Abstract
The radial spokes are required for Ca2+-initiated intraflagellar signaling, resulting in modulation of inner and outer arm dynein activity. However, the mechanochemical properties of this signaling pathway remain unknown. Here, we describe a novel nucleoside diphosphate kinase (NDK) from the Chlamydomonas flagellum. This protein (termed p61 or RSP23) consists of an N-terminal catalytic NDK domain followed by a repetitive region that includes three IQ motifs and a highly acidic C-terminal segment. We find that p61 is missing in axonemes derived from the mutants pf14 (lacks radial spokes) and pf24 (lacks the spoke head and several stalk components) but not in those from pf17 (lacking only the spoke head). The p61 protein can be extracted from oda1 (lacks outer dynein arms) and pf17 axonemes with 0.5 M KI, and copurifies with radial spokes in sucrose density gradients. Furthermore, p61 contains two classes of calmodulin binding site: IQ1 interacts with calmodulin-Sepharose beads in a Ca2+-independent manner, whereas IQ2 and IQ3 show Ca2+-sensitive associations. Wild-type axonemes exhibit two distinct NDKase activities, at least one of which is stimulated by Ca2+. This Ca2+-responsive enzyme, which accounts for ∼45% of total axonemal NDKase, is missing from pf14 axonemes. We found that purified radial spokes also exhibit NDKase activity. Thus, we conclude that p61 is an integral component of the radial spoke stalk that binds calmodulin and exhibits Ca2+-controlled NDKase activity. These observations suggest that nucleotides other than ATP may play an important role in the signal transduction pathway that underlies the regulatory mechanism defined by the radial spokes.
INTRODUCTION
The eukaryotic cilium/flagellum is a highly conserved motile organelle built around nine outer doublet microtubules and a central pair of singlet microtubules that form the 9 + 2 axoneme. Individual flagella provide for the motility of single cells such as mammalian sperm and Chlamydomonas, and ciliated epithelia are essential for fluid/mucus transport in many organs. Moreover, nodal cilia are required to define the left-right axis during mammalian development (Supp et al., 1997; Nonaka et al., 1998). Many cells also contain primary cilia that seem to play critical sensory roles during normal tissue function and development (Pazour and Witman, 2003). The power for ciliary/flagellar movement is generated by the inner and outer rows of dynein arms that associate with the A tubule of each microtubule doublet and transiently interact with the B tubule of the adjacent doublet to produce a linear force vector that results in the microtubules sliding with respect to each other. This sliding motion is then converted to a flagellar bend by other axonemal components, including the radial spoke and central pair microtubule complexes (see Mitchell (2000) for a review of Chlamydomonas flagellar structure and function).
In Chlamydomonas, control of flagellar waveform is mediated through Ca2+ signaling pathways (Bessen et al., 1980; Kamiya and Witman, 1984). Reactivation experiments have revealed that all the machinery necessary for waveform regulation is integrated within the demembranated flagellar axoneme (Bessen et al., 1980), and the central pair microtubule complex and radial spokes are thought to play key mechanochemical roles in this regulatory process (for recent review, see Smith and Yang, 2004). Cyclic AMP-dependent phosphorylation also exerts a regulatory effect on flagellar activity (Hasegawa et al., 1987; Habermacher and Sale, 1996) that involves the radial spoke and inner arm dynein systems. There is also accumulating evidence of a role for redox poise in activating flagellar motility in Chlamydomonas and both mammalian and sea urchin sperm (Ogawa et al., 1996; Patel-King et al., 1996; Aitken et al., 1997; Sadek et al., 2001, 2003; Harrison et al., 2002). Furthermore, isolated Chlamydomonas flagella have been reported to exhibit two distinct nucleoside diphosphate kinase1 (NDKase) activities, which catalyze the general reaction ATP + NDP → ADP + NTP; at least one of these enzymes has a marked preference for generating GTP (Watanabe and Flavin, 1976). Similarly, ND-Kase activity has been detected in sea urchin sperm flagella (Yanagisawa et al., 1968; Kobayashi et al., 1976), and there are also several mammalian NDKs (nm23-H5, -H7, -H8, and -H9) expressed either predominantly or exclusively in testis; for example, the human nm23-H5 NDK has recently been localized to the sperm flagellar axoneme (Munier et al., 1998, 2003). In sea urchin sperm outer arm dynein, the intermediate chain 1 (IC1) polypeptide consists of an N-terminal thioredoxin unit followed by three catalytic NDK modules (Ogawa et al., 1996); the mammalian homologue (Sptrx-2 [nm23-H8]) that is present in sperm also contains three NDK modules (Sadek et al., 2001). A second human protein (Txl-2 or nm23-H9) containing both thioredoxin and NDK units has been identified in the cilia of lung epithelia, the spermatid manchette, and sperm flagellar axoneme (Sadek et al., 2003). Together, these observations suggest that generation of nucleotides other than ATP may be of widespread significance for ciliary/flagellar function.
Although the purified outer dynein arm from Chlamydomonas flagella does not contain a polypeptide similar to sea urchin dynein IC1 (for recent review, see King (2002)), we (Patel-King et al., 2002) and others (Ikeda et al., 2003) recently identified a Chlamydomonas flagellar protein (here termed Rib72) consisting of three DM-10 domains (related to the N-terminal noncatalytic segment of human and rat NDK7 [nm23-H7 and nm23-R7]) followed by two consensus EF-hand motifs that could bind Ca2+. This intriguing molecule is tightly associated with the Chlamydomonas axoneme (Patel-King et al., 2002), and forms part of the protofilament ribbons within the wall of the A-tubules of each outer doublet (Ikeda et al., 2003).Because NDKs are the only proteins of known function in which the DM-10 domain has been identified, we suggested previously that Rib72 may represent a Ca2+-controlled regulatory component of a flagellar NTP synthesis system (Patel-King et al., 2002).
Because NDKase activity seems to be important for flagellar function and indeed may play a crucial and previously unconsidered role in motor regulation, we sought to identify NDK catalytic units within the Chlamydomonas flagellum. Here, we describe a 61-kDa polypeptide consisting of an N-terminal NDK catalytic module and a C-terminal domain containing three IQ motifs that bind calmodulin. We demonstrate that p61 is an integral component of the flagellar radial spokes, which transmit signals from the central pair microtubule complex to the dynein motors and are essential for the control of microtubule sliding within the flagellar axoneme (Smith and Sale, 1992). We also observe that a Ca2+-stimulated NDKase activity is missing in mutants lacking radial spokes, further suggesting that regulation of flagellar function through this structure may involve nucleotides other than ATP.
EXPERIMENTAL PROCEDURES
Molecular Biology
The full-length cDNA clone (AV645300) encoding Chlamydomonas p61 was identified in a BLAST search of the Chlamydomonas expressed sequence tag database by using the testis-specific nm23-H5 NDK (Munier et al., 1998) as the initial query sequence. This clone was obtained from the Kazusa DNA Research Institute (Chiba, Japan) (Asamizu et al., 1999). The entire 2.3-kb AV645300 clone was sequenced using the molecular core facility at the University of Connecticut Health Center. A 5′ section (140–718 base pairs) of this clone encoding the 5′-untranslated region (UTR) and NDK catalytic module were used to probe blots of Chlamydomonas genomic DNA and also RNA samples obtained before and 30 min after deflagellation. The 3′ portion of the clone has relatively low nucleotide complexity (71.2% GC content), which reflects the Ala-, Pro-, and Glu-rich regions in the C-terminal portion of p61; the use of this region as a probe resulted in unacceptably high backgrounds.
Computational Methods
Sequence searches of the nonredundant database were performed using BLAST. Analysis of the p61 region (scaffold #399) of the Chlamydomonas genome and identification of additional related proteins was performed using the BLAST interface at the Department of Energy Joint Genome Institute (Walnut Creek, CA) (http://genome.jgi-psf.org/chlre1/chlre1.home.html). The p61 cDNA sequence was assembled using the GCG suite of software. The repeat structure of p61 was identified and analyzed using COMPARE and DOTPLOT with a window size of 30 and stringency of 25. The COILS program was used to assess the probability of coiled coil regions (Lupas et al., 1991). The multiple sequence alignment was generated with ClustalW and used as the input for phylogenetic analysis. The molecular model for the catalytic domain of p61 was calculated using SWISSMODEL (Peitsch, 1996). The IQ motifs were identified using the Simple Modular Architecture Research Tool (http://dylan.embl-heidelberg.de).
