Abstract
Some of the coldest and driest permafrost soils on Earth are located in the high-elevation McMurdo Dry Valleys (MDVs) of Antarctica, but little is known about the permafrost microbial communities other than that microorganisms are present in these valleys. Here, we describe the microbiology and habitable conditions of highly unique dry and ice-cemented permafrost in University Valley, one of the coldest and driest regions in the MDVs (1700 m above sea level; mean temperature −23 °C; no degree days above freezing), where the ice in permafrost originates from vapour deposition rather than liquid water. We found that culturable and total microbial biomass in University Valley was extremely low, and microbial activity under ambient conditions was undetectable. Our results contrast with reports from the lower-elevation Dry Valleys and Arctic permafrost soils where active microbial populations are found, suggesting that the combination of severe cold, aridity, oligotrophy of University Valley permafrost soils severely limit microbial activity and survival.
Introduction
The McMurdo Dry Valleys (MDVs) of Antarctica can be subdivided into three microclimate zones on the basis of seasonal environmental conditions (Figure 1) (Marchant and Head, 2007). The coastal thaw zone (<400 m) has the highest mean summer air temperature (~−5 °C), snowfall (snow water equivalent) ranges between 25 and 100 mm per year and a wet active layer can develop in the summer with temperatures exceeding 0 °C for over 50 days annually. The stable upland zone, located at the highest elevations (~1000–2500 m), is the coldest and driest, with mean summer air temperature of ~−10 °C and snow water equivalent <10 mm per year. Both regions are separated by the intermediate mixed zone (Marchant and Head, 2007). The continuous aridity and cold throughout the MDVs result in a layer of dry cryotic soil of variable thickness (cm to m) overlaying ice-cemented permafrost (Lacelle et al., 2013; Marinova et al., 2013), and at elevations greater than ca. 1500 m no seasonal active layer develops.
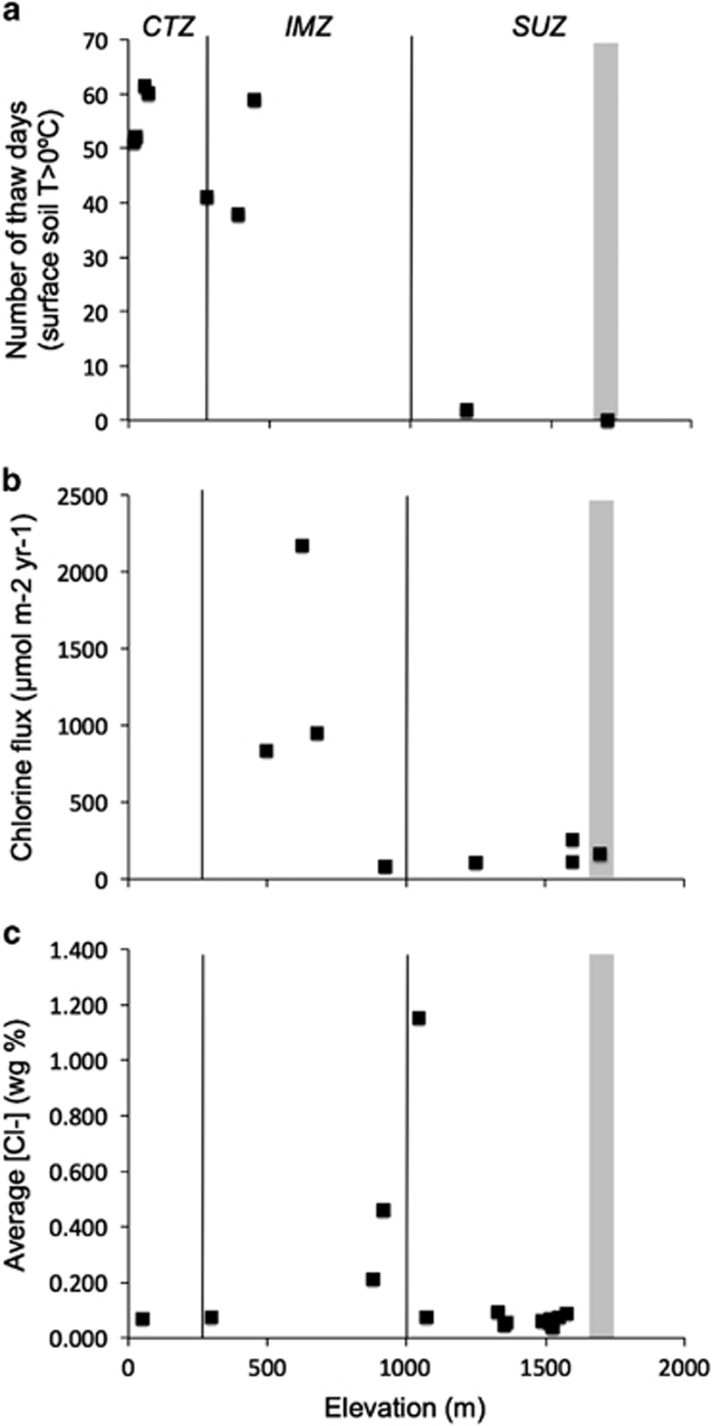
Figure 1.
Temperature and chlorine salt conditions in the McMurdo Dry Valleys. The combination of low temperature (a), low salt influx (b) and low salt concentrations (c) in the stable upland zone (SUZ) of the McMurdo Dry valleys do not permit for liquid water to exist in soils in University Valley (grey band). These conditions are met in the coastal thaw zone (CTZ) and intermediate mixed zone (IMZ). Temperature data compiled from the LTER climate database (http://www.mcmlter.org). Chlorine flux and chlorine concentration data are obtained from Witherow et al. (2006).
Previous soil microbial studies in the MDVs have focussed on the warmer and wetter coastal thaw zone and reported a wide diversity of microbial taxa, forming a simple but functional trophic structure with average soil respiration rates ∼20 times lower than Arctic tundra (cf. Cary et al., 2010). On the other hand, practically nothing is known regarding the microbiology of permafrost in the stable upland zone other than that microorganisms are present (Gilichinsky et al., 2007; Tamppari et al., 2012). Based on the capacity of some permafrost microorganisms to grow at temperatures as low as −15 °C and metabolize at −25 °C (Mykytczuk et al., 2013), we hypothesized that soils in University Valley, one of the coldest and driest in the stable upland zone, would also harbour a viable, active microbial ecosystem similar to other permafrost environments, and that these communities may be some of the most cold-adapted studied to date.
Materials and methods
Site description and field sampling
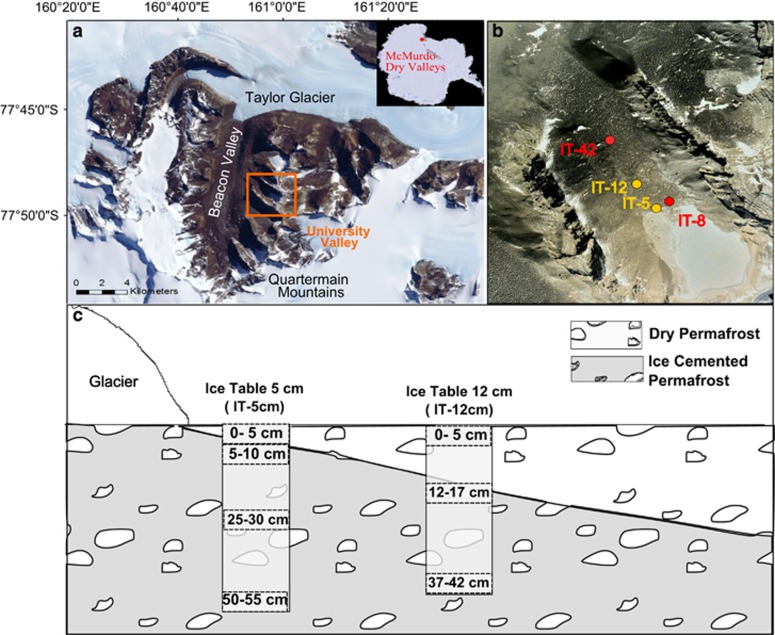
We examined the microclimate, physicochemistry and microbiology of permafrost soils in University Valley (1650–1800 m above sea level (a.s.l.)), located 450 m above Beacon Valley in the Quartermain Range (Figure 2). The northwest-facing valley is ~1.5 km long and ~500 m wide, and the ground surface is heterogeneous, with some areas covered with large sandstone and dolerite boulders, and other areas dominated by desert pavement. Polygonal patterned ground is pervasive in the valley (Heldmann et al., 2013; Lacelle et al., 2013), and a small glacier crowns the head of the valley. Ice-cemented permafrost soils are widespread beneath a layer of dry permafrost soils, and the depth to the ice table (that is, the interface between the dry permafrost soils and the ice-cemented permafrost soils) increases with distance from the glacier, being <1 cm close to the glacier and over 70 cm at the mouth of the valley (Lacelle et al., 2013; Marinova et al., 2013). Samples analysed in this study were located near the head of the valley close to the glacier, a shadowed region where the soil surface experiences few thaw hours (Supplementary Table S1), and where the uppermost 50 cm of ice content in the ice-cemented permafrost formed from water vapour diffusion into the cryotic soils rather than from liquid water (Mckay, 2009; Lacelle et al., 2013; Marinova et al., 2013). This area of the valley was selected for sampling because it is thought to be the harshest for microbial life.
