invasion response elements) in promoters of genes, which are involved in the dimorphic switch (Madhani and Fink, 1998b). One such FRE element is located in the promoter of the cell surface floccullin encoding gene FLO11, which is necessary for pseudohyphae formation and invasion (Lo and Dranginis, 1998). The FLO11 promoter integrates the MAP kinase cascade and cAMP/PKA filamentation signaling pathways (Pan and Heitman, 1999; Rupp et al., 1999). The agar adhesion phenotype observed for fol1Δ cells was found to be dependent on the MAPK-specific transcription factor Ste12p and on the cell wall protein Flo11p but not on the Tec1p transcription factor. Surprisingly the fol1Δ ste12Δ, fol1Δ tec1Δ, and fol1Δ flo11Δ double deletion strains showed the same extent of filamentous growth as the fol1Δ single deletion (Figure 8). This suggests that the internal starvation signal created by the fol1 deletion induces filamentous growth independent of Ste12p and Tec1p. In accordance with this result we could also not detect an increased phosphorylation level of the MAP kinases Kss1p and Fus3p, suggesting that the Kss1p and Fus3p are not constitutively activated in a fol1Δ deletion strain. We also tested the Phd1p transcription factor, which is thought to act independent of the cAMP and MAP kinase pathways (Palecek et al., 2002) and found that filamentous as well as invasive growth of fol1Δ cells was not dependent on Phd1p. This suggests that other signaling pathways possibly acting through Flo8p or other proteins may be relevant for filamentous growth as a consequence of the loss of C1 carrier and need to be tested. Likewise the flocullin Flo11p was found not to be necessary for filamentous growth of cells deleted for FOL1. The fact that Flo11p is not required for filamentous growth suggests that other mechanisms of cell-cell adhesion and/or cytokinesis are relevant for the filamentous growth phenotype. One such candidate could be Fig 2p a mating-specific adhesin, which when overexpressed can partially complement the loss of adhesion found in a FLO11 mutant strain (Guo et al., 2000). Alternatively a lack of mother-daughter separation can result in an increased cell-cell adhesion and indeed a link between septal maturation and cell adhesion has been observed (Pan and Heitman, 2000).
In contrast to filamentous growth of fol1Δ cells the adhesion phenotype of the fol1Δ mutant strain was dependent on Ste12p and Flo11p but not on Tec1p. We therefore tested whether deletion of FOL1 modulates the FLO11 promoter activity. Indeed in fol1Δ strains the FLO11 promoter activity was enhanced in a Ste12p-dependent manner in comparison to wt strains, demonstrating that the internal starvation signal induced by the depletion of C1 carrier is able to activate the expression of the most important cell-cell adhesin flocculin. More specifically the FLO11 promoter segments -800 to -1200 and -2000 to -2400 were induced by deletion of FOL1. Interestingly, it has been reported that Ste12p acts on the same segments of the FLO11 promoter (Rupp et al., 1999). However, Ste12p controls a third segment of the FLO11 promoter located at position -1600 to -2000, which was not activated in a fol1Δ strain. Thus our data imply that activation of FLO11 expression by deletion of FOL1 involves some but not all the FLO11 promoter elements regulated by the Ste12p pathway.
Our data show that deletion of the FOL1 gene results in Ste12p- and Flo11p-dependent but Tec1p- and Phd1p-independent adhesive growth, whereas filamentous growth of the fol1Δ strain requires neither Ste12p, Tec1, Phd1p, nor Flo11p. This behavior of the fol1Δ strain suggests that agar invasion and filamentation are brought about by different signals. Recently in a screen designed to find mutants showing increased invasiveness, strains were identified that promote agar invasion even when FLO11 was expressed at a constitutively low level. Specifically, haploid agar invasion, flocculation, diploid invasion, and diploid filamentation could be separated genetically (Palecek et al., 2000). Thus, although regulation of FLO11 transcription provides an important mechanism of invasive growth control, FLO11 upregulation is not essential for invasive growth and is yet not induced by all conditions that trigger the dimorphic switch. One or more FLO11-independent mechanisms are likely to exist for invasive growth (Palecek et al., 2000; Rua et al., 2001). It has been suggested that the invasive-growth response is triggered by many different physiological and genetic inputs (Breitkreutz et al., 2003). Similarly, our data suggest that there is a FLO11-independent mechanism(s) leading to fol1Δ-induced pseudohyphal growth.