Fusion Protein and Antibody Production
Residues 7–199, 7–424, 7–494, 424–494, and 7–586 of p61 were cloned into the pMAL-c2 vector across the XmnI/XbaI sites and expressed as C-terminal fusions with maltose-binding protein (MBP). These proteins were purified by amylose affinity chromatography and MBP-p61(7-199) used as the immunogen to inoculate rabbit CT220. The p61(7-199) segment was separated from MBP by digestion with Factor Xa and used to blot-purify a p61-specific antibody fraction from serum by using the method of Olmsted (1986). Fusion proteins begin at residue 7, because we were unable to amplify and subclone the appropriate regions when starting from residue 1.
Chlamydomonas Strains and Isolation of Flagellar Components
The following strains of Chlamydomonas reinhardtii were used in this study: cc124 (wild type), oda1 (lacks outer dynein arms and associated docking complex), ida1 (lacks inner arm I1), ida4 (lacks inner arm I2), pf14 (lacks radial spokes), pf17 (lacks radial spoke heads), pf18 (lacks the central pair microtubule complex), and pf24 (lacks radial spoke heads and several other components; assembles truncated stalks). Chlamydomonas strains were grown in R medium (Harris, 1989), deflagellated using dibucaine, and the flagella were isolated by standard methods (King, 1995). Axonemes were obtained by treating flagella with 1% IGEPAL CA-630 (replaces Nonidet P-40) to remove the membrane and either prepared directly for electrophoresis, or further fractionated by extraction with 0.6 M NaCl (King et al., 1986). From the deflagellation step onwards, a protease inhibitor cocktail (P-8340; Sigma-Aldrich, St. Louis, MO) was added to all solutions to yield final concentrations of 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 0.8 μM aprotinin, 20 μM leupeptin, 15 μM pepstatin A, and 14 μM N-(N-(l-3-trans-carboxyoxirane-2-carbonyl)-l-leucyl) agmatine (E-64). Radial spokes were solubilized from NaCl-extracted oda1 and pf17 axonemes by treatment with 0.5 M KI and purified by centrifugation in 5–20% sucrose density gradients as described by (Yang et al., 2001).
Calmodulin Binding Assay
One hundred micrograms each of the MBP-p61 fusion proteins were incubated for 2 h with 100 μl of calmodulin-Sepharose 4B beads (Amersham Biosciences, Piscataway, NJ) in 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 0.1% Tween 20, 2 mM CaCl2. Protein that did not bind was recovered after a brief centrifugation. The beads were then washed six times with buffer and resuspended in 200 μl of 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 0.1% Tween 20, 2 mM EGTA for 60 min to elute proteins bound in a Ca2+-dependent manner. After an additional wash with EGTA-containing buffer, protein that remained associated with the calmodulin-Sepharose beads in a Ca2+-independent manner was eluted with gel sample buffer.
Electrophoretic Methods
Routine analysis of fusion proteins was performed in 8% SDS polyacrylamide gels. Flagellar components were separated in 5–15% SDS polyacrylamide gradient gels and either stained with Coomassie Blue, or transferred to nitrocellulose and probed with antibody CT220 by using standard methods (Harrison et al., 1998). Polyclonal antibody against radial spoke protein-3 (RSP3) (Williams et al., 1989) was kindly provided by Drs. Dennis Diener and Joel Rosenbaum (Yale University, New Haven, CT). Antibody reactivity was detected using a peroxidase-conjugated secondary antibody and an enhanced chemiluminescent system (Amersham Biosciences).
Nucleoside Diphosphate Kinase Assay
NDKase activity was determined using the bioluminescent method described by Karamohamed et al. (1999). The assay is based on the transfer of the terminal γ-phosphate from GTP to ADP, and the subsequent utilization by luciferase of the ATP generated to yield light. A Tricine-based assay buffer system, pH 7.8, containing MgSO4, EDTA, dithiothreitol, and bovine serum albumin was used (assay kit FL-AAM; Sigma-Aldrich). ADP and GTP were both added to final concentrations of 0.25 mM, and the sample equilibrated for several minutes to allow any contaminating ATP to be used. The assay was started by addition of axonemes derived from either wild type or the pf14 mutant strain. Ca2+ and EGTA were added to the assay mix as appropriate. Light output was integrated >15-s intervals by using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA) and calibrated for each experiment with known amounts of ATP. All assays were performed in triplicate. Specific NDKase activity is expressed as micromoles of phosphate transferred per minute per milligram of protein. Addition of ADP alone generated negligible amounts of ATP, indicating that the axoneme and radial spoke preparations assayed did not contain a flagellar adenylate kinase.
RESULTS
Identification of a Flagellar Nucleoside Diphosphate Kinase
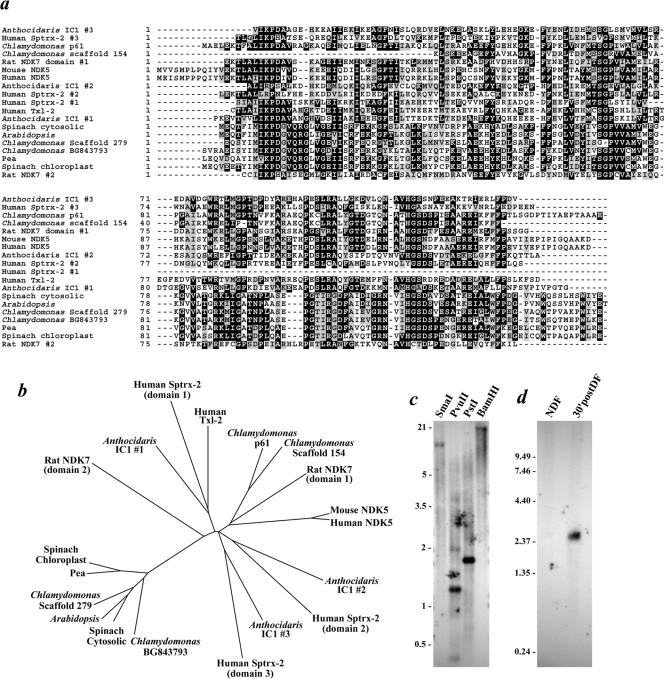
Chlamydomonas flagella have previously been demonstrated to contain an NDKase activity that exhibits a marked preference for generating GTP (Watanabe and Flavin, 1976). A BLAST search of the Chlamydomonas expressed sequence tag (EST) database by using the human testis-specific nm23-H5 NDK (NDK5; Munier et al., 1998) as the initial query sequence identified two potential NDK-encoding clones (AV645300 and BG843793). Furthermore, analysis of the recently released Chlamydomonas genome (version 1) uncovered two additional NDK genes (in scaffolds #154 and 279). Phylogenetic analysis (Figure 1, a and b) of the sequences available from the databases indicated that the BG843793 clone and the scaffold #279 sequence both encoded NDKs closely related to those in the cytoplasm and chloroplasts of higher plants. Consequently, neither was considered likely to represent a flagellar-specific NDK. In contrast, AV645300 encodes an NDK catalytic module most closely related to the testis-specific mammalian enzymes and to the NDK domains present within the IC1 polypeptide of sea urchin sperm outer arm dynein; the scaffold #154 sequence also groups with these proteins. Because it is present in the EST database and therefore presumably expressed in Chlamydomonas, the AV645300 clone was selected as a candidate to encode a flagellar NDK.
Figure 1.
NDK phylogeny and Northern/Southern blot analysis of p61. (a) Chlamydomonas ESTs AV645300 (encoding p61) and BG843793, the NDKs identified in scaffolds #154 and 279 of the Chlamydomonas genome, the NDK modules from the IC1 polypeptide of sea urchin dynein (Anthocidaris crassispina), human and murine NDK5, rat NDK7, human Sptrx-2, human Txl-2, and the cytosolic and chloroplast NDKs from spinach, pea, and Arabidopsis were aligned using ClustalW. Residues conserved in ≥30% of the sequences were shaded using BOXSHADE. (b) ClustalW alignment was used as the input to generate the neighbor-joining unrooted tree shown. The BG843793 EST and NDK encoded on scaffold 279 clearly group with the NDKs of higher plants. In contrast, AV645300 (p61) and the scaffold #154–9 NDK (p40) are more closely related to the testis-specific mammalian enzymes. (c) Southern blot analysis of Chlamydomonas genomic DNA restricted with SmaI, PvuII, PstI, and BamHI and probed with the 5′ region of the AV645300 EST clone. Single bands were detected in the SmaI-, PstI-, and BamHI-digested samples. (d) Northern analysis of Chlamydomonas RNA obtained from non-deflagellated cells (NDF) and from cells that had undergone flagella excision and been allowed to regenerate new flagella for 30 min (30′postDF). A 2.54-kb mRNA that is highly up-regulated after deflagellation was detected by the AV645300 probe.
