Abstract
Background
We evaluated whether preoperative biliary drainage was predictive of recurrence and survival among patients with resectable pancreatic cancer.
Methods
Patients with pancreatic cancer who were treated with upfront surgery between 2000 and 2012 were identified and stratified by preoperative percutaneous transhepatic cholangiogram-guided drainage (PTBD), placement of endoscopic stents (ERCP), or no biliary drainage (NBD). The primary endpoint was overall survival.
Results
We identified 193 patients with resectable pancreatic head cancer (33 PTBD; 96 ERCP; and 64 NBD). Key differences between the three groups were more patients who underwent >1 preoperative biliary procedures (p = 0.004) in the PTBD cohort. PTBD patients had a significant increase in hepatic recurrence rate compared with patients who did not undergo PTBD (44.8 vs. 23.3 %, p = 0.02). PTBD patients also had worse overall survival. Median and 5-year survival for PTBD, ERCP, and NBD patients were 17.5 months and 3 %, 22.4 months and 24 %, and 28.9 months and 32 %, respectively (p = 0.002). MVA revealed that percutaneous drainage was an independent predictor of worse overall survival [HR 1.76, 95 % CI (1.05–2.99), p = 0.03].
Conclusions
Patients with resectable pancreatic cancer who receive PTBD have more advanced disease, higher hepatic recurrence, and worse survival.
Keywords: Pancreatic cancer, PTBD, ERCP, Biliary drainage, Survival
Introduction
Pancreatic cancer is the second most common gastrointestinal malignancy, the fourth deadliest cancer in the USA and will account for an estimated 45,220 new cases and 38,460 deaths in 2013 [1]. Patients with pancreatic cancer have an extremely poor prognosis, with only 20 % of patients presenting with potentially curable, local disease at initial diagnosis, and 5-year outcomes among all patients ranging from 5 to 6 % [1].
Approximately 80 % of pancreatic cancers occur at the head of the pancreas and in close proximity to the biliary drainage system [2]. Given the high frequency of pancreatic head tumors, 35–75 % of patients will present with obstructive jaundice [3–5]. Bile obstruction leading to cholestasis has been shown to interfere with vitamin K-dependent clotting mechanisms, lead to cholangitis, and hepatotoxcity by means of bile extravasation, decreased hepatic blood flow, and reduced bile salts in the small bowel [2].
In an attempt to reduce the symptomatic and physiologic problems associated with hyperbilirubinemia in patients with pancreatic cancer, preoperative biliary decompression is often performed either endoscopically with an endoscopic retrograde cholangiopancreatography (ERCP)-guided stent, or by percutaneous transhepatic biliary drainage (PTBD). Despite the potential symptomatic and physiologic benefits of preoperative biliary drainage, routine preoperative biliary decompression remains a controversial issue [2, 6]. The placement of preoperative ERCP stents to decompress the biliary system has been associated with an increased risk of bactibilia, which, in turn, increases the risk of infectious complications and renal insufficiency [7]. Preoperative biliary drainage has been associated with increased rates of many other types of perioperative morbidity, without improving perioperative outcomes [8, 9]. As a result, cholangitis remains the only undisputed indication for immediate preoperative biliary decompression, while intense pruritus, a delay from diagnosis to surgery, and extreme jaundice remain other possible indications for preoperative biliary drainage [2].
Initial clinical studies comparing ERCP-guided biliary decompression and PTBD demonstrated an increase in surgical morbidity with PTBD raising questions as to whether percutaneous drainage should be performed [10]. While PTBD has been shown to improve pruritus symptoms, there is no improvement in patient's quality of life and PTBD potentially establishes a pancreaticodermal tract allowing for tumor cell seeding that can lead to recurrences [10–12]. Despite the controversy surrounding percutaneous drainage, PTBD is often performed in the event of ERCP failure [13]. We sought to evaluate whether PTBD in resectable pancreatic cancer patients influences hepatic recurrence rates and overall survival.
Methods and materials
Patient characteristics
We performed an institutional review board-approved study using our tumor registry of more than 2,000 pancreatic cancer patients from 2000 to 2012. We restricted our analysis to patients who underwent upfront surgical resection for carcinoma of the pancreatic head. Patients were excluded if they had in situ disease, metastatic disease, or unusual pancreatic tumor histologies including lymphoma, cystadenoma, intraductal papillary mucinous neoplasm, signet ring cell carcinoma, neuroendocrine tumors, islet cell tumors such as gastrinoma, insulinoma, glucagonoma, and VIPoma, or treatment with neoadjuvant chemotherapy and/or radiation therapy. All patients with biopsy-proven pancreatic head cancer underwent a preoperative staging work-up including an endoscopic ultrasound, a computed tomography scan with intravenous contrast of the thorax, abdomen and pelvis ± magnetic resonance imaging ± a positron emission tomography scan to determine resectability. All cases were presented at the gastrointestinal tumor board to determine resectability.
