Abstract
The oncolytic virotherapy field has made significant advances in the last decade, with a rapidly increasing number of early- and late-stage clinical trials, some of them showing safety and promising therapeutic efficacy. Targeting tumor vasculature by oncolytic viruses (OVs) is an attractive strategy that offers several advantages over nontargeted viruses, including improved tumor viral entry, direct antivascular effects, and enhanced antitumor efficacy. Current understanding of the biological mechanisms of tumor neovascularization, novel vascular targets, and mechanisms of resistance has allowed the development of oncolytic viral vectors designed to target tumor neovessels. While some OVs (such as vaccinia and vesicular stomatitis virus) can intrinsically target tumor vasculature and induce vascular disruption, the majority of reported vascular-targeted viruses are the result of genetic manipulation of their viral genomes. Such strategies include transcriptional or transductional endothelial targeting, “armed” viruses able to downregulate angiogenic factors, or to express antiangiogenic molecules. The above strategies have shown preclinical safety and improved antitumor efficacy, either alone, or in combination with standard or targeted agents. This review focuses on the recent efforts toward the development of vascular-targeted OVs for cancer treatment and provides a translational/clinical perspective into the future development of new generation biological agents for human cancers.
Keywords: vascular targeting, oncolytic virus, tumor angiogenesis
Introduction
The oncolytic virotherapy field has significantly expanded in the last decade, with ∼190 clinical trials using new viral vectors for the treatment of human malignancies, of which ∼11 are in advanced stages of development. As oncolytic viruses (OVs) have an intrinsic ability to infect, replicate in, and induce cytotoxicity in a cancer selective manner,1,2 they offer a potential advantage over standard anticancer therapies. OVs allow the introduction of therapeutic genes3 or modifications in the viral genome to modulate viral tropism and improve virus tumor-targeting abilities.4,5 They have been used in combination with either chemotherapy, radiation, or targeted therapies to improve antitumor efficacy.6,7
However, the true antitumor potential of OVs is limited by a number of host-derived factors, including viral neutralization by preexisting antibodies, sequestration by the reticuloendothelial system, inadequate intravenous tumor delivery, and limited intratumoral virus replication and spread.8,9 Among the recognized factors that limit viral entry into tumor tissues, the tumor endothelium represents a barrier to the efficient delivery of viral and nonviral therapeutic agents into tumor cells after systemic administration.4,10 Therefore, the development of oncolytic agents that target the tumor vasculature may be one way to circumvent the above obstacles and improve viral entry into tumor tissues, leading to improved antitumor activity.
Mechanisms of tumor neovascularization
When a tumor reaches a diameter of ∼2 mm3, it requires an independent blood supply to allow further growth.11 The mechanisms by which tumors induce new blood vessel formation include angiogenesis (new vessel sprouting from preexisting capillaries),12 vasculogenesis (the formation of de novo capillaries from bone marrow-derived endothelial progenitor cells),13 vessel cooption,14 and vasculogenic mimicry.15
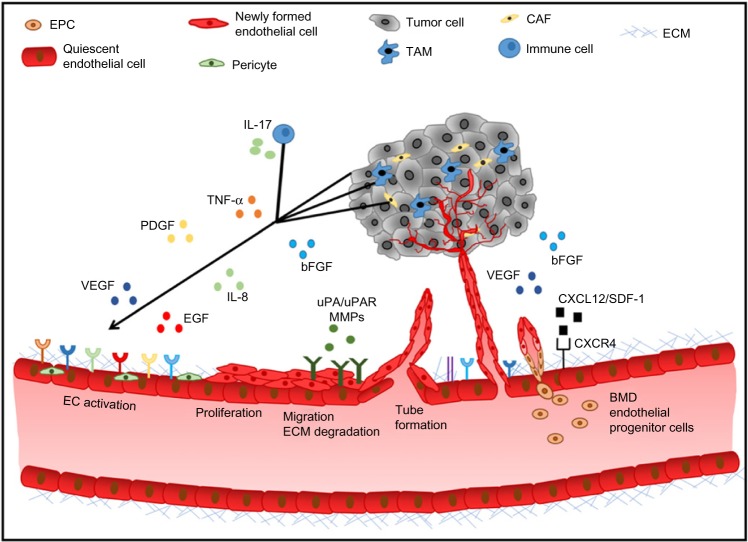
Tumor angiogenesis is regulated by a fine balance between endogenous pro- and antiangiogenic factors present in the tumor microenvironment (Figure 1). The expression of pro-angiogenic factors (vascular endothelial growth factor [VEGF], basic fibroblast growth factor [bFGF], platelet-derived growth factor [PDGF], epidermal growth factor [EGF], interleukin 8 [IL-8], the angiopoietins)16 by tumor or stromal cells is regulated by factors such as hypoxia, oncogene activation, or tumor suppressor silencing.17 Endogenous inhibitors of angiogenesis include thrombospondin-1,18 as well as peptides derived from plasma (angiostatin)19 or tumor stroma, such as endostatin, tumstatin, and canstatin,20 among others. When the balance favors pro-angiogenic factors, the “angiogenic switch” is “turned on”, leading to endothelial cell activation, proliferation, migration, matrix degradation, and capillary formation.21 The resulting tumor neovessels are structurally and functionally different from normal blood vessels, as they are leaky, tortuous, and disorganized. Tumor endothelial cells have aberrant morphology, lack pericytes, and have an abnormal basement membrane. These structural differences lead to an abnormal tumor microenvironment, hypoxia, acidosis, and an elevated tumor interstitial pressure, which contributes to impaired delivery of chemotherapy agents and resistance to standard therapies.22
Figure 1.
Tumor angiogenic cascade.
Notes: Quiescent endothelial cells become activated when stromal, tumor, and immune cell-derived pro-angiogenic factors are secreted into the tumor site leading to proliferation migration, ECM degradation, and tube formation from existing capillaries. BMD EPCs are recruited to the tumor site and differentiate into endothelial cells, forming de novo capillaries (vasculogenesis). The resulting tumor blood vessels are morphologically and functionally distinct from normal vasculature.
Abbreviations: bFGF, basic fibroblast growth factor; BMD, bone marrow-derived; CAF, cancer associated fibroblasts; EC, endothelial cell; ECM, extracellular matrix; EGF, epidermal growth factor; EPC, endothelial progenitor cell; IL-8, interleukin 8; IL-17, interleukin 17; MMP, matrix metallopeptidase; PDGF, platelet-derived growth factor; SDF-1, stromal cell-derived factor 1; TAM, tumor-associated macrophages; TNF-α, tumor necrosis factor alpha; uPA, urokinase plasminogen activator; uPAR, urokinase plasminogen activator receptor; VEGF, vascular endothelial growth factor.
The understanding of the above mechanisms has led to the development and FDA approval of agents that target angiogenesis pathways. Most of the clinically available angiogenesis inhibitors target the VEGF pathway, either by targeting the ligand (bevacizumab, aflibercept)23,24 or the receptor (ramucirumab, sorafenib, sunitinib, pazopanib, axitinib, regorafenib).25–28
Antiangiogenic therapies, however, have several limitations. One is related to side effects.29 Clinically important toxicities from these agents include vascular-related side effects, such as hypertension, thromboembolic, or bleeding events,30 which may be potentially severe, requiring close follow-up of patients treated with these drugs. Another limitation relates to the fact that these agents are cytostatic, and not cytotoxic; therefore, antiangiogenic agents are not curative.29 Moreover, even though these agents provide initial clinical benefit, the majority of patients eventually progress due to the development of acquired resistance.31,32 Mechanisms of resistance to antiangiogenic agents are reviewed elsewhere.33
Advances in the knowledge of tumor angiogenesis have allowed the development of OVs with the ability to target tumor vasculature. Of the ∼2,600 papers published on oncolytic virotherapy between 2005 and 2015, ∼5% of them focus on vascular targeting. The distinctive characteristics between normal and tumor vasculature have enabled scientists to develop several vascular-directed oncolytic viral strategies. Such strategies include: 1) unmodified OVs that have an endogenous ability to bind tumor blood vessels, 2) targeting tumor endothelial cell surface receptors by viral engineering (targeted viruses), 3) transcriptional targeting of the tumor vasculature, or 4) by the delivery of peptides/cytokines that inhibit angiogenesis by “armed” viral vectors. In addition, a number of studies have combined vascular-targeted viruses with other antiangiogenic or antitumor strategies, demonstrating enhanced efficacy.
