Abstract
Bone morphogenetic proteins (BMPs) act as growth regulators and inducers of differentiation. They transduce their signal via three different type I receptors, termed activin receptor-like kinase 2 (Alk2), Alk3, or bone morphogenetic protein receptor Ia (BMPRIa) and Alk6 or BMPRIb. Little is known about functional differences between the three type I receptors. Here, we have investigated consequences of constitutively active (ca) and dominant negative (dn) type I receptor overexpression in adult-derived hippocampal progenitor cells (AHPs). The dn receptors have a nonfunctional intracellular but functional extracellular domain. They thus trap BMPs that are endogenously produced by AHPs. We found that effects obtained by overexpression of dnAlk2 and dnAlk6 were similar, suggesting similar ligand binding patterns for these receptors. Thus, cell survival was decreased, glial fibrillary acidic protein (GFAP) expression was reduced, whereas the number of oligodendrocytes increased. No effect on neuronal differentiation was seen. Whereas the expression of Alk2 and Alk3 mRNA remained unchanged, the Alk6 mRNA was induced after impaired BMP signaling. After dnAlk3 overexpression, cell survival and astroglial differentiation increased in parallel to augmented Alk6 receptor signaling. We conclude that endogenous BMPs mediate cell survival, astroglial differentiation and the suppression of oligodendrocytic cell fate mainly via the Alk6 receptor in AHP culture.
INTRODUCTION
Bone morphogenetic proteins (BMPs) are pleiotropic cytokines and constitute the largest subfamily of the transforming growth factor-β (TGF-β) superfamily. Depending on concentration and cell type, they can act as growth regulators as well as inducers of differentiation (Panchision and McKay, 2002). They cause apoptosis in early central nervous system (CNS) precursor cells (Furuta et al., 1997), neuronal differentiation in midgestation CNS precursors (Li et al., 1998; Mehler et al., 2000), and glial differentiation in late embryonic or adult CNS precursors (Gross et al., 1996).
It is unclear how these molecules govern such a diverse pattern of action. The distinct profile of ligand and receptor expression in different regions of the brain at a given stage of development suggests actions specific for the BMP factor and receptor. Yet, it has been difficult to characterize signal transduction cascades that mediate distinct actions.
The BMP ligands are subdivided into groups, based on structural homologies. Thus, BMP2 and 4 belong to the decapentaplegic (dpp) subgroup; BMP5, 6, and 7 to the 60A subgroup; and GDF5 and 6 to yet another one (Kawabata et al., 1998). All BMPs signal via a tetrameric complex of two out of three type II receptors, namely, BMP receptor II (BMPRII), activin receptor type II (ActRII), and ActRIIB; and two out of three BMP type I receptors, namely, Alk 2, 3, and 6 (reviewed in Balemans and Van Hul, 2002). A given ligand can induce the formation of heteromeric complexes between different receptors and a given receptor can recognize different ligands, suggesting a redundancy in signaling (ten Dijke et al., 1994a,b; Rosenzweig et al., 1995; Nishitoh et al., 1996; Ebisawa et al., 1999). BMP7, for example, seems to bind preferentially to Alk2 and Alk6. BMP2 and 4, in contrast, have a stronger affinity to Alk3, but when the BMPRII is coexpressed, BMP7 also binds to Alk3 (ten Dijke et al., 1994a). Intracellularly, type I serine/threonine kinase receptors function as downstream components of type II receptors and phosphorylate Smad proteins. Alk3 and Alk6 phosphorylate Smad 1, 5, and 8. Alk2 was shown to phosphorylate only Smad1 and 5 (Aoki et al., 2001). Phosphorylated Smad proteins then form complexes with CoSmads (Smad4) and translocate into the nucleus. Here, they bind to DNA directly or indirectly via other DNA binding proteins and regulate the transcription of target genes (reviewed in von Bubnoff and Cho, 2001). Furthermore, TGF-β family ligands have been reported to activate mitogen-activated protein kinases of the p38, p46/54 c-Jun NH2 terminal kinase, and p42/p44 extracellular signal-regulated kinase families, but it is controversial whether these responses are direct or specific for such effects as mitogenesis or apoptosis (Hu et al., 1999; Iwasaki et al., 1999; Monzen et al., 1999; Nakamura et al., 1999; Kimura et al., 2000).
Attempts to show functional differences for Alk3 and 6 have failed in a number of studies carried out with overexpression of activated receptors (Kawakami et al., 1996; Zou et al., 1997; Chen et al., 1998a; Enomoto-Iwamoto et al., 1998; Yi et al., 2000; Timmer et al., 2002). However, experiments with overexpression of dominant negative (dn) receptors revealed that dnAlk6 but not dnAlk3 was able to block differentiation in osteoblasts, chondrocytes, or mesenchymal cells (Kawakami et al., 1996; Chen et al., 1998a; Enomoto-Iwamoto et al., 1998). Recently, Alk3 was found to promote proliferation in precursor cells throughout development, whereas apoptosis or differentiation seems to be linked to activation of the Alk6 receptor. It was suggested that the activities of these receptors are linked sequentially with Alk3 acting first and inducing the expression of Alk6 (Panchision et al., 2001).
We have investigated BMP signaling in adult-derived hippocampal progenitor cells (AHPs) by overexpression of dn-BMPRI (dnAlk2, 3, and 6). AHPs have the potential to give rise to neurons, astroglial cells, and oligodendrocytes. Because they have been shown to develop essential properties of functional CNS neurons in vitro (Song et al., 2002) and to integrate well into heterotopic regions after transplantation (Suhonen et al., 1996; Takahashi et al., 1998), they could serve as a cell source for transplantation therapy of Parkinson's disease. The striatum is a gliogenic region, where BMPs as known inducers of astroglial cell fate are highly expressed. Blocking signaling via the Alk6 receptor could be a strategy to manipulate progenitor cells before transplantation. Thus, in addition to addressing the fundamental question of differences and interactions in BMPRI signaling, this study was motivated by its possible implications for the therapeutic applications of AHPs.
MATERIALS AND METHODS
Cell Culture and Viral Infection
AHPs were kindly provided by Dr. F. Gage (Salk Institute, La Jolla, CA). The cells are derived from the adult rat hippocampus (Gage et al., 1995; Palmer et al., 1997). Cells were maintained as described previously (Palmer et al., 1997). Progenitor cells were used between passage 10 and 20. For propagation, cells were cultured in DMEM/Ham's F-12 (DMEM/F-12; 1:1) containing N2 supplements (Invitrogen, Carlsbad, CA), l-glutamine, and 20 ng/ml recombinant human basic fibroblast growth factor (bFGF) (PeproTech, Rocky Hill, NJ). For adenoviral infection and/or differentiation, cells were dissociated to single cells and plated on poly-ornithine (PORN) (Sigma-Aldrich, St. Louis, MO)/laminin (Invitrogen)-coated plates or chamber slides (Nalge Nunc, Naperville, IL) at a cell density of 2.7 × 104 cells/cm2 in medium lacking bFGF (differentiation medium).
For induction of Smad1/5-phosphorylation, human recombinant BMP2 (R&D Systems, Minneapolis, MN) or human recombinant BMP6 (kindly provided by Creative Biomolecules, Boston, MA) were added to the medium for 3 h before cell lysis and Western blotting on day in vitro (DIV)4.
For induction of Smad2-phosphorylation, human recombinant activinA (R&D Systems) or human recombinant TGF-β (R&D Systems) were added to the medium for 3 h before cell lysis and Western blotting on DIV4.
Noggin (R&D Systems) was added to some of the cultures on DIV2 and DIV4.
Adenoviral Vectors and Viral Infection of AHP Culture
The recombinant adenovirus encoding for LacZ (AdLacZ), the hemagglutinin (HA)-tagged constitutively active (ca) as well as dn Alk2, Alk3, and Alk6 receptor were described previously (Saito et al., 1985; Miyake et al., 1996). The adenovirus was propagated in 293 cells to produce high titer stocks. Cells were infected for 3–4 d, harvested, and lysed by five cycles of freeze/thawing. Lysates were centrifuged at 3000 rpm for 20 min at 4°C, and the stocks were kept at –80°C.
Experimental cultures were infected with adenoviral vectors on the second day after plating in differentiation medium (DIV2). Infection efficiency was monitored by staining for β-galactosidase and found to be 90% already at multiplicity of infection (moi) 50. The intensity of the staining per cell increased with higher mois.
Immunocytochemistry
On DIV5 or 6, i.e., 3 or 4 d after infection, the cells were fixed in 4% paraformaldehyde for 15 min at room temperature. After washing with phosphate-buffered saline (PBS), cells were incubated in Tris-buffered saline (TBS)-based blocking buffer containing 0.1% Triton X-100, 3% bovine serum albumin, and 3% normal horse serum for 1 h at room temperature. For detection of surface proteins, Triton X-100 was omitted in the blocking buffer. The cells were incubated with primary antibodies diluted in the respective blocking buffer overnight at 4°C. After three washes with TBS, cells were incubated with anti-rabbit Alexa Fluor 488 (Molecular Probes, Eugene, OR) or anti-mouse Alexa Fluor 594 (Molecular Probes) secondary antibodies at a 1:2000 dilution. For nuclear counterstain, cells were incubated in 50 ng/ml Hoechst 33258 (Sigma-Aldrich) for 20 min before being coverslipped in Dako fluorescent mounting medium (DakoCytomation Denmark A/S, Glostrup, Denmark). The following primary antibodies and dilutions were used: mouse monoclonal antimicrotubule-associated protein (MAP2ab), 1:100 (Sigma-Aldrich); mouse monoclonal antigalactocerebroside (galC), 1:100 (Roche Diagnostics, Mannheim. Germany); and rabbit polyclonal anti-glial fibrillary acidic protein (GFAP), 1:400 (DakoCytomation Denmark A/S).