Figure 2.
University Valley permafrost core locations. (a) University Valley placement in the Dry Valleys; (b) approximate locations of the two soil profiles used in this study (yellow) and meteorological stations (red) with depth to ice table indicated; (c) soil profiles studied are shown in solid rectangles, with sample depths analysed in dashed boxes.
We studied two cores of permafrost soils comprising both dry and ice-cemented permafrost (Figure 2); one profile was a 55 cm long core (designated IT-5 cm) as the depth to the ice table was 5 cm, and the second profile was a 42 cm long core collected further down valley where the depth to the ice table was 12 cm (designated IT-12 cm). The age of soils in both cores ranges between 104 and 105 years, with an average soil accumulation rate ca. 10-3 mm per year (Lacelle et al., 2013). The soils are largely derived from the weathering of Ferrar dolerite and Beacon Supergroup sandstone boulders originating from the valley walls (Tamppari et al., 2012; Lacelle et al., 2013).
Sample collection and processing
Climate data were obtained between December 2009 and February 2013 using an automated weather station set to record air and ground temperature and relative humidity (RH). Temperature and RH data were recorded with an HMP45C sensor linked to an automated Campbell Scientific (Logan, UT, USA) CR1000 data logger (accuracy: temperature±0.4 °C; RH ±2.5%), or with an Onset Hobo (Bourne, MA, USA) U23 ProV2 sensor (accuracy: temperature±0.2 °C; RH ±2.5%). All data loggers recorded hourly measurements. University Valley permafrost core samples were collected in the 2009 summer field season with a SIPRE corer (USA Cold Regions Research and Engineering Laboratory, Hanover, NH, USA), and dry soil above the IT-5 cm core was collected in the 2010 summer field season. The cores were 0.3 km apart from each other, and the coordinates for the IT-5 cm permafrost core are 77d51.970s S and 160d43.943s E and for the IT-12 cm core are 77d51.817s S and 160d43.524s E. Samples were shipped to McGill University in a thermally insulated box and maintained at −20 °C until processing. All sample handling and processing were carried out according to protocols developed for low biomass permafrost soils to minimize contamination. Initial core processing took place in a walk-in freezer held at −5 °C in a dedicated laminar flow hood where 1 cm of the outside of the core was removed with a sterilized chisel. An additional 1 cm of the outside core was removed in a biological safety cabinet at room temperature immediately before samples being weighed and aliquoted for analysis. Dedicated biological safety cabinets, which had been pretreated to remove DNA/RNA or cells, were used for sample processing, nucleic acid extraction and PCR preparation. Cryptoendoliths were sampled from the sandstone that makes up the North-West valley wall at 1875 m, at 77°S 51.745', 160°E 41.356'. Cryptoendoliths were sampled with a rock hammer that was wiped with ethanol, placed into sterile whirlpak bags and maintained at −20 °C until processing. Marambio samples were sampled from drill cuttings at 64°S 14.783', 56°W 39.131' in March 2011. The drill bit was sterilized with ethanol and the cuttings sampled aseptically using a falcon tube or sterilized scoop. Marambio samples were maintained below −10 °C until processing.
Nucleic acid extraction
Because of low biomass in soils, 10 g of soil was used per extraction per sample. Community DNA was extracted from 2 g of permafrost soil using the UltraClean Soil DNA Isolation kit (MoBio Laboratories Inc., Carlsbad, CA, USA), as described in the alternative protocol for maximum yield, and a bead beating step was added to aid lysis. For each sample, five extractions were performed and the resulting DNA was pooled and concentrated. DNA from cryptoendoliths was extracted from 100 mg of crushed rock, lysed with cetyltrimethyl ammonium bromide, RNase A and proteinase K and a bead beating step, followed by phenol/chloroform/isoamyl alcohol extraction at 60 °C and ethanol precipitation. Negative controls (H2O in place of soil) underwent identical handling during the extraction procedure and were used as templates for PCR using 16S rRNA gene primers (27 F and 1492 R) to ensure no contamination during extraction. Multiple attempts to extract RNA were carried out using the RNA Power Soil kit (MoBio Laboratories Inc.). Extractions using 2 g of soil were carried for a total of 20 g per sample and pooled. No RNA was detectable after extraction, nor was an amplifiable product found after a reverse transcription reaction on the extracted product.
Community profiling
DNA was sent for small subunit rDNA pyrosequencing analyses at the Research and Testing Laboratory (Lubbock, TX, USA) using the Roche 454 GS-FLX platform (Roche 454, Branford, CT, USA). Sample libraries were prepared with the following primers for bacterial 16S rRNA gene (28F-5′-GAGTTTGATCNTGGCTCAG-3′, 519R-5′-GTNTTACNGCGGCKGCTG-3′), archaeal 16S rRNA gene (349F-5′-GYGCASCAGKCGMGAAW-3′, 806R-5′-GGACTACVSGGGTATCTAAT-3′), Eukaryal/fungal internal transcribed region (ITS) (ITS1F-5′-CTTGGTCATTTAGAGGAAGTAA-3′, ITS4R-5′-TCCTCCGCTTATTGATATGC-3′) genes. Processing of sequences was performed in Mothur following the 454 SOP that is outlined in Schloss et al. (2009). Briefly, sequences were quality filtered by removing primer sequences, reads <150 bp long, sequences with ambiguous base calls and homopolymer repeats >8 bp. Bacteria and archaeal sequences were reduced to only unique sequences and aligned to the Mothur-interpreted Silva bacterial database (contains unique sequences from the small subunit Ref database (v.102)) and trimmed to equal size. Chimera removal using chimera.uchime within Mothur was used to further reduce sequencing error before clustering. Operational taxonomic units (OTUs) were clustered using average-neighbour clustering with a 97% cutoff. All sequences were classified with Mothur using the Ribosomal Database Project (RDP) training set (v. 9), and sequences that could not be classified to the kingdom of the target primer set were removed. Pre-alignment steps for fungal ITS sequences were as described above, except that unaligned sequences were pre-clustered at 99% nucleotide similarity, and were then hierarchically clustered into OTUs at 97%, 95% and 90% similarity with CD-HIT (Li and Godzik, 2006), with a word size of 8 nucleotides and default parameters. Output cluster files were reformatted as Mothur.list files that were used for downstream OTU analysis. Initial classification of sequences was performed in Mothur using the UNITE/QIIME 12_11 ITS reference database. Diversity indices were calculated using Mothur, and were subsampled as appropriate.