Here, we provide an update on the strategies toward the design of oncolytic viral vectors with the ability to affect tumor vasculature, focusing on the main OV platforms that have been reported to target tumor neovascularization.
Oncolytic viral platforms associated with vascular targeting or antivascular activity
Adenovirus and adeno-associated virus
Wild-type adenoviruses bind coxsackie virus-adenovirus receptor (CAR) and internalize using integrin receptors (avb3, avb5).34 Since CAR expression is highly variable in cancer and normal cells, adenoviruses require modifications in their genomes to increase tumor selectivity.35 Currently, there are ∼80 cancer clinical trials using adenoviruses as a platform, most of which are in Phases I and II.
Adenoviral vectors can be directed to tumor vasculature by either transcriptional or transductional retargeting (Table 1). VB-111 is a nonreplicating adenoviral vector (Ad-5, E1 deleted), containing a modified murine pre-proendothelin promoter (PPE-1-3X) and a Fas-chimera transgene (Fas and human TNF receptor 1). VB-111, which is undergoing early clinical evaluation,36 infects angiogenic vasculature, leading to improved antitumor effects in xenograft and syngeneic cancer models.37 Ad5ROBO4 is an E1- and E3-deleted adenovirus containing the endothelial human roundabout4 (ROBO4) enhancer/promoter, enabling the vector to target tumor endothelial cells.38
Table 1.
Adenoviral vectors
| Virus name | Mechanism | In vitro | In vivo | Delivery route | Reference |
|---|---|---|---|---|---|
| Retargeted | |||||
| FGF2-Ad-TK | FGF2 receptor targeting by a conjugate of FGF2 linked to a Fab’ fragment against the adenoviral knob region | N/A | ⇓ Tumor MVD and antitumor effects in HNSCC xenografts | IT | 40 |
| MHESpcAdluc | Polymer-coated Ad linked to monoclonal antibodies against human E-selectin | Infects umbilical cord ex vivo | N/A | N/A | 41 |
| PSGL-1-Fc-StrepGpcAdluc (in vivo) | Polymer-coated Ad linked to PSGL-1 | N/A | Co-localizes with CD31+ cells in HCC xenografts | IV | |
| Ad-uPAR-MMP-9 | Replication-deficient Ad expressing uPAR and MMP-9 antisense transcripts | ⇓ Cancer cell-induced EC capillary formation | ⇓ Tumor growth and lung metastasis in NSCLC xenografts | IT, IV | 50 |
| KOX/PEGbPHF | pH-sensitive oncolytic adenovirus expressing a VEGF transcriptional repressor | ⇓ VEGF expression ⇓ Vessel sprouting |
⇓ MVD and antitumor effects in rat glioma | IV | 43 |
| Ad5/35LacZ | CD 46 dependent endothelial binding | Efficient infection of endothelial cells | Rat HCC model: Colocalization of viral β-gal with CD46, CD31, Flk1 | Via hepatic artery | 42 |
| VB-111 | Ad-5 containing a pre- proendothelin 1 promoter (PPE-1-3X) and Fas-chimera transgene | N/A | Transgene expression and apoptosis in tumor-associated ECs | IV | 37 |
| Ad5ROBO4 | Replication-competent Ad5 containing ROBO4 promoter | N/A | RCC model: Tumor EC infection ⇓ MVD |
IV | 38 |
| Armed | |||||
| Ad5H2E-PPE1(3x)-ASMase | Endothelial targeted expression of acid sphingomyelinase | Specificity toward ECs | Melanoma; fibrosarcoma: Tumor EC-specific targeting ⇑ EC apoptosis |
IV | 112 |
| Ad-ΔB7-shVEGF | VEGF-specific shRNA expressing Ad vector | ⇓ Tube formation ⇓ Vessel sprouting (rat aortic ring assay) |
Matrigel plug assay ⇓ Vascularization ⇓ Tumor growth in multiple tumor models |
IT | 44 |
| Ad-ΔE1-U6shIL8 | Replication-competent Ad expressing IL-8 shRNA | ⇓ Migration and tube formation ⇓ Vessel sprouting (rat aortic ring assay) |
Breast cancer lung metastasis model; ⇓ VEGF and IL-8 ⇓ Tumor MVD |
IT | 113 |
| CRAd-S-5/3shMMP14 | Fiber-modified Ad vector expressing MMP14 shRNA | N/A | Glioma xenografts ⇓ CD31+ cells |
IC | 114 |
| Ad5/3-9HIF-Δ24-VEGFR-1-Ig | Hypoxia targeted Ad vector expressing soluble VEGF receptor 1-Ig fusion protein | Minimal HUVEC cytotoxicity | RCC xenografts: ⇓ Tumor size ⇓ Tumor MVD |
IT | 45 |
| Ad5/F35-XAF1 | Expression of tumor suppressor gene XAF1 | ⇓ VEGF mRNA and protein expression | HCC xenografts: ⇓ Tumor VEGF expression ⇓ Tumor MVD |
IT | 115 |
| ZD55-IL-18 | E1B gene deleted Ad expressing IL-18 | N/A | Melanoma xenografts: ⇓ VEGF ⇓ CD34 positive cells |
IT | 116 |
| EndoAngio-PRRA | Prostate-restricted Ad5 expressing Endostatin–angiostatin fusion protein | Conditioned media ⇓ EC proliferation, migration, and tube formation |
Prostate cancer xenografts: antitumor effects ⇓ Tumor MVD |
IT | 53 |
| pAd-2S03 | Ad expressing a scAb intrabody that inhibits mouse and human Tie-2 surface expression | Effective intrabody expression in ER of ECs | Human Kaposi’s sarcoma and colon carcinoma ⇓ Tumor MVD |
Peritumorally | 117 |
| Ad-eTie1-GALV | Ad encoding GALV fusogenic membrane glycoprotein regulated by human endothelial receptor tyrosine kinase (eTie1) | Heterocellular syncytia between tumor-associated endothelial cells and tumor cells | HEK 293 xenografts: heterocellular syncytia between infected ECs and HEK 293 cells lead to virus replication in vivo | IV | 118 |
| Combination | |||||
| Ad Flk1-Fc + Dl922/947 | Soluble VEGFR2 expressing Ad vector Oncolytic Ad virus selective for G1–S cell cycle checkpoint loss |
N/A | Prostate and colon cancer xenografts: ⇑ Antitumor effects ⇓ Tumor MVD |
IT IV |
47 |
| Ad-Endo + Ad-H101 | Replication-deficient Ad encoding human endostatin + replication-competent Ad | ⇓ ECs proliferation | NPC xenografts: combined treatment ⇑ Endostatin expression ⇑ Antitumor effects |
IT | 51 |
| dl922-947 + bevacizumab | E1 gene deleted Ad + anti-VEGF monoclonal antibody | N/A | Thyroid carcinoma; ⇑ Tumor viral distribution ⇓ MVD |
IT | 119 |
| AdVIL-24 + ionizing radiation | Ad5E1- and E3-deleted adenovirus expressing human IL-24 and GFP | N/A | NPC xenograft models: ⇓ Tumor MVD ⇓ VEGF expression ⇑ Antitumor effects |
IT | 59 |
Abbreviations: Ad, adenovirus; β-gal, β-galactosidase; EC, endothelial cell; ER, endoplasmic reticulum; FGF, fibroblast growth factor; GALV, gibbon ape leukemia virus; HEK, human embryonic kidney cells; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; IC, intracranial; IL, interleukin; IT, intratumoral; IV, intravenous; MMP-9, matrix metallopeptidase 9; MVD, microvessel density; N/A, not applicable; NPC, nasopharyngeal carcinoma; NSCLC, non-small-cell lung cancer; PPE-1-3X, pre-proendothelin promoter; PSGL, P-selectin glycoprotein ligand-1-Fc fusion; RCC, renal cell cancer; ROBO4, roundabout4; scAb, single chain antibody; shRNA, short hairpin RNA; uPAR, urokinase receptor; HUVEC, human umbilical vein endothelial cell; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; ⇑, increased; ⇓, decreased.