Microscopic Analysis
Cultures were analyzed by epi-illumination fluorescence microscopy. Color images were generated using a Zeiss fluorescent microscope attached to a Nikon digital camera. Images were processed using Easy Image Analysis 2000 software (Nikon, Tokyo, Japan). For every differentiation marker, a total number of 9000 cells was counted in three different wells in at least five randomly chosen fields of view per well.
Polymerase Chain Reaction (PCR)
Cells were infected at DIV2 and lysed for RNA extraction 6–48 h after the infection. To investigate ligand-induced receptor induction, cells received BMP2 or BMP6 at a concentration of 1, 10, and 50 ng/ml on DIV5 for 16 h and were lysed thereafter.
Total RNA was extracted by the guanidium thiocyanate method of Chomczynski and Sacchi (1987). For cDNA synthesis, random hexamer primers (Roche Diagnostics) were used. First strand cDNAs were synthesized from 1 μg of RNA by using M-MLV reverse transcriptase (Invitrogen). The PCR was carried out using standard protocol with Taq polymerase (Fermentas, Helsingborg, Sweden). The cycling parameters were as follows: denaturation at 94°C for 4 min followed by 25–32 cycles of denaturation at 94°C for 30 s, annealing step for 45 s, and 1 min 30 s at 72°C. The last cycle consisted of a final extension step at 72°C for 6 min. The following oligonucleotide primers were used: Alk 2 sense (s) 5′-TCT GTG CTA ATG ATG GCT CTC C and antisense (as) 5′-TTC TGC GAT CCA GGG AAG GAT TTC; Alk 3 s 5′-GGA GGA ATC GTG GAG GAA TAT and as 5′-CAT ACG CAA AGA ACA GCA TGTC;Alk 6 s 5′-CGG CTG AAT CAC AAC CAT TTG G and as 5′-CTA GAC ATC CAG AGG TGA CAA CAG; BMP 2/4 s 5′ TAT GTG GAC TTC AGT GAT GTG G and as 5′CAG AGT CTG CAC TAT GGC ATG GTT; BMP 5/6/7 s 5′ TGG CAG GAC TGG ATC ATT GCA C and as 5′ ATG GCG TGG TTG GTG GCA TTC AT; actin s 5′-AAG ATG ACC CAG ATC ATG TTT GAG and as 5′-AGG AGG AGC AAT GAT CTT GAT CTT; BMPRII s 5′-GGA GAA ATC AAA AGG GGA C and as 3′-CCT GTC AAC ATT CTG TAT CC; ActRII s 5′-CTG GAT ATC TAG CGA GAA C and as 3′-CAC ATA TTG CCC TCA CAG CA; and ActRIIB s 5′-GAG ACG ATG TCA CGA GGC C and as 3′-TCT CTG GAA GTT GAT GGC TC. All the PCR products were sequenced and thus verified. The number of cycles was chosen in such a way as to select PCR conditions on the linear portion of the reaction curve to avoid “saturation effect.” The samples were run on a 1% agarose gel containing ethidium bromide, and results were analyzed using a FLA 2000 (Fujifilm, Tokyo, Japan) plate reader.
Western Blotting
AHPs were cultured on PORN/laminin-coated 24-well plates at a cell density of 2.7 × 104 cells/cm2 as described and harvested on DIV4 for immunoblotting of p-Smad or on DIV6 for detection of the differentiation markers GFAP and β-III-tubulin. Cells were washed with PBS and lysed on ice in buffer containing 150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 0.5 mM EDTA, 1% NP-40, 0.5% CHAPS, and 0.1% SDS. Phenylmethylsulfonyl fluoride (PMSF) and aprotinin were added each time to a final concentration of 1 mM to inhibit protease activity. Cellular debris was removed by centrifugation. The concentration of each sample was determined by using DC Protein Assay (Bio-Rad, Hercules, CA). Eighty or 120 μg of protein, respectively, was separated on 12% SDS-PAGE and then electroblotted onto a polyvinylidene difluoride membrane by semidry transfer (Bio-Rad). After incubation with 5% bovine serum albumin (for detection of phospho-Smad) or with 5% nonfat skim milk (for differentiation markers) in Tris-buffered saline with 0.1% Tween 20, the membranes were probed with the respective primary antibodies: rabbit polyclonal anti-GFAP, 1:2000 (Dako Cytomation Denmark A/S); mouse monoclonal anti-β-III-tubulin, 1:500 (Sigma-Aldrich); mouse monoclonal anti-actin, 1:500 (Sigma-Aldrich); rabbit-polyclonal anti-phospho-Smad1/5, 1:2000; and rabbit-polyclonal anti-Alk6 (anti-p-Smad1/5 and anti-Alk6 were kindly provided by Dr. Heldin at Ludwig Institute, Uppsala, Sweden). Horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG were used as secondary antibodies at a concentration of 1:5000 (New England Biolabs, Beverly, MA). The rat monoclonal antibody against the HA-tag (3F10; Roche Diagnostics) was directly conjugated with horseradish peroxidase and used at a concentration of 1:500. The signals were detected by enhanced chemiluminescence (New England Biolabs).
In Vitro Kinase Assay
AHPs were cultured as described at a cell density of 2.7 × 104 cells/cm2 and infected on DIV2. Two days after infection, 50 ng/ml BMP6 was added to some of the dnAlk3-infected wells, and 30 min later cells were harvested in a buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 2.5 mM EDTA, 1% glycerol, 100 U/ml Trasylol, 1 mM PMSF, 1 mM dithiothreitol, and 0.1 mM sodium orthovanadate. The samples were mixed with protein A-Sepharose–coupled antibodies against the HA-tag bound to protein A-Sepharose for 3 h at 4°C. Immune complexes were precipitated, and in vitro kinase assays performed according to Mori et al., (1991)). The signals were detected by Image Analyzer (FLA 2000; Fuji, Tokyo, Japan).
Immunoprecipitation for Detection of Smad2 Phosphorylation
AHPs were cultured on PORN/laminin-coated 24-well plates at a cell density of 2.7 × 104 cells/cm2 as described and harvested on DIV4. Cells were washed with PBS and lysed on ice in buffer containing 50 mM Tris, pH 8, 1 mM EDTA, 120 mM NaCl, 0,5% NP-40, 10% glycerol, 1 mM PMSF, and 1 mM aprotinin. The lysates were cleared from insoluble material by centrifugation for 15 min at 15,000 rpm. Sample protein was incubated with an antibody raised against Smad2/3 (sc-6032; Santa Cruz Biotechnology, Santa Cruz, CA) for 3 h at 4°C, followed by precipitation by protein-G-plus agarose (sc-2002; Santa Cruz Biotechnology) overnight at 4°C. The immunoprecipitates were collected via centrifugation for 2 min at 1000 × g, washed four times with immunoprecipitation lysis buffer with PMSF and aprotinin, and boiled in sample buffer. The immunoprecipitates were separated using 12% SDS-PAGE, and Western blotting was performed as described above. Membranes were probed with either rabbit-polyclonal PS2 (1:1000 dilution) raised against phosphorylated Smad2 (Persson et al., 1998) or rabbit-polyclonal SED (1:500) raised against Smad2 (Nakao et al., 1997).
Lactate Dehydrogenase (LDH)-Cytotoxicity Assay
Four days after infection, i.e., on DIV6, cell death was determined by a method based on spectrophotometric measurement of LDH release by using the CytoTox 96 NonRadioactive Cytotoxicity Assay kit (Promega) according to the manufacturer's protocol. For each treatment group, maximal LDH release was determined after addition of Triton-X 100 to a final concentration of 10%. LDH concentration in the medium of three wells of Ad-dnAlk2-, 3-, or 6-infected cultures were expressed as percentages relative to the maximal LDH release in each treatment group and compared with the ratio of Ad-LacZ-infected control.
Statistics
All experiments were replicated at least three times. Overall significance was determined by submitting data to one-way analysis of variance. Significance of between-group differences was determined by Scheffé post hoc test. Differences were considered significant at p ≤ 0.05. * or + p ≤ 0.05; ** or ++ p ≤ 0.01; and *** or +++ p ≤ 0.001.
RESULTS
AHPs Express mRNA for BMP2/4 and BMP5/6/7 as Well as for the Type II Receptors BMPRII, ActRII, and ActRIIB and for the Type I Receptors Alk2, Alk3, and Alk6
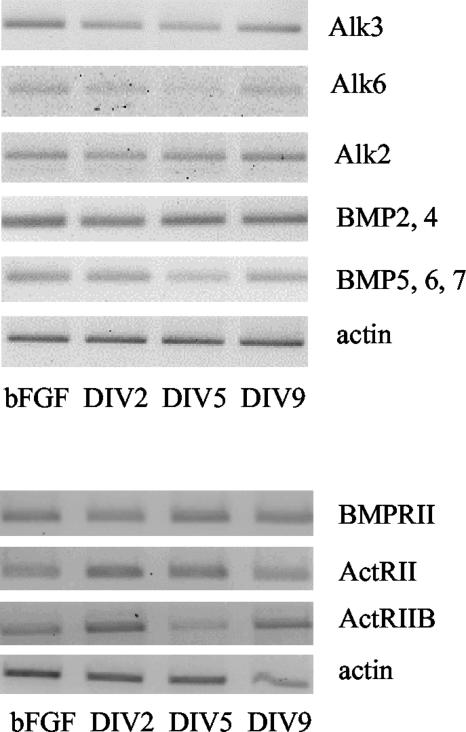
To establish whether BMPs play a significant role for AHPs under physiological conditions, we investigated the expression of BMP receptors and ligands in AHP cultures that were expanded in a bFGF containing medium as well as in cells grown in defined differentiation medium without bFGF for up to 9 d. We found mRNA for BMPRII, ActRII, ActRIIB, Alk2, Alk3, Alk6, BMP2/4, and BMP5/6/7 to be expressed at both early (DIV2) and late time points (DIV9) without any consistent changes over time. No difference in mRNA expression between expansion and differentiation condition could be detected for any of the investigated mRNAs. These results suggest that the BMP system has a functional role in the AHP culture. The expression pattern, however, does not seem to change dependent on different maturation stages or density conditions in the culture (Figure 1).