Southern blot analysis of PstI-, SmaI-, and BamHI-restricted genomic DNA by using the 5′ end of the AV645300 EST as a probe resulted in single bands, indicating that Chlamydomonas contains only one gene for this NDK (Figure 1c). A search of the Chlamydomonas genome also detected only a single 2763 base pair gene consisting of four exons within scaffold #399. Northern blotting provided further evidence that AV645300 indeed encodes a flagellar protein. The 2.54-kb mRNA for this clone was essentially undetectable in Chlamydomonas cells that had not been deflagellated, but was highly up-regulated 30 min after flagella excision as is characteristic of integral flagellar components (Figure 1d).
Properties of the p61 Nucleoside Diphosphate Kinase
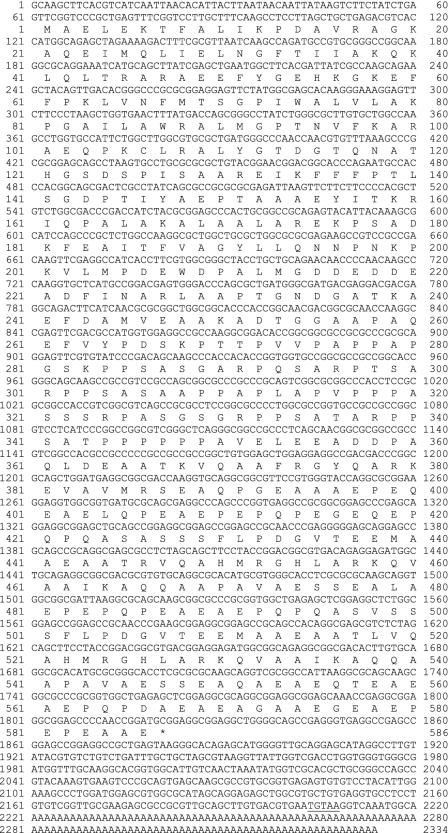
The entire AV645300 clone of ∼2.3 kb was sequenced and found to contain a single open reading frame encoding a 586-residue protein (termed p61) with a mass of 61,364 Da and a predicted pI of 4.48 (Figure 2). The clone contains an in-frame stop codon within the 5′-UTR, a perfect copy of the Chlamydomonas polyadenylation signal and a polyA tract at the 3′ end. The p61 protein has a NDK catalytic module at the N terminus. This domain (residues 1–200) shares 41% sequence identity (60% similarity) with human testis-specific nm23-H5 NDK (accession no. P56597). Sequence analysis and molecular modeling of residues 2–142 (Figure 3) indicates that this unit contains all the conserved NDK residues, including the active site motif (residues 118–126), that have been demonstrated previously from both structural and mutagenesis studies to be essential for catalysis (Tepper et al., 1994). In addition to multiple putative phosphorylation sites, p61 contains three IQ motifs that could potentially bind calmodulin. These motifs conform precisely to the (I/V/L)QXXX(K/R)XXXX(K/R) consensus (Rhoads and Friedberg, 1997) and are located within the C-terminal domain at residues 362–391, 440–469, and 512–541.
Figure 2.
p61 cDNA sequence. The entire 2.3-kb cDNA clone (AV645300) encoding full-length p61 was sequenced. The p61 protein consists of 586 residues and has a calculated mass of 61,394 Da and a pI of 4.48. The 5′-UTR contains a single in-frame stop codon and the putative polyadenylation signal in the 3′-UTR is underlined. The clone terminates in a polyA tract. This annotated sequence is available under GenBank/European Molecular Biology Laboratory/DNA Database of Japan accession no. AY452667.
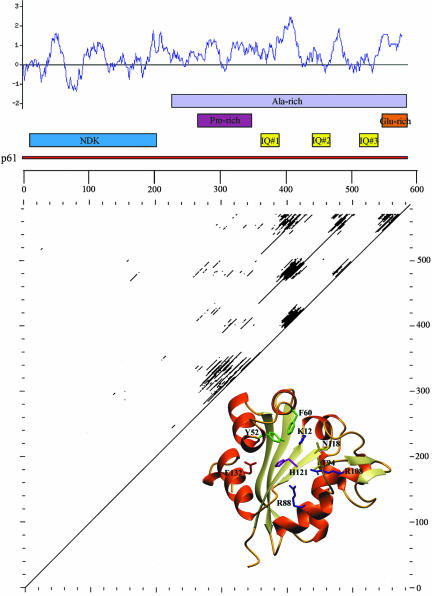
Figure 3.
Domain structure of p61. A map of p61 illustrating the location of the NDK domain, IQ motifs, and regions rich in specific amino acids is shown. A Kyte and Doolittle hydropathy plot is inserted above the map. Almost the entire C-terminal region is hydrophilic in nature. Bottom, dot plot comparison of p61 to illustrate the repetitive structure containing the three IQ motifs present within the C-terminal region of the protein. The inset at lower right is a molecular model for the p61 NDK domain (residues 2–142) built using SWISSMODEL. Indicated on the model are the residues known to be required for the catalytic activity of other NDKs (Tepper et al., 1994). All these active site residues are completely conserved in p61.
Further sequence analysis (Figure 3) revealed that the entire C-terminal portion of p61 (residues 221–586) is relatively hydrophilic and Ala-rich, containing 25% Ala overall. In addition, the regions encompassing residues 265–349 and 546–586 are Pro-rich (36.5% Pro) and Glu-rich (36.5% Glu), respectively. Because the C-terminal 40-residue Glu-rich domain also contains 32% Ala, this region is very likely helical. However, a high coiled coil prediction was obtained using the COILS program only with a scan window of 14 (but not 21 or 28), and thus this segment is unlikely to form a coiled coil (Lupas et al., 1991). The regions immediately surrounding the three IQ motifs are very similar. Indeed, in the case of IQ2 and IQ3, these motifs are embedded in ∼70-residue repeat sequences (residues 424–494 and 496–565) that share 92.8% identity.
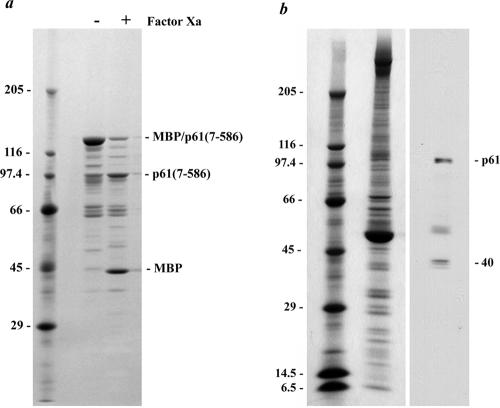
To further analyze this presumptive flagellar NDK, the N-terminal region (residues 7–199) of p61 was expressed as a C-terminal fusion with maltose-binding protein and used to immunize rabbit CT220. The resulting serum was blot-purified against p61 (residues 7–199) obtained by digestion of the fusion protein with factor Xa. We also generated a MBP fusion protein containing p61 residues 7–586. This polypeptide has a calculated molecular mass of 103,130 Da, but migrated with Mr = ∼135,000 (Figure 4). On digestion with factor Xa, this fusion protein yielded MBP (Mr = ∼40,000 as expected) and the fusion partner which had a Mr = ∼102,000 (Figure 4a). Thus, recombinant p61 migrates anomalously in SDS-polyacrylamide gels as has been found previously for several other axonemal proteins (e.g., DC1 of the outer arm docking complex; Koutoulis et al., 1997). Furthermore, the MBP-p61(7-586) protein was highly susceptible to proteolysis by endogenous proteases. Indeed, when we examined axoneme samples prepared in the absence of a comprehensive protease inhibitor cocktail, multiple bands ranging from Mr ∼102,000 to 20,000 were detected by the CT220 antibody (our unpublished data). However, when protease inhibitors were added to the isolation buffers, all bands except for those with Mr = ∼102,000 and 40,000 (Figure 4b) were dramatically reduced or absent, indicating that the larger band is highly susceptible to proteolysis. Because recombinant p61 exhibits anomalous electrophoretic mobility (see above), we conclude that the Mr = ∼102,000 band represents native p61. The CT220 antibody was raised against the conserved NDK region of p61, and thus it is likely that the Mr40,000 band represents a second flagellar NDK (most probably the Chlamydomonas ortholog of human nm23-H7 that is encoded on scaffold 154 and has a predicted mass of 39.9 kDa).