What are the signals and which receptors are used to activate the dimorphic switch in a fol1Δ strain? Thus far most of the receptors required for the dimorphic switch have not been identified. The Mep2p ammonium permease is used by the cells to detect nitrogen compounds, whereas Gpr1p a cell surface G-protein–coupled receptor seems to sense glucose, and mutations in either protein renders the cells unable to form filaments in diploid strains or to grow invasively in haploid strains (Lorenz and Heitman, 1998; Lorenz et al., 2000b). Similarly amino acid starvation was found as a nutritional signal that induces FLO11 gene expression and induces adhesive growth in a GCN4-dependent manner (Braus et al., 2003). Also, metabolic enzymes are thought to regulate directly or indirectly filamentous phenotypes, e.g., mutations in the NADP-linked glutamate dehydrogenase GDH3, in the phosphoglucose isomerase PGI1, or in the alcohol dehydrogenase ADH1 constitutively activate invasive growth in haploids and pseudohyphal growth in diploids (Wilkinson et al., 1996; Palecek et al., 2000). However, it is completely unclear which metabolite is sensed in a fol1Δ cell depleted of C1 carrier and what the possible receptor(s) are. Starvation for C1 carrier affects a variety of biosynthetic pathways including the synthesis of purines, methionine, and glycine. Thus it is likely that more than one signal is activated in cells starving for folate and that the combined read-out of these signals results in the filamentous and adhesive growth seen for fol1Δ cells.
Acknowledgments
We thank Ursula Fleig for extensive discussions, critical reading, and editing of the manuscript and Sabine Klein for FACS analysis. This work was supported by the FAZIT-Stiftung and by a contract awarded to J.H.H. in the context of the EUROFAN project of the EC (BIO4-CT95-0080).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0680. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0680.
References
- Allegra, C.J., Boarman, D., Kovacs, J.A., Morrison, P., Beaver, J., Chabner, B.A., and Masur, H. (1990). Interaction of sulfonamide and sulfone compounds with Toxoplasma gondii dihydropteroate synthase. J. Clin. Invest. 85, 371-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames, B.N., Gold, L.S., and Willett, W.C. (1995). The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 92, 5258-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appling, D.R. (1991). Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 5, 2645-2651. [DOI] [PubMed] [Google Scholar]
- Barclay, B.J., Huang, T., Nagel, M.G., Misener, V.L., Game, J.C., and Wahl, G.M. (1988). Mapping and sequencing of the dihydrofolate reductase gene (DFR1) of Saccharomyces cerevisiae. Gene 63, 175-185. [DOI] [PubMed] [Google Scholar]
- Bateman, A. et al. (2002). The Pfam protein families database. Nucleic Acids Res. 30, 276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly, A.M., Berglez, J.M., Patel, O., Castelli, L.A., Hankins, E.G., Coloe, P., Hopkins Sibley, C., and Macreadie, I.G. (2001). Folic acid utilisation related to sulfa drug resistance in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 204, 387-390. [DOI] [PubMed] [Google Scholar]
- Bayly, A.M., and Macreadie, I.G. (2002). Cytotoxicity of dihydropteroate in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 213, 189-192. [DOI] [PubMed] [Google Scholar]
- Blakley, R.L., and Benkovic, S.J. (1984). Folates and pterins. Chemistry and biochemistry of folates, Vol. 1, New York: John Wiley & Sons.