Preoperative biliary drainage
Patients were initially diagnosed with, or suspected of having, pancreatic cancer at nearby community hospitals and were referred to Moffitt Cancer Center for additional management. The majority of patients (95 %) presenting with obstructive jaundice underwent biliary drainage with either ERCP or PTBD prior to surgery with the remaining going straight to surgery. Patients who experienced ERCP stent occlusion had either ERCP stent revision (n = 12) or PTBD placed (n = 1). Patients treated with PTBD at any point were included in the PTBD group for analysis, and patients treated with at least one successful ERCP-guided stent placement without subsequent PTBD placement were included in the ERCP-guided stent group. ERCP-guided stents included plastic, uncovered metal, partially covered metal, or fully covered metal stents.
Surgery
Patients with pancreatic head tumors underwent pancreaticoduodenectomy with or without a pylorus-sparing procedure. All but two patients underwent regional lymph node sampling or dissection. Postoperative CA19-9 levels were recorded in 143 (74 %) cases.
Adjuvant therapy
Following surgery, patients received chemoradiation with or without neoadjuvant or adjuvant chemotherapy, chemotherapy alone, or no adjuvant therapy. Adjuvant therapy was initiated within 4 months from the time of surgery in all except two cases (149 of 151 cases).
Patients treated with chemotherapy alone received single-agent gemcitabine. Patients treated with chemotherapy followed by radiation were treated in a similar fashion to the Radiation Therapy Oncology Group (RTOG) 9704 protocol with one month of gemcitabine followed by concurrent chemoradiation with continuous infusion 5-FU or gemcitabine, followed by adjuvant gemcitabine. Patients treated with chemoradiation alone received concurrent radiation with 5-FU or gemcitabine. The median radiation dose was 50 Gy (range 45–55.8 Gy) in 180–200 cGy daily fractions for a median of 28 fractions (range 25–33) to the pancreatic tumor bed and regional lymphatics; a minority of patients received a boost to the tumor bed (median 0 Gy, range 0–14.4 Gy).
Endpoints and statistical analysis
The primary endpoint was overall survival, defined as the interval from surgery to date of death. Secondary endpoints included recurrence rates and perioperative morbidity and mortality. Recurrences were recorded as the site of first failure locally, in the liver, or at all other sites. All recurrences were included for those who failed at >1 site as the first failure (7 patients, 3.6 %). Recurrence information was available for 175 patients (90.7 %); nine patients (4.7 %) died within 90 days of surgery; and nine patients (4.7 %) died >90 days post-surgery without clear imaging or pathologic documentation of their pancreatic cancer status.
Perioperative complications (<30 days following surgery) were recorded, including pancreatic leak, gastrojejunostomy leak, atrial fibrillation, pulmonary embolism, blood clot with bowel necrosis, wound infection, wound dehiscence, postoperative hemorrhage, and pancreatic or enterocutaneous fistula. 30-, 60-, and 90-day mortality rates were recorded.
Statistical analysis was performed using SPSS® version 21.0 (IBM®, Chicago, IL). Continuous variables were compared using both Wilcoxon rank sum test and the Kruskal–Wallis test as appropriate. Pearson's Chi-square test was used to compare categorical variables. Actuarial rates of overall survival were calculated using the Kaplan–Meier method and the log-rank test. A Cox multivariate model was performed for overall survival, including all clinical, histopathologic, and treatment variables. Time from diagnosis to surgery was included in the model and defined as either the time from initial diagnosis, or the time from failed ERCP procedure to surgery in patients treated PTBD. Continuous variables for inclusion in the multivariate model were split at clinically meaningful cut-points; postoperative CA19-9 level was split at <90 and ≥90. All statistical tests were two-sided, and an α (type I) error <0.05 was considered statistically significant.
Results
We identified 193 patients who met inclusion criteria [33 PTBD; 96ERCP; and 64with nobiliary drainage (NBD)] with a median follow-up of 42 months (range 3–156 months). Patient characteristics are presented in Table 1. PTBD patients underwent more preoperative procedures than the ERCP or NBD patients (18.2, 13.5, 0 %, respectively, p = 0.004). There were also significant differences in pathologic T3/4 tumor status (81.8 % PTBD, 88.5 % ERCP, and 67.2 % NBD, p = 0.004) and median time from surgery to adjuvant treatment (63 days PTBD, 56 days ERCP, and 72 days NBD, p = 0.04) between the groups. There were no other significant differences in patient characteristics.
Table 1.
