Abstract
Background
Air pollution is linked to increased emergency room visits for headache and migraine patients frequently cite chemicals or odors as headache triggers, but the association between air pollutants and headache is not well-understood. We previously reported that nasal administration of environmental irritants acutely increases meningeal blood flow via a TRPA1-dependent mechanism, involving the trigeminovascular system. Here, we examine whether chronic environmental irritant exposure sensitizes the trigeminovascular system.
Methods
Male rats were exposed to acrolein, a TRPA1 agonist, or room air by inhalation for 4 days prior to meningeal blood flow measurements. Some animals were injected daily with a TRPA1 antagonist, AP-18 or vehicle prior to inhalation exposure. Trigeminal ganglia were isolated following blood flow measurements for immunocytochemistry and/or qPCR determination of TRPV1, TRPA1 and CGRP levels.
Results
Acrolein inhalation exposure potentiated blood flow responses to both TRPA1 and TRPV1 agonists compared to room air. Acrolein exposure did not alter TRPV1 or TRPA1 mRNA levels or TRPV1 or CGRP immunoreactive cell counts in the trigeminal ganglion. Acrolein sensitization of trigeminovascular responses to a TRPA1 agonist was attenuated by pre-treatment with AP-18.
Interpretation
These results suggest trigeminovascular sensitization as a mechanism for enhanced headache susceptibility after chemical exposure.
Keywords: headache, trigeminal ganglion, TRPA1, TRPV1, acrolein
INTRODUCTION
Headache is one of the most common neurological disorders, yet remains poorly understood and undertreated. Migraineurs worldwide report similar headache triggers including air pollutants and odors (1). Specifically, increased air pollution is correlated with an increase in emergency room visits for headache symptoms (2–4) and poor air quality and odors are commonly reported triggers of migraine (1). Multiple Chemical Sensitivity (MCS (5)) is an acquired disorder associated with chemical exposure. MCS patients report headache and other symptoms, although the link between chemical exposure and the induction of headache remains unknown.
Activation of the trigeminovascular system is thought to play a critical role in headache. Release of the inflammatory peptides substance P and calcitonin gene-related peptide (CGRP) from trigeminal sensory neuron endings (6) produces neurogenic inflammation and vasodilatation in the dura (7;8), which are believed to be mechanistically linked to headache pain. We have previously demonstrated that nasal administration of environmental irritants stimulates meningeal vasodilatation acutely via a TRPA1 receptor-dependent mechanism (9). This suggests a molecular mechanism for acute environmental irritant-induced headache, but what remains unclear is whether previous airborne chemical exposure may sensitize the trigeminovascular system and predispose subjects to headache.
TRPA1 receptors have been shown to act as sensors of many environmental irritants including acrolein (10), formaldehyde (11), and umbellulone (12), and of pungent plant ingredients such as mustard oil (10;13), cinnamaldehyde (13), and allicin (10;14). TRPA1 is an excitatory nonselective cation channel co-localized with CGRP in a subset of sensory neurons (14;15), including those of the trigeminal ganglion (TG) (16). TRPA1 receptors are expressed in trigeminal sensory neurons innervating the nasal mucosa (17) where they may be activated by inhalants. TRPA1 receptors are usually co-localized with TRPV1 receptors (15;18;19) and the two receptors have been shown to interact functionally (20). We have demonstrated that TRPV1 expressing neurons are important for TRPA1 responses in the nasal-meningeal pathway (21). Herein we examine the functional role of both receptors in meningeal vasodilatation following irritant exposure.
It has been proposed that sensitization of the headache pain pathway may occur through repeated or sustained activation of peripheral nociceptors (22;23) but this process is not well-understood. Here we address the question of whether prolonged and repeated in vivo inhalation exposure to non-toxic levels of the TRPA1 agonist acrolein changes trigeminovascular responsiveness. Furthermore, we explore whether changes in responsiveness are linked to changes in TRPA1, TRPV1 and/or CGRP expression.
Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Indiana University School of Medicine and followed the ethical guidelines of International Association for the Study of Pain (24). Experiments were performed on 131 adult male (170–250 g) Sprague-Dawley rats (Harlan Bioproducts, IN). Rats were housed in pairs with a standard light and dark cycle with free access to food and water.
Inhalation Exposure
Rats were exposed to acrolein by mixing acrolein gas (Air Liquide, Plumsteadville, PA) and room air to obtain the desired concentration. The acrolein dosage (0.3 ppm) was chosen because it produced minimal or no detectable harmful effects in previous studies (25–27) and in our preliminary studies. In addition, it is equivalent to the limit for short-term exposure recommended by National Institute of Occupational Safety and Health. Separate inhalation chambers (Braintree Scientific, Inc; 5.5 L total volume) and tubing were used for acrolein and control groups to avoid cross-contamination. The flow rate was maintained at 1.5 L/min and temperature and humidity were monitored in the chamber. Rats were exposed to acrolein (0.32 ± 0.06 ppm (n = 52) 4 hours per day for 4 days while control animals were exposed to room air with the same paradigm. Cumulative acrolein exposure for each animal was determined with monitoring badges placed in the chamber (Advanced Chemical Sensors Inc, Boca Raton, FL). On day 5 approximately 22 hours after the last inhalation exposure, laser Doppler flowmetry was conducted followed by tissue collection. Selected groups of rats received daily injections of vehicle (9% DMSO in sterile saline; 200 μl injection volume i.p.; n =45) or AP-18 (200 μg/kg; Sigma; n = 28) diluted in vehicle 30 min before inhalation chamber exposure. Animals exposed to room air gained 12% in both saline and AP-18 injected groups over the course of the experiment, whereas those animals exposed to acrolein demonstrated a minor (4%) weight loss in both saline and AP-18 injected groups after the first exposure day. However, the rates of weight gain after the initial exposure were identical in all groups.