FGF2-Ad-TK, an adenovirus retargeted to FGF2 that expresses the herpes simplex virus thymidine kinase (HSV-tk) reduces tumor microvessel density (MVD), induces apoptosis and antitumor effects in vivo.39,40 Other adenoviral vectors, designed to target tumor endothelium via endothelial selectins41 or CD46,42 show important in vivo antitumor and antiangiogenic effects. KOX/PEGbPHF is an adenoviral vector coated with a pH-sensitive block copolymer, expressing a VEGF promoter-targeting transcriptional repressor (KOX), which targets the acidic tumor microenvironment and inhibits tumor growth and angiogenesis in vitro and in vivo.43
Armed adenoviruses
A large number of “armed” adenoviruses have been designed to suppress angiogenic factors, especially VEGF. This has been achieved by either introducing shRNAs against VEGF,44 soluble VEGF receptors,45–48 or VEGF promoter-targeted artificial zinc-finger proteins.49 Ad-uPAR-MMP-9 is a replication-deficient adenovirus expressing antisense urokinase receptor (uPAR) and antisense Matrix metallopeptidase (MMP)-9, and therefore inhibits the expression of these important angiogenic targets in tumor tissues.50 Other adenoviral vectors exert antiangiogenic effects in vitro and in vivo by expressing anti-angiogenic molecules, including endostatin,51 angiostatin,52 or an endostatin/angiostatin fusion.53 Armed adenoviruses can reach the tumor vasculature via circulating endothelial progenitor (CEP) cells. Infection of CEPs ex vivo with an adenovirus expressing soluble CD-115 resulted in significant antitumor effects and inhibition of tumor neovasculature in prostate cancer xenografts.54
Adeno-associated viral vectors have been designed to target angiogenesis by expressing bevacizumab (AAVrh10. BevMab),55 endostatin, thrombospondin-1,56 or plasminogen kringle 5.57 These agents have shown successful induction of antiangiogenic and antitumor effects in vivo.
Combination strategies
Bevacizumab, an anti-VEGF monoclonal antibody, given before treatment with CRAd-S-pk7, a conditionally replicating adenovirus with selectivity to glioma cells, induces MMP-2 activity, extracellular matrix degradation, and increased intratumoral viral distribution.58 AdVIL-24, an E1- and E3-deleted adenovirus expressing both human IL-24 and green fluorescent protein (GFP), in combination with ionizing radiation, was associated with decreased tumor VEGF expression, decreased microvessel density, and in vivo antitumor effects in a nasopharyngeal carcinoma.59 Other combination strategies are presented in Table 1.
Herpes simplex virus
The majority of Herpes simplex virus type 1 (HSV-1) vectors used for oncolytic virotherapy are replication-competent with genome modifications. For example, deletion of both the copies of γ34.5 gene is commonly performed to reduce neurovirulence. The gene product of γ 34.5, ICP34.5, directs protein phosphatase 1 to specifically dephosphorylate eIF2α, leading to inhibition of the protein synthesis shutoff.60 ICP6 gene encodes for the large subunit of ribonucleotide reductase, and it is needed to replicate in nondividing neurons. Inactivation of these genes allows efficient tumor cell specificity as it will only replicate in dividing cells.61
There are ∼18 clinical trials using HSV in cancer patients, with some vectors in advanced stages of clinical development.62
There is no clear consensus as to whether unmodified HSV-1 vectors have endogenous vascular binding abilities. While some studies have shown that oncolytic HSV-1 has a truly innate ability to infect murine and human endothelial cells in vitro and in vivo,63,64 other reports suggest that HSV-1 may actually elicit a potent angiogenic response.65–67 Most of the recombinant “antiangiogenic” HSV-1 vectors are armed viruses targeting pro-angiogenic factors or expressing angiogenesis inhibitors (Table 2).
Table 2.
Herpes simplex virus
| Virus | Mechanism | In vitro | In vivo | Delivery route | Reference |
|---|---|---|---|---|---|
| Recombinant | |||||
| G207 | HSV-1 gene disruption of ICP6 + LacZ gene | ECs sensitive to replicative and cytotoxic effects Disrupts endothelial cell tubes |
Matrigel plug assay: ⇓ Vessel perfusion Rhabdomyosarcoma: ⇓ Tumor vasculature |
IT | 120 |
| Armed | |||||
| RAMBO | Expression of vasculostatin under the control of IE4/5 | ⇓ EC migration ⇓ Tube formation |
Glioma xenografts: ⇓ Reduction in tumor vascular volume fraction and MVD |
IT | 70 |
| bG47Δ-PF4 | Expression of soluble platelet factor-4 | ⇓ Migration ⇑ EC apoptosis |
Glioma xenografts Murine MPNSTs: ⇓ Tumor growth and ⇓ MVD |
IT | 69 |
| rQT3 | HSV-1 expressing TIMP-3 | Efficient EC replication and cytotoxic effects | Neuroblastoma xenografts: MPNST xenografts ⇓ Tumor growth and MVD ⇓ CEP recruitment to tumor site |
IT | 71 |
| NV1042 | HSV carrying the murine IL-12 gene | CM of virus infected SCCs | Murine SCC model: ⇓ Tumor growth ⇓ Tumor MVD |
IT | 121 |
| ⇓ ECs tube formation | ⇑ Levels of IFN-γ and IFN-inducible protein 10 | ||||
| T-TSP-1 | HSV expressing human TSP-1 | N/A | Gastric cancer xenografts: ⇓ CD31 staining in tumors |
IT | 68 |
| VAE | HSV F expressing Endo–Angio fusion protein | N/A | Glioma xenografts; ⇓ Tumor MVD ⇓Tumor growth |
IT | 73 |
| Combination | |||||
| G47Δ-mAngio + G47Δ-mIL12 | G47Δ viruses expressing murine angiostatin and IL-12 | ⇓ EC tube formation | Glioma xenografts: ⇑ Tumor virus distribution ⇓ Tumor VEGF ⇓ Tumor MVD |
IT | 74 |
| G207 + hrR3 + erlotinib | HSV-1 mutant vectors EGFR TKI | EC cytotoxicity andviral replication | MPNST xenografts: Tumor EC targeting ⇓ Pro-angiogenic gene expression ⇓ Tumor MVD ⇑ Antitumor effects |
IT | 76 |
| cRGD + HrR3 | Angiostatic peptide Mutant HSV-1 vector |
N/A | Rat glioma: ⇓ Tumor vascular permeability ⇑ Survival |
IT | 78 |
| HF10 + bevacizumab | Mutant HSV-1 strain HF Anti-VEGF monoclonal Ab | N/A | Breast cancer xenografts: ⇑ Tumor virus distribution ⇓ MVD ⇑ Antitumor effects |
IT | 77 |
| NV1042 + vinblastine | oHSV secreting IL-12+ MDA | ⇓ EC tube formation | Prostate cancer xenografts; ⇓ CD31 staining in tumors ⇓ Tumor burden |
IT | 75 |
Abbreviations: Angio, angiostatin; CEP, circulating endothelial progenitor; ECs, endothelial cells; MPNSTs, malignant peripheral nerve sheath tumors; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; Endo, endostatin; HSV-1, Herpes simplex virus type 1; IFN, interferon; IL, interleukin; IT, intratumoral; MDA, microtubule disrupting agent; MVD, microvessel density; N/A, not applicable; PF4, platelet factor-4; TIMP-3, tissue inhibitor of metalloproteinases; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; ⇑, increased; ⇓, decreased.