Patient characteristics
| Variable | Level | PTBD; N (%) | ERCP; N (%) | NBD; N (%) | p value |
|---|---|---|---|---|---|
| Median age (years, range) | 67 (44–86) | 69 (25–90) | 69 (37–88) | 0.31 | |
| Sex | Male | 13 (39.4) | 53 (55.2) | 32 (50.0) | 0.29 |
| Female | 20 (60.6) | 43 (44.8) | 32 (50.0) | ||
| Number of biliary procedures | ≤1 | 27 (81.8) | 83 (86.5) | 64 (100) | 0.004 |
| >1 | 6 (18.2) | 13 (13.5) | 0 (0) | ||
| Vein resection | 2 (6.1) | 9 (9.4) | 7 (10.9) | 0.74 | |
| Median tumor size (cm, range) | 2.5 (1.2–5.5) | 3.0 (0.5–12.0) | 3.0 (0.8–6.0) | 0.66 | |
| Pathologic tumor stage | 1/2 | 6 (18.2) | 11 (11.5) | 21 (32.8) | 0.004 |
| 3/4 | 27 (81.8) | 85 (88.5) | 43 (67.2) | ||
| Median nodes positive (range) | 2 (0.10) | 2 (0.16) | 1 (0.25) | 0.07 | |
| Median nodes removed (range) | 16 (0.29) | 12 (1.45) | 13 (0.49) | 0.23 | |
| Pathologic nodal stage | 0 | 6 (18.2) | 32 (33.3) | 27 (42.2) | 0.06 |
| 1 | 27 (81.8) | 64 (66.7) | 37 (57.8) | ||
| Tumor grade | Well | 4 (12.1) | 13 (13.5) | 11 (17.2) | 0.17 |
| Moderate | 19 (57.6) | 59 (61.5) | 42 (65.6) | ||
| Poor/Undifferentiated | 8 (24.2) | 24 (25.0) | 8 (12.5) | ||
| Unknown | 2 (6.1) | 0 (0) | 3 (4.7) | ||
| Surgical margins | Negative | 27 (81.8) | 80 (83.3) | 55 (85.9) | 0.85 |
| Positive | 6 (18.2) | 16 (16.7) | 9 (14.1) | ||
| Postoperative CA19–9 > 90 | No | 17 (51.5) | 56 (58.3) | 41 (64.1) | 0.54 |
| Yes | 5 (15.2) | 13 (13.5) | 11 (17.2) | ||
| Unknown | 11 (33.3) | 27 (28.1) | 12 (18.8) | ||
| Median time from surgery to adjuvant treatment (days, range) | 63 (30–99) | 56 (21–195) | 72 (22–202) | 0.043 | |
| Adjuvant treatment | None | 8 (24.2) | 21 (21.9) | 13 (20.3) | 0.94 |
| Chemoradiation | 21 (63.6) | 58 (60.4) | 39 (60.9) | ||
| Chemo | 4 (12.1) | 17 (17.7) | 12 (18.8) |
PTBD percutaneous biliary drainage, ERCP endoscopic retrograde cholangiopancreatography, NBD no biliary drainage, RT radiation therapy, y years
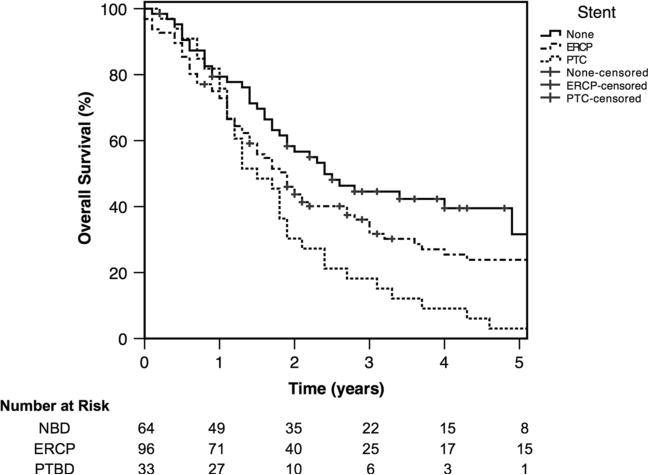
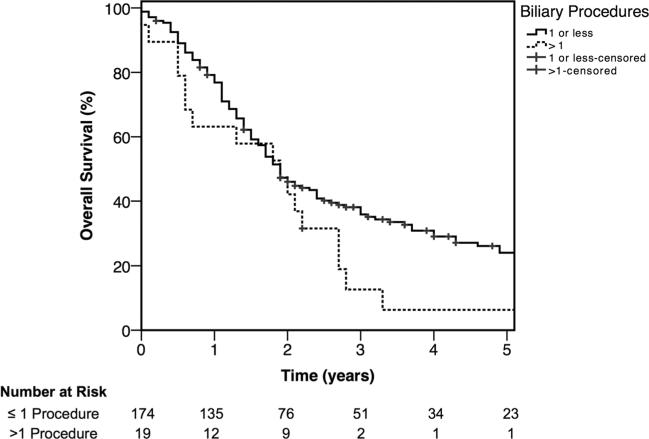
Kaplan–Meier analysis of overall survival by method of preoperative biliary drainage is displayed in Fig. 1. PTBD patients had worse overall survival. Median and 5-year survival for PTBD, ERCP, and NBD patients were 17.5 months and 3 %, 22.4 months and 24 %, and 28.9 months and 32 %, respectively (p = 0.002). Figure 2 shows overall survival stratified by number of biliary procedures. Patients who had more than one biliary procedure had a significantly worse overall survival. Three-year and 5-year overall survival for patients with ≤1 versus >1 procedure was 37.2 and 23.9 % versus 12.6 and 6.3 %, respectively (p = 0.04).