Laser Doppler flowmetry
Laser Doppler flowmetry was performed as previously described (9). Male rats were anesthetized with ketamine/xylazine (80 and 10 mg/kg body weight, respectively), followed by additional doses of ketamine/xylazine (40 and 5 mg/kg body weight) as needed. Body temperature was maintained at 37° C with a homeothermic blanket. For the measurement of meningeal blood flow, the skull was fixed in a stereotaxic frame and a cranial window prepared (9) with the dura left intact. Dural blood flow was measured with a laser Doppler flowmeter (TSI, MN). A needle type probe was placed over a large branch of the middle meningeal artery (MMA), distant from visible cortical blood vessels and the cranial window kept moist with synthetic interstitial solution (SIF) consisting of: 135 mM NaCl, 5 mM KCl, 5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM D-glucose (pH 7.3). Blood flow was sampled at 1Hz with a Digidata 1320 interface using Axoscope software (Axon Instruments, CA).
Blood flow drug administration
To stimulate the nasal mucosa, 25 μl of test compound or vehicle solution was applied over a 30 sec period at a site 2 mm into the right nostril using a Pipetman pipette (9;28). Solutions of mustard oil (Sigma) were prepared fresh daily by diluting in SIF to the desired concentrations. Stock solutions of capsaicin (10 mM; Sigma) were dissolved in ethanol and stored at −20°C and then diluted to the desired concentration with SIF prior to use. A thirty minute stabilization period preceded all test drug applications to ensure steady basal blood flow measurements. Each animal in blood flow experiments (Figures 1, 4–6) was given nasal saline as a vehicle for mustard oil testing or saline containing 0.0001% ethanol as a vehicle for capsaicin testing 15 min before administering the TRP agonist. Saline or vehicle controls produced less than 2% change in blood flow on average consistent with our previous published results (9;21) (data not shown). Thirty minutes after TRP agonist administration, 50 μl of 3 M KCl was administered to the dura to assess the integrity of meningeal blood vessel responses. Some rats received a single injection of saline two hours before or AP-18 either two hours or 27 hours before blood flow drug administration. Following completion of the blood flow experiments, each rat was decapitated and the brain removed. The underlying left TG was rapidly removed, frozen on dry ice and stored at −80°C for later qPCR analysis. The right TG was fixed in situ by placement of the skull into 4% paraformaldehyde in 0.1 M PBS (pH 7.4).
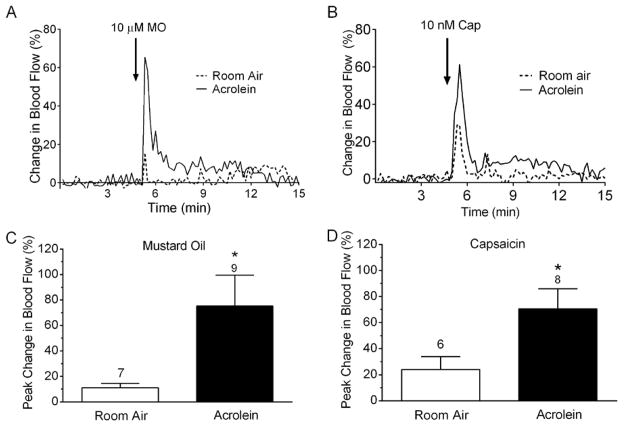
Figure 1.
Blood flow changes in the middle meningeal artery after nasal administration of mustard oil (A, C) or capsaicin (B, D) after exposure to acrolein vapor or room air. (A, B) Representative traces of middle meningeal blood flow in response to nasally administered mustard oil (A) or capsaicin (B) after acrolein vapor or room air. Laser Doppler flowmetry measurements were collected at 1 Hz and filtered at 0.1 Hz for graphical representation. Arrows indicate nasal administration of agonist. (B, D) Compared to room air-exposed animals, blood flow response to nasal mustard oil or capsaicin was significantly increased in acrolein-exposed animals. Values are means ± S.E.M. Number of animals per group is indicated. P < 0.05 compared to blood flow changes in room air-exposed animals.
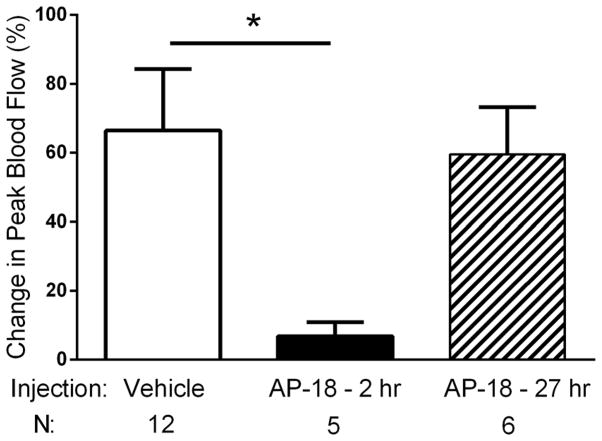
Figure 4.