Armed HSV vectors and combination strategies
The great majority of in vivo experiments using armed oncolytic HSVs have used the intratumoral route of administration. Treatment with T-TSP-1, an HSV-1 vector expressing thrombospondin-1 is associated with reduced tumor MVD and improved antitumor effects.68 bG47ΔPF4 induces antiangiogenic effects in vitro and in vivo by expression of soluble platelet factor-4 (PF4), in models of glioblastoma and peripheral nerve sheet tumors.69 Other armed oncolytic HSV vectors have shown antiangiogenic and antitumor effects in vitro and in vivo by the expression of vasculostatin,70 TIMP-3,71 angiostatin, endostatin,72,73 or IL-12.74
Zhang et al demonstrated that combined treatment with recombinant HSV-1 vectors carrying murine angiostatin (G47Δ–mAngio) and IL-12 (G47Δ-mIL12) on a human glioma xenograft model was associated with improved survival compared to treatment with each individual virus.74 Combination of NV1042, an oncolytic HSV expressing IL-12, and vinblastine has superior antiangiogenic and anti-tumor effects in human prostate cancer xenograft model, when compared to the parental virus alone, or the parent virus and vinblastine.75 Oncolytic HSVs have been successfully combined with agents like erlotinib,76 bevacizumab,77 and RGD (arginylglycylaspartic acid) peptides78 in models of malignant peripheral nerve sheet tumors, breast cancer, and gliomas, respectively.
Vaccinia virus
Currently, there are ∼56 clinical trials using vaccinia virus (VV), some of them showing promising results.79 The biology and pathogenesis of this viral vector has been extensively characterized.80 VV is known to intrinsically target tumor vasculature and induce vascular collapse after intravenous administration.81 Another proposed antiangiogenic mechanism include VEGF downregulation during active viral infection.82
JX-594 is an oncolytic VV (OVV) engineered to target cells with Ras/MAPK activation and to express the human granulocyte-monocyte colony-stimulating factor (hGM-CSF) and β-galactosidase (β-gal) transgenes. In vivo, JX-594 replicates in tumor-associated endothelial cells, leading to disruption of tumor blood flow and hypoxia, while normal vessels are not affected.81 In early phase trials, JX-594 showed satisfactory tolerability, viral replication, transgene expression, and importantly, antivascular effects, as evidenced by disruption of tumor perfusion in patients with hepatocellular carcinoma.81
Armed VV vectors and combination strategies
Armed OVVs can target the VEGF pathway, either by expressing single chain antibodies against VEGF83 or soluble VEGF receptor 1.84 Other vectors express endostatin-angiostatin fusion protein,85 or interferon beta.86 All of the above viruses induce antiangiogenic and antitumor effects in vivo after intravenous administration (Table 3). CXCL12 and its receptor CXCR4 is a chemokine system that has been associated with angiogenesis, vasculogenesis, and tumor progression.87 OVV-CXCR4-A-Fc is an OVV that delivers a CXCR4 antagonist expressed in the context of the murine Fc fragment of IgG2a. Intravenous administration of this viral vector resulted in inhibition of tumor growth and vascular disruption in murine mammary cancer, effects associated with decreased levels of CXC12, VEGF, and circulating endothelial progenitor cells (CEPs).88
Table 3.
Vaccinia virus
| Virus | Mechanism | In vitro | In vivo effects | Delivery route | Reference |
|---|---|---|---|---|---|
| Armed | |||||
| vvDD-SR-RFP | VV expressing human somatostatin receptor subtype 2 and RFP | ⇑ Viral replication after VEGF stimulation | Colon cancer xenografts Colocalization of the viral RFP within CD31+ cells ⇓ CD31 staining |
IP | 122 |
| JX-795 | VV B18R deletion mutant expressing IFN-β | N/A | Murine mammary carcinoma: ⇓ Tumor perfusion ⇑ Antitumor effects In vivo tumor ECs targeting |
IV; IT | 86 |
| GLV-1h446 | Expression of scAb against VEGF and FAP | N/A | Feline mammary carcinoma; ⇓ Tumor growth ⇓ Tumoral VEGF levels and angiogenesis |
IV | 83 |
| vvdd-VEGFR-1-Ig | Expression of soluble VEGFR 1 | N/A | RCC (human and murine) ⇑ Antitumor effects ⇓ MVD |
IV | 84 |
| OVV-CXCR4-A-mFc | Expression of CXCR4 antagonist | N/A | Murine breast cancer; ⇓ Tumor perfusion ⇓ Tumor CXCL 12, VEGF ⇓ BMD endothelial and myeloid cells |
IV | 88 |
| JX-594 | hGM-CSF expressing VV, targeted toward cells with Ras/MAPK activation | Efficient EC replications EC cytotoxicity | Murine breast and colon cancer: Selective tumor EC targeting ⇑ Antivascular cytokines Disrupts vasculature |
IV | 81 |
| VVhEA | LV strain of VV expressing the Endo–Angio fusion gene | ⇓ EC tube formation | Pancreatic cancer xenografts: ⇓ Tumor MVD ⇑ Antitumor effects |
IV; IT | 85 |
| Combination | |||||
| vvDD-luc + Ad Flk1-Fc or vvDD-luc + sunitinib | vvDD-luc Ad expressing Flk1-Fc Anti-VEGFR TKI |
vvDD alone Inhibits ECs proliferation |
Murine breast cancer; Enhancement of antitumor effects and improved survival with combination treatments |
IV | 82 |
| JX-594 + sorafenib | VV + VEGFR/RAF TKI | N/A | Human HCC; murine melanoma; Combination improves antitumor efficacy |
IV | 89 |
| VVDD-EGFP + PDT | TK and VGF-deleted VV expressing EGFP + PDT | N/A | Murine neuroblastoma, human SCC: PDT enhances viral replication ⇑ Antitumor effects |
IV | 90 |
Abbreviations: RFP, red fluorescent protein; Angio, angiostatin; Ad, adenovirus; BMD, bone marrow-derived; ECs, endothelial cells; Endo, endostatin; HCC, hepatocellular carcinoma; hGM-CSF, human granulocyte-macrophage colony-stimulating factor; IFN-β, Interferon beta; FAP, fibroblast activation protein; IP, intraperitoneal; IT, intratumoral; IV, intravenous; LV, Lister vaccine; MVD, microvessel density; N/A, not applicable; PDT, photodynamic therapy; RCC, renal cell cancer; MAPK, mitogen-activated protein kinase; scAb, single chain antibody; SCC, squamous cell carcinoma; TK, thymidine kinase; VEGF, vascular endothelial growth factor; VGF, vaccinia growth factor; VV, vaccinia virus; VEGFR, VEGF receptor; EGFP, enhanced green fluorescent protein; ⇑, increased; ⇓, decreased.
JX-594 has been combined with sorafenib in murine cancer models and in patients with hepatocellular carcinoma. The combination was well tolerated and associated with decreased tumor perfusion and objective responses.89 Combination of OVV with either adenoviral vectors expressing FLK1 Fc or Sunitinib was associated with improved antitumor effects in models of murine mammary cancer in vivo.82 Gil et al combined OVV with photodynamic therapy (PDT), showing that vascular disruption caused by PDT led to higher viral titers and improved antitumor in murine models of neuroblastoma and squamous cell carcinoma.90
Vesicular stomatitis virus
Vesicular stomatitis virus (VSV) is a negative-stranded RNA virus that induces potent and rapid in vitro and in vivo antitumor effects.91 Currently, there is one Phase I clinical trial using VSV as an oncolytic vector in patients with hepatocellular carcinoma. The oncolytic ability of VSV is based on the knowledge that most cancer cells possess an impaired antiviral response induced by type I interferon, making them more susceptible to VSV infection than normal cells.92 The low density lipoprotein receptor has been recognized as the major cell surface receptor for VSV in human and mouse cells.93
Oncolytic VSV has been shown to directly bind to tumor vasculature, reduce vascular perfusion due to clot formation, and decrease microvessel density.94 Attempts have been made to design oncolytic VSVs displaying endothelial targeting peptides, such as echistatin of RGD peptides. However, endothelial infection in vivo could not be demonstrated in tumors treated by the targeted viruses.95 Studies combining oncolytic VSV and other vascular targeted agents have shown enhanced antitumor effects (Table 4).