Identification of isolates
DNA was extracted from isolates grown in liquid culture (tryptic soy broth) at room temperature using a phenol/chloroform/isoamyl alcohol extraction at 60 °C and ethanol precipitation. The 16S rRNA gene was amplified using PCR using primers (27F, 5′-AGAGTTTGATCCTGGCTCAG-3′ 758R 5′-CTACCAGGGTATCTAATCC-3′) for bacteria and the ITS region for fungi (ITS1, 5′-TCCGTAGGTGAACCTGCGG-3′ 5.8S, 5′-CGCTGCGTTCTTCATCG-3′) and resulting PCR product Sanger sequenced. Sequences were compared against the GenBank database using the BLASTn (http://blast.ncbi.nlm.nih.gov/Blast.cgi) algorithm and Classifier tool of RDPII (http://pyro.cme.msu.edu/) for identification of isolates.
Phylogenetic tree of archaea
Representative archaeal sequences were obtained after OTU analysis as described above (97% cutoff), and sequences that contained a minimum of 10 reads were compared with the GenBank database using the BLASTn algorithm. Top matches with environmental metadata were used for downstream analysis. All sequences were aligned using ClustalX (http://www.clustal.org/clustal2) and trimmed to the same length (170 bp), and a maximum likelihood phylogenetic tree (bootstrap value 500) was constructed in MEGA6.06 (http://www.megasoftware.net/).
Cell enumeration and isolation
Total cell counts on dry and ice-cemented permafrost were enumerated with DTAF (5-(4,6-dichlorotriazinyl) aminofluorescein) stain as described by Steven et al. (2008). Other fluorescence-based staining techniques were used, including DAPI (4',6-diamidino-2-phenylindole), Live/Dead BacLight viability stain (Molecular Probes Inc., Invitrogen, Eugene, OR, USA) and catalyzed activated reporter deposition-fluorescent in situ hybridization analyses, as carried out in the study of Niederberger et al. (2010); however, cells were not easily or unambiguously discernible using these methods and were not chosen for presentation or enumeration. To determine viable heterotrophic cell counts and isolate a diversity of bacteria, a dilution series was used with 11 different solid media. The solid media chosen were R2A agar; 1:2 R2A agar (supplemented with 1.5% agar (m/v)); mineral salts agar with no carbon source as well as augmented with yeast, tryptone and soluble starch (MSM+YTS; 0.25 g each per l); tryptone soy agar (TSA); 1:10 TSA supplemented with 1.5% agar (m/v); BG11 media for photoautotrophs (incubated in light), and R2A, 1:2 R2A, TSA and 1:10 TSA supplemented with 5% NaCl (m/v). Agar plates were incubated at 5 and 20 °C aerobically for all media, anaerobically (0 p.p.m. (O2)) at 25 °C in an anaerobic chamber (COY Laboratory Products, Inc., Grass Lake, MI, USA) and at 0 °C for media supplemented with 5% NaCl. All media were adjusted to neutral pH and plate counts were performed in triplicate, except for 0 °C incubations and anaerobic incubations that were carried out in duplicate. A total of four ice-cemented permafrost and two dry soil samples were used. As only one isolate was obtained using solid media directly, enrichment techniques were attempted. The first enrichment technique consisted of a 1-month incubation of ice-cemented permafrost at 5 °C with no amendment before plating on the Medias' described above at 5 °C. Enrichments for sulphate-reducing bacteria (Postgate medium) and perchlorate-reducing bacteria (per l: NH4Cl, 0.25 g; NaClO3, 1.03 g; CH3CO2Na, 1.36 g; NaH2PO4, 0.6 g; KCl, 0.1 g; NaHCO3, 2.5 g, 10 ml trace mineral and vitamin solution) were carried out at 25 °C anaerobically. Liquid aerobic heterotroph enrichments were also carried out on dry and ice-cemented permafrost at 5 °C and 20 °C in 0.1% Na4P2O7, R2A, TSA, 1:10 TSA, BG11 (incubated in light) and R2A, TSA and 1:10 TSA supplemented with 5% NaCl (m/v). Soil was incubated in liquid media and sampled at 2-week intervals for 6 weeks and then plated in duplicate on R2A, TSA and on solid media identical to the liquid enrichments being used at 5 °C (MSM+YTS media were used for 0.1% Na4P2O7 enrichments).
Radiorespiration assay for heterotrophic activity
Permafrost (5 g) was added to individual microcosms as in Steven et al. (2007a, b). Each microcosm was performed in triplicate, and included triplicate sterilized controls (autoclaved twice for 2 h at 120 °C and 1.0 atm, with a 24 h period between autoclavings). Microcosms were spiked with 0.045 mCi ml−1 (~100 000 disintegrations per mi of 1−14C acetic acid. Cold acetic acid was added to a final concentration of 15 mM acetic acid per microcosm in a total volume of 40 μl. The CO2 trap consisted of 1 M KOH for microcosms incubated at 5 °C and −5 °C, and 1 M KOH+20% v/v ethylene glycol for microcosms incubated at −10 °C and −15 °C. For nutrient-amended microcosms, 3 g of ice-cemented permafrost samples (IT-12 surface dry permafrost) was used for each microcosm, and spiked with cold and radioactive acetate as described. Nitrogen and phosphorus was added at a concentration of 1 mg g−1 wet soil, in the form of NH4Cl and Ca(H2PO4)2 respectively. Stationary-phase Planococcus halocryophilus OR1 biomass incubated at −5 °C in tryptic soy broth amended with 5% salt and 2% glycerol was pelleted, and rinsed 3 times with 0.1% Na4P2O7 buffer to remove residual media. A total of 106 cells in 50 μl buffer was added to individual microcosms. Amended samples were measured after 30 days of incubation at −5 °C. Measurements of radioactivity were determined by liquid scintillation spectrometry on a Beckman Coulter (Fullerton, CA, USA) LS 6500 Multi-purpose Scintillation Counter.
In-situ soil gas flux measurements
Soil CO2 flux was measured in the 2013 summer field season in University Valley with a Los Gatos Ultraportable Greenhouse Gas Analyzer that is able to detect 0.01–100 p.p.m. CH4 and 200–20 000 p.p.m. CO2 (Los Gatos Research, Mountain View, CA, USA). Two PVC soil collars were inserted to a depth of 6 cm at ~IT-5 cm and ~IT-10 cm sites, and allowed to equilibrate with the soil for 24 h before measurements being taken. Collars remained in place throughout the experiment, minimizing disturbance while enabling repeated measurements of CO2 flux from the same soil surface. The closed gas exchange system was customized with a LiCor-8100 chamber (Lincoln, NE, USA), and measurements were taken over 5 and 10 min intervals to measure small changes in chamber CO2 concentrations following efflux from soil. Measurements were taken for 2 consecutive days, three times daily in the morning, noon and night.
Soil analysis
The soils were analysed for total carbon and total nitrogen by combustion at 900 °C with a Carlo Erba (Milan, Italy) Flash EA 1112 NC Soils Analyzer that has an analytical error of ±1%. Gravimetric moisture content was measured as a percentage of dry weight. Net, 20 g of soil was oven dried at 100 °C for 48 h and weighed using a Mettler (Greifensee, Switzerland) AE 163 analytical balance with an accuracy of ±0.02 mg. The pH of soils was measured using a 1:2 slurry of soil/deionized water with a Fisher Scientific pH electrode (Fisher Scientific, Pittsburgh, PA, USA), with an efficiency slope of >95%.
Experimental determination of unfrozen water content
We determined the unfrozen water content of three bulk soil samples from University Valley collected near the head of the valley using Decagon 5TE (Pullman, WA, USA) three-in-one soil temperature, moisture and conductivity sensors. Soils were initially dried at 105 °C, and ∼500 cm3 of bulk soils from each site were placed in 1 l beakers. The soils were saturated with distilled water (18 Ω) and the Decagon 5TE sensor placed in the wet soils. The beakers were then placed in a Burnsco environmental test chamber (Arnprior, ON, Canada) and the ambient temperature in the chamber was programmed to decrease from +2 °C to –20 °C at steps of 1 °C every 1.5 h. After reaching –20 °C, the ambient temperature was increased to +2 °C at a rate of 1 °C every 1 h. This allowed determining the unfrozen water content during the cooling and warming of the soils as a slight hysteresis has been shown to occur. The Decagon 5TE sensors recorded temperature and apparent dielectric constant (Ka) at 1 min interval. To ensure the best accuracy in unfrozen water content, the measured apparent dielectric constant was first calibrated with the soils used in the experiments following the method described by Starr and Paltineanu (2002). The unfrozen water content (%wt; gH2O/g soils) is reported using the calibration curve and the accuracy is ±0.5%. The sensor accuracy for soil temperature is ±1 °C.