Effects of systemic AP-18 on nasal administration of 30 μM mustard oil. AP-18 administered two hours before nasal mustard oil, significantly attenuated peak blood flow responses compared to saline administration 2 hours before nasal mustard oil (*, p < 0.05). In contrast, AP-18 administered 27 hours before nasal mustard oil had no effect on blood flow responses. Values are means ± S.E.M. Number of animals per group is indicated. Note that 30 μM mustard oil is used in this experiment because the animals have not been exposed to acrolein.
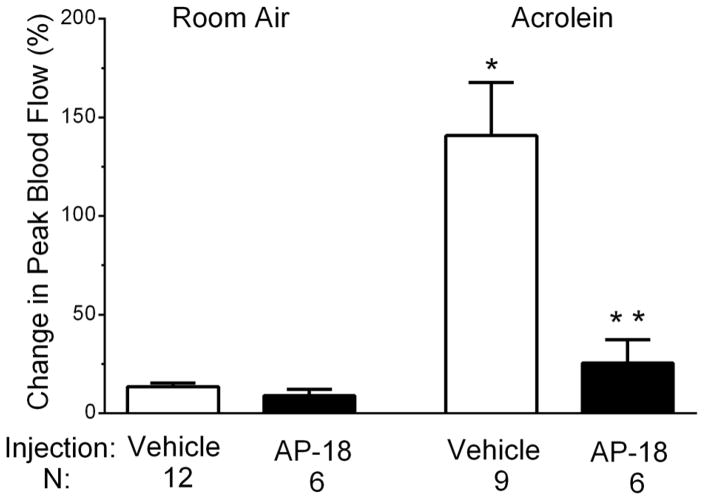
Figure 6.
Effects of daily administration of the TRPA1 antagonist AP-18 prior to each inhalation exposure on blood flow changes in response to nasal administration of 10 nM capsaicin. Compared to vehicle treated controls, blood flow response to nasal mustard oil was decreased in AP-18 injected animals in acrolein exposed animals (**, p < 0.05) but not in room air exposed animals. Values are means ± S.E.M. Number of animals per group is indicated.
Immunocytochemistry
To determine whether irritant exposure altered the number of TRPV1- or CGRP-positive TG neurons, immunocytochemistry was performed. Following overnight fixation, the TG was removed from the skull and placed in PBS and then cryoprotected in 10% sucrose for 2 hrs followed by 20% sucrose in PBS overnight at 4°C. Tissues were cut to 35 μm in thickness and collected free-floating in PBS. For immunofluorescent labeling, sections were rinsed in PBS and blocked in 4% normal goat serum and 0.025% Triton-X100 in PBS for 1 hr. The sections were then incubated with the primary antibody (rabbit anti-TRPV1 C-terminus (29); dilution 1:7500 or mouse anti-CGRP (Sigma) dilution 1:2000) diluted in blocking solution overnight at 4°C. Subsequently, sections were rinsed in PBS and incubated with the secondary antibody (Dylight 549-conjugated to goat anti-rabbit IgG, dilution 7.5 μg/ml, Jackson ImmunoResearch or Alexa Fluor 488 goat anti-mouse, dilution 7.5 μg/ml, Jackson ImmunoResearch) for 1 hr at room temperature. The sections were rinsed, mounted on slides, and treated with Prolong Gold antifade reagent (Life Technologies) before coverslips were affixed. Fluorescent images were acquired with a 4x objective and cell counts conducted in Photoshop (Adobe). TG sections containing all 3 subdivisions were used for cell counts. No reactivity was observed in control sections in which primary antibody was omitted.
RNA isolation and quantitative RT-PCR
RNA from homogenized TG tissue lysate (25 – 30 mg) was isolated and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Genomic DNA was removed from isolated RNA with TURBO DNAse (Life Technologies, Foster City, CA) and yield and purity were determined on a Nanodrop ND-1000 Spectrophotometer (Thermoscientific, Franklin, MA). A260/A280 ratios were between 2.0 and 2.2 for all samples. Single-stranded cDNA was synthesized from 1 μg mRNA using reverse transcriptase (Superscript II reverse transcriptase, Life Technologies) and Oligo(dT)12–18 primers (Life Technologies).
Quantitative PCR (qPCR) reactions were run in triplicate on an ABI PRISM 7900HT Sequence Detection System (Life Technologies). The cDNA was amplified for quantitative RT-PCR with SYBR Green PCR Master mix (Life Technologies) and gene specific primers (TRPV1 − 800 nM, TRPA1 – 200 nM or β-actin – 400 nM). The primers for amplification of rat TRPV1 (Trpv1, Ref NM_031982.1) message were as follows: TRPV1 forward (5′-AGG ACC CAG GCA ACT GTG-3′, TM =58° C) and TRPV1 reverse (5′-ATC CCT CAG AAG GGG AAC C-3′, TM = 56° C). These primers span exons 15 and 16, align with nucleotides 2456– 2474 and 2362 – 2379 and produce a 113 bp product. The primers for amplification of rat TRPA1 (Trpa1, Ref NM_207608.1) were as follows: TRPA1 forward (5′-GCC CCT GTC TCT GTA AAT AAC C-3′, TM = 55° C) and TRPA1 reverse (5′-CTT GTG TCG CTG ATG TCT TG-3′, TM = 54° C). These primers span exons 11and 12, align with nucleotides 1276–1297 and 1402–1421 and yield a 146 bp product. The primers for β-actin (Actb, Ref NM_031144.2) were as follows: β-Actin forward (5′-CAC TTT CTA CAA TGA GCT GCG-3′, TM= 54° C) and β-actin reverse (5′-CTG GAT GGC TAC GTA CAT GG-3′, TM = 55° C). The primers span exons 4 and 5, align with nucleotides 345–365 and 473–492 and yield a 148 bp product. A mixture of cDNA template, SYBR Green Master mix and forward and reverse primers was treated with uracil N-glycosylase (Life Technologies) before undergoing the following protocol: 50°C for 2 min, 95°C for 10 min, then 45 cycles of 95°C for 15 s, 60°C for 1 min, followed by 1 cycle of 95°C for 15 s, 60°C for 15s, and 95°C for 15 s. The PCR products were analyzed with ABI PRISM sequence detection software. The specificity of these amplifications was verified by melt curve analysis with detection of only a single peak. Reactions containing no reverse transcriptase (RT) or no template were also run as additional negative controls.