Fig. 1.
Kaplan–Meier overall survival plot of patients treated with preoperative biliary drainage by percutaneous transhepatic catheterization (PTBD), endoscopic retrograde cholangiopancreatography (ERCP)-guided stent placement, or those who did not receive biliary drainage (NBD; p = 0.002)
Fig. 2.
Kaplan–Meier overall survival plot of patients treated with ≤1 or >1 preoperative biliary procedure (p = 0.04)
Variables associated with more advanced disease as demonstrated by positive lymph node status are shown in Table 2. On univariate Cox analysis, pathologic T3/4 status, moderate and poor/undifferentiated tumor grades, number of nodes examined, and PTBD were all associated with nodal positivity compared with their respective counterparts (p = 0.002, p = 0.049, p = 0.005, p = 0.01, p = 0.02, respectively). However, on multivariate analysis, only pathologic T3/4 tumor stage (hazard ratio (HR) 2.31, 95 % confidence interval (CI) 1.01–5.28, p = 0.048) was associated with involved lymph nodes. There was also a trend toward N1 nodal status with PTBD (HR 2.68, 95 % CI 0.94–7.67, p = 0.07), suggesting that PTBD is a surrogate for more locally advanced disease.
Table 2.
Univariate and multivariate analysis for node positivity
| Variable | Level | UV OR (95 % CI) | UV p value | MV OR (95 % CI) | MV p value |
|---|---|---|---|---|---|
| Tumor size | 1.08 (0.85, 1.37) | 0.51 | 0.99 (0.77, 1.27) | 0.95 | |
| Tumor stage | T1/2 | Ref | |||
| T3/4 | 3.12 (1.50, 6.46) | 0.002 | 2.31 (1.01, 5.28) | 0.048 | |
| Tumor grade | Well | Ref | |||
| Moderate | 2.31 (1.00, 5.31) | 0.049 | 1.99 (0.83, 4.78) | 0.12 | |
| Poor/undifff | 4.62 (1.58, 13.50) | 0.005 | 3.15 (1.00, 9.94) | 0.0497 | |
| Unknown | 1.73 (0.25, 12.01) | 0.58 | 1.51 (0.17, 13.7) | 0.71 | |
| Nodes examined | 1.05 (1.01, 1.09) | 0.01 | 1.03 (0.99, 1.07) | 0.21 | |
| Preoperative biliary drainage | NBD | Ref | |||
| ERCP | 1.50 (0.76, 2.80) | 0.26 | 1.13 (0.55, 2.32) | 0.74 | |
| PTBD | 3.28 (1.19, 9.05) | 0.02 | 2.68 (0.94, 7.67) | 0.07 |
UV univariate, OR odds ratio, MV multivariate; CI confidence interval, Ref reference, PTBD percutaneous biliary drainage, ERCP endoscopic retrograde cholangiopancreatography, NBD no biliary drainage
Table 3 displays the Cox regression multivariable analysis for overall survival. The method of preoperative biliary drainage was an independent predictor of overall survival; PTBD patients had significantly increased mortality (HR 1.77, 95 % CI 1.05–2.99, p = 0.03), while ERCP-guided stent patients did not have a similar increase in mortality (HR 1.41, 95 % CI 0.92–2.15, p = 0.11) compared with patients who received no preoperative biliary drainage. Other factors influencing mortality included having >1 preoperative biliary procedures performed (HR 1.72, 95 % CI 1.00–2.96, p = 0.049), a pathologic nodal N1 status (HR 2.68, 95 % CI 1.76–4.08, p < 0.001), a postoperative CA19–9 > 90 status (HR 3.06, 95 % CI 1.76–5.33, p < 0.001), and the method of adjuvant treatment. Treatment with both adjuvant chemoradiation therapy and adjuvant chemotherapy alone was associated with improved survival compared with patients who received no adjuvant treatment (HR 0.39, 95 % CI 0.24–0.63, p < 0.001 and HR 0.35, 95 % CI 0.18–0.66, p = 0.001, respectively).
Table 3.