- Blount, B.C., Mack, M.M., Wehr, C.M., MacGregor, J.T., Hiatt, R.A., Wang, G., Wickramasinghe, S.N., Everson, R.B., and Ames, B.N. (1997). Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 94, 3290-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke, J.D., LaCroute, F., and Fink, G.R. (1984). A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197, 345-346. [DOI] [PubMed] [Google Scholar]
- Borges-Walmsley, M.I., and Walmsley, A.R. (2000). cAMP signalling in pathogenic fungi: control of dimorphic switching and pathogenicity. Trends Microbiol. 8, 133-141. [DOI] [PubMed] [Google Scholar]
- Braus, G.H., Grundmann, O., Brückner, S., and Mösch, H.-U. (2003). Amino acid starvation and Gcn5p regulate adhesive growth and FLO11 gene expression in Saccharomyces cerevisiae. Mol. Biol. Cell 14, 4272-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreutz, A., Boucher, L., Breitkreutz, B.J., Sultan, M., Jurisica, I., and Tyers, M. (2003). Phenotypic and transcriptional plasticity directed by a yeast mitogen-activated protein kinase network. Genetics 165, 997-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali, B.M., Doyle, T.C., Botstein, D., and Fink, G.R. (1998). Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae. Mol. Biol. Cell 9, 1873-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant, J. (1999). Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 15, 365-391. [DOI] [PubMed] [Google Scholar]
- Cherest, H., Thomas, D., and Surdin-Kerjan, Y. (2000). Polyglutamylation of folate coenzymes is necessary for methionine biosynthesis and maintenance of intact mitochondrial genome in Saccharomyces cerevisiae. J. Biol. Chem. 275, 14056-14063. [DOI] [PubMed] [Google Scholar]
- Cormack, B.P., Bertram, G., Egerton, M., Gow, N.A., Falkow, S., and Brown, A.J. (1997). Yeast-enhanced green fluorescent protein (yEGFP)a reporter of gene expression in Candida albicans. Microbiology 143, 303-311. [DOI] [PubMed] [Google Scholar]
- Cossins, E.A., and Chen, L. (1997). Folates and one-carbon metabolism in plants and fungi. Phytochemistry 45, 437-452. [DOI] [PubMed] [Google Scholar]
- Cullen, P.J., and Sprague, G.F., Jr. (2000). Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. USA 97, 13619-13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas, W.S., Gowen, J.E., Ray, P.H., Cox, M.J., and Dev, I.K. (1992). Cloning, sequencing, and enhanced expression of the dihydropteroate synthase gene of Escherichia coli MC4100. J. Bacteriol. 174, 5961-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza, L., Shen, Y., and Bognar, A.L. (2000). Disruption of cytoplasmic and mitochondrial folylpolyglutamate synthetase activity in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 376, 299-312. [DOI] [PubMed] [Google Scholar]
- Dickinson, J.R. (1996). `Fusel' alcohols induce hyphal-like extensions and pseudohyphal formation in yeasts. Microbiology 142, 1391-1397. [DOI] [PubMed] [Google Scholar]
- D'Souza, C.A., and Heitman, J. (2001). Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25, 349-364. [DOI] [PubMed] [Google Scholar]
- Duesterhoeft, A., Klonierung von Restriktions-Modifikationssystemen aus Herpetosiphon giganteus. PhD thesis. Justus-Liebig-University, Giessen, 1990.
- Duthie, S.J. (1999). Folic acid deficiency and cancer: mechanisms of DNA instability. Br. Med. Bull. 55, 578-592. [DOI] [PubMed] [Google Scholar]
- Entian, K.-D., and Kötter, P. (1998). Yeast mutant and plasmid collections. In: Methods in Microbiology, Vol. 26, ed. A.J.P. Brown, M. Tuite, San Diego: Academic Press, 431-450. [Google Scholar]
- Gavrias, V., Andrianopoulos, A., Gimeno, C.J., and Timberlake, W.E. (1996). Saccharomyces cerevisiae TEC1 is required for pseudohyphal growth. Mol. Microbiol. 19, 1255-1263. [DOI] [PubMed] [Google Scholar]
- Gimeno, C.J., and Fink, G.R. (1994). Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol. Cell. Biol. 14, 2100-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno, C.J., Ljungdahl, P.O., Styles, C.A., and Fink, G.R. (1992). Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68, 1077-1090. [DOI] [PubMed] [Google Scholar]
- Girgis, S., Suh, J.R., Jolivet, J., and Stover, P.J. (1997). 5-Formyltetrahydrofolate regulates homocysteine remethylation in human neuroblastoma. J. Biol. Chem. 272, 4729-4734. [DOI] [PubMed] [Google Scholar]
- Greene, N.D., and Copp, A.J. (1997). Inositol prevents folate-resistant neural tube defects in the mouse [see comments]. Nat. Med. 3, 60-66. [DOI] [PubMed] [Google Scholar]