Real-time quantitative PCR data was analyzed with the ΔΔCT method as described by Livak and Schmittgen (2001) (30). TRPA1 or TRPV1 transcript levels were compared in trigeminal ganglia of room air (control) and acrolein exposed animals. TRPV1 or TRPA1 values were normalized to β-actin and calibrated to control data using the ΔΔCT method. β-actin was used as a reference gene and its level was not altered across these experimental conditions. The quantification cycle (Cq) was defined as the number of cycles required to attain a fluorescence threshold of 0.2 units.
Data collection and statistics
For blood flow experiments, data was collected at 1 Hz and filtered at 0.1 Hz for graphical representation. Basal blood flow was determined as the mean flow rate measured during a 4 minute period prior to drug application and the effects of test compounds were calculated by comparing the peak response after drug or saline administration to the basal blood flow. Changes in blood flow were calculated relative to the basal blood flow for each animal, averaged within treatment groups and expressed as percent changes. Comparison of blood flow changes and immunoreactive cell counts in the control and acrolein exposed groups were performed using a two-tailed Student’s t-test or ANOVA, respectively. qPCR results were calculated with the ΔΔCT method as described (30). qPCR data are presented as relative expression levels. Graphical presentation and statistical analysis was performed using GraphPad Prism software (GraphPad, CA). Data values are presented as means ± SEM. The significance level for all tests was set at p < 0.05.
Results
Acrolein exposure potentiates meningeal blood flow in response to TRPA1 and TRPV1 agonists
Airborne chemical exposure is cited as a common trigger for migraine headache and has been linked to MCS an acquired disorder whose symptoms include headache. Therefore we examined the effect of 4 days of periodic inhalation exposure (see Methods) to acrolein, a TRPA1 agonist and irritant common in indoor and outdoor pollution, on subsequent trigeminovascular responsiveness. As shown in Figure 1A and C, daily repeated acrolein exposure significantly potentiated meningeal blood flow responses to acute nasal administration of a sub-maximal concentration of mustard oil (10 μM). Representative traces of meningeal blood flow changes in response to nasal administration of mustard oil following room air or acrolein conditioning are shown in Figure 1A. The blood flow changes are rapid and brief, peaking within the first 1–2 min before returning toward basal values within 10–15 minutes. The data comparing peak blood flow in response to mustard oil in all animals conditioned to either acrolein or room air is summarized in Figure 1C. Acrolein exposure significantly increased blood flow responses to mustard oil compared to room air controls (75 ± 24% (n = 9) vs 11 ± 3% (n = 7), p = 0.037).
Because TRPA1 and TRPV1 receptors interact functionally (20) and TRPV1 expressing neurons are important for TRPA1 responses in the nasal-meningeal pathway (21), we also examined the effect of acrolein conditioning on blood flow responses to capsaicin, a TRPV1 agonist. As shown in Figure 1B and D, acrolein exposure significantly potentiated meningeal blood flow responses to acute nasal administration of a sub-maximal concentration of capsaicin (10 nM). Representative traces of meningeal blood flow changes in response to nasal administration of capsaicin in animals periodically conditioned to room air or acrolein are shown in Figure 1B. The summarized data (Figure 1D) demonstrate that acrolein exposure significantly increased blood flow responses to capsaicin compared to room air controls (71 ± 15% (n = 8) vs 24 ± 10% (n = 6), p = 0.036).