Patient characteristics with univariate and multivariate analysis of overall survival
| Variable | Level | Median OS (m) | UV HR (95 % CI) | UV p value | MV HR (95 % CI) | MV p value |
|---|---|---|---|---|---|---|
| Age (years)a | 1.02 (1.00, 1.03) | 0.04 | 1.01 (0.99, 1.02) | 0.38 | ||
| Gender | Male | 23.8 | Ref | |||
| Female | 20.8 | 1.12 (0.86, 1.66) | 0.29 | 1.11 (0.77, 1.58) | 0.58 | |
| Biliary drainage | NBD | 28.9 | Ref | |||
| ERCP | 22.4 | 1.40 (0.95, 2.08) | 0.09 | 1.41 (0.92, 2.15) | 0.11 | |
| PTBD | 17.5 | 2.23 (1.40, 3.55) | 0.001 | 1.77 (1.05, 2.99) | 0.03 | |
| Biliary procedures | ≤1 | 22.6 | Ref | |||
| >1 | 22.5 | 1.66 (1.01, 2.73) | 0.046 | 1.72 (1.00, 2.96) | 0.049 | |
| Tumor grade | Well | 28.9 | Ref | |||
| Moderate | 21.7 | 1.16 (0.72, 1.87) | 0.54 | 1.07 (0.64, 1.78) | 0.80 | |
| Poor/undiff | 15.8 | 1.30 (0.74, 2.27) | 0.36 | 0.96 (0.52, 1.74) | 0.88 | |
| Unknown | 29.9 | 1.17 (0.40, 3.43) | 0.77 | 1.47 (0.46, 4.64) | 0.51 | |
| Vein resection | No | 23.2 | Ref | |||
| Yes | 16.9 | 1.35 (0.78, 2.35) | 0.29 | 1.09 (0.60, 2.01) | 0.78 | |
| Tumor size (cm)* | 1.12 (1.00, 1.24) | 0.04 | 1.10 (0.96, 1.27) | 0.18 | ||
| Nodal status | N0 | 48.2 | Ref | |||
| N1 | 17.5 | 2.49 (1.69, 3.65) | <0.001 | 2.68 (1.76, 4.08) | <0.001 | |
| Nodes removed | ≤15 | 23.3 | Ref | |||
| >15 | 22.4 | 1.25 (0.89, 1.76) | 0.21 | 0.86 (0.58, 1.28) | 0.46 | |
| Surgical margins | Negative | 23.2 | Ref | |||
| Positive | 17.3 | 1.30 (0.85, 2.01) | 0.23 | 1.41 (0.87, 2.28) | 0.16 | |
| Postoperative CA19–9 | ≤90 | 27.9 | Ref | |||
| >90 | 10.3 | 2.81 (1.79, 4.42) | <0.001 | 3.06 (1.76, 5.33) | <0.001 | |
| Unknown | 22.4 | 1.04 (0.71, 1.54) | 0.83 | 0.78 (0.50, 1.20) | 0.25 | |
| Adjuvant treatment | None | 13.2 | Ref | |||
| Chemoradiation | 24.7 | 0.61 (0.41, 0.90) | 0.01 | 0.39 (0.24, 0.63) | <0.001 | |
| Chemotherapy | 20.8 | 0.74 (0.43, 1.28) | 0.29 | 0.35 (0.18, 0.66) | 0.001 |
OS overall survival, HR hazard ratio, CI confidence interval, Ref reference, NBD no biliary drainage, PTBD percutaneous biliary drainage, ERCP endoscopic retrograde cholangiopancreatography, Ref reference,
Continuous variable
The site of first recurrence was then assessed with respect to PTBD status (Table 4). Patients treated with PTBD had significantly increased rates of hepatic metastases (44.8 vs. 23.3 %, p = 0.02) and any site of metastasis (79.3 vs. 56.2 %, p = 0.02) compared with patients who received an ERCP-guided stent or no biliary drainage. There was no difference in rate of local recurrence, or other site of recurrence (i.e., lung or peritoneum) between the two groups (p = 0.21, p = 0.68, respectively).
Table 4.
Site of first recurrence
| Recurrence site | PTBD; N (%) | Other; N (%) | p value |
|---|---|---|---|
| Liver | 13 (44.8) | 34 (23.3) | 0.02 |
| Local | 4 (13.8) | 10 (6.8) | 0.21 |
| Other | 8 (27.6) | 46 (31.5) | 0.68 |
| Overall | 23 (79.3) | 82 (56.2) | 0.02 |
PTBD percutaneous biliary drainage
PTBD patients, ERCP-guided stent patients, and those without biliary drainage were then compared with respect to surgical complications (Table 5). PTBD patients had a higher rate of wound infections than both ERCP patients and patients with biliary drainage (24.2 vs. 13.5 vs. 4.7 %, p = 0.02). There were no other significant differences in perioperative morbidity between the groups. Rates of postoperative mortality significantly differed between the three groups at 30-day post-surgery (p = 0.04), but did not differ at 60- and 90-day post-surgery. Patients who received an ERCP-guided stent had an increased 30-day mortality rate compared with the other two groups; six patients (6.3 %) who received an ERCP-guided stent died within 30 days of surgery compared with no patients who received percutaneous biliary drainage or no biliary drainage.
Table 5.