- Griffiths, G., McDowall, A., Back, R., and Dubochet, J. (1984). On the preparation of cryosections for immunocytochemistry. J. Ultrastruct. Res. 89, 65-78. [DOI] [PubMed] [Google Scholar]
- Gueldener, U., Heinisch, J., Koehler, G.J., Voss, D., and Hegemann, J.H. (2002). A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 30, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldener, U., Heck, S., Fiedler, T., Beinhauer, J.D., and Hegemann, J.H. (1996). A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24, 2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, B., Styles, C.A., Feng, Q., and Fink, G.R. (2000). A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc. Natl. Acad. Sci. USA 97, 12158-12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann, C., Rohdich, F., Schmidt, E., Bacher, A., and Richter, G. (1998). Biosynthesis of pteridines in Escherichia coli. Structural and mechanistic similarity of dihydroneopterin-triphosphate epimerase and dihydroneopterin aldolase. J. Biol. Chem. 273, 17418-17424. [DOI] [PubMed] [Google Scholar]
- Holmes, W.B., and Appling, D.R. (2002). Cloning and characterization of methenyltetrahydrofolate synthetase from Saccharomyces cerevisiae. J. Biol. Chem. 277, 20205-20213. [DOI] [PubMed] [Google Scholar]
- Huang, T., Barclay, B.J., Kalman, T.I., von Borstel, R.C., and Hastings, P.J. (1992). The phenotype of a dihydrofolate reductase mutant of Saccharomyces cerevisiae. Gene 121, 167-171. [DOI] [PubMed] [Google Scholar]
- Huxley, C., Green, E.D., and Dunham, I. (1990). Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet. 6, 236. [DOI] [PubMed] [Google Scholar]
- Iliades, P., Berglez, J., Meshnick, S., and Macreadie, I. (2003). Promoter strength of folic acid synthesis genes affects sulfa drug resistance in Saccharomyces cerevisiae. Microb. Drug Resist. 9, 249-255. [DOI] [PubMed] [Google Scholar]
- Jardine, O., Gough, J., Chothia, C., and Teichmann, S.A. (2002). Comparison of the small molecule metabolic enzymes of Escherichia coli and Saccharomyces cerevisiae. Genome Res. 12, 916-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko, Y., Hayashi, N., Toh-e, A., Banno, I., and Oshima, Y. (1987). Structural characteristics of the PHO8 gene encoding repressible alkaline phosphatase in Saccharomyces cerevisiae. Gene 58, 137-148. [DOI] [PubMed] [Google Scholar]
- Kron, S.J., Styles, C.A., and Fink, G.R. (1994). Symmetric cell division in pseudohyphae of the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 5, 1003-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagosky, P.A., Taylor, G.R., and Haynes, R.H. (1987). Molecular characterization of the Saccharomyces cerevisiae dihydrofolate reductase gene (DFR1). Nucleic Acids Res. 15, 10355-10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts, M.G., Bauer, F.F., Marmur, J., and Pretorius, I.S. (1996). Muc1, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc. Natl. Acad. Sci. USA 93, 8419-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler, K.B., Davidson, R.C., D'Souza, C., Harashima, T., Shen, W.C., Wang, P., Pan, X., Waugh, M., and Heitman, J. (2000). Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64, 746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, J.G., and Haynes, R.H. (1979). Isolation and characterization of yeast mutants auxotrophic for 2′-deoxythymidine 5′-monophosphate. Mol. Gen. Genet. 168, 141-151. [DOI] [PubMed] [Google Scholar]
- Liu, H., Styles, C.A., and Fink, G.R. (1993). Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science 262, 1741-1744. [DOI] [PubMed] [Google Scholar]
- Lo, W.S., and Dranginis, A.M. (1998). The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol. Biol. Cell 9, 161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, P., and Lacks, S.A. (1993). A bifunctional protein in the folate biosynthetic pathway of Streptococcus pneumoniae with dihydroneopterin aldolase and hydroxymethyldihydropterin pyrophosphokinase activities. J. Bacteriol. 175, 2214-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, M.C., Cutler, N.S., and Heitman, J. (2000a). Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell 11, 183-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, M.C., and Heitman, J. (1998). The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 17, 1236-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, M.C., Pan, X., Harashima, T., Cardenas, M.E., Xue, Y., Hirsch, J.P., and Heitman, J. (2000b). The G protein-coupled receptor gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics 154, 609-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden, K., and Snyder, M. (1998). Cell polarity and morphogenesis in budding yeast. Annu. Rev. Microbiol. 52, 687-744. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D., and Fink, G.R. (1997). Combinatorial control required for the specificity of yeast MAPK signaling. Science 275, 1314-1317. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D., and Fink, G.R. (1998a). The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 8, 348-353. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D., and Fink, G.R. (1998b). The riddle of MAP kinase signaling specificity. Trends Genet. 14, 151-155. [DOI] [PubMed] [Google Scholar]