Results and discussion
Climate data were obtained between December 2009 and February 2013 using an automated weather station. Air climate and soil environmental data are summarized in Supplementary Table S1. The mean annual air temperature was −23.4±0.9 °C, with the maximum hourly value always below −2.8 °C. The mean annual soil surface temperature ranged between −23.5 °C and −26.5 °C, with the mean daily values remaining below 0 °C, irrespective of ice table depth; as such the soils are perennially cryotic lacking a seasonal thaw layer. Maximum hourly ground surface temperatures rose above 0 °C only for a few hours during clear summer days due to solar insolation, accounting for <1% of the year (<80 total h per year). The current climate conditions have likely persisted since the Eemian interglacial period, ca. 150 000 years ago (Lacelle et al., 2013), and extremely cold and dry conditions in this region have likely prevailed since the mid-Miocene (Marchant et al., 2013).
Soil samples were found to be highly oligotrophic (0.01%−0.05% total carbon, undetectable to 0.09% total nitrogen) and near neutral pH (7.5−8) (Supplementary Table S2). Total soluble solutes (<5 g l−1) were primarily of a SO4–NO3 geochemical composition (Lacelle et al., 2013). Very low microbial biomass was found by direct microscopic cell counts (1.4−5.7 × 103 cells per g soil) in both the dry and ice-cemented permafrost using DTAF stain as described by Steven et al. (2008). Comparatively, 2 orders of magnitude higher cell counts (1.2−4.5 × 105 cells per g soil) were detected in the active layer and permafrost soils from the Antarctic Peninsula. Other fluorescence-based staining techniques were used, including DAPI, Live/Dead BacLight viability staining and catalyzed activated reporter deposition-fluorescent in situ hybridization analyses, as carried out in the study of Niederberger et al. (2010); however, cells were not easily or unambiguously discernible using these methods and thus could not be accurately quantified. Close visual inspection of the cores showed no signs of biofilm microbial life including at the ice table that we had hypothesized to be a potentially habitable niche. Culturing was carried out using a variety of enrichment techniques and media previously used successfully in Arctic and Antarctic permafrost (Gilichinsky et al., 2007; Steven et al., 2007a) and included oligotrophic media as well as mineral salts media with no carbon added (see Materials and methods). Aerobic and anaerobic culturing from 6 soil samples along the two permafrost profiles yielded only 6 heterotrophic isolates on over 1000 agar plates in 2 years (Table 1); 2/6 of the soil samples yielded no culturable isolates (IT-5cm, 41 cm depth; IT-12, 28 cm depth). With the exception of one Chaetothyriales fungal isolate, the other 5 strains required liquid enrichment steps before isolation on media and ~3−5 months of incubation at 5 °C before colonies appeared on agar plates, indicating that the isolated organisms may have been in dormant or damaged states. Two strains of the 6 isolates (Rhodotorula and Rhodococcus spp.), isolated from within the ice-cemented permafrost (IT-12 cm, 37 cm depth), were both capable of sub-zero growth at −10 °C and −5 °C, respectively. We note that this ice-cemented permafrost is constantly at an ambient temperature of ~−25 °C, and the age of the soils is estimated to be in the order of 105 years (Lacelle et al., 2013). Attempts to enrich or isolate photoautotrophs, sulphate-reducing bacteria, perchlorate-reducing bacteria, methanotrophic or methanogenic bacteria were not successful. Both direct microbial and culturable bacterial counts from University Valley were 4−5 orders of magnitude below those encountered in Arctic permafrost and in the surface soils of mid- and low-elevation MDVs (Cowan et al., 2002; Gilichinsky et al., 2007; Steven et al., 2008) (Supplementary Table S3).
Table 1. Cultured isolates from University Valley.
| Isolate | Environment of closest BLAST match | % Similarity | Temp. growth range (°C)a | Salinity growth range (% NaCl) | Isolation media | Enrichment step before isolation | Sample | GenBank accession number |
|---|---|---|---|---|---|---|---|---|
| Rhodococcus sp. (Bacteria) | Cloud water (1465 m), France (HQ256820.1) | 97% | −5–30 | 0– 7 | R2A, 5 °C | 0.1% Na4P2O7, 2 weeks | IT-12, 37–42 cm | KM279631 |
| Methylobacterium sp. (Bacteria) | Moss phyllosphere (NR_117561.1) | 96% | 5– 30 | 0–5 | TSA+5% NaCl | TSA+5% NaCl, 2 weeks | IT-5, 5–10 cm | KM279632 |
| Rhodotorula sp. (Fungi) | Lake Vostok accretion ice, Antarctica (EU108797.1) | 99% | −10–30 | 0–15 | R2A, 5 °C | 0.1% Na4P2O7, 2 weeks | IT-12, 37–42 cm | KM279633 |
| Sphingomonas sp. (Bacteria) | Surface Soil, South Korea (NR_043171.1) | 95% | 5–25 | 0–5 | R2A | TSA+5% NaCl, 2 weeks | IT-12, 12–15 cm | KM279634 |
| Unidentified Chaetothyrialesb (Fungi) | University Valley sandstone endolith collected 1980/81 (GU250317.1) | 99% | 0–25 | ND | 1:10 TSA, 5 °C, 50 days; 1:2 R2A, TSA, 5 °C, 40 days | No enrichment | IT-12, surface soil; IT-5, 5–10 cm | KM279635 |
| Bacillus sp. (Bacteria) | Taibai mountain, China (KJ589539) | 99% | ND–25 | ND | R2A, anaerobic conditions | Perchlorate-reducing media | IT-5, 5–10 cm | KM279636 |
Abbreviations: ND, Not determined; Temp, temperature; TSA, tryptone soy agar.
No strains were found to grow at 37 °C.
Cannot identify to the genus level.