Table 4.
Vesicular stomatitis virus
| Virus | Virus design | In vitro | In vivo effects | Delivery route | Reference |
|---|---|---|---|---|---|
| Recombinant | |||||
| VSV-GFP | Δ51 VSV-expressing GFP HR strain of wt Indiana virus | N/A | Murine colon cancer; Infects and induces clot formation in tumor vasculature ⇓ Tumor perfusion ⇓ MVD |
IV | 94 |
| Combination | |||||
| VSVΔ51 + ZD6126 | Mutant VSV ZD6126: vascular disrupting agent |
N/A | Human HNSCC: Combination therapy: ⇑ Viral replication ⇑ Antitumor effects |
IV | 96 |
| rVSV-F + DSM | VSV-expressing NDV fusion protein + embolization with degradable starch microspheres | N/A | Rat HCC; ⇑ Tumor necrosis ⇑ Apoptosis ⇓ MVD (CD 31) |
Via hepatic artery | 98 |
| VSV (M51R) + Sunitinib | Attenuated mutant VSV Anti-VEGFR TKI |
N/A | Prostate, breast, and kidney tumors Increased antitumor effects of the combination |
IT | 97 |
Abbreviations: DSM, degradable starch microspheres; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; HR, heat resistant; IV, intravenous; MVD, microvessel density; N/A, not applicable; NDV, New castle disease virus; VEGFR, vascular endothelial growth factor receptor; VSV, vesicular stomatitis virus; wt, wild-type; ⇑, increased; ⇓, decreased.
Combination strategies
Combination of intravenous VSVΔ51 with the vascular disrupting agent ZD6126 or with radiation therapy demonstrated enhanced antitumor effects, compared to each agent alone.96 ZD6126 increased viral delivery via vascular disruption and decreased interstitial fluid pressure. Sunitinib in combination with oncolytic VSV are associated with significant antitumor effects in models of prostate, breast, and kidney cancer, compared to each agent alone.97 Finally, in a hepatocellular carcinoma model, combination of embolization and rVSV-F, a recombinant VSV expressing the Newcastle Disease Virus fusion protein, resulted in decreased tumor MVD, improved antitumor effects, and improved survival.98
Measles virus
Measles virus (MV) is a negative-stranded RNA virus that belongs to the family of Paramyxoviridae.99 The Edmonston vaccine strain of MV (MV-Edm) has oncoselectivity and promising antitumor activity in vitro and in mouse xenograft models. Currently, there are approximately seven active clinical trials using MVs as oncolytic vectors showing satisfactory results in terms of safety and promising antitumor effects.100,101 Three endogenous MV receptors have been identified: CD46 (ubiquitously expressed in cells), SLAM (expressed on immune cells),102 and Nectin-4. Nectin-4 is considered the epithelial receptor for this viral agent.103
Targeted and armed oncolytic MV vectors
Oncolytic MV vectors have been engineered to target vasculature by displaying vascular-targeted ligands as C-terminal extensions of the MV-H protein (Table 5). The first reported vascular-targeted oncolytic MV is MV-ERV, which displays echistatin, a disintegrin that binds with high affinity to integrin αvβ3.104 This agent was shown to bind and infect endothelial cells in vitro and vasculature in vivo and induced potent antitumor effects.105 An MV vector displaying RGD peptides able to bind endothelial cells via αvβ3 and α5β1 (MV-RGD) was shown to target neovessels in the ear pinna angiogenesis model.106
Table 5.
Measles virus
| Virus | Mechanism | In vitro | In vivo | Delivery route | Reference |
|---|---|---|---|---|---|
| Targeted | |||||
| MV-ERV | MV displaying echistatin | Infects and ⇓ EC tube formation Infects newly formed blood vessels in chorioallantoic membrane assays |
Multiple myeloma tumors; ⇓ Tumor volume |
IT | 105 |
| MV-RGD MV-echistatin | MV displaying RGD and echistatin peptides as C-terminal extensions of MV-H protein | N/A | Infection of neovessels in ear pinna angiogenesis model and CAM assays Myeloma xenografts: MV-N colocalizes with CD31+ cells |
IV | 106 |
| MV-h-uPA MV-m-PA |
MV-Edm fully retargeted against human or murine uPAR | Efficiently infects and replicates in VEGF stimulated ECs Infects endothelial capillaries |
Breast cancer MV-N colocalizes with CD31+ cells |
IV | 107 |
| Armed | |||||
| MV-E:A | Expression of Angio and Endo | CM of infected cancer ⇓ EC tube formation ⇓ ECs migration |
Medulloblastoma xenografts: ⇓ Tumor microvessels |
IT | 110 |
| MV-mIFN-β | Expression of murine IFN-β | N/A | Mesothelioma xenografts: ⇓ Tumor MVD (CD31) |
IT | 111 |
Abbreviations: Angio, angiostatin; EC, endothelial cell; Endo, endostatin; IFN-β, interferon beta; IT, intratumoral; IV, intravenous; MV, measles virus; MVD, microvessel density; N/A, not applicable; CAM, chick chorioallantoic membrane; uPA, urokinase plasminogen activator; uPAR, urokinase receptor; VEGF, vascular endothelial growth factor; ⇓, decreased.
MV-uPA is a fully retargeted oncolytic MV directed against the uPAR. This vector was generated by displaying the aminoterminal fragment of human or mouse urokinase into the C-terminus of a mutant MV-H unable to bind to CD46 or SLAM.107 In vitro, MV-human-uPA efficiently infected and replicated in human umbilical vein endothelial cells stimulated with VEGF. MV-mouse-uPA was able to infect murine tumor vasculature, as evidenced by MV-N and CD31 colocalization in tumor tissues.107 Both retargeted viruses induce species-specific antitumor and antimetastatic effects.108,109
MV-E:A encodes human or murine endostatin/angiostatin fusion protein (MV-hE:A and MV-mE:A, respectively).110 In vivo, intratumoral injection of the recombinant viruses in a medulloblastoma xenograft model was associated with decreased MVD. MV-mIFNβ is a murine interferon beta expressing MV, which was found to decrease microvessel density (measured by CD31 expression) after intratumoral administration in malignant mesothelioma.111
Conclusion and future directions
Recent studies have shown proof of concept that vascular targeting by OVs is a feasible and promising strategy, associated with significant antiangiogenic and antitumor effects. Angiogenic pathways previously thought to be targetable only by small molecules or antibodies can now be targeted by redesigned OVs. This strategy is unique and offers an advantage over current antiangiogenic agents. In addition to targeting tumor vasculature, OVs exert potent oncolytic and immunomodulatory effects, which may help overcome tumor-resistance mechanisms.
However, there are challenges to the clinical development of vascular-targeted viruses. One of the main translational questions involves the safety of vascular targeting by an OV. As animal studies are not always predictive of the clinical scenario, it is extremely important that preclinical and clinical testing of “antiangiogenic” OVs take into account the potential for toxicity to normal vasculature. Experience from currently approved antiangiogenic agents shows that “tumor endothelial” targeted agents are associated with significant off-target effects in normal vasculature. Therefore, extensive preclinical toxicity studies will be required, focusing on the virus effects on vasculature, before moving such agents into the clinic. Clinical trials of such agents will require careful planning in regard to trial design, dosing schedule, patient selection, and methods to monitor the OVs’ safety and biological effects. This can be achieved by multidisciplinary discussion among scientists, clinical investigators, ethics committees, and regulatory agencies.