- Madhani, H.D., Styles, C.A., and Fink, G.R. (1997). MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91, 673-684. [DOI] [PubMed] [Google Scholar]
- McNeil, J.B., Bognar, A.L., and Pearlman, R.E. (1996). In vivo analysis of folate coenzymes and their compartmentation in Saccharomyces cerevisiae. Genetics 142, 371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg, D., Muller, R., and Funk, M. (1994). Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22, 5767-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg, D., Muller, R., and Funk, M. (1995). Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156, 119-122. [DOI] [PubMed] [Google Scholar]
- Nardese, V., Gütlich, M., Brambilla, A., and Carbone, M.L. (1996). Disruption of the GTP-cyclohydrolase I gene in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 218, 273-279. [DOI] [PubMed] [Google Scholar]
- Niedenthal, R., Riles, L., Güldener, U., Klein, S., Johnston, M., and Hegemann, J.H. (1999). Systematic analysis of S. cerevisiae chromosome VIII genes. Yeast 15, 1775-1796. [DOI] [PubMed] [Google Scholar]
- Niedenthal, R.K., Riles, L., Johnston, M., and Hegemann, J.H. (1996). Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast 12(8), 773-786. [DOI] [PubMed] [Google Scholar]
- Palecek, S.P., Parikh, A.S., and Kron, S.J. (2000). Genetic analysis reveals that FLO11 upregulation and cell polarization independently regulate invasive growth in Saccharomyces cerevisiae. Genetics 156, 1005-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek, S.P., Parikh, A.S., and Kron, S.J. (2002). Sensing, signalling and integrating physical processes during Saccharomyces cerevisiae invasive and filamentous growth. Microbiology 148, 893-907. [DOI] [PubMed] [Google Scholar]
- Pan, X., and Heitman, J. (1999). Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 4874-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, X., Harashima, T., and Heitman, J. (2000). Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr. Opin. Microbiol. 3, 567-572. [DOI] [PubMed] [Google Scholar]
- Pan, X., and Heitman, J. (2000). Sok2 regulates yeast pseudohyphal differentiation via a transcription factor cascade that regulates cell-cell adhesion. Mol. Cell. Biol. 20, 8364-8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, W.R. (1994). Using the FASTA program to search protein and DNA sequence databases. Methods Mol. Biol. 24, 307-331. [DOI] [PubMed] [Google Scholar]
- Planta, R.J. et al. (1999). Transcript analysis of 250 novel yeast genes from chromosome XIV. Yeast 15, 329-350. [DOI] [PubMed] [Google Scholar]
- Rebeille, F., Macherel, D., Mouillon, J.M., Garin, J., and Douce, R. (1997). Folate biosynthesis in higher plants: purification and molecular cloning of a bifunctional 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase/7,8-dihydropteroate synthase localized in mitochondria. EMBO J. 16, 947-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeille, F., Douce, R. (1999). Folate synthesis and compartmentation in higher plants. In: Regulation of Primary Metabolic Pathways in Plants, eds. N.T. Kruger, S.A. Hill, R.G. Ratcliffe, Dordrecht, The Netherlands: Kluwer Academic Publishers, 53-99.
- Roberts, R.L., and Fink, G.R. (1994). Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8, 2974-2985. [DOI] [PubMed] [Google Scholar]
- Roland, S., Ferone, R., Harvey, R.J., Styles, V.L., and Morrison, R.W. (1979). The characteristics and significance of sulfonamides as substrates for Escherichia coli dihydropteroate synthase. J. Biol. Chem. 254, 10337-10345. [PubMed] [Google Scholar]
- Rua, D., Tobe, B.T., and Kron, S.J. (2001). Cell cycle control of yeast filamentous growth. Curr. Opin. Microbiol. 4, 720-727. [DOI] [PubMed] [Google Scholar]