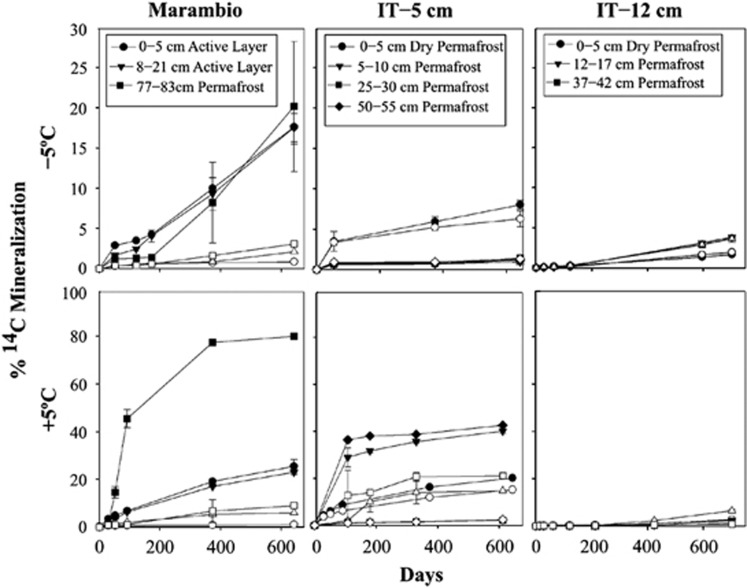
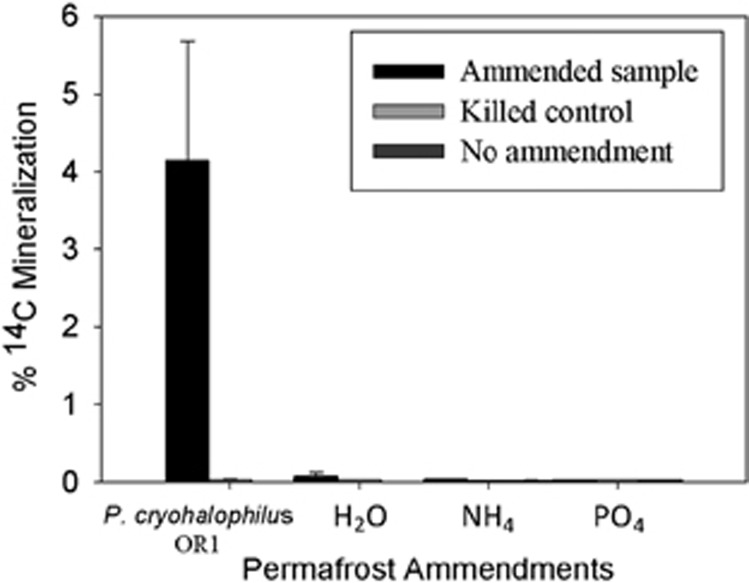
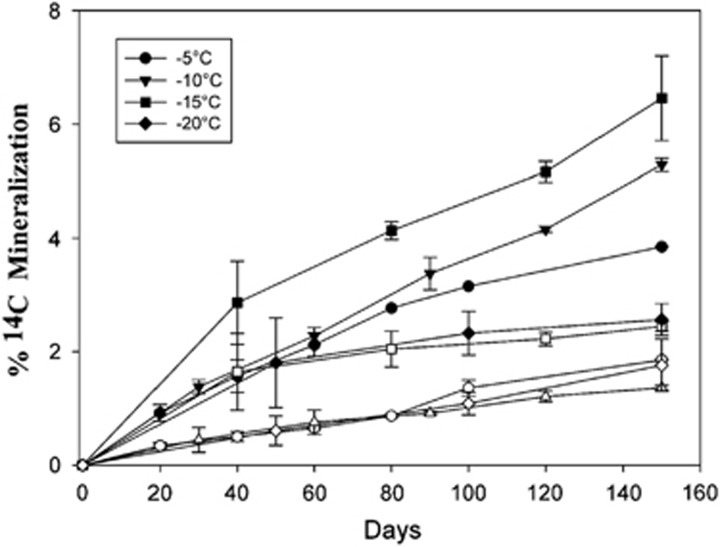
Radiorespiration assays (Steven et al., 2007b) (using 14C acetate as a C source) were carried out on 7 permafrost soil microcosms incubated at 5 °C, −5 °C, −10 °C and −15 °C for ~600 days (Figure 3) to detect microbial activity. For comparison, radiorespiration assays were also conducted with sandstone samples colonized by cryptoendolithic communities collected from the valley walls, and in permafrost and active layer soils from the Antarctic Peninsula. CO2 respiration in University Valley soil microcosms was not detected in any of the soils tested above abiotically produced background levels at sub-zero temperatures (P<0.05, paired t-test, 1 tailed) relevant to in situ conditions (Figure 3). In 4 of the 7 soil samples tested (all depths tested in the IT-12 cm core and the 25–30 cm sample in the IT-5 cm core), radiorespiration activity was not detected at any of the sub-zero temperatures or at +5 °C. It is possible that CO2 respiration at sub-zero temperatures did occur in these low biomass soils but was below the detection limits of this assay, although incubations were carried out for over 600 days in an attempt to detect low signal. It is also possible that microorganisms in these soils were not able to metabolize acetate, although acetate radiorespiration was clearly detected in three samples at +5 °C, indicating that the capacity for these populations to metabolize acetate can occur under favourable conditions, and that some University Valley permafrost soils have small, viable microbial populations. Low N and P levels were not limiting respiration as NH4 and PO4 additions had no effect on mineralization when incubated at −5 °C (Figure 4). Addition of water to dry soil samples in a 1:1 ratio also had no effect on mineralization rates. When dry soil samples from University Valley were amended with 106 cells per g soil of P. halocryophilus sp. OR1, an Arctic bacterium that reproduces at −15 °C and metabolizes at −25 °C in Arctic permafrost soil (Mykytczuk et al., 2013), high levels of mineralization were observed at −5 °C after 30 days, indicating that the lack of detectable microbial activity at sub-zero temperatures was not due to soil toxicity. In contrast, relatively higher respiration rates were measured in the sandstone cryptoendolithic communities as low as −20 °C (Figure 5), demonstrating that viable microbial communities exist within the porous sandstone rock. In addition, ~20% mineralization of 14C acetate was measured at −5 °C in soils from the Antarctic Peninsula, similar to values observed in Arctic permafrost (Steven et al., 2008) (Figure 3). During the 2013 summer field campaign, in situ CO2 and CH4 fluxes were not detected (detection limit 0.001 p.p.m. CH4 and 200 p.p.m. CO2) in University Valley soils at two sites where the ice table was located at 42 cm depth (IT-42) and 5 cm depth (IT-5 cm) (data not shown).
Figure 3.
Microbial activity assay in dry and ice-cemented permafrost samples. The 14C-labelled acetate radiorespiration assays. Open symbols represent abiotic CO2 production in killed controls. Standard error is reported here. Not shown are assays carried out at −10 °C and −15 °C that exhibited no detectable mineralization.
Figure 4.
Nutrient- and biomass-amended radiorespiration assays at −5 °C. The 30-day 14C-labelled acetate radiorespiration assays amended with 106 cells P. halocryophilus OR1, 3 ml H20, 1 mg g−1 soil of NH4Cl or 1 mg g−1 soil of Ca(H2PO4)2. Assays were carried out in triplicate and error bars denote s.e.
Figure 5.
Sub-zero respiration of 14C acetate measured in cryptoendoliths. Open symbols are killed controls and represent abiotic release of CO2. Bars denote s.e. of triplicate.
The permafrost microbial community structure was determined by pyrosequencing of the 16S rRNA gene for Bacteria and Archaea and the 18S ITS region for Fungi. Multiple attempts to extract RNA were carried out on a total of 20 g of soil, but no RNA was detectable after extraction, nor was an amplifiable product found after a reverse transcription reaction on the extracted product. This is likely because of the extremely low biomass and negligible activity, suggesting that the extracted DNA may be largely derived from dormant or dead organisms. As observed in other cold and temperate hyperarid soils (Lee et al., 2012; Stomeo et al., 2012; Crits-Christoph et al., 2013) community composition was cosmopolitan, highly variable (Supplementary Figure S1) and bacterial diversity (H' =1.1–4.3, 1673 unique OTUs) was high considering the extremely low viable and total microbial counts (Supplementary Table S4). Overall, Proteobacteria dominated the bacterial community, mainly comprising Gammaproteobacteria (25%) and Betaproteobacteria (9%). Firmicutes, Actinobacteria and Bacteroidetes were also variably present in samples. OTUs related to bacterial genera commonly associated with soil (Burkholderia, Ralstonia, Sphingomonas, Bradyrhizobium) and aquatic/marine environments (Alcanivorax, Pelagibacter, Gillisia) were found, although University Valley contains no aquatic habitats. Existing genera represented a diverse suite of potential metabolisms, with aerobic heterotrophy the most predominant; also detected were taxa associated with fermentation (Clostridia, Anaerolineae), methylotrophy (Methylobacteria, Methylophilus, Methylobacillus), sulphate reduction (Desulfovibrio), perchlorate reduction (Dechloromonas), sulphur and sulphite oxidation (Sulfuricella, Sulfitobacteria), nitrite oxidation (Nitrospira) and phototrophy; Cyanobacteria-Chloroplast and Rhodobacteraceae were found in the deeper ice-cemented permafrost samples. The genus (or order in the case of Chaetothyriales) of all cultured isolates in this study was found in the pyrosequencing results. Our molecular survey is consistent with the few diversity surveys reported for Dry Valley permafrost to date (Gilichinsky et al., 2007; Bakermans et al., 2014). Clone libraries in Taylor Valley (Bakermans et al., 2014) (elevation 21 m a.s.l.) and lower Beacon Valley permafrost (1000 m a.s.l.) (Gilichinsky et al., 2007) reported the presence of Proteobacteria, Gemmatimonadales, Chloroflexi and Acidobacteria, ubiquitous soil organisms that we also detected. Beacon Valley permafrost soils were found to be dominated by Gammaproteobacteria, results that are similar to one core in University Valley (IT-5 cm) but not in any depths of the other core profile (IT-12 cm). Previous work on surface soils in other Dry Valley soils have reported similarities at the phylum level, but high heterogeneity at the OTU level (Lee et al., 2012) that we also observed in University Valley. The differences in species composition found in surface soils of different Dry Valleys are likely driven by local physicochemical conditions (Lee et al., 2012), although allochthonous sources and dispersal of microorganisms through aeolian processes also likely play a role in community composition.