Figure 4.
p61 migrates anomalously in SDS-polyacrylamide gels. (a) Twenty micrograms of the MBP-p61(7-586) fusion protein was electrophoresed in a 5–15% acrylamide gradient gel, both before and after incubation with factor Xa, and stained with Coomassie Blue. The intact fusion protein has a calculated mass of 103,130 Da but migrated with Mr ∼135,000. The minor bands derive from cleavage of MBP-p61(7-586) by endogenous bacterial proteases. After factor Xa cleavage of the fusion protein, MBP migrated at ∼Mr 40,000 as observed previously, whereas the p61(7-586) segment (calculated mass 60,662 Da) had Mr ∼100,000. (b) Wild-type axonemes (∼100 μg) prepared in the presence of a protease inhibitor cocktail were electrophoresed in a 5–15% acrylamide gradient gel and either stained with Coomassie Blue (left) or blotted and probed with the CT220 antibody (right). Two prominent bands of Mr102,000 and 40,000 were obtained. The top band corresponds to p61, which migrates anomalously. The diffuse band located above tubulin is a proteolytic fragment of p61.
p61 Is Missing from pf14 and pf24 Axonemes
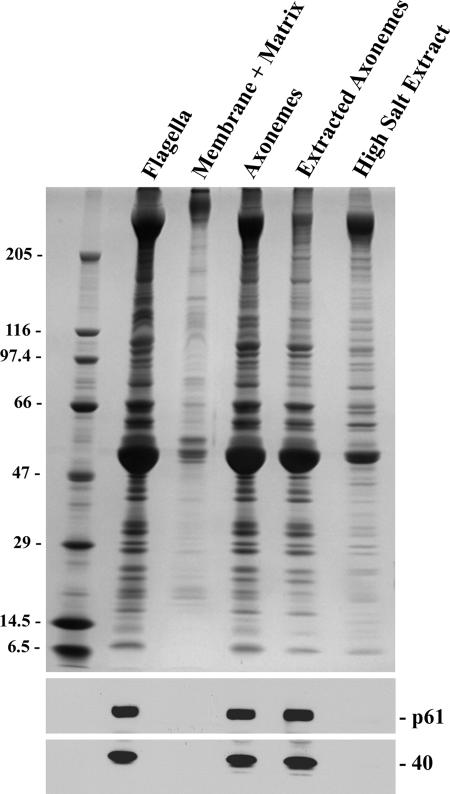
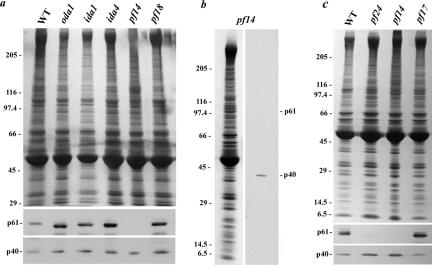
Fractionation of Chlamydomonas flagella revealed that p61 and the Mr 40,000 protein are integral components of the axoneme and remained tightly associated with that structure after both detergent treatment to remove the flagellar membrane and soluble matrix components, and extraction with 0.6 M NaCl, which is used to solubilize the dynein arms (Figure 5). In an initial effort to ascertain the axonemal location of p61, we used the CT220 antibody to probe axoneme samples prepared from wild-type Chlamydomonas and mutant strains lacking the outer dynein arm and docking complex (oda1) or inner arms I1 (ida1) and I2 (ida4), the radial spokes (pf14) and the central pair microtubule complex (pf18). The Mr 40,000 band detected by CT220 was present in all samples (Figure 6, a–c). In contrast, p61 and its proteolytic breakdown products were completely missing from pf14 axonemes (Figure 6, a and b). Further analysis revealed that p61 was present in axonemes derived from pf17, which lack only the radial spoke head (Figure 6c), suggesting that p61 is an integral component of the radial spoke stalk. Interestingly, p61 was almost completely absent from pf24 axonemes; this strain is defective for RSP2 and lacks the spoke head and several other components resulting in a truncated stalk (Huang et al., 1981; Yang et al., 2004). This suggests that p61 associates with the radial spoke stalk near RSP2 in the region distal to the doublet microtubules.
Figure 5.
NDKs are integral axonemal components. Chlamydomonas flagella were treated with a nonionic detergent to remove the flagellar membrane and soluble matrix components, yielding microtubule-based axonemes that were subsequently extracted with 0.6 M NaCl. Equivalent samples of each fraction were separated in a 5–15% acrylamide gradient gel and either stained with Coomassie Blue (top) or blotted to nitrocellulose and probed with CT220 antibody (bottom). Both p61 and the Mr 40,000 band are integral axonemal components that are almost completely resistant to extraction by high ionic strength buffers containing 0.6 M NaCl.
Figure 6.
p61 is missing in mutant axonemes lacking radial spokes. (a) Axonemes were obtained from wild-type Chlamydomonas (WT) and from mutants lacking outer (oda1) and inner (ida1 and ida4) dynein arms, radial spokes (pf14), and the central pair microtubule complex (pf18). After electrophoresis, samples were stained with Coomassie Blue (top) or examined for CT220 immunoreactivity (bottom). The Mr 40,000 protein (p40) was detected in all samples, whereas p61 was absent only in pf14 axonemes. (b) Axonemes from the pf14 mutant were prepared in the presence of a comprehensive protease inhibitor cocktail. The sample was electrophoresed and stained with Coomassie Blue (left) or blotted and probed with CT220 (right). These axonemes contain the Mr 40,000 band. However, p61 and its breakdown product migrating just above tubulin (see Figure 4b) were missing. (c) To assess the location of p61 within flagellar radial spokes, axonemes were prepared from wild type, pf14, pf17 (lack the spoke head), and pf24 (no spoke head and truncated stalk) axonemes. The p61 protein was present in pf17, but levels were drastically reduced in pf24 (upon prolonged exposure, a very weak p61 band was detected in this mutant). This suggests that p61 is located within the distal portion of the radial spoke stalk.
p61 Copurifies with RSP3
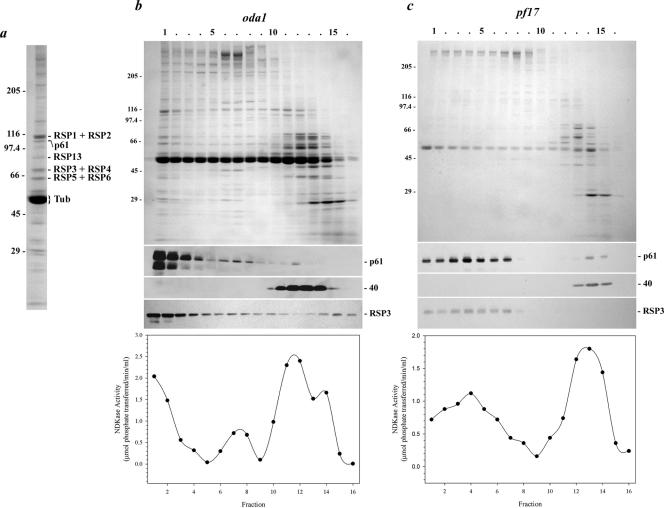
To test whether p61 is indeed a radial spoke component, we first treated axonemes from the mutant oda1 (lacks outer arm dynein and docking complex) with 0.6 M NaCl to remove additional dynein components. Subsequently, these treated axonemes were extracted with 0.5 M KI to solubilize the radial spokes as described by Yang et al. (2001). After dialysis to remove KI, the extract was sedimented in a 5–20% sucrose density gradient (Figure 7, a and b). Both the Mr 40,000 protein and p61 were solubilized under these conditions. The majority of p61 sedimented near the bottom of the gradient (in fractions 1–3), which is characteristic of isolated radial spokes (Yang et al., 2001); there was also a much smaller fraction of p61 present in fractions 6–8. In contrast, the Mr 40,000 protein was found near the top of the gradient in fractions 11–14. To determine whether p61 comigrated with bona fide radial spoke proteins, we probed the same fractions with an antibody against RSP3 (Williams et al., 1989). The RSP3 peak was found close to the bottom of the gradient as described previously (Yang et al., 2001) and coincided with p61. Based on immunoblot analysis and/or by comparison with the electrophoretic patterns observed by Yang et al. (2001), p61 and several other RSP proteins could be readily identified in the peak sucrose gradient fractions (Figure 7a). Densitometric analysis using the RSP3 + RSP4 band as a standard indicates that p61 is present at a ratio of 0.85:1. Thus, p61 seems to be a stoichiometric component of the radial spokes.