Figure 1.
mRNA expression of BMP ligands, BMP type I, and BMP type II receptors in AHP culture. Cells were either cultured in proliferation medium and serially passaged (bFGF) or plated on PORN/laminin-coated dishes in differentiation medium without bFGF and monitored over time (DIV2–9).
Adenoviral Vectors Efficiently Infect AHPs
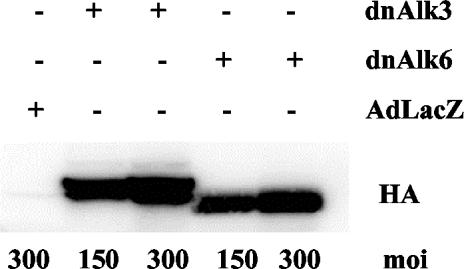
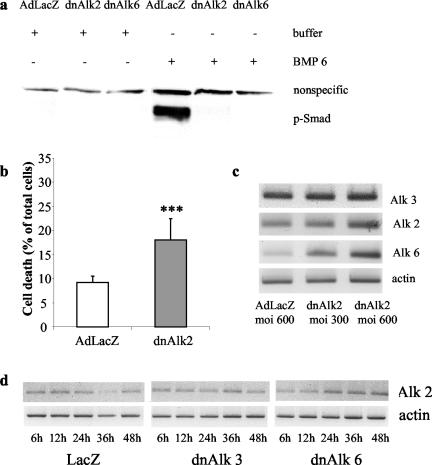
Infection efficiency of adenoviral vectors was monitored by infecting AHPs with adenovirus carrying the LacZ gene (AdLacZ) and consecutive staining for β-gal. Addition of the virus at the second day after plating resulted in positive β-gal staining in 90% of the cells at 48 h after infection at moi of 50 (our unpublished data). Staining intensity increased with increasing mois. Successful infection and exogenous protein expression with adenoviral vectors carrying the genes for the dominant negative BMP receptors, Alk3, and Alk6, was demonstrated by Western blots for the tagged protein HA (Figure 2). HA expression was detectable already 12 h after infection, increased until the second day and could be monitored thereafter for the following 7–10 d (our unpublished data).
Figure 2.
Efficiency of adenoviral infection in AHP culture demonstrated by immunoblotting for HA protein. Cells were cultured in differentiation medium, infected at DIV2 and cultured until DIV6. Eighty micrograms of protein from whole cell lysates was separated by SDS-PAGE and blotted with anti-HA. AdLacZ does not carry any HA-tag.
Overexpression of caAlk3 Results in a Similar Differentiation Pattern as Overexpression of caAlk6
caAlk receptors are characterized by a point mutation in the intracellular domain that leads to a constant phosphorylation of Smad1/5/8 in a ligand-independent manner. When caAlk3 receptor was overexpressed in AHPs for 4–5 d, GFAP-positive cells changed dramatically in number and morphology. Almost no astrocytes had developed at this early time point (DIV4) in Adeno-LacZ–infected control cultures. In caAlk3-expressing cultures, in contrast, numerous flat GFAP-positive cells that morphologically resembled type I astrocytes were found. The overexpression of caAlk3 did not have any detectable effect on number or morphology of MAP2ab-expressing neurons. Overexpression of caAlk6 resulted in a picture almost identical to caAlk3 overexpression. Slight differences in cell morphology could be seen, but attempts to define these differences with the help of different antibodies failed (Figure 3).
Figure 3.
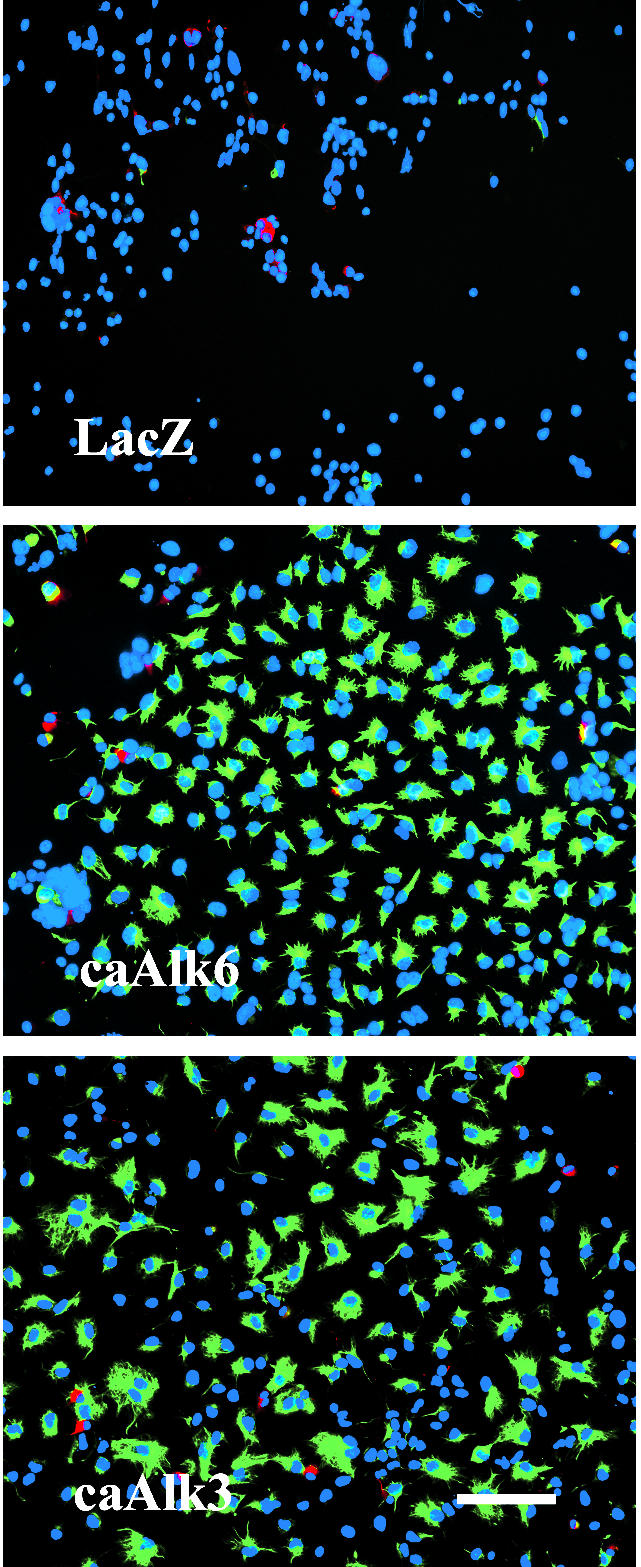
GFAP- and MAP2ab-positive cells after infection of AHPs with adenovirus carrying the sequence for caAlk3 or caAlk6. Cells were infected with adeno-caAlk3 (bottom) or adeno-caAlk6 (middle) (moi 20) on DIV2, fixed with 4% paraformaldehyde on DIV5, and immunocytochemically stained for the astroglial marker GFAP (green) and the neuronal marker MAP2ab (red). Cell nuclei (blue) were counterstained using Hoechst33528. Bar, 100 μm.
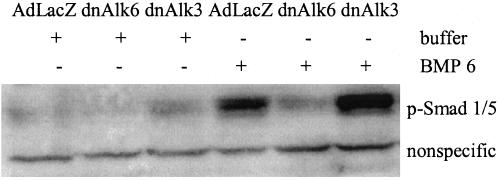
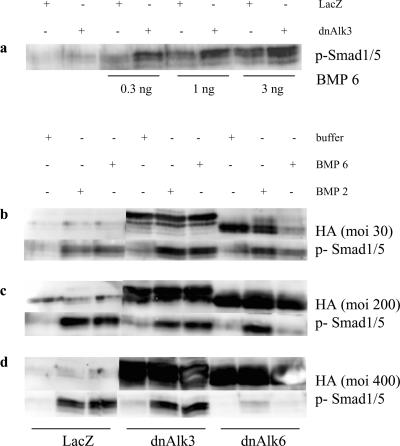
Overexpression of dn Alk6 but Not dnAlk3 Blocks BMP6 Signaling in AHP Cultures
Signaling through the endogenously expressed Alk3 and 6 receptors could be reduced or prevented, respectively, by infecting AHP cultures with adenovirus carrying the gene for the dnAlk6. dnAlks consist of a mutated nonfunctional intracellular receptor kinase domain and a functional extracellular part. Overexpression of dnAlks thus depletes the medium from BMPs that would be available for the endogenous receptor under physiological/uninfected conditions. Blockage of signaling was demonstrated 2 d after infection of AHP cultures. For this purpose, BMP6 was added at 50 ng/ml to the infected cultures for 3 h before lysis. Western blots of those lysates showed strong expression of phosphorylated Smad1/5, the downstream signaling molecule for BMP receptors, in cultures that were infected with AdLacZ (Figure 4). In cultures infected with the dnAlk6, no phosphorylated Smad1/5 could be detected after stimulation with BMP6, indicating that the added ligands were bound to the overexpressed nonfunctional receptor and thus not available for the endogenous Alk3 or Alk6 receptor. The detection system used here demonstrated efficient blockage already at mois of 150 that further increases at mois of 300 (our unpublished data). Overexpression of dnAlk3 at comparable titers, however, did not prevent Smad1/5 phosphorylation after stimulation with BMP6. Judging from the HA protein expression, the amount of expressed receptor was comparable between dnAlk3 and 6 infected cultures (Figure 2), so that we ascribed the difference in functional impairment to a higher affinity of the added ligands toward Alk6. The ligands would thus be able to bind to endogenous Alk6 even under overexpression of Alk3.