Acrolein exposure does not change the number of TRPV1 or CGRP immunoreactive neurons or TRPV1 or TRPA1 message levels in the trigeminal ganglion
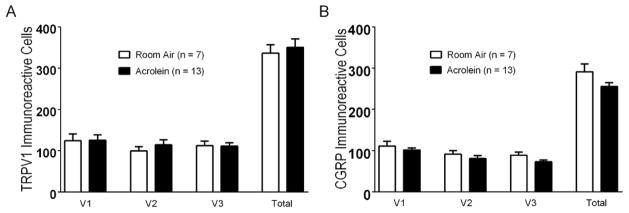
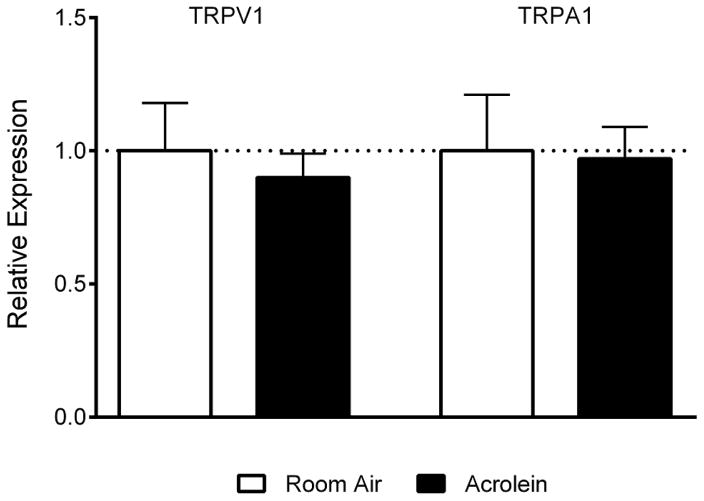
Sensitization of pain pathways has been linked to increased protein or message levels of important pain signaling molecules such as TRPV1 (31) or CGRP (32). To determine whether altered TRPV1 or CGRP expression in the TG underlies the enhanced blood flow response, we compared TRPV1 and CGRP immunoreactive cell numbers between acrolein- and room air-exposed animals (Figure 2). Neither TRPV1 (Figure 2A) or CGRP (Figure 2B) immunoreactive cell counts were altered by the acrolein exposure paradigm. Immunoreactive TRPV1 cell counts in acrolein exposed TG’s were similar to those of controls (350 ± 20.16 (n =13) vs 336.29 ± 20.41 (n= 7), p = 0.53) as were CGRP expressing cells (255.3 ± 9.90 (n=13) vs 291.30 ± 18.99 (n=7), p = 0.078). Messenger RNA levels for TRPA1 or TRPV1 in trigeminal ganglia from animals exposed to room air or acrolein were also determined by qPCR (Fig. 3). TRPV1 mRNA levels were not changed in acrolein exposed animals relative to room air exposed animals (0.90 ± 0.09 (n=20) vs 1.0 ± 0.18 (n=10), p = 0.58). Likewise, TRPA1 mRNA levels were not changed upon acrolein exposure (0.97 ± 0.12 (n=20) vs 1.0 ± 0.21 (n=10), p = 0.89). As no changes in TRPV1 or CGRP immunoreactive cells or in TRPA1 or TRPV1 mRNA levels were detected we conclude that expression changes of these molecules are not critical in the functional sensitization at the whole ganglion level.
Figure 2.
TRPV1 receptor and CGRP immunoreactivity in trigeminal ganglia of rats exposed to acrolein or room air. (A) TRPV1 receptor immunoreactive cell counts do not differ in V1, V2 or V3 divisions or total trigeminal ganglion in rats exposed to acrolein compared to room air. (B) CGRP immunoreactive cell counts do not differ in V1, V2 or V3 divisions or total trigeminal ganglion in rats exposed to acrolein compared to room air. Cell counts per section are represented as mean ± S.E.M. Number of animals per group is indicated.
Figure 3.
Relative expression levels of TRPV1 and TRPA1 mRNA do not change in trigeminal ganglia of rats exposed to acrolein vs room air. Each samples values are normalized to β-actin values using the ΔΔCT method and averaged across groups. Values are represented as mean ± S.E.M. N = 10–20 animals per group.
AP-18, a short acting TRPA1 antagonist blocks meningeal blood flow changes when administered 2 hours but not 27 hours before nasal administration of mustard oil
We hypothesize that TRPA1 receptor activation mediates the sensitization of blood flow responses after acrolein. To test this, we administered TRPA1 receptor antagonists or vehicle controls systemically during each acrolein inhalation exposure. To demonstrate the efficacy of antagonists to interfere with TRPA1-dependent blood flow we first examined the ability of systemic AP-18, a potent but short acting TRPA1 antagonist to acutely block blood flow stimulated by nasal administration of 30 μM mustard oil. AP-18 has been shown to acutely blunt responses in complete Freund’s adjuvant or formalin pain tests (33;34). AP-18 (0.2 mg/kg, i.p.) administered two hours before blood flow measurement in response to nasal mustard oil nearly abolished blood flow compared to saline (Figure 4, 7 ± 4 (n = 5) vs. 66 ± 18 ( n = 12), p = 0.007). In contrast, when given 27 hours before blood flow measurements, AP-18 had no effect on mustard oil stimulated blood flow (60 ± 14 (n = 6) vs. 66 ± 18 ( n = 12), p = 0.80) compared to saline injected animals. This suggests that AP-18 while effective acutely (when given immediately before the inhalation periods) is no longer effective when blood flow is measured the day after inhalation exposures.