Finally, combination studies using vascular-targeted viruses and standard/targeted therapies should be carefully evaluated in appropriate preclinical models that closely resemble human cancers. This will ensure safe and effective translation of this highly attractive strategy into a novel clinical therapeutic option.
Acknowledgments
JR Merchan was supported by the Sylvester Comprehensive Cancer Center and by a grant from the National Institutes of Health (5R01CA149659-05).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanford MM, Bell JC, Vaha-Koskela MJ. Novel oncolytic viruses: riding high on the next wave? Cytokine Growth Factor Rev. 2010;21(2–3):177–183. doi: 10.1016/j.cytogfr.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Hermiston TW, Kuhn I. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 2002;9(12):1022–1035. doi: 10.1038/sj.cgt.7700542. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Deisseroth A. Tumor vascular targeting therapy with viral vectors. Blood. 2006;107(8):3027–3033. doi: 10.1182/blood-2005-10-4114. [DOI] [PubMed] [Google Scholar]
- 5.Thorne SH, Hermiston T, Kirn D. Oncolytic virotherapy: approaches to tumor targeting and enhancing antitumor effects. Semin Oncol. 2005;32(6):537–548. doi: 10.1053/j.seminoncol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Gao L, Yeagy B, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10(4):371–379. [PubMed] [Google Scholar]
- 7.Sze DY, Reid TR, Rose SC. Oncolytic virotherapy. J Vasc Interv Radiol. 2013;24(8):1115–1122. doi: 10.1016/j.jvir.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Wong HH, Lemoine NR, Wang Y. Oncolytic viruses for cancer therapy: overcoming the obstacles. Viruses. 2010;2(1):78–106. doi: 10.3390/v2010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith E, Breznik J, Lichty BD. Strategies to enhance viral penetration of solid tumors. Hum Gene Ther. 2011;22(9):1053–1060. doi: 10.1089/hum.2010.227. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9(3):253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- 11.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2(3):213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang Z, Frerich JM, Huntoon K, et al. Tumor derived vasculogenesis in von Hippel-Lindau disease-associated tumors. Sci Rep. 2014;4:4102. doi: 10.1038/srep04102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartha K, Rieger H. Vascular network remodeling via vessel cooption, regression and growth in tumors. J Theor Biol. 2006;241(4):903–918. doi: 10.1016/j.jtbi.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Seftor RE, Hess AR, Seftor EA, et al. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181(4):1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Biochim Biophys Acta. 2014;1846(1):161–179. doi: 10.1016/j.bbcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 18.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6(1):1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y. Therapeutic potentials of angiostatin in the treatment of cancer. Haematologica. 1999;84(7):643–650. [PubMed] [Google Scholar]
- 20.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 21.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev. 2011;37(1):63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96(9):1157–1158. doi: 10.1136/bjophthalmol-2011-300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 25.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 26.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26(29):4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 28.Zhu AX, Finn RS, Mulcahy M, et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res. 2013;19(23):6614–6623. doi: 10.1158/1078-0432.CCR-13-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayson GC, Hicklin DJ, Ellis LM. Antiangiogenic therapy – evolving view based on clinical trial results. Nat Rev Clin Oncol. 2012;9(5):297–303. doi: 10.1038/nrclinonc.2012.8. [DOI] [PubMed] [Google Scholar]
- 30.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khattak M, Larkin J. Sequential therapy with targeted agents in metastatic renal cell carcinoma: beyond second-line and overcoming drug resistance. World J Urol. 2014;32(1):19–29. doi: 10.1007/s00345-012-1013-z. [DOI] [PubMed] [Google Scholar]
- 32.McCarty JH. Glioblastoma resistance to anti-VEGF therapy: has the challenge been MET? Clin Cancer Res. 2013;19(7):1631–1633. doi: 10.1158/1078-0432.CCR-13-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soudais C, Boutin S, Hong SS, et al. Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J Virol. 2000;74(22):10639–10649. doi: 10.1128/jvi.74.22.10639-10649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanerva A, Hemminki A. Adenoviruses for treatment of cancer. Ann Med. 2005;37(1):33–43. doi: 10.1080/07853890410018934. [DOI] [PubMed] [Google Scholar]
- 36.Brenner AJ, Cohen YC, Breitbart E, et al. Phase I dose-escalation study of VB-111, an antiangiogenic virotherapy, in patients with advanced solid tumors. Clin Cancer Res. 2013;19(14):3996–4007. doi: 10.1158/1078-0432.CCR-12-2079. [DOI] [PubMed] [Google Scholar]
- 37.Reddi HV, Madde P, Cohen YC, et al. Antitumor activity of VB-111, a novel antiangiogenic virotherapeutic, in thyroid cancer xenograft mouse models. Genes Cancer. 2011;2(10):993–995. doi: 10.1177/1947601912437933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu ZH, Kaliberov S, Sohn RE, Kaliberova L, Curiel DT, Arbeit JM. Transcriptional targeting of primary and metastatic tumor neovasculature by an adenoviral type 5 roundabout4 vector in mice. PLoS One. 2013;8(12):e83933. doi: 10.1371/journal.pone.0083933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Malley BW, Jr, Chen SH, Schwartz MR, Woo SL. Adenovirus-mediated gene therapy for human head and neck squamous cell cancer in a nude mouse model. Cancer Res. 1995;55(5):1080–1085. [PubMed] [Google Scholar]
- 40.Saito K, Khan K, Sosnowski B, Li D, O’Malley BW., Jr Cytotoxicity and antiangiogenesis by fibroblast growth factor 2-targeted Ad-TK cancer gene therapy. Laryngoscope. 2009;119(4):665–674. doi: 10.1002/lary.20127. [DOI] [PubMed] [Google Scholar]
- 41.Bachtarzi H, Stevenson M, Subr V, Seymour LW, Fisher KD. E-selectin is a viable route of infection for polymer-coated adenovirus retargeting in TNF-alpha-activated human umbilical vein endothelial cells. J Drug Target. 2011;19(8):690–700. doi: 10.3109/1061186X.2010.547585. [DOI] [PubMed] [Google Scholar]
- 42.Shinozaki K, Suominen E, Carrick F, et al. Efficient infection of tumor endothelial cells by a capsid-modified adenovirus. Gene Ther. 2006;13(1):52–59. doi: 10.1038/sj.gt.3302598. [DOI] [PubMed] [Google Scholar]
- 43.Choi JW, Jung SJ, Kasala D, et al. pH-sensitive oncolytic adenovirus hybrid targeting acidic tumor microenvironment and angiogenesis. J Control Release. 2015;205:134–143. doi: 10.1016/j.jconrel.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo JY, Kim JH, Kwon YG, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15(2):295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 45.Guse K, Diaconu I, Rajecki M, et al. Ad5/3-9HIF-Delta24-VEGFR-1-Ig, an infectivity enhanced, dual-targeted and antiangiogenic oncolytic adenovirus for kidney cancer treatment. Gene Ther. 2009;16(8):1009–1020. doi: 10.1038/gt.2009.56. [DOI] [PubMed] [Google Scholar]
- 46.Jin K, He K, Teng F, et al. FP3: a novel VEGF blocker with antiangiogenic effects in vitro and antitumour effects in vivo. Clin Transl Oncol. 2011;13(12):878–884. doi: 10.1007/s12094-011-0749-z. [DOI] [PubMed] [Google Scholar]
- 47.Thorne SH, Tam BY, Kirn DH, Contag CH, Kuo CJ. Selective intratumoral amplification of an antiangiogenic vector by an oncolytic virus produces enhanced antivascular and anti-tumor efficacy. Mol Ther. 2006;13(5):938–946. doi: 10.1016/j.ymthe.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Yu DC, Lee JS, Yoo JY, et al. Soluble vascular endothelial growth factor decoy receptor FP3 exerts potent antiangiogenic effects. Mol Ther. 2012;20(5):938–947. doi: 10.1038/mt.2011.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang YA, Shin HC, Yoo JY, Kim JH, Kim JS, Yun CO. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol Ther. 2008;16(6):1033–1040. doi: 10.1038/mt.2008.63. [DOI] [PubMed] [Google Scholar]