Figure 4.
Smad1/5 phosphorylation after infection of AHPs with adenovirus carrying the sequence for dnAlk3 and dnAlk6. Cells were infected (moi 300) at DIV2. At DIV4, 50 ng/ml BMP6 was added to some of the cultures, and cell lysates were collected 3 h later. Eighty micrograms of protein from whole cell lysates was separated on SDS-PAG and blotted with anti-phospho-Smad1/5.
Because of the inability of adenovirus to be stably integrated into the host cell DNA, the ratio of dnAlk to functional endogenous Alk receptors decreased continuously over time (our unpublished data). Due to this fact, we chose DIV2, 3, and 4 after infection as time points for evaluation of direct effects from the mutated receptors, corresponding to maximal impairment of Alk6 signaling judged by p-Smad1/5 Western blotting.
Blockage of Alk3 Signaling Induces the Expression of Alk6
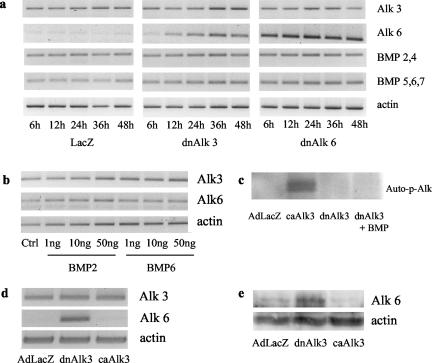
If BMPs or their receptors have an important functional role in AHPs, blockage of receptor signaling can be expected to induce an up-regulation of one or more key molecules of the respective signaling chain. We therefore investigated the expression of mRNA for BMP2/4, BMP5/6/7 and the two closely related BMP type I receptors Alk3 and Alk6 after infection with either dnAlk3, dnAlk6, or AdLacZ. Interestingly, the Alk6 receptor was found to be the molecule undergoing the most obvious dynamic changes under the different culture conditions. Whereas it was only very weakly expressed in control-infected cultures, it increased time dependently after infection with dnAlk3 (Figure 5a). Because BMP2 has been described to induce Alk6 expression in progenitor cultures (Panchision et al., 2001), one can speculate that the Alk6 receptor up-regulation monitored here is the result of an indirect rather than a direct coupling of these two receptors. BMP ligands could be up-regulated first to compensate for the ligand depletion in the medium. The higher amount of BMPs would then induce the expression of the respective receptor. Changes in mRNA expression for BMP ligands belonging to the BMP2/4 could not be detected. The reverse transcription (RT)-PCR for ligands belonging to the BMP5/6/7 group, in contrast, suggests a modest mRNA up-regulation (Figure 5a). The addition of BMP2 or BMP6 at different concentrations did, however, not induce any detectable changes in the mRNA expression of any of the receptors (Figure 5b). The increased expression of mRNA for BMP5/6/7 ligands and for the Alk6 receptor after dnAlk3 infection might thus be independent from each other. It is impossible to investigate possible feedback mechanisms for Alk6 after infection with dnAlk6, because the human Alk6 sequence was not available when we initiated this study and the rat specific primers thus recognize both the endogenous and the viral DNA for Alk6. The stronger band seen in Figure 5a is most likely representing overexpressed DNA after viral infection. Primers for the Alk3 receptor, however, are specific for the endogenous rat sequence, explaining why no stronger signal can be seen for Alk3 after dnAlk3 infection in Figure 5a.
Figure 5.
Induction of the Alk6 receptor in AHP culture. (a) Time-dependent mRNA expression of BMP ligands and type I receptors in AHP culture after infection with dnAlk3 and dnAlk6. AHPs were infected (moi 300) on DIV2, and lysates were harvested at different time points after infection. (b) mRNA expression of the Alk3 and Alk6 receptor in AHP culture after ligand addition. At DIV3, ligands were added and cells harvested 12 h later. (c) Autophosphorylation of BMP receptors, demonstrating inactivity of dnAlk3 receptor kinase. Cells were infected (moi 300) on DIV2. BMP6 was added to some of the infected wells on DIV4, and 30 min later cells were harvested. Lysates were immunoprecipitated with anti-HA antibodies, in vitro kinase assay was performed, and the signal was detected by Image Analyser. (d) mRNA expression of Alk3 and Alk6 after infection with adenovirus carrying the sequence for dnAlk3 or constitutively active Alk3 (caAlk3). Cells were infected (moi 300) on DIV2, and lysates were harvested 24 h later. (e) Protein expression of Alk6 after infection with adenovirus carrying the sequence for dnAlk3 or constitutively active Alk3 (caAlk3). Cells were infected (moi 300) on DIV2, and lysates were harvested 48 h later. One hundred twenty micrograms of protein from whole cell lysates was separated by SDS-PAGE and blotted with anti-Alk6 and actin.
Induction of Alk6 has been described to occur downstream of the activated Alk3 (Panchision et al., 2001). We were therefore surprised to find it to be induced in the opposite situation after Alk3 receptor blockage. To prove the inactivity of the dnAlk3 receptor kinase, in vitro kinase assays were carried out. Receptors were immunoprecipitated with an antibody against HA and the incorporation of 32P-labeled γ-ATP was detected. Cell lysates from cultures overexpressing an HA-tagged caAlk3 receptor served as a positive control. A negative control was included from cultures overexpressing LacZ that is not HA tagged. As shown in Figure 5c, BMP stimulated receptors from cultures overexpressing HA-tagged dnAlk3 did not incorporate 32P-labeled γ-ATP. We furthermore monitored the mRNA expression of Alk6 at 36 h after viral mediated overexpression of the caAlk3 receptor. Contrary to what has been described previously, we did not find any induction of Alk6 mRNA expression (Figure 5d). A Western blot for the Alk6 receptor confirmed our RT-PCR data and showed translation of the Alk6 mRNA into protein (Figure 5e).
Receptor Binding Patterns Differ for BMP2 and BMP6
The increase in Alk6 receptor expression observed after dnAlk3 overexpression explains why our Western blots, displayed in Figure 4 and Figure 6a, showed a stronger band for p-Smad1/5 in dnAlk3-overexpressing cells compared with the AdLacZ-infected control cultures. The added BMP6 ligands indeed seem to have a strong affinity for the Alk6 receptor, therefore being able to bypass the blocked pathway and find newly induced receptors available to transduce their signal.
Figure 6.
Smad1/5 phosphorylation induced by BMP6 and BMP2 after infection of AHPs with adenovirus carrying the sequence for dnAlk3 or dnAlk6. (a) Cells were infected with adenovirus carrying the sequence for LacZ or dnAlk3 (moi 300) at DIV2. At DIV4, different concentrations of BMP6 were added, and cell lysates were collected 3 h later. Eighty micrograms of protein from whole cell lysates was separated by SDS-PAGE and blotted with anti-phospho-Smad1/5. (b–d) Cells were infected with different amount of adenovirus (b, moi 50; c, moi 200; and d, moi 400) carrying the sequence for LacZ, dnAlk3, or dnAlk6 at DIV2. At DIV4, 50 ng/ml BMP6 or BMP2 was added, and cell lysates were collected 3 h later and processed as described for a.
Stimulation with BMP2, however, resulted in a slightly different picture (Figure 6, b–d). Whereas a very low titer of dnAlk6 (moi 30) was enough to efficiently reduce Smad1/5-phosphorylation after addition of BMP6 (Figure 6b), BMP2-induced phosphorylation of Smad1/5 was unimpaired at titers up to moi 200 (Figure 6c). Most likely, BMP2 transduced its signal via the Alk3 receptor in this situation. At high titer of moi 400, however, the BMP2 induced Smad1/5-signal was reduced as well, suggesting a certain affinity of BMP2 also for the Alk6 receptor (Figure 6d). In summary, one can conclude that compared with BMP6, the affinity of BMP2 is higher for the Alk3 receptor and lower for the Alk6 receptor.
Blockage Obtained by dnAlk Receptors Is Specific for BMP Signaling
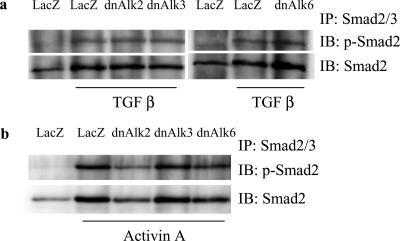
So far, BMPs are the only growth factors known to bind to and signal via the BMP type I receptors Alk2, 3, and 6. Type II receptors, however, are shared with activins, another group of TGF-β family member proteins (Balemans and Van Hul, 2002). Overexpression of dnBMP type I receptors might engage ActRII or ActRIIa into the nonfunctional complex and thus indirectly impair activin signaling. To investigate whether pathways of other growth factors within the TGF-β family were influenced by overexpression of dnAlk2, 3, or 6, we monitored the phosphorylation of their downstream signal mediator Smad2 after stimulation with the respective growth factor. Neither TGF-β nor activin-induced Smad2 phosphorylation seemed to be affected by overexpression of the dn receptors (Figure 7). We thus consider changes occurring in the infected cultures to be specific for the BMP signaling pathway.
Figure 7.