Pretreatment with TRPA1 antagonist blocks acrolein-induced sensitization to TRPA1 and TRPV1 agonists
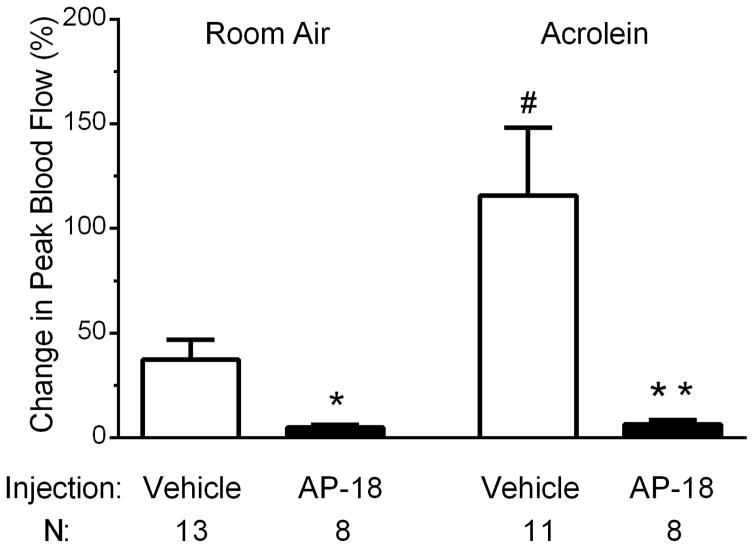
To assess the involvement of TRPA1 in the sensitization phenomenon, we examined whether pretreatment with a TRPA1 antagonist during acrolein conditioning inhalations would block the subsequent potentiation. AP-18, was injected daily 30 min before placing an animal in the inhalation chamber. Control animals received an injection of vehicle only. Blood flow experiments were conducted approximately 22 hours after the last inhalation period and in the absence of vehicle or AP-18 injections. Similar to the data in Figure 1, in vehicle injected animals, conditioning acrolein exposure potentiates the blood flow responses to 10 μM MO relative to room air exposure (Figure 5, 116 ± 32% (n=11) vs 37 ± 9% (n=13), p = 0.020). AP-18 pre-treatment significantly inhibited responses to 10 μM MO in both room air exposed and acrolein exposed animals (Figure 5) compared to vehicle injected animals (room air exposed, 5 ± 1% (n=8) vs 37 ± 9% (n=13), p = 0.015; acrolein exposed, 6 ± 2% (n=8) vs 116 ± 32% (n=11), p = 0.011). Interestingly, AP-18 also reduced MO responses in animals exposed to room air. While the mechanism is unclear, it is unlikely to involve a change in receptor gene expression as AP-18 did not alter message levels of TRPA1 or TRPV1 in room air or acrolein exposed animals in the trigeminal ganglion (data not shown).
Figure 5.
Effects of daily administration of TRPA1 antagonist (AP-18) prior to each inhalation exposure on blood flow changes in response to 10 μM mustard oil. In vehicle-injected animals the blood flow response to nasal mustard oil was significantly increased in acrolein-exposed animals compared to room-air exposed (#, p < 0.05). Compared to vehicle treated controls, blood flow response to nasal mustard oil was decreased in AP-18 injected animals in room air (*, p < 0.05) and acrolein exposed animals (**, p < 0.05). Values are means ± S.E.M. Number of animals per group is indicated.
We also tested whether pre-treatment with AP-18 attenuated the acrolein-induced potentiation of blood flow in response to capsaicin. A separate group of animals received AP-18 or vehicle injections prior to exposure to acrolein or room air and their subsequent blood flow response to nasally administered capsaicin recorded. Similar to the results in Figure 1, vehicle-injected animals displayed a sensitized response to capsaicin (10 nM) following acrolein chamber exposure (Figure 6). This increase was significant when comparing acrolein-exposed animals to those receiving room air (141 ± 27% (n=9) vs 14 ± 2% (n=12), p < 0.0001). Blood flow responses to 10 nM capsaicin were not reduced in animals injected with AP-18 and exposed to room air when compared to the vehicle injected animals (9 ± 3% (n=6) vs 14 ± 2% (n=12), p = 0.191). However, blood flow responses to 10 nM capsaicin were attenuated in animals injected with AP-18 and exposed to acrolein compared to the vehicle injected animals exposed to acrolein (26 ± 12% (n=6) vs 141 ± 27% (n=9), p = 0.006).
Discussion
Headache is the most common symptom of air pollution and studies have demonstrated that poor air quality is correlated with increases in ER visits due to headache (2–4;35), but few clinical studies have addressed the mostly anecdotal observations. In our previous studies (9;21) we suggested that inhalation of environmental irritants may excite trigeminal neurons supplying the nose and airways through TRPA1 receptors and subsequently cause trigeminovascular activation leading to headache. Intranasal administration of the irritants mustard oil or capsaicin induced dilatation of meningeal blood vessels via a CGRP-dependent mechanism (9). Ablation of TRPV1 expressing neurons in the trigeminal ganglia significantly decreased irritant induced meningeal vasodilatation and TRPA1 and CGRP expression in the trigeminal ganglia (21), consistent with the notion that TRP receptor activation and CGRP release from the trigeminovascular network of neurons are contributing mechanisms of headache.
Headache, as well as other symptoms, is associated in Multiple Chemical Sensitivity and Sick Building Syndrome, acquired disorders strongly linked to chemical exposure (5). Usually such chemical exposures are via inhalation and lead to hypersensitivity to environmental irritants following either acute or chronic exposures. Like air pollution exposure, the pathophysiological mechanisms that induce headache in these syndromes are unknown, but may involve sensitization of peripheral neural elements. We examined whether chronic exposure to environmental irritants would induce sensitization, by exposing rats to the environmental irritant acrolein, a TRPA1 receptor agonist, through inhalation. Consistent with this hypothesis, daily acrolein exposure sensitized the trigeminovascular response to intranasal TRP agonist administered 24 hours later. Interestingly, trigeminovascular responses to both the TRPA1 agonist mustard oil and the TRPV1 receptor agonist capsaicin were enhanced by acrolein exposure. No changes in tissue expression levels of either TRPA1 or TRPV1 or of CGRP-positive cell counts in the whole ganglion were observed following acrolein exposure. These data suggest that if the molecular locus of sensitization to activation of either TRP receptor subtype is in the sensory neuron that the convergence may occur in signaling events downstream of and critical to both receptors. As we could not quantitatively assess expression in subpopulations, we cannot rule out changes in message or protein levels specific to only the nasal-meningeal pathway. An alternate explanation for the observed sensitization may be agonist-induced membrane trafficking of TRPA1 receptors as has been previously described (36). While a complete understanding of the mechanism of sensitization will require further studies, the fact that daily administration of the TRPA1 receptor antagonist, AP-18, prior to acrolein exposure eliminated agonist sensitization strongly implicates TRPA1 activation in the sensitization phenomenon itself, regardless of the molecular element manifesting the sensitization.