Figure 7.
p61 copurifies with radial spoke protein 3.(a) Coomassie Blue-stained lane of isolated radial spokes (from the sucrose gradient shown in b). The p61 protein and several RSP components were identified by immunoblot analysis and/or by comparison with the electrophoretic analysis performed by Yang et al. (2001). (b) Axonemes from the mutant oda1 were treated with 0.6 M NaCl to remove inner arm dyneins, and the radial spokes were subsequently extracted with 0.5 M KI. This extract was then sedimented in a 5–20% sucrose density gradient. Fractions were electrophoresed in a 5–15% acrylamide gradient gel and either stained with Coomassie Blue (top) or blotted to nitrocellulose and probed with CT220 (middle) and the RSP3 antibody (bottom). Most of the p61 and RSP3 proteins were found together in fractions 1–3. In contrast, the Mr 40,000 band detected by antibody CT220 sedimented near the top of the gradient in fractions 11–14. NDKase activity of each fraction is shown in the lower panel, and precisely copurified with p61 near the bottom of the gradient and also with p40 toward the top of the gradient. (c) Radial spokes lacking the spoke head were extracted from pf17 axonemes and sedimented in a 5–20% sucrose gradient. Both p61 and RSP3 were present nearer to the top of the gradient in fractions 1–7; sedimentation of the Mr 40,000 band was unaffected by the pf17 mutation. NDKase activity also was shifted up the gradient and again followed the p61 profile.
To further confirm that p61 is an integral component of the radial spokes, we purified this structure from the mutant pf17, which assembles a radial spoke that lacks five head components (Huang et al., 1981) and consequently sediments more slowly in sucrose gradients (Yang et al., 2001). In these samples, p61 was found in fractions 1–7 (peak was in fraction 4) and again precisely comigrated with RSP3 (Figure 7c). The migration of the Mr 40,000 band from pf17 axonemes was unaffected, and this protein remained near the top of the gradient in fractions 13–15. Thus, we conclude that p61 is indeed an integral component of the flagellar radial spoke stalk.
p61 Interacts with Calmodulin In Vitro
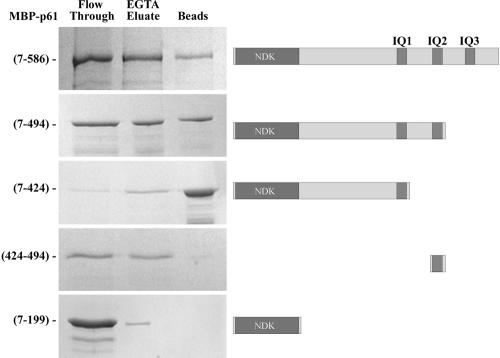
Sequence analysis (Figure 3) revealed that p61 contains three canonical IQ motifs (IQ2 and IQ3 are identical) that could potentially act to bind calmodulin (Rhoads and Friedberg, 1997). To test this, we examined whether the MBP fusion proteins containing the NDK domain alone [p61(7-199)] or with various IQ motif combinations [NDK+IQ1, p61(7-424); NDK+IQ1+IQ2, p61(7-494); NDK+IQ1+IQ2+IQ3, p61(7-586)] would interact specifically with calmodulin immobilized on Sepharose 4B beads. We found that ∼60% of total added MBP-p61(7-586) adhered to the calmodulin resin in the presence of Ca2+ and that ∼70% of bound protein was eluted after addition of 2 mM EGTA (Figure 8). In contrast, <10% of the truncated p61(7-199) fusion protein was found associated with the calmodulin-Sepharose beads; all of this presumably nonspecifically bound protein was eluted by washing with EGTA. Because IQ motifs usually bind calmodulin in a Ca2+-independent manner, we next tested whether all motifs within p61 exhibited the same properties. We observed that 87% of a construct containing IQ1 alone bound calmodulin in a Ca2+-independent manner. However, addition of one or both other IQ motifs caused significant amounts (∼50% with IQ2) of the fusion protein to be eluted by EGTA. Furthermore, when IQ2 alone was fused to MBP, the resulting protein bound calmodulin only in the presence of Ca2+ and was essentially completely eluted by EGTA. These observations suggest that p61 can bind calmodulin through each of the three IQ motifs. However, whereas association with calmodulin via IQ1 is Ca2+-independent, interaction through IQ2 and IQ3 is disrupted upon EGTA addition.
Figure 8.
p61 binds calmodulin through both Ca2+-dependent and Ca2+-independent mechanisms. Equimolar amounts of the full-length MBP-p61(7-586), the truncated MBP-p61(7-199), MBP-p61(7-424), MBP-p61(7-494) fusion proteins, and a MBP protein to which only the IQ2 region (p61 residues 424–494) was attached, were incubated with calmodulin-Sepharose beads in the presence of 2 mM Ca2+. Protein that did not bind was recovered after a brief centrifugation (lane marked flow through). After several washes, protein bound in a Ca2+-dependent manner was eluted with buffer containing 2 mM EGTA (EGTA eluate). Any protein that remained associated through a Ca2+-independent interaction was obtained by incubating the beads in gel sample buffer (lane marked beads). Samples were electrophoresed in an 8% acrylamide gel and stained with Coomassie Blue. Maps illustrating the domain structure of the various constructs are shown at right.
Radial Spokes Contain a Ca2+-regulated Nucleoside Diphosphate Kinase
To assess if radial spokes indeed exhibit NDKase activity in situ and to determine whether that activity is Ca2+ stimulated, we first examined the enzymatic properties of flagellar axonemes prepared from wild-type Chlamydomonas and from the mutant pf14. Our assay system tested for the transfer of the terminal phosphate of GTP to ADP and the subsequent utilization of generated ATP by luciferase. Although Chlamydomonas flagella contain an adenylate kinase activity, most of this activity is readily solubilized (Watanabe and Flavin, 1976). Direct measurement of ATP production from ADP in the absence of an NTP revealed that the detergent-extracted flagellar axonemes used here contained negligible adenylate kinase activity. We observed that wild-type axonemes in the presence of 1 mM EGTA exhibited a specific NDKase activity of 0.46 ± 0.03 μmol of phosphate transferred/min/mg protein (values given are the mean ± SD for samples from three separate axoneme preparations). This value is close to the activity of 0.53 μmol of phosphate transferred/min/mg protein reported previously by Watanabe and Flavin (1976) for flagella extracted using low ionic strength dialysis. Replacement of EGTA with 1 mM Ca2+ resulted in a significant increase in axonemal NDKase to 0.81 ± 0.10 μmol of phosphate transferred/min/mg protein. Axonemes prepared from pf14 had a somewhat lower total specific activity of 0.25 ± 0.08 μmol of phosphate transferred/min/mg protein in the presence of EGTA. This value increased to 0.34 ± 0.06 μmol of phosphate transferred/min/mg protein upon Ca2+ addition. Because pf14 axonemes lack p61, these observations suggest that the p61 NDK accounts for ∼45% of axonemal NDKase activity. Furthermore, this activity seems to be stimulated several-fold in the presence of Ca2+, whereas the NDKase activity remaining in pf14 axonemes was affected to a lesser extent by this cation. We also have observed that in addition to GTP, the axonemal NDKases can use other nucleotides, including CTP, ITP, TTP, and UTP.
To further test whether isolated radial spokes indeed contain a functional NDKase, we determined the enzyme activity present after sucrose gradient fractionation of 0.5 M KI extracts from oda1 and pf17 axonemes. We observed that peaks of NDKase activity in the various fractions precisely coincided with radial spoke-associated p61 and also with p40 nearer the top of the gradient (Figure 7, b and c; bottom). The p61 and p40 NDK peaks from the oda1 KI extract sucrose gradient (fractions 1 and 12) had specific activities of 6.2 and 8.7 μmol of phosphate transferred/min/mg protein, respectively.