Smad2 phosphorylation induced by TGF-β or activinA after infection of AHPs with adenovirus carrying the sequence for dnAlk2, dnAlk3, or dnAlk6. Cells were infected (moi 300) at DIV2. At DIV4, growth factors were added to some of the cultures and cell lysates were collected 3 h later. Lysates were immunoprecipitated with anti-Smad2/3 antibodies, separated by SDS-PAGE, and blotted with anti-Smad2 and anti-pSmad2. (a) Result after stimulation with 50 ng/ml TGF-β. (b) Result after stimulation with 50 ng/ml activin A.
Overexpression of dnAlk6 Transiently Reduces GFAP Expression While Increasing the Number of Oligodendrocytes in Cultures of AHPs
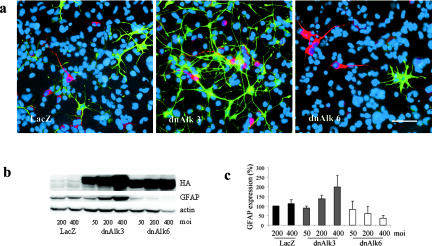
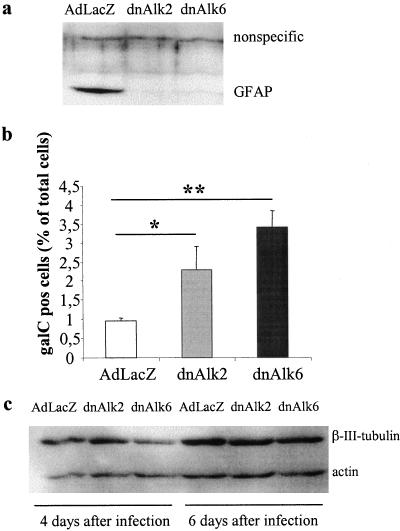
In the experiments described above, stimulation of the AHP culture with exogenously added ligand was necessary to increase the amount of p-Smad above the sensitivity threshold for detection by Western blotting. Smadsignaling far below that threshold seems yet to be present and functionally important in AHP cultures grown without addition of ligand. Serially passaged AHPs are a population of nestin-positive neural progenitors when grown in medium containing bFGF. However, upon the differentiating condition used in our experiments (deprivation of bFGF from the medium), they undergo differentiation to neurons, astroglia and oligodendrocytes. Overexpression of dnAlk6 decreased the amount of GFAP in the culture at a time point consistent with high expression levels of dominant negative receptors. The decrease in comparison to Adeno-LacZ–infected control cultures was most obvious around DIV4 after infection. Results obtained by immunocytochemistry (Figure 8a) were confirmed by densitometric analysis of Western blots: GFAP signals were normalized to the levels of actin present in the same sample and expressed as percent of the control-sample infected with LacZ (Figure 8, b and c). A virus titer-dependent reduction of GFAP after infection with dnAlk6 was measured. Furthermore, consistent with the induction of the Alk6 receptor after infection with Adeno-dnAlk3, the GFAP signal detected by Western blotting was dose dependently increased in the corresponding samples. Immunocytochemistry revealed more GFAP-positive cells compared with LacZ-infected control cultures. Astrocytes in the dnAlk3-infected cultures displayed a fibrous shape with long processes. This morphology precluded accurate quantification of GFAP-positive cell numbers.
Figure 8.
Expression of differentiation markers after infection of AHPs with adenovirus carrying the sequence for dnAlk3 or dnAlk6. (a) GFAP- and MAP2ab-positive cells. Cells were infected with adeno-LacZ (left), adeno-dnAlk3 (middle) or adeno-dnAlk6 (right) (moi 300) on DIV2, fixed with 4% paraformaldehyde on DIV5, and immunocytochemically stained for the glial marker GFAP (green) and the neuronal marker MAP2ab (red). Cell nuclei (blue) were counterstained using Hoechst33528. Bar, 50 μm. (b) Dose-dependent GFAP expression. Cells were infected at different mois on DIV2, and cell lysates were harvested on DIV4. Eighty micrograms of protein were separated by SDS-PAGE, and immunoblotting was done using anti-GFAP antibodies. (c) Semiquantitation of GFAP expression standardized against actin expression. Bars represent mean values of three different experiments ± SD.
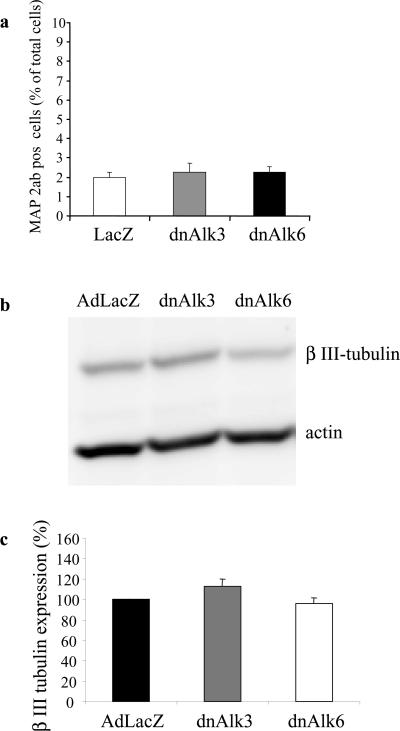
No significant changes in the number of neurons assessed by counting MAP2ab-positive cells were noticed at this early time point (Figures 8a and 9a). These results were confirmed by densitometric analysis of Western blots for expression of the neuronal marker β-tubulin normalized to actin-levels as described above (Figure 9b and c).
Figure 9.
Expression of neuronal marker after infection of AHPs with adenovirus carrying the sequence for dnAlk3 or dnAlk6. (a) Cell counts of immunocytochemically stained MAP2ab-positive cells as percentage of total cells. Cells were infected (moi 300) on DIV2 and fixed with 4% paraformaldehyde on DIV5. A total number of 9000 cells was counted in three different wells in at least five randomly chosen fields of view per well. (b) Expression of β-III-tubulin. Cells were infected (moi 300) on DIV2, and cell lysates were harvested on DIV6. Eighty micrograms of protein was separated by SDS-PAGE, and immunoblotting was done using anti-β-III-tubulin antibodies. (c) Semiquantitation of β-III-tubulin expression standardized against actin expression. Bars represent mean values of three different experiments ± SD.
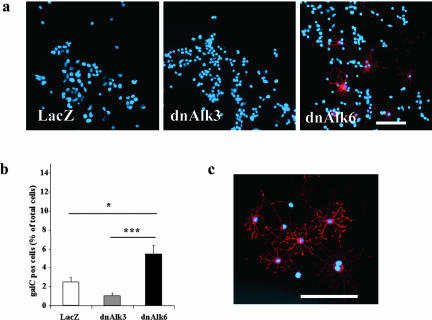
Oligodendrocytes were found to be significantly increased in cultures infected with dnAlk6. Results from cell counts after immunocytochemistry for the mature oligodendrocyte marker galC are displayed in Figure 10. In spite of consistently lower counts of galC-positive cells in cultures overexpressing dnAlk3, the numbers failed to reach statistical significance compared with LacZ-infected controls.
Figure 10.
Oligodendrocytes after infection of AHPs with adenovirus carrying the sequence for dnAlk3 or dnAlk6. Cells were infected (moi 300) on DIV2, fixed with 4% paraformaldehyde on DIV5, and immunocytochemically stained for galC (red). Cell nuclei (blue) were counterstained using Hoechst33528. (a) GalC expression in AHP culture. Bar, 100 μm. (b) Number of galC-positive cells as percentage of total cells in AHP culture. A total number of 9000 cells was counted in three different wells in at least five randomly chosen fields of view per well. Values represent means ± SD; *p < 0.05, ***p < 0.001; analysis of variance post hoc Scheffé. (c) Morphology of galC-positive oligodendrocytes in dnAlk6-overexpressing AHP culture. Bar, 100 μm.
Survival of a Subpopulation of AHPs Is Mediated via the Alk6 Receptor
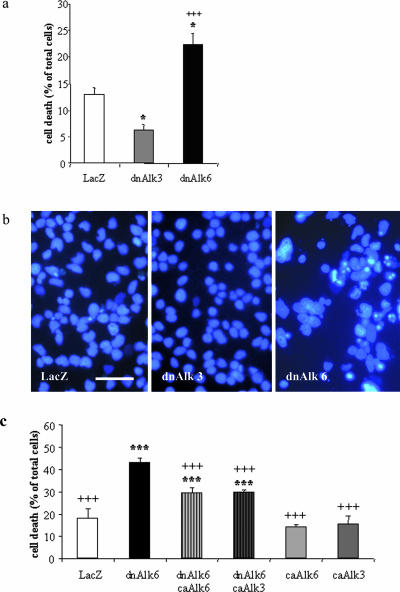
It was obvious from just monitoring the cells under the microscope in the phase-contrast view over time that a significant number of cells was dying around DIV4 after infection with dnAlk6. Nuclear staining with Hoechst done on DIV4 after infection showed many apoptotic nuclei in dnAlk6-overexpressing cultures (Figure 11b). Cell death was quantified by LDH measurements (Figure 11a). Interestingly, cell death was reduced after induction of the endogenous Alk6 receptor via dnAlk3 and increased after its blockage via dnAlk6. To show that the increased expression of Alk6 after dnAlk3 overexpression has an important functional role for survival, we measured cell death in cultures infected with adenovirus carrying the caAlk6 receptor. In support of our hypothesis, the overexpression of caAlk6 partially rescued cells in dnAlk6-overexpressing cultures. The caAlk3 receptor also had a positive effect on cell survival in these cultures (Figure 11c). Thus, both type I receptors are able to mediate cell survival when activated. The cellular context in AHP culture does, however, determine that BMP ligands and/or type II receptors preferentially activate the Alk6 receptor.