One potential confound relates to whether chronic exposure to TRPA1 agonists may induce general tissue damage, yet few studies have investigated this question. Hazari and colleagues observed acute cardiac pathophysiology following short term exposure of rats to acrolein (37;38). They reported that a single diesel exhaust or acrolein exposure increased the sensitivity and incidence of arrhythmia, both immediately and 24 hrs post exposure. Although we did not monitor heart function in our study, the concentration of acrolein we employed was 10-fold less than in their studies. Olfactory mucosal damage and respiratory epithelial metaplasia has been reported after sub-chronic exposure to acrolein levels over 0.67 ppm, while lower levels resulted in only minor epithelial changes (26;27). Together these studies suggest that while exposure to high levels of acrolein, either acute or chronic, may induce tissue injury and inflammatory responses, exposure to lower concentrations such as employed here induces minimal damage.
To our knowledge this is the first demonstration of sensitization of trigeminovascular responses after environmental irritant exposure. As we observed no changes in TRPA1 or TRPV1 mRNA expression levels or numbers of TRPV1 and CGRP expressing cells in the TG it seems more likely that posttranslational or other downstream events may be important. For example, PKA/PLC signaling and membrane trafficking of the TRPA1 receptor were implicated in the reduced pain thresholds observed after repeated injections of mustard oil in mice (36). Likewise, repeated application of mustard oil to the nasal cavity in humans produced sensitization when a second stimulus was delivered shortly after the first stimulus, however the mechanism was not explored (39). Our observation that acrolein exposure potentiates capsaicin as well as mustard oil responses highlights the complexity of TRPA1-TRPV1 interactions in sensory neurons (reviewed by Akopian, 2011(20)). TRPA1 and TRPV1 can form heteromultimers which exhibit unique desensitization properties such that TRPV1 controls the density of TRPA1 on the cell surface (20). In addition, both receptors are modulated by inflammatory mediators, such as bradykinin. It is possible that the acrolein exposure paradigm used here could result in release of such inflammatory mediators which would subsequently activate and sensitize trigeminal neurons.
The irritant exposure paradigm used here produces long-lasting excitation of the trigeminovascular response and bears some similarities to previously described chronic migraine models where repeated activation of peripheral trigeminal nerve terminals produces long-lasting hyperexcitabilty (40;41). In these models, other attributes of chronic migraine which reflect central sensitization were observed including changes in behavior (periorbital allodynia) and in signaling molecules such as c-FOS or neurotransmitters in the trigeminal nucleus caudalis. It is not clear whether the sensitization observed after irritant exposure reflects both central and peripheral sensitization or just peripheral because the nasal-meningeal pathway underlying the phenomenon is not yet well-defined. In this regard utilizing double retrograde labeling from the nose and meninges we observed that single trigeminal neurons do not project axons to both nasal epithelium and meninges (21). Instead separate populations of cell bodies innervating the nose or meninges reside next to each other in the trigeminal ganglion, thus we hypothesize that the nasal-meningeal pathway for trigeminovascular activation is through intraganglionic transmission. Alternatively, a central relay cannot be definitively ruled out at this time. Future studies will determine the duration and mechanism of the sensitization as well as whether irritant exposure produces characteristics of central sensitization.
Taken together our results suggest a mechanism for the enhanced headache susceptibility after airborne chemical exposure. This novel model may allow us to gain a better understanding of migraine triggers and perhaps the progression to chronic migraine.
Clinical and public health implications.
Air pollution and chemical odors are widely cited headache triggers
Inhalation exposure to environmental irritants sensitizes trigeminovascular responses to painful stimuli
TRPA1 antagonists attenuate trigeminovascular sensitization induced by environmental irritants
Acknowledgments
We would like to thank Dr. Richard P. Kraig for use of the laser Doppler flowmeter. This work was supported by a grant from the NIEHS (ES017430) to JHH and GSO.
Footnotes
Conflict of Interest Statement
The Author(s) declare that there is no conflict of interest.