DISCUSSION
Studies on both sea urchin sperm and Chlamydomonas revealed that demembranated flagella exhibit NDKase activity, suggesting that nucleotides other than ATP may be of functional importance (Yanagisawa et al., 1968; Kobayashi et al., 1976; Watanabe and Flavin, 1976). However, apart from the identification of NDK modules in the IC1 polypeptide from sea urchin sperm outer arm dynein (Ogawa et al., 1996), the intraflagellar location of these enzymes and the role they might play in motility has remained almost completely unexplored. Here, we describe a functional Ca2+/calmodulin-regulated NDK (termed p61 or RSP23)2 from flagella of Chlamydomonas that is present in the radial spoke stalk, suggesting that intraflagellar Ca2+ signaling involves production of nucleotides other than ATP.
p61(RSP23) Is a Component of the Radial Spoke Stalk
The p61 (RSP23) protein contains a single NDK catalytic module at the N terminus; this region contains all the residues that in other NDKs have been shown to be necessary for catalytic activity (Tepper et al., 1994). Indeed, we observed that radial spokes contain ∼45% of the total axonemal NDKase activity. The C-terminal 381-residue section has a high Ala content (∼25% overall) and consists of several distinct regions, including an 85-residue Pro-rich domain adjacent to the catalytic module. This is followed by a triple repeat structure, each section of which contains an IQ motif that could potentially bind calmodulin. The protein terminates in a highly acidic 40-residue region.
We prepared an antibody against the catalytic domain of p61 and found that it detected two bands of Mr 102,000 and 40,000 in flagella. The Mr 102,000 band was identified as p61 after analysis of recombinant protein. The anomalous migration of p61 in SDS gels is likely caused by the Ala-, Pro-, and Glu-rich C-terminal domain because the recombinant catalytic NDK module alone migrated with the predicted Mr. This polypeptide was absent from pf14 mutant axonemes, which do not assemble the entire radial spoke, and from pf24 axonemes that lack the distal region of the spoke stalk and the head. However, p61 was not missing in any of the other mutant strains that we analyzed, including pf17 that only lacks the radial spoke head domain. As is characteristic of the radial spokes, p61 could be extracted from the axoneme only with a strong chaotrope (0.5 M KI) but not with 0.6 M NaCl that is used to solubilize the dynein arms (Yang et al., 2001). Furthermore, after KI extraction from both oda1 and pf17 axonemes, p61 copurified in sucrose gradients with the radial spoke cAMP-dependent protein kinase-anchoring protein RSP3. Thus, we conclude that p61 is an integral component of the radial spoke stalk and is likely located in the region distal to the doublet microtubules.
Interconnection of Ca2+ Signaling with NTP Synthesis
Several Ca2+-binding proteins have been identified within the Chlamydomonas flagellum. These include a light chain (LC4) of outer arm dynein (King and Patel-King, 1995); an outer arm docking complex protein (DC3) (Casey et al., 2003); centrin, which is associated with a subset of inner dynein arms (Piperno et al., 1992); calmodulin (Gitelman and Witman, 1980; Van Eldik et al., 1980; Witman and Minervini, 1982; for review, see Otter, 1989); and the protofilament ribbon component Rib72 (Patel-King et al., 2002; Ikeda et al., 2003). Identification of Ca2+-binding proteins associated with individual dynein heavy chains, and analysis of the microtubule-binding properties of mutant dyneins lacking individual motor units (Sakato and King, 2003) suggests that Ca2+ signals may impinge directly on particular heavy chains. This does not, however, preclude the possibility that Ca2+ also signals through additional mechanisms. Indeed, Yang et al. (2001) recently reported that one pool of flagellar calmodulin is present within the radial spokes, although the stoichiometry remains to be ascertained. Furthermore, pharmacological studies support a role for radial spoke calmodulin in controlling dynein-driven microtubule sliding possibly through a calmodulin-dependent protein kinase (Smith, 2002).
The C-terminal domain of p61 contains three IQ motifs that are predicted to bind calmodulin (Rhoads and Friedberg, 1997). Indeed, we found that the full-length recombinant p61 protein bound calmodulin-Sepharose, whereas the NDK catalytic domain alone did not. Interestingly, p61 contains two classes of IQ motif that exhibit distinct interactions with calmodulin. IQ1 acts as a canonical Ca2+-independent binding domain. However, IQ2 and IQ3 (which are identical) bound calmodulin through Ca2+-sensitive interactions. Although, IQ motifs are generally considered to represent Ca2+-independent calmodulin binding sites, there have been several previous reports of Ca2+-dependent interactions via these segments (e.g., myosin V; Trybus et al., 1999 and IRS-1; Munshi et al., 1996). Thus, we suggest that at least some of the calmodulin present within the radial spokes is likely permanently associated with p61 through IQ1. This is consistent with our observation that the radial spoke-associated NDK accounted for much of the axonemal Ca2+-stimulated NDKase activity. It is also possible that soluble calmodulin present in the flagellar matrix can bind to the additional IQ motifs within p61 after a Ca2+ increase. Because flagellar axonemes contain multiple calmodulin and Ca2+-binding proteins, analysis of reconstituted p61/calmodulin will be required to demonstrate that Ca2+ regulation of NDKase activity occurs directly through the p61 C-terminal domain.
Modulation of p61 activity in response to a Ca2+ signal would lead to a local increase in GTP (or other NTP) levels (Figure 9). This suggestion further implies that downstream GTP-binding proteins are present in the axoneme that could transduce this signal (see below). This hypothesis does not of course preclude the possibility that calmodulin also associates with other components of this large macromolecular complex. For example, radial spoke protein 2 (RSP2) contains two 1-8-14 calmodulin-binding motifs (see Rhoads and Friedberg (1997) for motif description) and has recently also been shown to bind calmodulin in a Ca2+-dependent manner (Yang et al., 2004).
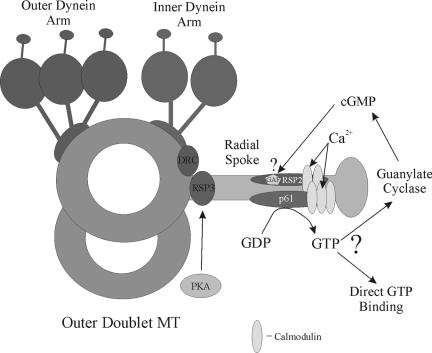
Figure 9.
Model for Ca2+-dependent GTP signaling through the radial spokes. The diagram illustrates the location of structures associated with a single flagellar doublet microtubule. Within the radial spoke stalk, RSP3 is located at the base and is thought to anchor cAMP-dependent protein kinase (PKA), resulting in phosphorylation of inner dynein arm proteins. The p61 protein and associated calmodulin (CaM) are also present in the stalk. Ca2+ binding results in activation of the p61 NDK module to generate GTP (or some other NTP), which subsequently could be used either by a GTP-binding switch protein, a specific GTPase or a guanylate cyclase. Intriguingly, the calmodulin-associated RSP2 protein, which contains a GAF domain that might potentially bind cGMP and act as a downstream target for p61-generated GTP, is also a component of the radial spoke (Yang et al., 2004) and is apparently located close to p61. The dynein regulatory complex (DRC) is located near the base of the radial spokes and inner arms.
What Role Might GTP Play in Flagellar Function?
The biochemical studies of (Watanabe and Flavin, 1976) on Chlamydomonas flagella revealed the presence of two distinct soluble NDKs (one of which likely derived from cell body contamination) and also an insoluble NDKase activity; these enzymes had a significant preference for generating GTP. Our data support their observation that most Chlamydomonas flagellar NDKase activity is tightly associated with the axoneme. The small soluble fraction described by (Watanabe and Flavin, 1976) may have derived from proteolysis of p61, as we observed a soluble p61-derived fragment (Mr∼20,000) containing the NDK module in flagella prepared in the absence of a comprehensive inhibitor cocktail (data not shown). Alternatively, it may represent a flagellar pool of the standard cytosolic NDK.
There are several distinct possibilities for what the GTP (or other nucleotides) generated by these enzymes in response to a Ca2+ signal might be used for. First, as originally suggested by Watanabe and Flavin (1976), flagellar GTP could be used to recharge tubulin dimers that have dissociated from the doublet and/or central pair microtubules and thus are in a nonpolymerizable state as they contain GDP. Although this remains a formal possibility, the identification of intraflagellar transport as a mechanism for replenishing axonemal components (Kozminski et al., 1993), our observation that p61 is present exclusively in the radial spokes that are arrayed along the entire axonemal length, and the fact that the radial spoke-defective mutants lacking p61 make full-length flagella all argue against this model.