- 50.Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4(9):1399–1408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu RY, Zhou L, Zhang YL, et al. An oncolytic adenovirus enhances antiangiogenic and antitumoral effects of a replication-deficient adenovirus encoding endostatin by rescuing its selective replication in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2013;442(3–4):171–176. doi: 10.1016/j.bbrc.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 52.Hajitou A, Grignet C, Devy L, et al. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. FASEB J. 2002;16(13):1802–1804. doi: 10.1096/fj.02-0109fje. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Liu YH, Lee SJ, Gardner TA, Jeng MH, Kao C. Prostate-restricted replicative adenovirus expressing human endostatin-angiostatin fusion gene exhibiting dramatic antitumor efficacy. Clin Cancer Res. 2008;14(1):291–299. doi: 10.1158/1078-0432.CCR-07-0867. [DOI] [PubMed] [Google Scholar]
- 54.Lucas T, Abraham D, Untergasser G, et al. Adenoviral-mediated endothelial precursor cell delivery of soluble CD115 suppresses human prostate cancer xenograft growth in mice. Stem Cells. 2009;27(9):2342–2352. doi: 10.1002/stem.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie Y, Hicks MJ, Kaminsky SM, Moore MA, Crystal RG, Rafii A. AAV-mediated persistent bevacizumab therapy suppresses tumor growth of ovarian cancer. Gynecol Oncol. 2014;135(2):325–332. doi: 10.1016/j.ygyno.2014.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Xu J, Lawler J, Terwilliger E, Parangi S. Adeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatin. Clin Cancer Res. 2007;13(13):3968–3976. doi: 10.1158/1078-0432.CCR-07-0245. [DOI] [PubMed] [Google Scholar]
- 57.Bui Nguyen TM, Subramanian IV, Xiao X, Nguyen P, Ramakrishnan S. Adeno-associated virus-mediated delivery of kringle 5 of human plasminogen inhibits orthotopic growth of ovarian cancer. Gene Ther. 2010;17(5):606–615. doi: 10.1038/gt.2010.15. [DOI] [PubMed] [Google Scholar]
- 58.Thaci B, Ulasov IV, Ahmed AU, Ferguson SD, Han Y, Lesniak MS. Anti-angiogenic therapy increases intratumoral adenovirus distribution by inducing collagen degradation. Gene Ther. 2013;20(3):318–327. doi: 10.1038/gt.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, Zhang Y, Sun P, Xie Y, Xiang J, Yang J. Enhanced therapeutic efficacy of adenovirus-mediated interleukin-24 gene therapy combined with ionizing radiotherapy for nasopharyngeal carcinoma. Oncol Rep. 2013;30(3):1165–1174. doi: 10.3892/or.2013.2550. [DOI] [PubMed] [Google Scholar]
- 60.Orvedahl A, Alexander D, Tallóczy Z, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1(1):23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 62.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 63.Benencia F, Courreges MC, Conejo-García JR, et al. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther. 2005;16(6):765–778. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 64.Key NS, Vercellotti GM, Winkelmann JC, et al. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci U S A. 1990;87(18):7095–7099. doi: 10.1073/pnas.87.18.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurozumi K, Hardcastle J, Thakur R, et al. Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61. Mol Ther. 2008;16(8):1382–1391. doi: 10.1038/mt.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aghi M, Rabkin SD, Martuza RL. Angiogenic response caused by oncolytic herpes simplex virus-induced reduced thrombospondin expression can be prevented by specific viral mutations or by administering a thrombospondin-derived peptide. Cancer Res. 2007;67(2):440–444. doi: 10.1158/0008-5472.CAN-06-3145. [DOI] [PubMed] [Google Scholar]
- 67.Sahin TT, Kasuya H, Nomura N, et al. Impact of novel oncolytic virus HF10 on cellular components of the tumor microenviroment in patients with recurrent breast cancer. Cancer Gene Ther. 2012;19(4):229–237. doi: 10.1038/cgt.2011.80. [DOI] [PubMed] [Google Scholar]
- 68.Tsuji T, Nakamori M, Iwahashi M, et al. An armed oncolytic herpes simplex virus expressing thrombospondin-1 has an enhanced in vivo antitumor effect against human gastric cancer. Int J Cancer. 2013;132(2):485–494. doi: 10.1002/ijc.27681. [DOI] [PubMed] [Google Scholar]
- 69.Liu TC, Zhang T, Fukuhara H, et al. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther. 2006;14(6):789–797. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Hardcastle J, Kurozumi K, Dmitrieva N, et al. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol Ther. 2010;18(2):285–294. doi: 10.1038/mt.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahller YY, Vaikunth SS, Ripberger MC, et al. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res. 2008;68(4):1170–1179. doi: 10.1158/0008-5472.CAN-07-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berto E, Bozac A, Volpi I, et al. Antitumor effects of non-replicative herpes simplex vectors expressing antiangiogenic proteins and thymidine kinase on Lewis lung carcinoma establishment and growth. Cancer Gene Ther. 2007;14(9):791–801. doi: 10.1038/sj.cgt.7701058. [DOI] [PubMed] [Google Scholar]
- 73.Zhang G, Jin G, Nie X, et al. Enhanced antitumor efficacy of an oncolytic herpes simplex virus expressing an endostatin-angiostatin fusion gene in human glioblastoma stem cell xenografts. PLoS One. 2014;9(4):e95872. doi: 10.1371/journal.pone.0095872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W, Fulci G, Wakimoto H, et al. Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models. Neoplasia. 2013;15(6):591–599. doi: 10.1593/neo.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Passer BJ, Cheema T, Wu S, Wu CL, Rabkin SD, Martuza RL. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer Gene Ther. 2013;20(1):17–24. doi: 10.1038/cgt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahller YY, Vaikunth SS, Currier MA, et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther. 2007;15(2):279–286. doi: 10.1038/sj.mt.6300038. [DOI] [PubMed] [Google Scholar]
- 77.Tan G, Kasuya H, Sahin TT, et al. Combination therapy of oncolytic herpes simplex virus HF10 and bevacizumab against experimental model of human breast carcinoma xenograft. Int J Cancer. 2015;136(7):1718–1730. doi: 10.1002/ijc.29163. [DOI] [PubMed] [Google Scholar]
- 78.Kurozumi K, Hardcastle J, Thakur R, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99(23):1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 79.Guse K, Cerullo V, Hemminki A. Oncolytic vaccinia virus for the treatment of cancer. Expert Opin Biol Ther. 2011;11(5):595–608. doi: 10.1517/14712598.2011.558838. [DOI] [PubMed] [Google Scholar]
- 80.Jefferson A, Cadet VE, Hielscher A. The mechanisms of genetically modified vaccinia viruses for the treatment of cancer. Crit Rev Oncol Hematol. 2015;95(3):407–416. doi: 10.1016/j.critrevonc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Breitbach CJ, Arulanandam R, De Silva N, et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73(4):1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 82.Hou W, Chen H, Rojas J, Sampath P, Thorne SH. Oncolytic vaccinia virus demonstrates antiangiogenic effects mediated by targeting of VEGF. Int J Cancer. 2014;135(5):1238–1246. doi: 10.1002/ijc.28747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang T, Wang H, Chen NG, Frentzen A, Minev B, Szalay AA. Expression of anti-VEGF antibody together with anti-EGFR or anti-FAP enhances tumor regression as a result of vaccinia virotherapy. Mol Ther Oncolytics. 2015;2:15003. doi: 10.1038/mto.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guse K, Sloniecka M, Diaconu I, et al. Antiangiogenic arming of an oncolytic vaccinia virus enhances antitumor efficacy in renal cell cancer models. J Virol. 2010;84(2):856–866. doi: 10.1128/JVI.00692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tysome JR, Briat A, Alusi G, et al. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Ther. 2009;16(10):1223–1233. doi: 10.1038/gt.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4(12):e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2015 May 11; doi: 10.1038/onc.2015.139. Epub. [DOI] [PubMed] [Google Scholar]