Figure 11.
Cell death after infection of AHPs with adenovirus carrying the sequence for dnAlk3 or dnAlk6. (a) Cell death as percentage of total cells determined by quantification of LDH release. Cells were infected (moi 300) on DIV2. On DIV6, cell death was determined by spectrophotometric measurement of LDH release by using the CytoTox 96 NonRadioactive Cytotoxicity Assay kit (Promega). Values represent means ± SD; *p < 0.05 in comparison with control; +++p < 0.001 in comparison with dnAlk3; analysis of variance post hoc Scheffé. (b) Morphology of cell nuclei after Hoechst33528 staining. Cells were infected on DIV2 and fixed with 4% paraformaldehyde on DIV5. Bar, 40 μm. (c) Decrease of cell death after overexpression of caAlk3 and caAlk6 together with dnAlk6. Cells were coinfected with adenovirus carrying the sequence for dnAlk6 (moi 300) and caAlk3 (moi 20) or caAlk6 (moi 20). On DIV6, cell death was determined as described for a. Values represent means ± SD; ***p < 0.001 in comparison with LacZ control; +++p < 0.001 in comparison with dnAlk6; analysis of variance post hoc Scheffé.
Overexpression of dnAlk2 Results in a Picture That Is Similar to Overexpression of dnAlk6
The specificity of the intracellular signals by type I receptors is determined by a specific region in the serine/threonin kinase domain, termed the L45 loop (Feng and Derynck, 1997; Persson et al., 1997). The structures of the L45 loop of Alk3 and Alk6 are identical to each other (Chen et al., 1998b; Chen and Massague, 1999), and it has therefore been the focus of many studies to show differences and/or similarities between these two type I receptors. The L45 loop of the third type I receptor, Alk2, is most divergent from the other TGF-β and BMP type I receptors (Chen and Massague, 1999). Yet, Alk2 has been shown to transduce BMP signals via phosphorylation of the same Smad-molecules as Alk3 and 6. To make the picture of feedback mechanisms and biological effects after BMP signal impairment more complete, we have repeated the investigations described above after overexpression of dnAlk2.
The results were most similar to those obtained by overexpressing dn Alk6, suggesting similar binding affinities for BMP ligands for the Alk2 and the Alk6 receptor. Thus, Smad1/5-phosphorylation was absent in cultures stimulated with BMP6 and infected with adenovirus carrying the sequence for dnAlk2 (Figure 12a), suggesting that the ligands were trapped by the overexpressed nonfunctional receptors. The absence of signaling is reflected in increase in cell death (Figure 12b).
Figure 12.
Effects of adenoviral-mediated overexpression of dnAlk2 in AHP culture. Cells were infected (moi 300) at DIV2. (a) Smad1/5 phosphorylation in AHP culture after infection with adenovirus carrying the sequence for dnAlk2 and dnAlk6. The assay was carried out as described for Figure 4. (b) Cell death in AHP culture after infection with adenovirus carrying the sequence for dnAlk2. The assay was carried out as described for Figure 12. Values represent means + SD; ***p < 0.001 in comparison with control (ctrl); analysis of variance post hoc Scheffé. (c) moi-dependent expression of mRNA for Alk2, 3, and 6 after infection with adenovirus carrying the sequence for dnAlk2. Cells were infected on DIV2, and lysates were harvested 24 h later. (d) Time-dependent expression of mRNA for Alk2 after infection with adenovirus carrying the sequence for dnAlk3 or dnAlk6. AHPs were infected (moi 300) on DIV2, and lysates were harvested at different time points after infection.
Interestingly, PCR analysis of cultures infected with dnAlk2 revealed a dose-dependent up-regulation of the Alk6 receptor (Figure 12c), whereas the Alk2 receptor mRNA expression did not change after infection with dnAlk2, dnAlk3, or dnAlk6, respectively (Figure 12d). The up-regulated Alk6 receptor remains, however, inactive after dnAlk2 infection, because ligands are bound to the overexpressed dnAlk2 receptor and thus not available to stimulate the Alk6 receptor. The affinity of the up-regulated Alk6 receptor to endogenous BMP ligands is not high enough to easily overcome the binding strength between the dnAlk2 receptor and the ligands. Therefore, dnAlk2 infected cultures showed a decrease in GFAP expression (Figure 13a) and an increased number of oligodendrocytes compared with control cultures infected with AdLacZ (Figure 13b). The expression of β-tubulin was not significantly affected (Figure 13c). Cultures infected with dnAlk6 were included to make comparison between these conditions feasible.
Figure 13.
Expression of differentiation markers after infection of AHPs with adenovirus carrying the sequence for dnAlk2 or dnAlk6. (a) Expression of the glial marker GFAP. Cells were infected (moi 300) on DIV2, and cell lysates were harvested on DIV4. Eighty micrograms of protein was separated by SDS-PAGE, and immunoblotting was done using anti-GFAP antibodies. (b) Number of oligodendrocytes. The assay was carried out as described for Figure 12. Values represent means ± SD; *p < 0.05, **p < 0.01; analysis of variance post hoc Scheffé. (c) Expression of β-III-tubulin. Cells were infected (moi 300) on DIV2, and cell lysates were harvested on DIV6, i.e., 4 d after infection, and on DIV8, i.e., 6 d after infection.
The BMP Antagonist Noggin Mimics Effects Obtained by Overexpression of dnAlk2 and dnAlk6
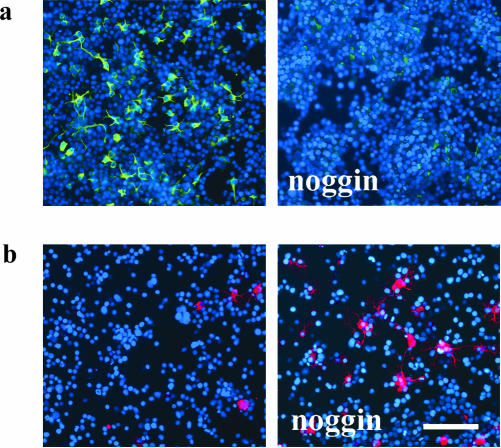
Noggin is one of many known polypeptides that bind to BMPs and consequently prevent their activation of BMP receptors (reviewed in Wilson and Hemmati-Brivanlou, 1997). Addition of 600 ng/ml noggin to the culture medium of AHP cells on DIV2 increased the number of galC-positive oligodendrocytes while decreasing GFAP expression (Figure 14). It thus confirmed our hypothesis that changes observed in cultures overexpressing dnAlk2 or dnAlk6 were due to BMP ligand depletion. In line with results described above, no change in the number of MAP2ab-positive neurons was observed. In contrast to experiments with dnAlk2 or Alk6, however, survival of AHP was not impaired in noggin treated cultures (our unpublished data). One has to consider, though, that noggin is likely to be unstable in culture and thus most likely does not deplete the medium from ligands as efficiently as the continuously synthesized dnAlk receptors.
Figure 14.
Expression of differentiation markers after addition of noggin in AHP culture. Noggin was added to the AHP culture medium at a concentration of 600 ng/ml on DIV2 and DIV4. On DIV6, cells were fixed in 4% paraformaldehyde and immunocytochemically stained for differentiation markers. Cell nuclei (blue) were counterstained using Hoechst33528. Bar, 100 μm. (a) Expression of the glial marker GFAP (green). (b) Expression of galC, representing mature oligodendrocytes (red).
DISCUSSION
We have shown here that endogenously produced BMPs play a significant role for cell survival and the astroglial differentiation of glial precursors in AHP culture. In contrast, they suppress the number of differentiated oligodendrocytes. No correlation between neuronal development and the functionality of the BMP signaling system could be shown. Experiments with constitutively active Alk3 and Alk6 receptors indicated that both type I receptors have the potential to induce any of the described effects. Overexpression of the corresponding dominant negative receptors did, however, suggest that differences in the affinity of the respective type I receptor toward BMP ligands as well as differential receptor regulation determine a specific role for each receptor in the process of differentiation and survival. In AHP culture, it is the Alk6 receptor that undergoes the most dynamic changes in response to diminished BMP signaling and that has a high affinity to BMP ligands important for survival.
BMPs are morphogens and their effects are thus highly dependent on the ligand's concentration or on the level of receptor activation. For activin, a morphogen closely related to BMPs, it has been shown that differences in occupancy of the activin receptor lead to activation of different genes (Dyson and Gurdon, 1998). The overexpression of constitutively active BMP receptors induces a strong and permanent signal that is likely to override intracellular pathways active at physiological ligand concentrations. The overexpression of dominant negative receptors, in contrast, makes it possible to modify ligand concentrations at a more physiological range. The extracellular part of the receptor functions as a trap for ligands and depletes them from the medium. The overexpressed receptor might also deplete the cell membrane from type II receptors by recruiting them into a tetrameric complex. Binding patterns of different BMP ligands toward different type I receptors are known to overlap and type II receptors are shared between Alk2, 3, and 6. One therefore has to consider, that the overexpression of one of the dn type I receptors might indirectly impair signaling via the others. Results obtained in this system have therefore to be interpreted carefully.