Postoperative complications
| Postoperative complications | PTBD; N (%) | ERCP; N (%) | NBD, N (%) | p value |
|---|---|---|---|---|
| Pancreatic leak | 2 (6.1) | 8 (8.3) | 7 (10.9) | 0.71 |
| G-J leak | 1 (3) | 2 (2.1) | 0 (0) | 0.44 |
| Atrial fibrillation | 3 (9.1) | 3 (3.1) | 7 (10.9) | 0.13 |
| Pulmonary embolism | 0 (0) | 3 (3.1) | 0 (0) | 0.21 |
| Abscess | 1 (3) | 3 (3.1) | 1 (1.6) | 0.82 |
| Wound infection | 8 (24.2) | 13 (13.5) | 3 (4.7) | 0.02 |
| Wound dehiscence | 0 (0) | 1 (1) | 0 (0) | 0.60 |
| Anastomotic bleed | 1 (3) | 4 (4.2) | 4 (6.3) | 0.74 |
| Stricture | 0 (0) | 1 (1) | 0 (0) | 0.60 |
| Enterocutaneous fistula | 0 (0) | 1 (1) | 0 (0) | 0.60 |
| SMA clot with bowel necrosis | 0 (0) | 1 (1) | 0 (0) | 0.60 |
| Peritonitis | 0 (0) | 2 (2.1) | 2 (1.8) | 0.36 |
| 30-day mortality | 0 (0) | 6 (6.3) | 0 (0) | 0.04 |
| 60-day mortality | 0 (0) | 6 (6.3) | 1 (1.6) | 0.14 |
| 90-day mortality | 1 (3) | 7 (7.3) | 1 (1.6) | 0.22 |
PTBD percutaneous biliary drainage, ERCP endoscopic retrograde cholangiopancreatography, NBD no biliary drainage, G-J gastrojejunostomy, SMA superior mesenteric artery
Of 193 patients who underwent surgery, six patients died within 30 days of surgery and nine died within 90 days. All nine patients who died within 90 days of surgery presented with jaundice; one went directly to surgery without stent placement, one had incomplete ERCP drainage prior to surgery, and seven experienced complete biliary drainage prior to surgery (one PTBD and six ERCP). There were multiple causes of acute mortality among these nine patients. One patient developed an intraoperative portal vein hemorrhage eventually leading to cardiac arrest. Another patient developed a blood clot postoperatively in the superior mesenteric artery causing bowel ischemia. A third patient developed bilateral lower extremity deep vein thromboses with subsequent fatal pulmonary emboli. Three patients developed postoperative hemorrhage involving the gastroduodenal artery. Two other patients developed pancreatic leaks found by drain chemistry, followed by respiratory failure requiring intubation and deconditioning; both patients recovered briefly and were discharged to rehabilitation facilities, but passed away shortly after from unknown causes. The final patient had poor return of bowel function following surgery with placement of a gastrostomy tube for nutritional support, but passed away soon after discharge from unknown causes. No patients who died within 90 days of surgery had documented preoperative cholangitis, elevated creatinine (>1.5 mg/dL), or hypoalbuminemia (<3.5 g/dL).
Discussion
We demonstrate an overall survival detriment with preoperative PTBD in jaundiced patients with resectable pancreatic cancer. The worse overall survival with PTBD was likely multifactorial; PTBD patients had a trend toward an increased rate of pathologic N1 tumor status, which was also an independent risk factor for poor overall survival. Compared with patients who did not require preoperative biliary drainage, PTBD patients had the highest risk of death, with a relative risk of 1.77, suggesting that the placement of a percutaneous drain itself likely increases the risk of mortality. The independent risk of death with PTBD might also be a result of an increased rate of hepatic metastases with percutaneous drainage, possibly from tumor seeding along the drainage tract.
A prospective study by Speer et al. [10] in 1987 first demonstrated that preoperative endoscopic stent insertion, rather than percutaneous stenting, leads to a higher success rate of relief of jaundice and a lower 30-day mortality rate (15 vs. 30 %, respectively). The increased 30-day mortality with percutaneous drainage in the study resulted from an increased rate of liver hemorrhage and bile leaks with the percutaneous, transhepatic approach. Our findings did not support the increased 30-day mortality with PTBD, and in fact, we found a lower incidence of 30-day mortality in PTBD patients compared with ERCP patients (0 vs. 6.3 %, respectively). These contrasting results are likely a result of improved imaging, procedural and surgical techniques over the past three decades; a 30-day mortality of 15 % with endoscopic stent placement and 30 % following percutaneous stent placement would be considered high by today's standards [13–15]. Speer et al. [10] also assessed only short-term outcomes between the different methods of biliary drainage and did not assess whether PTBD patients had different rates of metastases or late-mortality compared with ERCP patients. In contrast, the current study assessed both perioperative mortality and late recurrence and mortality rates.
Cases of tumor seeding in the liver or skin along the biliary catheter tract have previously been reported in the literature [11, 12]. In one report, an autopsy of an individual with initial metastatic tumor seeding at the tumor wall demonstrated numerous additional metastases along the catheter tract [11]. Tumor seeding may have also been responsible for the finding of a high rate of hepatic metastases in PTBD patients in the current study. However, definitively proving that hepatic metastases were a result of tumor seeding in the liver would be extremely difficult, given that the liver is a common site of pancreatic tumor metastasis.