Archaeal sequences (H'=not determined −2.7) (Supplementary Table S4) were primarily composed of Halobacteria and Methanomicrobia (only detected in surface soils) and related to sequences previously detected in saline and alpine/cold environments (Supplementary Figure S2). Recovered fungal sequences (H'=0.04–1.7) were dominated by Dothideomycetes (54% of total reads) composed of the orders Capnodiales and Pleosporales. Eurotiomycetes (order Chaetothyriales) were only detected in surface soil samples; Eurotiomycetes include black yeast fungi known to be both desiccation and ultraviolet light resistant, and found in hot and cold desert lithic habitats (Selbmann et al., 2005; Ruibal et al., 2009).
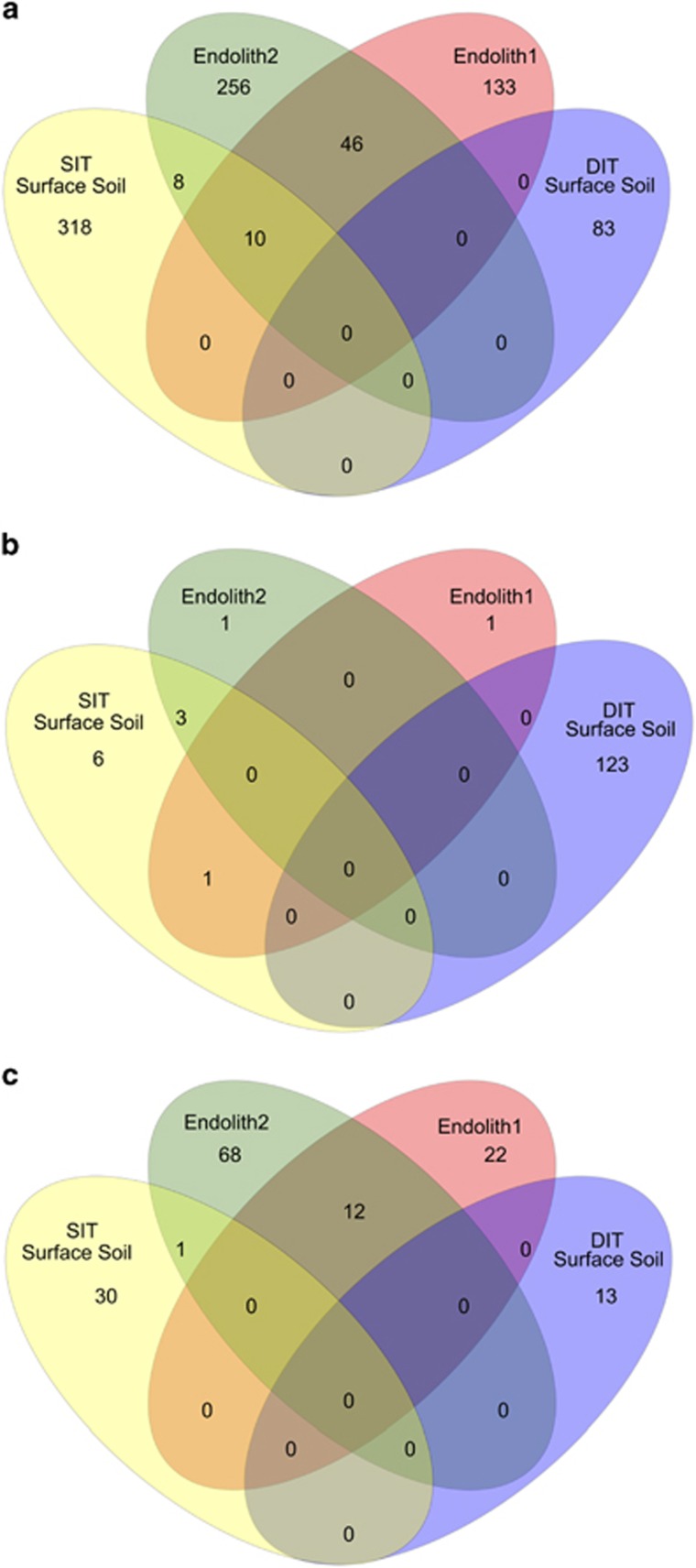
OTUs related to halophilic genera in both Archaea (Halobacterium, Haloarcula, Halorubrum) and Bacteria (Halomonas) could be indicative of selective pressures favourable to microorganisms capable of inhabiting briny films in permafrost. However, the absence of metabolic activity in the radiorespiration assays and the co-occurrence of halophilic organisms with taxa related to phototrophy and marine environments, as well as the presence of obligate anaerobes in aerobic surface soils, is more consistent with mixed allochthonous sources of microorganisms (Hopkins et al., 2006; Nkem et al., 2006). The most obvious allochthonous source of microorganisms is the sandstone (Beacon Supergroup) cryptoendolithic community colonizing part of the valley walls. However, pyrosequencing of two cryptoendolith samples showed they shared few bacterial, archaeal or fungal OTUs in common with University Valley permafrost soils (Figure 6), and the most abundant taxonomic groups in the sandstone were poorly represented (<1%) or completely absent. The disparity between the cryptoendolithic and the valley floor microbial communities suggests that airborne bacteria from other regions could be accumulating over time through aeolian/snow deposition and become preserved in the dry, cryotic soils, diluting much of the cryptoendolithic signature. An exception was the yeast strain isolated from dry and ice-cemented soils that was most closely related to a previously isolated University Valley cryptoendolith Chaetothyriales strain (Table 1) (Selbmann et al., 2005), suggesting that some cryptoendolith microorganisms are indeed seeding the soils on the valley floor and maintaining their viability within the permafrost.
Figure 6.
Shared OTUs between cryptoendolith and surface soils. Bacterial (a), archaeal (b) and fungal (c) shared OTUs based on 16S rRNA gene sequences. OTUs were defined at a 97% cutoff.
Based on our radiorespiration assays and the extremely low culturable and total microbial biomass observed, and given the continuous and prolonged dryness and subfreezing temperatures (Supplementary Table S1), it is unlikely that microbial communities in the permafrost soils analysed here metabolize at any time of the year, even during the austral summer, because of a lack of available water. The presence of salts and solutes in permafrost may reduce the freezing point of water and facilitate the formation of small but habitable brine veins within the soil matrix (Gilichinsky et al., 2003).