Our experiments show striking similarities between the Alk2 and the Alk6 receptor. Addition of external ligand suggests that both of them bind BMP6 more efficiently than BMP2. This is in line with earlier reports of experiments done in other systems/with other cell types (ten Dijke et al., 1994a). Overexpression of both dnAlk2 and dnAlk6 impairs smad1/5 phosphorylation in our culture system and interferes with cell survival. This demonstrates that both receptors are capable of engaging molecules important for survival into a nonfunctional receptor complex. In contrast, the ability of Alk3 to recruit these molecules seems comparably weak. Even after overexpression of dnAlk3, these molecules are available for the functional Alk2 and/or Alk6 receptor and therefore cell survival is not impaired. One can thus hypothesize that Alk2 and Alk6 due to their higher affinity toward signaling partners are the predominant signal mediators under physiological conditions. Interestingly, those two receptors seem to be regulated differently. Whereas the Alk2 receptor mRNA expression remained stable under various culture conditions, levels of Alk6 receptor mRNA expression changed. An increase of Alk6 mRNA was seen after overexpression of dnAlk2 as well as after overexpression of dnAlk3. The up-regulation after dnAlk2 expression remained without biological effect, indicating that the Alk6 receptor did not manage to recruit binding partners trapped in the dnAlk2 receptor complex. The up-regulation of Alk6 after dnAlk3 expression, in contrast, resulted in strong Smad1/5 phosphorylation, increased cell survival as well as increased GFAP expression. This is the exact mirror image to the opposite scenario of impaired Alk6 signaling after dnAlk6 overexpression and thus confirms the important role of the Alk6 receptor for survival and GFAP expression in AHP culture.
The mechanism that leads to up-regulation of the Alk6 receptor remains unclear. Judging from dnAlk2- and -3–overexpressing cultures, the mRNA for the Alk6 receptor increases when BMP signaling is impaired. The addition of noggin to the medium, however, did not have any effect on receptor mRNA expression. This might be due to a less efficient signaling blockage. Alternatively, receptor up-regulation could be dependent on a specific balance between different types of BMP receptors and different BMP ligands. Because noggin binds BMP2, 4, 5, 6, and 7 with about the same affinity, cell culture conditions might not be comparable between dn-overexpressing and noggin-containing cultures. Interestingly, Panchision et al. (2001) described an up-regulation of Alk6 in the opposite scenario, i.e., increased BMP signaling. According to the model suggested in their article, the Alk6 receptor lies downstream of Alk3 and is induced upon increased Alk3 signaling as well as after stimulation of the cells with BMP2. Neither overexpression of a constitutively active Alk3 receptor nor addition of BMPs to our culture system changed Alk6 mRNA expression. These discrepancies might be due to the different cell types used. Panchision et al. (2001)base their model on embryonic cells and suggest that it is applicable to cells that switch from proliferation to differentiation. The AHPs used here differ in their intrinsic properties from embryonic cells and have a much more limited capacity to proliferate. In fact, they undergo differentiation and need BMP signaling in this process for survival. Panchision et al. (2001)discuss that the spectrum of mechanisms to regulate BMP receptor expression is broader than suggested by their model. Down-regulation of the BMP receptor by ligand has, for example, been described previously (Lecuit and Cohen, 1998; Entchev et al., 2000; Teleman and Cohen, 2000; Gurdon and Bourillot, 2001). Our finding that Alk6 has a higher capacity than Alk3 to engage important signaling molecules is in line with Panchision's hypothesis of Alk6 acting downstream of Alk3. Whether the up-regulation is due to a receptor-receptor interaction or indirectly mediated via other partners in this complex signaling system will be the focus of future studies.
The influence of BMPs on cell fate in neural progenitor culture has been investigated previously (Zhu et al., 1999; Lillien and Raphael, 2000; Mehler et al., 2000; Nakashima et al., 2001; Sauvageot and Stiles, 2002). It is believed that BMPs induce neuronal cell fate in early and astroglial cell fate in late progenitors (Mehler et al., 2000; Sauvageot and Stiles, 2002). In adult subventricular zone stem cells, BMPs inhibit neurogenesis (Lim et al., 2000). In AHP cultures, in contrast, BMPs seem to exert their gliogenic effect without being antineurogenic. Neither β-III-tubulin expression nor the number of MAP2ab-positive cells showed any stable differences between control and any of the dn receptor-overexpressing cultures. Neuronal development, however, is known to be a relatively slow process. Short-term monitoring of our cultures was dictated by unstable transgene expression. We can thus not totally exclude that an increase in neurons might have become obvious at later time points.
Western blotting and immunostaining of cultures in which BMP6-induced Smad1/5 signaling was blocked (overexpressing dnAlk2 or dnAlk6) showed a lack of GFAP. Because it has been described that the Alk6 receptor induces differentiation, one possible interpretation was that the block in BMP signaling kept the cells cycling. Results from proliferation assays obtained by incorporation of [3H]thymidine into the DNA of AHP cells, however, did not confirm this hypothesis (our unpublished data). Furthermore, other differentiation markers were unchanged (MAP2ab) or increased (galC). The disappearance of GFAP was paralleled by a larger number of oligodendrocytes in comparison with control culture. It is therefore likely that BMPs under normal AHP culture condition suppress oligodendroglia development while promoting astroglial fate. This hypothesis is in line with previous studies (Gross et al., 1996; Mabie et al., 1997; Mehler et al., 2000). Grinspan et al. (2000) showed in forebrain culture that BMPs instruct an oligodendrocyte preprogenitor to adopt astroglial cell fate. The significant increase in cell death in cultures with diminished BMP signaling could further account for the strong decrease of GFAP expression. Unfortunately, cell fragments around the apoptotic nuclei did not stain positive for any differentiation marker, so that we cannot specify which type of cells are dying. The fact that the addition of noggin reduced GFAP expression without inducing cell death shows that cell death and reduced GFAP expression are not necessarily interrelated. They might, however, represent different points on the same scale: a very weak BMP signal could ensure survival, a slightly stronger induce GFAP expression.
Results obtained in this study clearly do not encourage adenoviral vector-mediated overexpression of the dominant negative Alk6 receptor in combination with AHP transplants. The goal of increasing neurogenesis could not be reached. Whether a decrease in glial cells and/or GFAP expression is favorable in transplantation or not is still controversial. Recently, astrocytes have been in the center of attention as inducers of neurogenesis (Seri et al., 2001; Song et al., 2002). Another problematic issue is the increased cell death that results from overexpression of dnAlk6.
In conclusion, endogenously produced BMPs were shown to be indispensable for suppression of oligodendrocytic cell fate, astroglial differentiation and cell survival in the AHP culture. None of the dn receptors induced an increase in neurogenesis, voting against the application of dnBMP receptors in combination with transplantation of AHPs. Differences exist in the affinity of the three type I receptors toward BMP ligands. Compared with Alk3, Alk6 and Alk2 have a higher ability to engage molecules important for survival and glial differentiation. The Alk6 receptor is the only out of the three type I receptors that undergoes dynamic changes in response to impaired BMP signaling. This, together with the observation that the level of GFAP in- and decreases in parallel to the expression of functional Alk6 receptor, suggests that the Alk6 receptor is the main mediator of glial cell differentiation in AHP culture.
Acknowledgments
We thank Dr. P. Kaplan at the Creative Biomolecules, now known as Curis, for generous gift of recombinant BMP6; K. Gabrielson for technical assistance; and W. Wang for sequence verification of PCR products. This work was supported by grants from the Swedish Research Council, Cancerfonden, Barncancerfonden, The Inga and Arne Lundberg Foundation, The Swedish Foundation for International Cooperation in Research and Higher Education (STINT), and the Swedish Institute. A.B. was partly supported by the Boehringer Ingelheim Foundation. A.Z. was partly supported by the Socrates Erasmus Programme. R.F. was partly supported by Adlerbertska Hospitiefonden.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0584. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0584.