Preoperative biliary drainage remains a controversial issue, and two recent reviews of the literature, along with a randomized controlled trial, showed that preoperative biliary drainage with either ERCP stents or PTBD lead to an increase in perioperative morbidity, without significantly affecting or improving perioperative outcomes compared with patients who proceeded directly to surgery [2, 8, 9]. Yet not all stents have equal efficacy, and plastic stents have been shown to occlude more rapidly than metal stents and are unable to maintain patency long enough for neoadjuvant chemoradiotherapy for pancreatic carcinoma [16, 17]. Recent retrospective and prospective studies suggest that endoscopic placement of short expandable metal stents (SEMS) provides a safe and effective method of improving jaundice in patients with resectable or borderline resectable pancreatic carcinoma [18, 19]. A recent trial assessing the safety and efficacy of SEMS for biliary decompression in resectable pancreatic cancer prior to neoadjuvant chemotherapy found promising results, with only 15 % of stents malfunctioning at 260 days following placement (13 % occluded and 2 % migrated) [19]. Covered SEMS have also been shown to maintain patency longer than uncovered SEMS, and they are more easily removed than uncovered SEMS, making them a good option in patients with resectable pancreatic cancer [20]. A recent meta-analysis comparing covered and uncovered SEMS found that covered SEMS maintain patency longer in patients with distal malignant obstruction [20].
Studies have demonstrated an increased rate of perioperative morbidity following ERCP stent placement, including early stent occlusion, need for stent exchange, pancreatitis, cholangitis, perforation, hemorrhage, bile leak, and wound infection [2, 9]. The current study did not include ERCP stent occlusion or stent exchange as an endpoint, since we focused only on morbidity in the postoperative period. PTBD patients did have an increased rate of wound infection compared with ERCP-stented patients, who in turn had an increased rate of wound infection compared with patients who did not receive biliary drainage (24.2, 13.5, 4.7 %, respectively, Table 5). Speer et al. [10] demonstrated an increased risk of liver hemorrhage and bile leak with PTBD, which lead to an increased rate of perioperative mortality. The current study does not support this finding. We instead found a similar rate of postoperative pancreatic/bile leak between PTBD, ERCP-stented, and patients without biliary drainage (6.1, 8.3, 10.9 %, respectively), along with a similar rate of intraoperative or postoperative hemorrhage between the three groups (3.0, 4.2, 6.3 %, respectively).
Given the present findings that patients who undergo PTBD with resectable pancreatic cancer have a high risk of metastasis and poor survival, these patients might benefit from preoperative local or systemic therapies. The current standard of care following upfront resection for pancreatic cancer is either adjuvant chemotherapy alone [21] or adjuvant chemotherapy combined with chemoradiation [22]. However, even with resection and subsequent therapy, survival outcomes remain poor and in the range of 15–20 % [23]. In the current study, we found that PTBD patients had an even worse 5-year survival rate at 3 %. For patients with such a poor prognosis, neoadjuvant therapy with either chemotherapy or chemoradiation provides a promising opportunity to both increase the chance of cure by addressing aggressive local or systemic disease spread early and to identify patients who have aggressive tumors and can be spared the morbidity of surgery [24].
This study was limited by its retrospective nature and by its inherent selection bias. The study also took place over a 12-year period, over which time advances in diagnostic, procedural, surgical, and adjuvant therapies likely improved outcomes in patients with resectable pancreatic cancer. Despite this, the study did include a large, extremely homogeneous and consistent population of patients with resectable pancreatic carcinoma, treated at a single institution with relatively consistent techniques among physicians from diagnosis to treatment. With respect to the rate of positive margins in this study, margin positivity at our institution has dropped in recent years to 16 % (2000–2012) from a previous cumulative rate of 27 %, as reported during an earlier time period from 1987 to 2006 [25].
In conclusion, PTBD patients had an independently worse overall survival, likely a result of advanced locoregional disease on presentation, as well as an increased rate of hepatic metastases.
Footnotes
Disclosures Tobin J. Strom, Jason B. Klapman, Gregory M. Springett, Kenneth L. Meredith, Sarah E. Hoffe, Junsung Choi, Pamela Hodul, Mokenge P. Malafa and Ravi Shridhar have no conflicts of interest or financial ties to disclose.