A second more likely possibility is that GTP generated in response to a Ca2+ signal is utilized either directly by a GTP-binding switch protein or other specific GTPase, or by a guanylate cyclase which could convert GTP to cGMP and lead to second messenger signaling (Figure 9). Intriguingly, Chlamydomonas RSP2 contains a GAF (cyclic GMP, adenylyl cyclase, and FhlA) domain (Yang et al., 2004), some of which are known to bind cyclic nucleotides and act as regulatory modules (for recent review, see Hurley, 2003). Furthermore, because both RSP2 and p61 are missing in the pf24 mutant, they are likely located close together within the spoke superstructure. Although cGMP-mediated effects on flagellar motility have not previously been reported in Chlamydomonas, studies in Paramecium have revealed a role for cGMP in control of both swimming speed and direction of the ciliary power stroke (Bonini and Nelson, 1988) and have identified proteins phosphorylated in a cGMP-dependent manner (Ann and Nelson, 1995). Furthermore, cyclic nucleotidegated channels control Ca2+ entry into mammalian sperm (Wiesner et al., 1998).
A third possibility derives from the observation that both sea urchin and mammalian sperm contain modular proteins consisting of thioredoxin modules and catalytic NDK domains (Ogawa et al., 1996; Sadek et al., 2001, 2003); the sea urchin protein is known to be an integral component of the outer dynein arm. This suggests an interconnection between flagellar redox state and NDKase activity. In Chlamydomonas, flagellar thioredoxins have only been found associated with the α and β heavy chains of the outer dynein arm (Patel-King et al., 1996). However, it is possible that redox changes, Ca2+ signaling and NDKase activity are interconnected through additional flagellar components in this organism. Finally, initial proteomic studies of human cilia have identified at least one enzyme (pyruvate dehydrogenase) normally associated with intermediary metabolism (Ostrowski et al., 2002). Consequently, because GTP plays key roles in several metabolic pathways, it is possible that some of these reactions also occur in the flagellar matrix.
Other Chlamydomonas NDKs
In addition to p61, we identified three other NDKs within the Chlamydomonas genome. Two of these are single NDK modules with no associated domains and clearly group with the canonical cytosolic and chloroplast enzymes of higher plants. The third NDK (encoded on genome scaffold #154) has a predicted size of 39.9 kDa and is most closely related to p61 and the mammalian testis-specific NDKs. Analysis of the genome sequence indicates that this protein contains an N-terminal DM-10 domain similar to the three found in Rib72 (Patel-King et al., 2002; Ikeda et al., 2003), followed by two catalytic NDK modules. Although no ESTs have so far been identified for this sequence in Chlamydomonas, our antibody against the catalytic domain of the p61 NDK does detect an axonemal component of appropriate size (Mr 40,000), suggesting that this protein also may function in flagella. Furthermore, the apparent mammalian ortholog (nm23-H7) is expressed in a testis-specific manner (Munier et al., 2003).
In conclusion, we have described here a NDK within the Chlamydomonas flagellar radial spokes that binds calmodulin and is regulated in a Ca2+-dependent manner. These data suggest a mechanism for integrating intraflagellar Ca2+ signaling through the radial spokes with the production of NTPs. Consequently, we predict that Chlamydomonas flagella contain signaling protein(s) that specifically bind nucleotides other than ATP. We are currently seeking to identify such axonemal components by photoaffinity labeling with biotinylated nucleotide analogs.
Acknowledgments
We thank Dr. Pinfen Yang (Marquette University, Milwaukee, WI) for helpful discussions, Drs. Dennis Diener and Joel Rosenbaum (Yale University) for providing antibody against radial spoke protein 3, and Dr. Peter Setlow (University of Connecticut Health Center) for the use of a luminometer. This study was supported by grant GM-51293 from the National Institutes of Health. S.M.K. is an investigator of the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-04-0352. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-04-0352.
Abbreviations used: MBP, maltose-binding protein; NDK, nucleoside diphosphate kinase; RSP, radial spoke protein; UTR, untranslated region.
Footnotes
Nomenclature: a recent report (Yang et al., 2001) identified 22 distinct components (including calmodulin and the LC8 dynein light chain) within Chlamydomonas flagellar radial spokes. These proteins have been termed RSP1-22. In an effort to retain a consistent naming system, we propose that p61, which we demonstrate here is also an integral radial spoke component, be henceforth termed RSP23.
References
- Aitken, R.J., Harkiss, D., Knox, W., Paterson, M., and Irvine, D.S. (1997). A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J. Cell Sci. 111, 645-656. [DOI] [PubMed] [Google Scholar]
- Ann, K.-S., and Nelson, D.L. (1995). Protein substrates for cGMP-dependent protein phosphorylation in cilia of wildtype and atalanta mutants of Paramecium. Cell Motil. Cytoskeleton 30, 252-260. [DOI] [PubMed] [Google Scholar]
- Asamizu, E., Nakamura, Y., Sato, S., Fukuzawa, H., and Tabata, S. (1999). A large scale structural analysis of cDNAs in a unicellular green alga, Chlamydomonas reinhardtii. I. Generation of 3433 non-redundant expressed sequence tags. DNA Res. 6, 369-373. [DOI] [PubMed] [Google Scholar]
- Bessen, M., Fay, R.B., and Witman, G.B. (1980). Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J. Cell Biol. 86, 446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini, N., and Nelson, D. (1988). Differential regulation of Paramecium ciliary motility by cAMP and cGMP. J. Cell Biol. 106, 1615-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, D., Inaba, K., Pazour, G., Takada, S., Wakabayashi, K., Wilkerson, C., Kamiya, R., and Witman, G. (2003). DC3, the 21-kD subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol. Biol. Cell 14, 3650-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman, S.E., and Witman, G.B. (1980). Purification of calmodulin from Chlamydomonas: calmodulin occurs in cell bodies and flagella. J. Cell Biol. 87, 764-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermacher, G., and Sale, W.S. (1996). Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas. J. Cell Sci. 109, 1899-1907. [DOI] [PubMed] [Google Scholar]
- Harris, E.H. (1989). The Chlamydomonas Sourcebook, San Diego: Academic Press.
- Harrison, A., Olds-Clarke, P., and King, S.M. (1998). Identification of the t complex-encoded cytoplasmic dynein light chain Tctex1 in inner arm I1 supports the involvement of flagellar dyneins in meiotic drive. J. Cell Biol. 140, 1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, A., Sakato, M., Tedford, H.W., Benashski, S.E., Patel-King, R.S., and King, S.M. (2002). Redox-based control of the γ heavy chain ATPase from Chlamydomonas outer arm dynein. Cell Motil. Cytoskeleton 52, 131-143. [DOI] [PubMed] [Google Scholar]
- Hasegawa, E., Hayashi, H., Asakura, S., and Kamiya, R. (1987). Stimulation of in vitro motility of Chlamydomonas axonemes by inhibition of cAMP-dependent phosphorylation. Cell Motil. 8, 302-311. [DOI] [PubMed] [Google Scholar]
- Huang, B., Piperno, G., Ramanis, Z., and Luck, D. (1981). Radial spokes of Chlamydomonas flagella: genetic analysis of assembly and function. J. Cell Biol. 88, 80-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley, J. (2003). GAF domains: cyclic nucleotides come full circle. Science's STKE 2003, pe1. [DOI] [PubMed] [Google Scholar]
- Ikeda, K., Brown, J., Yagi, T., Norrander, J., Hirono, M., Eccleston, E., Kamiya, R., and Linck, R. (2003). Rib72, a conserved protein associated with the ribbon compartment of flagellar A-microtubules and potentially involved in the linkage between outer doublet microtubules. J. Biol. Chem. 278, 7725-7734. [DOI] [PubMed] [Google Scholar]
- Kamiya, R., and Witman, G.B. (1984). Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J. Cell Biol. 98, 97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamohamed, S., Nordstrom, T., and Nyren, P. (1999). Real-time bioluminometric method for detection of nucleoside diphosphate kinase activity. BioTechniques 26, 728-733. [DOI] [PubMed] [Google Scholar]
- King, S.M. (1995). Large-scale isolation of Chlamydomonas flagella. Methods Cell Biol. 47, 9-12. [DOI] [PubMed] [Google Scholar]
- King, S.M. (2002). Dynein motors: structure, mechanochemistry and regulation. In: Molecular Motors, ed. M. Schliwa, Weinheim: Wiley-VCH Verlag, 45-78.