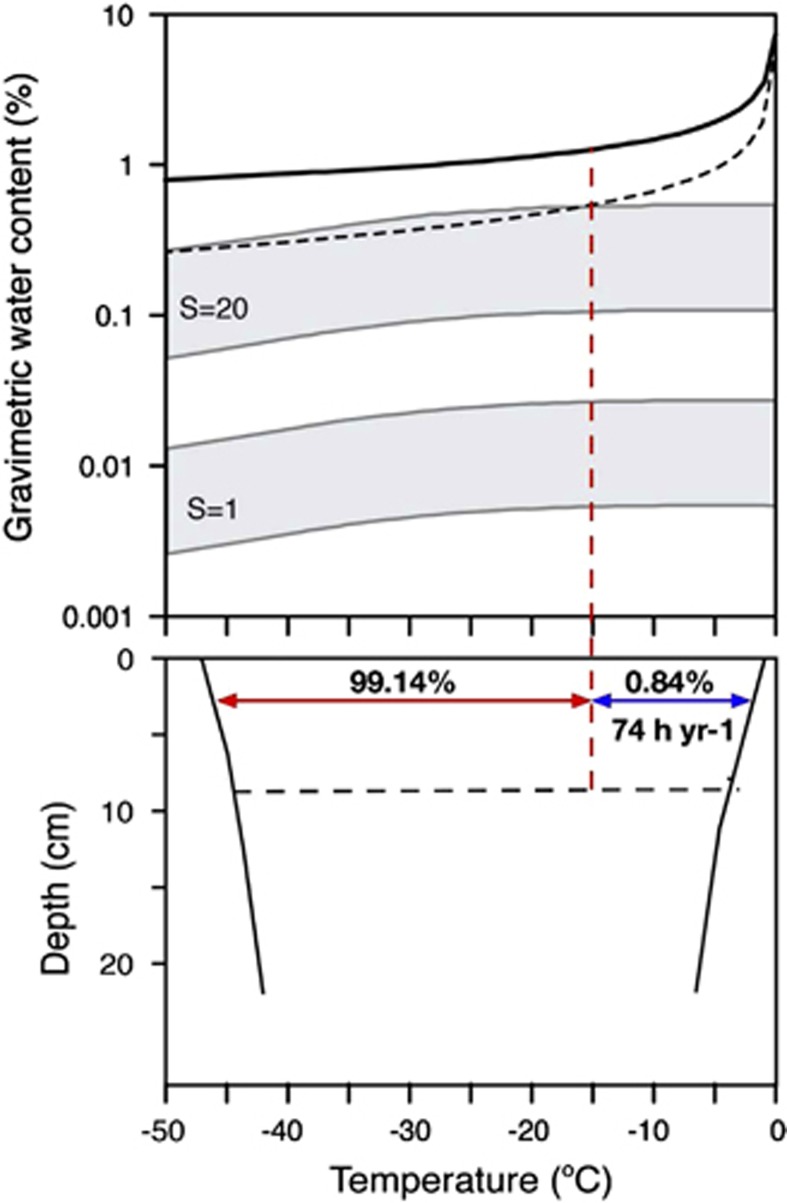
Thin films of water do not appear to be a relevant source of water in University Valley. Measured average unfrozen water content as a function of soil temperature is shown in Figure 7 for two bulk soil samples from University Valley. We computed the amount of water that would be strongly bound to soil particles (that is, interfacial water) using the Langmuir's absorption model, and a specific surface area of soil particles of 20 m2 g−1, as measured in Beacon Valley (Sizemore and Mellon, 2008), and 1 m2 g−1, as a hypothetical lower value. Unfrozen water content curves described a rapid transition from bulk (thin films) to interfacial water at −15 °C, with a progressive decrease of the latter as hydrogen bonds were slowly overcoming van Der Waals bonds at the mineral surfaces under cooling permafrost temperature. A considerable amount of unfrozen bulk water (>10%) was found to exist in the soils only at temperatures above −1 °C. Using the climate data (Supplementary Table S1), we calculated that bulk water (that is, when temperature > −15 °C, RH >95%) is present in the dry soils for ∼0.84% of the year (74 h per year). This is in contrast with conditions on colonized faces of sandstone boulders measured in Linneaus Terrace (1650 m a.s.l.), a dry valley with similar climate conditions as University Valley. In Linneaus Terrace, the colonized area of the boulders was shown to be moist for more than 700 h per year (Friedmann et al., 1993). In contraposition to soils in the stable upland zone, soils in the coastal thaw zone and the intermediate mixed zone are more likely to contain habitable liquid brine veins because of increased concentrations of salts and a higher marine influence (Figure 1). This, in addition to experiencing warmer temperatures and receiving more precipitation in the form of snow, makes the lower- and mid-elevation dry valley soils more hospitable and habitable.
Figure 7.
Unfrozen water content in University Valley soils. (Top) Gravimetric (unfrozen) water content as a function of temperature in University Valley soils. The black dotted line includes the error in the sensors. Grey areas show the region for interfacial water for two values of specific surface area of soil particles. Soil water content transitions from bulk to interfacial at −15 °C (red dotted line, see Supplementary Text for details). (Bottom) Yearly envelope of soil temperatures near the head of the valley as a function of depth. The black dotted line shows the ice table at 8 cm depth. Bulk water is present in the dry soils above the ice table for up to 74 h per year.
These results help us to place the known limits of life to grow and metabolize in an environmental context. We cannot rule out the possibility that microbial activity in University Valley permafrost soils might occur under natural conditions but below current detection limits. However, the same assay methodologies provide unambiguous evidence of active microbial activity at similar cold ambient temperatures in other permafrost soils from the Arctic and in lower elevation Antarctic Dry Valleys. For example, 14C acetate mineralization assays have detected a significant amounts of activity in permafrost soils from the Canadian high Arctic (Steven et al., 2008), Siberia (Rivkina et al., 2000) and in a lower elevation Antarctic Dry Valley (Taylor Valley) (Bakermans et al., 2014) within 50, 160 and 180 days respectively at −5 °C. Soils from the hyper-arid core of the Atacama Desert have cell numbers and culturable counts similar to University Valley permafrost (Supplementary Table S3), but small, viable microbial communities are activated and detected when Atacama soils are wetted (Navarro-González et al., 2003; Crits-Christoph et al., 2013). Our results suggest that microorganisms in the University Valley permafrost soils analysed here are not exposed to sufficiently long and frequent clement conditions to allow for metabolism or growth. Instead, our results suggest that a fundamental threshold may be crossed in some University Valley permafrost soils, where the combination of permanently subfreezing temperatures, low water activity, oligotrophy and age are severely constraining the evolution of functional cold-adapted organisms. As such, the microorganisms detected in this study may represent a transient inoculum rather than an established, cold-adapted microbial community. The depauperate nature of these soils is in contrast with the sandstone boulders and cliffs that are colonized by cryptoendolithic organisms, and our findings add to a growing body of evidence that extant life in extremely dry, cold or hot deserts is limited to specialized lithic habitats at very small scales (Davila et al., 2008; Pointing et al., 2009).
Dry permafrost as observed in University Valley is rare on Earth, likely only occurring in the McMurdo Dry Valleys (Bockheim and Tarnocai, 1998; Bockheim et al., 2007), but is commonplace in the northern polar regions of Mars at the Phoenix landing site (Levy et al., 2009). Thus, our results have implications for our understanding of the cold limits of life in terrestrial environments, with potential implications for habitability models of Mars near surface permafrost and other icy worlds.
Acknowledgments
This work was supported by NASA ASTEP program and with field support via NSF/OPP (project B-302-M). Support was provided by the Natural Sciences and Engineering Research Council (NSERC) Discovery Grant Program, NSERC Northern Supplements Program and NSERC CREATE Canadian Astrobiology Training Program (CATP). We thank Jon Rask at NASA Ames for providing the permafrost and active layer samples from the Marambio Antarctic site. We thank the Whalen laboratory, McGill University for soil analyses. Pyrosequencing data sets have been deposited in the NCBI Sequence Read Archive (SRA) under project PRJNA240343.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Bakermans C, Skidmore ML, Douglas S, McKay CP. (2014). Molecular characterization of bacteria from permafrost of the Taylor Valley, Antarctica. FEMS Microbiol Ecol 89: 331–346. [DOI] [PubMed] [Google Scholar]
- Bockheim J, Tarnocai C. (1998). Nature, occurrence and origin of dry permafrost. Proceedings of the Seventh International Conference on Permafrost: Yellowknife, Canada.