References
- Aoki, H., Fujii, M., Imamura, T., Yagi, K., Takehara, K., Kato, M., and Miyazono, K. (2001). Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J. Cell Sci. 114, 1483-1489. [DOI] [PubMed] [Google Scholar]
- Balemans, W., and Van Hul, W. (2002). Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev. Biol. 250, 231-250. [PubMed] [Google Scholar]
- Chen, D., Ji, X., Harris, M.A., Feng, J.Q., Karsenty, G., Celeste, A.J., Rosen, V., Mundy, G.R., and Harris, S.E. (1998a). Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J. Cell Biol. 142, 295-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.G., Hata, A., Lo, R.S., Wotton, D., Shi, Y., Pavletich, N., and Massague, J. (1998b). Determinants of specificity in TGF-beta signal transduction. Genes Dev. 12, 2144-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.G., and Massague, J. (1999). Smad1 recognition and activation by the ALK1 group of transforming growth factor-beta family receptors. J. Biol. Chem. 274, 3672-3677. [DOI] [PubMed] [Google Scholar]
- Chomczynski, P., and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156-159. [DOI] [PubMed] [Google Scholar]
- Dyson, S., and Gurdon, J.B. (1998). The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell 93, 557-568. [DOI] [PubMed] [Google Scholar]
- Ebisawa, T., Tada, K., Kitajima, I., Tojo, K., Sampath, T.K., Kawabata, M., Miyazono, K., and Imamura, T. (1999). Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 112, 3519-3527. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto, M., Iwamoto, M., Mukudai, Y., Kawakami, Y., Nohno, T., Higuchi, Y., Takemoto, S., Ohuchi, H., Noji, S., and Kurisu, K. (1998). Bone morphogenetic protein signaling is required for maintenance of differentiated phenotype, control of proliferation, and hypertrophy in chondrocytes. J. Cell Biol. 140, 409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entchev, E.V., Schwabedissen, A., and Gonzalez-Gaitan, M. (2000). Gradient formation of the TGF-beta homolog Dpp. Cell 103, 981-991. [DOI] [PubMed] [Google Scholar]
- Feng, X.H., and Derynck, R. (1997). A kinase subdomain of transforming growth factor-beta (TGF-beta) type I receptor determines the TGF-beta intracellular signaling specificity. EMBO J. 16, 3912-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta, Y., Piston, D.W., and Hogan, B.L. (1997). Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124, 2203-2212. [DOI] [PubMed] [Google Scholar]
- Gage, F.H., Coates, P.W., Palmer, T.D., Kuhn, H.G., Fisher, L.J., Suhonen, J.O., Peterson, D.A., Suhr, S.T., and Ray, J. (1995). Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Proc. Natl. Acad. Sci. USA 92, 11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspan, J.B., Edell, E., Carpio, D.F., Beesley, J.S., Lavy, L., Pleasure, D., and Golden, J.A. (2000). Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J. Neurobiol. 43, 1-17. [PubMed] [Google Scholar]
- Gross, R.E., Mehler, M.F., Mabie, P.C., Zang, Z., Santschi, L., and Kessler, J.A. (1996). Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17, 595-606. [DOI] [PubMed] [Google Scholar]
- Gurdon, J.B., and Bourillot, P.Y. (2001). Morphogen gradient interpretation. Nature 413, 797-803. [DOI] [PubMed] [Google Scholar]
- Hu, P.P., Shen, X., Huang, D., Liu, Y., Counter, C., and Wang, X.F. (1999). The MEK pathway is required for stimulation of p21(WAF1/CIP1) by transforming growth factor-beta. J. Biol. Chem. 274, 35381-35387. [DOI] [PubMed] [Google Scholar]
- Iwasaki, S., Iguchi, M., Watanabe, K., Hoshino, R., Tsujimoto, M., and Kohno, M. (1999). Specific activation of the p38 mitogen-activated protein kinase signaling pathway and induction of neurite outgrowth in PC12 cells by bone morphogenetic protein-2. J. Biol. Chem. 274, 26503-26510. [DOI] [PubMed] [Google Scholar]
- Kawabata, M., Imamura, T., and Miyazono, K. (1998). Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 9, 49-61. [DOI] [PubMed] [Google Scholar]
- Kawakami, Y., Ishikawa, T., Shimabara, M., Tanda, N., Enomoto-Iwamoto, M., Iwamoto, M., Kuwana, T., Ueki, A., Noji, S., and Nohno, T. (1996). BMP signaling during bone pattern determination in the developing limb. Development 122, 3557-3566. [DOI] [PubMed] [Google Scholar]
- Kimura, N., Matsuo, R., Shibuya, H., Nakashima, K., and Taga, T. (2000). BMP2-induced apoptosis is mediated by activation of the TAK1–p38 kinase pathway that is negatively regulated by Smad6. J. Biol. Chem. 275, 17647-17652. [DOI] [PubMed] [Google Scholar]
- Lecuit, T., and Cohen, S.M. (1998). Dpp receptor levels contribute to shaping the Dpp morphogen gradient in the Drosophila wing imaginal disc. Development 125, 4901-4907. [DOI] [PubMed] [Google Scholar]
- Li, W., Cogswell, C.A., and LoTurco, J.J. (1998). Neuronal differentiation of precursors in the neocortical ventricular zone is triggered by BMP. J. Neurosci. 18, 8853-8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillien, L., and Raphael, H. (2000). BMP and FGF regulate the development of EGF-responsive neural progenitor cells. Development 127, 4993-5005. [DOI] [PubMed] [Google Scholar]
- Lim, D.A., Tramontin, A.D., Trevejo, J.M., Herrera, D.G., Garcia-Verdugo, J.M., and Alvarez-Buylla, A. (2000). Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713-726. [DOI] [PubMed] [Google Scholar]
- Mabie, P.C., Mehler, M.F., Marmur, R., Papavasiliou, A., Song, Q., and Kessler, J.A. (1997). Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J. Neurosci. 17, 4112-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler, M.F., Mabie, P.C., Zhu, G., Gokhan, S., and Kessler, J.A. (2000). Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev. Neurosci. 22, 74-85. [DOI] [PubMed] [Google Scholar]
- Miyake, S., Makimura, M., Kanegae, Y., Harada, S., Sato, Y., Takamori, K., Tokuda, C., and Saito, I. (1996). Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl. Acad. Sci. USA 93, 1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzen, K., et al. (1999). Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol. Cell. Biol. 19, 7096-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, S., Claesson-Welsh, L., and Heldin, C.H. (1991). Identification of a hydrophobic region in the carboxyl terminus of the platelet-derived growth factor beta-receptor which is important for ligand-mediated endocytosis. J. Biol. Chem. 266, 21158-21164. [PubMed] [Google Scholar]
- Nakamura, K., Shirai, T., Morishita, S., Uchida, S., Saeki-Miura, K., and Makishima, F. (1999). p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp. Cell Res. 250, 351-363. [DOI] [PubMed] [Google Scholar]
- Nakao, A., Roijer, E., Imamura, T., Souchelnytskyi, S., Stenman, G., Heldin, C.H., and ten Dijke, P. (1997). Identification of Smad2, a human Mad-related protein in the transforming growth factor beta signaling pathway. J. Biol. Chem. 272, 2896-2900. [DOI] [PubMed] [Google Scholar]
- Nakashima, K., Takizawa, T., Ochiai, W., Yanagisawa, M., Hisatsune, T., Nakafuku, M., Miyazono, K., Kishimoto, T., Kageyama, R., and Taga, T. (2001). BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc. Natl. Acad. Sci. USA 98, 5868-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh, H., Ichijo, H., Kimura, M., Matsumoto, T., Makishima, F., Yamaguchi, A., Yamashita, H., Enomoto, S., and Miyazono, K. (1996). Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J. Biol. Chem. 271, 21345-21352. [DOI] [PubMed] [Google Scholar]
- Palmer, T.D., Takahashi, J., and Gage, F.H. (1997). The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 8, 389-404. [DOI] [PubMed] [Google Scholar]
- Panchision, D.M., and McKay, R.D. (2002). The control of neural stem cells by morphogenic signals. Curr. Opin. Genet. Dev. 12, 478-487. [DOI] [PubMed] [Google Scholar]
- Panchision, D.M., Pickel, J.M., Studer, L., Lee, S.H., Turner, P.A., Hazel, T.G., and McKay, R.D. (2001). Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 15, 2094-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, U., et al. (1998). The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation Transforming growth factor (TGF-beta)-specific signaling by chimeric TGF-beta type II receptor with intracellular domain of activin type IIB receptor. FEBS Lett. 434, 83-87. [DOI] [PubMed] [Google Scholar]
- Persson, U., Souchelnytskyi, S., Franzen, P., Miyazono, K., ten Dijke, P., and Heldin, C.H. (1997). Transforming growth factor (TGF-beta)-specific signaling by chimeric TGF-beta type II receptor with intracellular domain of activin type IIB receptor. J. Biol. Chem. 272, 21187-21194. [DOI] [PubMed] [Google Scholar]
- Rosenzweig, B.L., Imamura, T., Okadome, T., Cox, G.N., Yamashita, H., ten Dijke, P., Heldin, C.H., and Miyazono, K. (1995). Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 92, 7632-7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, I., Oya, Y., Yamamoto, K., Yuasa, T., and Shimojo, H. (1985). Construction of nondefective adenovirus type 5 bearing a 2.8-kilobase hepatitis B virus DNA near the right end of its genome. J. Virol. 54, 711-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageot, C.M., and Stiles, C.D. (2002). Molecular mechanisms controlling cortical gliogenesis. Curr. Opin. Neurobiol. 12, 244-249. [DOI] [PubMed] [Google Scholar]
- Seri, B., Garcia-Verdugo, J.M., McEwen, B.S., and Alvarez-Buylla, A. (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 21, 7153-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H., Stevens, C.F., and Gage, F.H. (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417, 39-44. [DOI] [PubMed] [Google Scholar]
- Suhonen, J.O., Peterson, D.A., Ray, J., and Gage, F.H. (1996). Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 383, 624-627. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Palmer, T.D., Takahashi, J., and Gage, F.H. (1998). Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol. Cell Neurosci. 12, 340-348. [DOI] [PubMed] [Google Scholar]
- Teleman, A.A., and Cohen, S.M. (2000). Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103, 971-980. [DOI] [PubMed] [Google Scholar]
- ten Dijke, P., Yamashita, H., Ichijo, H., Franzen, P., Laiho, M., Miyazono, K., and Heldin, C.H. (1994a). Characterization of type I receptors for transforming growth factor-beta and activin. Science 264, 101-104. [DOI] [PubMed] [Google Scholar]
- ten Dijke, P., Yamashita, H., Sampath, T.K., Reddi, A.H., Estevez, M., Riddle, D.L., Ichijo, H., Heldin, C.H., and Miyazono, K. (1994b). Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 269, 16985-16988. [PubMed] [Google Scholar]
- Timmer, J.R., Wang, C., and Niswander, L. (2002). BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 129, 2459-2472. [DOI] [PubMed] [Google Scholar]
- Wilson, P.A., and Hemmati-Brivanlou, A. (1997). Vertebrate neural induction: inducers, inhibitors, and a new synthesis. Neuron 18, 699-710. [DOI] [PubMed] [Google Scholar]
- von Bubnoff, A., and Cho, K.W. (2001). Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev. Biol. 239, 1-14. [DOI] [PubMed] [Google Scholar]
- Yi, S.E., Daluiski, A., Pederson, R., Rosen, V., and Lyons, K.M. (2000). The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127, 621-630. [DOI] [PubMed] [Google Scholar]
- Zhu, G., Mehler, M.F., Mabie, P.C., and Kessler, J.A. (1999). Developmental changes in progenitor cell responsiveness to cytokines. J. Neurosci. Res 56, 131-145. [DOI] [PubMed] [Google Scholar]
- Zou, H., Wieser, R., Massague, J., and Niswander, L. (1997). Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 11, 2191-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]