- King, S.M., Otter, T., and Witman, G.B. (1986). Purification and characterization of Chlamydomonas flagellar dyneins. Methods Enzymol. 134, 291-306. [DOI] [PubMed] [Google Scholar]
- King, S.M., and Patel-King, R.S. (1995). Identification of a Ca2+-binding light chain within Chlamydomonas outer arm dynein. J. Cell Sci. 108, 3757-3764. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., Kaji, K., and Sakai, H. (1976). Nucleoside diphosphate kinase and the flagellar movement of glycerinated spermatozoa. J. Biochem. 79, 413-418. [DOI] [PubMed] [Google Scholar]
- Koutoulis, A., Pazour, G.J., Wilkerson, C.G., Inaba, K., Sheng, H., Takada, S., and Witman, G.B. (1997). The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J. Cell Biol. 137, 1069-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski, K.G., Johnson, K.A., Forscher, P., and Rosenbaum, J.L. (1993). A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 90, 5519-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas, A., Van Dyke, M., and Stock, J. (1991). Predicting coiled coils from protein sequences. Science 252, 1162-1164. [DOI] [PubMed] [Google Scholar]
- Mitchell, D.R. (2000). Chlamydomonas flagella. J. Phycol. 36, 261-273. [Google Scholar]
- Munier, A., Feral, C., Milon, L., Pinon, V., Gyapay, G., Capeau, J., Guellaen, G., and Lacombe, M.-L. (1998). A new nm23 homologue (nm23–H5) specifically expressed in testis germinal cells. FEBS Lett. 434, 289-294. [DOI] [PubMed] [Google Scholar]
- Munier, A., Serres, C., Kann, M., Boissan, M., Lesaffre, C., Capeau, J., Fouquet, J., and Lacombe, M. (2003). Nm23/NDP kinases in human male germ cells: role in spermiogenesis and sperm motility? Exp. Cell Res. 289, 295-306. [DOI] [PubMed] [Google Scholar]
- Munshi, H., Burks, D., Joyal, J., White, M., and Sacks, D. (1996). Ca2+ regulates calmodulin binding to IQ motifs in IRS-1. Biochemistry 35, 15883-15889. [DOI] [PubMed] [Google Scholar]
- Nonaka, S., Tanaka, Y., Okada, Y., Takeda, S., Harada, A., Kanai, Y., Kido, M., and Hirokawa, N. (1998). Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829-837. [DOI] [PubMed] [Google Scholar]
- Ogawa, K., Takai, H., Ogiwara, A., Yokota, E., Shimizu, T., Inaba, K., and Mohri, H. (1996). Is outer arm dynein intermediate chain 1 multifunctional? Mol. Biol. Cell 7, 1895-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted, J.B. (1986). Analysis of cytoskeletal structures using blot-purified monospecific antibodies. Methods Enzymol. 134, 467-472. [DOI] [PubMed] [Google Scholar]
- Ostrowski, L., Blackburn, K., Radde, K., Moyer, M., Schlatzer, D., Moseley, A., and Boucher, R. (2002). A proteomic analysis of human cilia: identification of novel components. Mol. Cell Proteomics 1, 451-465. [DOI] [PubMed] [Google Scholar]
- Otter, T. (1989). Calmodulin and the control of flagellar movement. In: Cell Movement. The Dynein ATPases, vol. 1, ed. F. Warner, P. Satir, and I. Gibbons, New York: Alan R. Liss, Inc., 281-298. [Google Scholar]
- Patel-King, R.S., Benashki, S.E., Harrison, A., and King, S.M. (1996). Two functional thioredoxins containing redox-sensitive vicinal dithiols from the Chlamydomonas outer dynein arm. J. Biol. Chem. 271, 6283-6291. [DOI] [PubMed] [Google Scholar]
- Patel-King, R.S., Benashski, S.E., and King, S.M. (2002). A bipartite Ca2+-regulated nucleoside-diphosphate kinase system within the Chlamydomonas flagellum. The regulatory subunit p72. J. Biol. Chem. 277, 34271-34279. [DOI] [PubMed] [Google Scholar]
- Pazour, G., and Witman, G. (2003). The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 15, 105-110. [DOI] [PubMed] [Google Scholar]
- Peitsch, M.C. (1996). ProMod and Swiss-Model: internet-based tools for automated comparative protein modeling. Biochem. Soc. Trans. 24, 274-279. [DOI] [PubMed] [Google Scholar]
- Piperno, G., Mead, K., and Shestak, W. (1992). The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J. Cell Biol. 118, 1455-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads, A., and Friedberg, F. (1997). Sequence motifs for calmodulin recognition. FASEB J. 11, 331-340. [DOI] [PubMed] [Google Scholar]
- Sadek, et al. (2003). Characterization of human thioredoxin-like 2. A novel microtubule-binding thioredoxin expressed predominantly in the cilia of lung airway epithelia and spermatid manchette and axoneme. J. Biol. Chem. 278, 13133-13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek, C.M., Damdimopoulos, A.E., Pelto-Huikko, M., Gustafsson, J.A., Spyrou, G., and Miranda-Vizuete, A. (2001). Sptrx-2, a fusion protein composed of one thioredoxin and three tandemly repeated NDP-kinase domains is expressed in human testis germ cells. Genes Cells 6, 1077-1090. [DOI] [PubMed] [Google Scholar]
- Sakato, M., and King, S.M. (2003). Calcium regulates ATP-sensitive microtubule binding by Chlamydomonas outer arm dynein. J. Biol. Chem. 278, 43571-43579. [DOI] [PubMed] [Google Scholar]
- Smith, E., and Yang, P. (2004). The radial spokes and central apparatus: mechanochemical transducers that regulate flagellar motility. Cell Motil. Cytoskeleton 57, 8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E.F. (2002). Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol. Biol. Cell 13, 3303-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E.F., and Sale, W.S. (1992). Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science 257, 1557-1559. [DOI] [PubMed] [Google Scholar]
- Supp, D.M., Witte, D.P., Potter, S.S., and Brueckner, M. (1997). Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature 389, 963-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper, A.D., Dammann, H., Bominaar, A.A., and Veron, M. (1994). Investigation of the active site and the conformational stability of nucleoside diphosphate kinase by site-directed mutagenesis. J. Biol. Chem. 269, 32175-32180. [PubMed] [Google Scholar]
- Trybus, K., Krementsova, E., and Freyzon, Y. (1999). Kinetic characterization of a monomeric unconventional myosin V construct. J. Biol. Chem. 274, 27448-27456. [DOI] [PubMed] [Google Scholar]
- Van Eldik, L.J., Piperno, G., and Watterson, D.M. (1980). Similarities and dissimilarities between calmodulin and a Chlamydomonas flagellar protein. Proc. Natl. Acad. Sci. USA 77, 4779-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, T., and Flavin, M. (1976). Nucleotide-metabolizing enzymes in Chlamydomonas flagella. J. Biol. Chem. 251, 182-192. [PubMed] [Google Scholar]
- Wiesner, B., Weiner, J., Middendorf, R., Hagan, V., Kaupp, U., and Weyand, I. (1998). Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. J. Cell Biol. 142, 473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, B., Velleca, M., Curry, A., and Rosenbaum, J. (1989). Molecular cloning and sequence analysis of the Chlamydomonas gene coding for radial spoke protein 3, flagellar mutation pf14 is an ochre allele. J. Cell Biol. 109, 235-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman, G., and Minervini, N. (1982). Role of calmodulin in the flagellar axoneme: effect of phenothiazines on reactivated axonemes of Chlamydomonas. Cell Motil. Suppl. 1, 199-204. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, T., Hasegawa, S., and Mohri, H. (1968). The bound nucleotides of the isolated microtubules of sea urchin sperm flagella and their possible role in flagellar movement. Exp. Cell Res. 52, 86-100. [DOI] [PubMed] [Google Scholar]
- Yang, P., Diener, D.R., Rosenbaum, J.L., and Sale, W.S. (2001). Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J. Cell Biol. 153, 1315-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, P., Yang, C., and Sale, W. (2004). Flagellar radial spoke protein 2 is a calmodulin-binding protein required for motility in Chlamydomonas reinhardtii. Eukaryotic Cell 3, 72-81. [DOI] [PMC free article] [PubMed] [Google Scholar]