- Bockheim JG, Campbell IB, McLeod M. (2007). Permafrost distribution and active-layer depths in the McMurdo Dry Valleys, Antarctica. Permafrost Periglac Process 18: 217–227. [Google Scholar]
- Cary SC, McDonald IR, Barrett JE, Cowan DA. (2010). On the rocks: the microbiology of Antarctic Dry Valley soils. Nat Rev Microbiol 8: 129–138. [DOI] [PubMed] [Google Scholar]
- Cowan D, Russell N, Mamais A, Sheppard D. (2002). Antarctic Dry Valley mineral soils contain unexpectedly high levels of microbial biomass. Extremophiles 6: 431–436. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph A, Robinson CK, Barnum T, Fricke WF, Davila AF, Jedynak B et al. (2013). Colonization patterns of soil microbial communities in the Atacama Desert. Microbiome 1: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila AF, Gómez-Silva B, de Los Rios A, Ascaso C, Olivares H, McKay CP et al. (2008). Facilitation of endolithic microbial survival in the hyperarid core of the Atacama Desert by mineral deliquescence. J Geophys Res 113. [Google Scholar]
- Friedmann E, Kappen L, Meyer M, Nienow J. (1993). Long-term productivity in the cryptoendolithic microbial community of the Ross Desert, Antarctica. Microb Ecol 25: 51–69. [DOI] [PubMed] [Google Scholar]
- Gilichinsky D, Rivkina E, Shcherbakova V, Laurinavichuis K, Tiedje J. (2003). Supercooled water brines within permafrost-an unknown ecological niche for microorganisms: a model for astrobiology. Astrobiology 3: 331–341. [DOI] [PubMed] [Google Scholar]
- Gilichinsky D, Wilson G, Friedmann E, McKay C, Sletten R, Rivkina E et al. (2007). Microbial populations in Antarctic permafrost: biodiversity, state, age, and implication for astrobiology. Astrobiology 7: 275–311. [DOI] [PubMed] [Google Scholar]
- Heldmann J, Pollard W, McKay C, Marinova M, Davila A, Williams K et al. (2013). The high elevation Dry Valleys in Antarctica as analog sites for subsurface ice on Mars. Planet Space Sci 85: 53–58. [Google Scholar]
- Hopkins D, Sparrow A, Novis P, Gregorich E, Elberling B, Greenfield L. (2006). Controls on the distribution of productivity and organic resources in Antarctic Dry Valley soils. Proc R Soc B Biol Sci 273: 2687–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacelle D, Davila AF, Fisher D, Pollard WH, DeWitt R, Heldmann J et al. (2013). Excess ground ice of condensation–diffusion origin in University Valley, Dry Valleys of Antarctica: evidence from isotope geochemistry and numerical modeling. Geochim Cosmochim Acta 120: 280–297. [Google Scholar]
- Lee CK, Barbier BA, Bottos EM, McDonald IR, Cary SC. (2012). The Inter-Valley Soil Comparative Survey: the ecology of Dry Valley edaphic microbial communities. ISME J 6: 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JS, Head JW, Marchant DR. (2009). Cold and dry processes in the Martian Arctic: Geomorphic observations at the Phoenix landing site and comparisons with terrestrial cold desert landforms. Geophys Res Lett 36. [Google Scholar]
- Li WZ, Godzik A. (2006). Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–1659. [DOI] [PubMed] [Google Scholar]
- Marchant D, Mackay S, Lamp J, Hayden A, Head J. (2013). A review of geomorphic processes and landforms in the Dry Valleys of southern Victoria Land: implications for evaluating climate change and ice-sheet stability. Geo Soc London Spec Publ 381: 319–352. [Google Scholar]
- Marchant DR, Head JW III. (2007). Antarctic dry valleys: Microclimate zonation, variable geomorphic processes, and implications for assessing climate change on Mars. Icarus 192: 187–222. [Google Scholar]
- Marinova MM, Mckay CP, Pollard WH, Heldmann JL, Davila AF, Andersen DT et al. (2013). Distribution of depth to ice-cemented soils in the high-elevation Quartermain Mountains, McMurdo Dry Valleys, Antarctica. Antarct Sci 25: 575–582. [Google Scholar]
- Mckay CP. (2009). Snow recurrence sets the depth of dry permafrost at high elevations in the McMurdo Dry Valleys of Antarctica. Antarct Sci 21: 89–94. [Google Scholar]
- Mykytczuk NC, Foote SJ, Omelon CR, Southam G, Greer CW, Whyte LG. (2013). Bacterial growth at -15 °C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J 7: 1211–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-González R, Rainey FA, Molina P, Bagaley DR, Hollen BJ, de la Rosa J et al. (2003). Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science 302: 1018–1021. [DOI] [PubMed] [Google Scholar]
- Niederberger TD, Perreault NN, Tille S, Lollar BS, Lacrampe-Couloume G, Andersen D et al. (2010). Microbial characterization of a subzero, hypersaline methane seep in the Canadian High Arctic. ISME J 4: 1326–1339. [DOI] [PubMed] [Google Scholar]
- Nkem JN, Wall DH, Virginia RA, Barrett JE, Broos EJ, Porazinska DL et al. (2006). Wind dispersal of soil invertebrates in the McMurdo Dry Valleys, Antarctica. Polar Biol 29: 346–352. [Google Scholar]
- Pointing SB, Chan Y, Lacap DC, Lau MCY, Jurgens JA, Farrell RL. (2009). Highly specialized microbial diversity in hyper-arid polar desert. Proc Natl Acad Sci USA 106: 19964–19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkina EM, Friedmann EI, McKay CP, Gilichinsky DA. (2000). Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microb 66: 3230–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruibal C, Gueidan C, Selbmann L, Gorbushina A, Crous P, Groenewald J et al. (2009). Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Stud Mycol 64: 123–133-S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB et al. (2009). Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb 75: 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbmann L, De Hoog G, Mazzaglia A, Friedmann E, Onofri S. (2005). Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Stud Mycol 51: 1–32. [Google Scholar]
- Sizemore HG, Mellon MT. (2008). Laboratory characterization of the structural properties controlling dynamical gas transport in Mars-analog soils. Icarus 197: 606–620. [Google Scholar]
- Starr JL, Paltineanu IC. (2002). Capacitance Devices. In: Dane JH, Topp GC (eds) Methods of Soil Analysis, Part 4, Physical Methods. SSSA: Madison, WI, USA, pp 463–474. [Google Scholar]
- Steven B, Briggs G, McKay CP, Pollard WH, Greer CW, Whyte LG. (2007. a). Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using culture-dependent and culture-independent methods. FEMS Microbiol Ecol 59: 513–523. [DOI] [PubMed] [Google Scholar]
- Steven B, Niederberger TD, Bottos EM, Dyen MR, Whyte LG. (2007. b). Development of a sensitive radiorespiration method for detecting microbial activity at subzero temperatures. J Microbiol Methods 71: 275–280. [DOI] [PubMed] [Google Scholar]
- Steven B, Pollard WH, Greer CW, Whyte LG. (2008). Microbial diversity and activity through a permafrost/ground ice core profile from the Canadian high Arctic. Environ Microbiol 10: 3388–3403. [DOI] [PubMed] [Google Scholar]
- Stomeo F, Makhalanyane TP, Valverde A, Pointing SB, Stevens MI, Cary CS et al. (2012). Abiotic factors influence microbial diversity in permanently cold soil horizons of a maritime-associated Antarctic Dry Valley. FEMS Microbiol Ecol 82: 326–340. [DOI] [PubMed] [Google Scholar]
- Tamppari L, Anderson R, Archer P, Douglas S, Kounaves S, Mckay C et al. (2012). Effects of extreme cold and aridity on soils and habitability: McMurdo Dry Valleys as an analogue for the Mars Phoenix landing site. Antarct Sci 24: 211–228. [Google Scholar]
- Witherow RA, Lyons WB, Bertler NA, Welch KA, Mayewski PA, Sneed SB et al. (2006). The aeolian flux of calcium, chloride and nitrate to the McMurdo Dry Valleys landscape: evidence from snow pit analysis. Antarct Sci 18: 497–505. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.