- 88.Gil M, Seshadri M, Komorowski MP, Abrams SI, Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110(14):E1291–E1300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heo J, Breitbach CJ, Moon A, et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol Ther. 2011;19(6):1170–1179. doi: 10.1038/mt.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gil M, Bieniasz M, Seshadri M, et al. Photodynamic therapy augments the efficacy of oncolytic vaccinia virus against primary and metastatic tumours in mice. Br J Cancer. 2011;105(10):1512–1521. doi: 10.1038/bjc.2011.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17(4):516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 92.Hastie E, Grdzelishvili VZ. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol. 2012;93(pt 12):2529–2545. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2013;110(18):7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Breitbach CJ, De Silva NS, Falls TJ, et al. Targeting tumor vasculature with an oncolytic virus. Mol Ther. 2011;19(5):886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ammayappan A, Peng KW, Russell SJ. Characteristics of oncolytic vesicular stomatitis virus displaying tumor-targeting ligands. J Virol. 2013;87(24):13543–13555. doi: 10.1128/JVI.02240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alajez NM, Mocanu JD, Krushel T, Bell JC, Liu FF. Enhanced vesicular stomatitis virus (VSVDelta51) targeting of head and neck cancer in combination with radiation therapy or ZD6126 vascular disrupting agent. Cancer Cell Int. 2012;12(1):27. doi: 10.1186/1475-2867-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jha BK, Dong B, Nguyen CT, Polyakova I, Silverman RH. Suppression of antiviral innate immunity by sunitinib enhances oncolytic virotherapy. Mol Ther. 2013;21(9):1749–1757. doi: 10.1038/mt.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Altomonte J, Braren R, Schulz S, et al. Synergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in rats. Hepatology. 2008;48(6):1864–1873. doi: 10.1002/hep.22546. [DOI] [PubMed] [Google Scholar]
- 99.Galanis E. Therapeutic potential of oncolytic measles virus: promises and challenges. Clin Pharmacol Ther. 2010;88(5):620–625. doi: 10.1038/clpt.2010.211. [DOI] [PubMed] [Google Scholar]
- 100.Galanis E, Hartmann LC, Cliby WA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70(3):875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Myers R, Harvey M, Kaufmann TJ, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19(7):690–698. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Msaouel P, Iankov ID, Dispenzieri A, Galanis E. Attenuated oncolytic measles virus strains as cancer therapeutics. Curr Pharm Biotechnol. 2012;13(9):1732–1741. doi: 10.2174/138920112800958896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Delpeut S, Noyce RS, Richardson CD. The tumor-associated marker, PVRL4 (nectin-4), is the epithelial receptor for morbilliviruses. Viruses. 2014;6(6):2268–2286. doi: 10.3390/v6062268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar CC, Huiming-Nie CPR, Malkowski M, Maxwell E, Catino JJ, Armstrong L. Biochemical characterization of the binding of echistatin to integrin αvβ3 receptor. J Pharmacol Exp Ther. 1997;283(2):843–853. [PubMed] [Google Scholar]
- 105.Hallak LK, Merchan JR, Storgard CM, Loftus JC, Russell SJ. Targeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regression. Cancer Res. 2005;65(12):5292–5300. doi: 10.1158/0008-5472.CAN-04-2879. [DOI] [PubMed] [Google Scholar]
- 106.Ong HT, Trejo TR, Pham LD, Oberg AL, Russell SJ, Peng KW. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol Ther. 2009;17(6):1012–1021. doi: 10.1038/mt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jing Y, Tong C, Zhang J, et al. Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 2009;69(4):1459–1468. doi: 10.1158/0008-5472.CAN-08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jing Y, Bejarano MT, Zaias J, Merchan JR. In vivo anti-metastatic effects of uPAR retargeted measles virus in syngeneic and xenograft models of mammary cancer. Breast Cancer Res Treat. 2015;149(1):99–108. doi: 10.1007/s10549-014-3236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jing Y, Zaias J, Duncan R, Russell SJ, Merchan JR. In vivo safety, biodistribution and antitumor effects of uPAR retargeted oncolytic measles virus in syngeneic cancer models. Gene Ther. 2014;21(3):289–297. doi: 10.1038/gt.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hutzen B, Bid HK, Houghton PJ, et al. Treatment of medulloblastoma with oncolytic measles viruses expressing the angiogenesis inhibitors endostatin and angiostatin. BMC Cancer. 2014;14:206. doi: 10.1186/1471-2407-14-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li H, Peng KW, Dingli D, Kratzke RA, Russell SJ. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17(8):550–558. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stancevic B, Varda-Bloom N, Cheng J, et al. Adenoviral transduction of human acid sphingomyelinase into neo-angiogenic endothelium radiosensitizes tumor cure. PLoS One. 2013;8(8):e69025. doi: 10.1371/journal.pone.0069025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoo JY, Kim JH, Kim J, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008;15(9):635–651. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 114.Ulasov I, Borovjagin AV, Kaverina N, et al. MT1-MMP silencing by an shRNA-armed glioma-targeted conditionally replicative adenovirus (CRAd) improves its anti-glioma efficacy in vitro and in vivo. Cancer Lett. 2015;365(2):240–250. doi: 10.1016/j.canlet.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 115.Zhu LM, Shi DM, Dai Q, et al. Tumor suppressor XAF1 induces apop-tosis, inhibits angiogenesis and inhibits tumor growth in hepatocellular carcinoma. Oncotarget. 2014;5(14):5403–5415. doi: 10.18632/oncotarget.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng JN, Pei DS, Mao LJ, et al. Oncolytic adenovirus expressing interleukin-18 induces significant antitumor effects against melanoma in mice through inhibition of angiogenesis. Cancer Gene Ther. 2010;17(1):28–36. doi: 10.1038/cgt.2009.38. [DOI] [PubMed] [Google Scholar]
- 117.Popkov M, Jendreyko N, McGavern DB, Rader C, Barbas CF., 3rd Targeting tumor angiogenesis with adenovirus-delivered anti-Tie-2 intrabody. Cancer Res. 2005;65(3):972–981. [PubMed] [Google Scholar]
- 118.Chen HH, Cawood R, El-Sherbini Y, et al. Active adenoviral vascular penetration by targeted formation of heterocellular endothelial-epithelial syncytia. Mol Ther. 2011;19(1):67–75. doi: 10.1038/mt.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Libertini S, Iacuzzo I, Perruolo G, et al. Bevacizumab increases viral distribution in human anaplastic thyroid carcinoma xenografts and enhances the effects of E1A-defective adenovirus dl922-947. Clin Cancer Res. 2008;14(20):6505–6514. doi: 10.1158/1078-0432.CCR-08-0200. [DOI] [PubMed] [Google Scholar]
- 120.Cinatl J, Jr, Michaelis M, Driever PH, et al. Multimutated herpes simplex virus g207 is a potent inhibitor of angiogenesis. Neoplasia. 2004;6(6):725–735. doi: 10.1593/neo.04265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wong RJ, Chan MK, Yu Z, et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res. 2004;10(13):4509–4516. doi: 10.1158/1078-0432.CCR-04-0081. [DOI] [PubMed] [Google Scholar]
- 122.Ottolino-Perry K, Tang N, Head R, et al. Tumor vascularization is critical for oncolytic vaccinia virus treatment of peritoneal carcinomatosis. Int J Cancer. 2014;134(3):717–730. doi: 10.1002/ijc.28395. [DOI] [PubMed] [Google Scholar]