- Rupp, S., Summers, E., Lo, H.J., Madhani, H., and Fink, G. (1999). MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 18, 1257-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh, W., Jr., Flatauer, L.J., Bardwell, A.J., and Bardwell, L. (2001). Specificity of MAP kinase signaling in yeast differentiation involves transient versus sustained MAPK activation. Mol. Cell 8, 683-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martinez, C., and Perez-Martin, J. (2001). Dimorphism in fungal pathogens: Candida albicans and Ustilago maydis–similar inputs, different outputs. Curr. Opin. Microbiol. 4, 214-221. [DOI] [PubMed] [Google Scholar]
- Sen-Gupta, M., Güldener, U., Beinhauer, J., Fiedler, T., and Hegemann, J.H. (1997). Sequence analysis of the 33 kb long region between ORC5 and SUI1 from the left arm of chromosome XIV from Saccharomyces cerevisiae. Yeast 13, 849-860. [DOI] [PubMed] [Google Scholar]
- Sherman, F. (1991). Getting started with yeast. Methods Enzymol. 194, 3-21. [DOI] [PubMed] [Google Scholar]
- Sherman, F., and Hicks, J. (1991). Micromanipulation and dissection of asci. Methods Enzymol. 194, 21-37. [DOI] [PubMed] [Google Scholar]
- Slock, J., Stahly, D.P., Han, C.Y., Six, E.W., and Crawford, I.P. (1990). An apparent Bacillus subtilis folic acid biosynthetic operon containing pab, an amphibolic trpG gene, a third gene required for synthesis of para-aminobenzoic acid, and the dihydropteroate synthase gene. J. Bacteriol. 172, 7211-7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechmann, A., and Cavalier-Smith, T. (2002). Rooting the eukaryote tree by using a derived gene fusion. Science 297, 89-91. [DOI] [PubMed] [Google Scholar]
- Suckow, M., and Hollenberg, C.P. (1998). The activation specificities of wild-type and mutant Gcn4p in vivo can be different from the DNA binding specificities of the corresponding bZip peptides in vitro. J. Mol Biol. 276, 887-902. [DOI] [PubMed] [Google Scholar]
- Talarico, T.L., Ray, P.H., Dev, I.K., Merrill, B.M., and Dallas, W.S. (1992). Cloning, sequence analysis, and overexpression of Escherichia coli folK, the gene coding for 7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase. J. Bacteriol. 174, 5971-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki, H., Miwa, T., Shinozaki, M., Saito, M., Yun, C.W., Yamamoto, K., and Kumagai, H. (2000). GPR1 regulates filamentous growth through FLO11 in yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 267, 164-168. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu, K.T. (1989). Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem. J. 21, 163-171. [DOI] [PubMed] [Google Scholar]
- Triglia, T., and Cowman, A.F. (1994). Primary structure and expression of the dihydropteroate synthetase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 91, 7149-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe, F., Ballantine, S.P., and Delves, C.J. (1993). The multifunctional folic acid synthesis fas gene of Pneumocystis carinii encodes dihydroneopterin aldolase, hydroxymethyldihydropterin pyrophosphokinase and dihydropteroate synthase. Eur. J. Biochem 216, 449-458. [DOI] [PubMed] [Google Scholar]
- Volpe, F., Ballantine, S.P., and Delves, C.J. (1995). Two domains with amino-acid sequence similarity are required for dihydroneopterin aldolase function in the multifunctional folic acid synthesis Fas protein of Pneumocystis carinii. Gene 160, 41-46. [DOI] [PubMed] [Google Scholar]
- Volpe, F., Dyer, M., Scaife, J.G., Darby, G., Stammers, D.K., and Delves, C.J. (1992). The multifunctional folic acid synthesis fas gene of Pneumocystis carinii appears to encode dihydropteroate synthase and hydroxymethyldihydropterin pyrophosphokinase. Gene 112, 213-218. [DOI] [PubMed] [Google Scholar]
- Wach, A., Brachat, A., Pohlmann, R., and Philippsen, P. (1994). New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793-1808. [DOI] [PubMed] [Google Scholar]
- Wilkinson, B.M., James, C.M., and Walmsley, R.M. (1996). Partial deletion of the Saccharomyces cerevisiae GDH3 gene results in novel starvation phenotypes. Microbiology 142(Pt 7), 1667-1673. [DOI] [PubMed] [Google Scholar]
- Zelikson, R., and Luzzati, M. (1977). Mitochondrial and cytoplasmic distribution in Saccharmoyces cerevisiae of enzymes involved in folate-coenzyme-mediated one-carbon-group transfer. A genetic and biochemical study of the enzyme deficiencies in mutants tmp3 and ade3. Eur J. Biochem. 79, 285-292. [DOI] [PubMed] [Google Scholar]
- Zimmer, T., Kaminski, K., Scheller, U., Vogel, F., and Schunck, W.H. (1995). In vivo reconstitution of highly active Candida maltosa cytochrome P450 monooxygenase systems in inducible membranes of Saccharomyces cerevisiae. DNA Cell Biol. 14, 619-628. [DOI] [PubMed] [Google Scholar]