Contributor Information
Tobin J. Strom, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA
Jason B. Klapman, Gastrointestinal Tumor Program, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
Gregory M. Springett, Gastrointestinal Tumor Program, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
Kenneth L. Meredith, Department of Surgery, University of Wisconsin Hospital and Clinic-Madison, Madison, WI, USA
Sarah E. Hoffe, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA
Junsung Choi, Department of Interventional Radiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Pamela Hodul, Gastrointestinal Tumor Program, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Mokenge P. Malafa, Gastrointestinal Tumor Program, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
Ravi Shridhar, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Bonin EA, Baron TH. Preoperative biliary stents in pancreatic cancer. J Hepato-Biliary-Pancreat Sci. 2011;18:621–629. doi: 10.1007/s00534-011-0403-8. [DOI] [PubMed] [Google Scholar]
- 3.Uchida H, Shibata K, Iwaki K, Kai S, Ohta M, Kitano S. Ampullary cancer and preoperative jaundice: possible indication of the minimal surgery. Hepatogastroenterology. 2009;56:1194–1198. [PubMed] [Google Scholar]
- 4.Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17:991–997. doi: 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of pancreatic cancer in symptomatic patients in primary care: a large case–control study using electronic records. Br J Cancer. 2012;106:1940–1944. doi: 10.1038/bjc.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bellis M, Palaia R, Sandomenico C, Di Girolamo E, Cascella M, Fiore F. Is preoperative endoscopic biliary drainage indicated for jaundiced patients with resectable pancreatic cancer? Curr Drug Targets. 2012;13:753–763. doi: 10.2174/138945012800564167. [DOI] [PubMed] [Google Scholar]
- 7.Sivaraj SM, Vimalraj V, Saravanaboopathy P, Rajendran S, Jeswanth S, Ravichandran P, Vennilla R, Surendran R. Is bactibilia a predictor of poor outcome of pancreaticoduodenectomy? Hepatobiliary Pancreat Dis Int. 2010;9:65–68. [PubMed] [Google Scholar]
- 8.Fang Y, Gurusamy KS, Wang Q, Davidson BR, Lin H, Xie X, Wang C. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2012;9:CD005444. doi: 10.1002/14651858.CD005444.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 10.Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57–62. doi: 10.1016/s0140-6736(87)92733-4. [DOI] [PubMed] [Google Scholar]
- 11.Chapman WC, Sharp KW, Weaver F, Sawyers JL. Tumor seeding from percutaneous biliary catheters. Annals of surgery. 1989;209:708–713. doi: 10.1097/00000658-198906000-00008. discussion 713–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu Y, Yasui K, Kato T, Yamamura Y, Hirai T, Kodera Y, Kanemitsu Y, Ito S, Shibata N, Yamao K, Ohhashi K. Implantation metastasis along the percutaneous transhepatic biliary drainage sinus tract. Hepatogastroenterology. 2004;51:365–367. [PubMed] [Google Scholar]
- 13.Neal CP, Thomasset SC, Bools D, Sutton CD, Garcea G, Mann CD, Rees Y, Newland C, Robinson RJ, Dennison AR, Berry DP. Combined percutaneous-endoscopic stenting of malignant biliary obstruction: results from 106 consecutive procedures and identification of factors associated with adverse outcome. Surg Endosc. 2010;24:423–431. doi: 10.1007/s00464-009-0586-0. [DOI] [PubMed] [Google Scholar]
- 14.Rai R, Dick R, Doctor N, Dafnios N, Morris R, Davidson BR. Predicting early mortality following percutaneous stent insertion for malignant biliary obstruction: a multivariate risk factor analysis. Eur J Gastroenterol Hepatol. 2000;12:1095–1100. doi: 10.1097/00042737-200012100-00005. [DOI] [PubMed] [Google Scholar]
- 15.Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33:213–221. doi: 10.1016/j.ctrv.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Elwir S, Sharzehi K, Veith J, Moyer MT, Dye C, McGarrity T, Mathew A. Biliary stenting in patients with malignant biliary obstruction: comparison of double layer, plastic and metal stents. Dig Dis Sci. 2013;58:2088–2092. doi: 10.1007/s10620-013-2607-z. [DOI] [PubMed] [Google Scholar]
- 17.Decker C, Christein JD, Phadnis MA, Wilcox CM, Varadarajulu S. Biliary metal stents are superior to plastic stents for preoperative biliary decompression in pancreatic cancer. Surg Endosc. 2011;25:2364–2367. doi: 10.1007/s00464-010-1552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui AA, Mehendiratta V, Loren D, Kowalski T, Fang J, Hilden K, Adler DG. Self-expanding metal stents (SEMS) for preoperative biliary decompression in patients with resectable and borderline-resectable pancreatic cancer: outcomes in 241 patients. Dig Dis Sci. 2013;58:1744–1750. doi: 10.1007/s10620-012-2482-z. [DOI] [PubMed] [Google Scholar]
- 19.Aadam AA, Evans DB, Khan A, Oh Y, Dua K. Efficacy and safety of self-expandable metal stents for biliary decompression in patients receiving neoadjuvant therapy for pancreatic cancer: a prospective study. Gastrointest Endosc. 2012;76:67–75. doi: 10.1016/j.gie.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 20.Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74(321–327):e321–323. doi: 10.1016/j.gie.2011.03.1249. [DOI] [PubMed] [Google Scholar]
- 21.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. J Am Med Assoc. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 22.Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Rich TA, Willett CG. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–1756. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 24.Hoffe S, Rao N, Shridhar R. Neoadjuvant vs adjuvant therapy for resectable pancreatic cancer: the evolving role of radiation. Semin Radiat Oncol. 2014;24:113–125. doi: 10.1016/j.semradonc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Helm JF, Centeno BA, Coppola D, Druta M, Park JY, Chen DT, Hodul PJ, Kvols LK, Yeatman TJ, Carey LC, Karl RC, Malafa MP. Outcomes following resection of pancreatic adeno-carcinoma: 20-year experience at a single institution. Cancer Control. 2008;15:288–294. doi: 10.1177/107327480801500403. [DOI] [PubMed] [Google Scholar]