Literature cited
- 1.Friedman DI, De ver Dye T. Migraine and the environment. Headache. 2009 Jun;49(6):941–52. doi: 10.1111/j.1526-4610.2009.01443.x. [DOI] [PubMed] [Google Scholar]
- 2.Nattero G, Enrico A. Outdoor pollution and headache. Headache. 1996 Apr;36(4):243–5. doi: 10.1046/j.1526-4610.1996.3604243.x. [DOI] [PubMed] [Google Scholar]
- 3.Szyszkowicz M. Ambient air pollution and daily emergency department visits for headache in Ottawa, Canada. Headache. 2008 Jul;48(7):1076–81. doi: 10.1111/j.1526-4610.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 4.Szyszkowicz M. Air pollution and daily emergency department visits for headache in Montreal, Canada. Headache. 2008 Mar;48(3):417–23. doi: 10.1111/j.1526-4610.2007.00808.x. [DOI] [PubMed] [Google Scholar]
- 5.Cullen MR. The worker with multiple chemical sensitivities: an overview. Occup Med. 1987 Oct;2(4):655–61. [PubMed] [Google Scholar]
- 6.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001 Sep 13;413(6852):203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 7.Messlinger K, Hanesch U, Kurosawa M, Pawlak M, Schmidt RF. Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can J Physiol Pharmacol. 1995 Jul;73(7):1020–4. doi: 10.1139/y95-143. [DOI] [PubMed] [Google Scholar]
- 8.Schwenger N, Dux M, de CR, Carr R, Messlinger K. Interaction of calcitonin gene-related peptide, nitric oxide and histamine release in neurogenic blood flow and afferent activation in the rat cranial dura mater. Cephalalgia. 2007 Jun;27(6):481–91. doi: 10.1111/j.1468-2982.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 9.Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011 Jan;152(1):38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006 Mar 24;124(6):1269–82. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 11.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007 Aug 14;104(33):13525–30. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De SG, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012 Feb;135(Pt 2):376–90. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- 13.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004 Mar 25;41(6):849–57. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 14.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12248–52. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003 Mar 21;112(6):819–29. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 16.Huang D, Li S, Dhaka A, Story GM, Cao YQ. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plevkova J, Kollarik M, Poliacek I, Brozmanova M, Surdenikova L, Tatar M, et al. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol. 2013 Jul;115(2):268–74. doi: 10.1152/japplphysiol.01144.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002 Mar 8;108(5):705–15. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 19.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004 Jan 15;427(6971):260–5. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 20.Akopian AN. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr Pharm Biotechnol. 2011 Jan 1;12(1):89–94. doi: 10.2174/138920111793937952. [DOI] [PubMed] [Google Scholar]
- 21.Kunkler PE, Ballard CJ, Pellman JJ, Zhang L, Oxford GS, Hurley JH. Intraganglionic Signaling as a Novel Nasal-Meningeal Pathway for TRPA1-Dependent Trigeminovascular Activation by Inhaled Environmental Irritants. PLoS One. 2014;9(7):e103086. doi: 10.1371/journal.pone.0103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996 Dec 12;384(6609):560–4. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 23.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998 Feb;79(2):964–82. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983 Jun;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 25.Lyon JP, Jenkins LJ, Jr, Jones RA, Coon RA, Siegel J. Repeated and continuous exposure of laboratory animals to acrolein. Toxicol Appl Pharmacol. 1970 Nov;17(3):726–32. doi: 10.1016/0041-008x(70)90047-5. [DOI] [PubMed] [Google Scholar]
- 26.Cassee FR, Groten JP, Feron VJ. Changes in the nasal epithelium of rats exposed by inhalation to mixtures of formaldehyde, acetaldehyde, and acrolein. Fundam Appl Toxicol. 1996 Feb;29(2):208–18. doi: 10.1006/faat.1996.0024. [DOI] [PubMed] [Google Scholar]
- 27.Dorman DC, Struve MF, Wong BA, Marshall MW, Gross EA, Willson GA. Respiratory tract responses in male rats following subchronic acrolein inhalation. Inhal Toxicol. 2008 Feb;20(3):205–16. doi: 10.1080/08958370701864151. [DOI] [PubMed] [Google Scholar]
- 28.Gottselig R, Messlinger K. Noxious chemical stimulation of rat facial mucosa increases intracranial blood flow through a trigemino-parasympathetic reflex--an experimental model for vascular dysfunctions in cluster headache. Cephalalgia. 2004 Mar;24(3):206–14. doi: 10.1111/j.1468-2982.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Xu P, Cuascut FX, Hall AK, Oxford GS. Activin acutely sensitizes dorsal root ganglion neurons and induces hyperalgesia via PKC-mediated potentiation of transient receptor potential vanilloid I. J Neurosci. 2007 Dec 12;27(50):13770–80. doi: 10.1523/JNEUROSCI.3822-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Pei L, Lin CY, Dai JP, Yin GF. Facial pain induces the alteration of transient receptor potential vanilloid receptor 1 expression in rat trigeminal ganglion. Neurosci Bull. 2007 Mar;23(2):92–100. doi: 10.1007/s12264-007-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caviedes-Bucheli J, Arenas N, Guiza O, Moncada NA, Moreno GC, Diaz E, et al. Calcitonin gene-related peptide receptor expression in healthy and inflamed human pulp tissue. Int Endod J. 2005 Oct;38(10):712–7. doi: 10.1111/j.1365-2591.2005.01006.x. [DOI] [PubMed] [Google Scholar]
- 33.Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maione S, Costa B, Piscitelli F, Morera E, De CM, Comelli F, et al. Piperazinyl carbamate fatty acid amide hydrolase inhibitors and transient receptor potential channel modulators as “dual-target” analgesics. Pharmacol Res. 2013 Oct;76:98–105. doi: 10.1016/j.phrs.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Szyszkowicz M, Stieb DM, Rowe BH. Air pollution and daily ED visits for migraine and headache in Edmonton, Canada. Am J Emerg Med. 2009 May;27(4):391–6. doi: 10.1016/j.ajem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009 Nov 25;64(4):498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, et al. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011 Jul;119(7):951–7. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazari MS, Griggs J, Winsett DW, Haykal-Coates N, Ledbetter A, Costa DL, et al. A single exposure to acrolein desensitizes baroreflex responsiveness and increases cardiac arrhythmias in normotensive and hypertensive rats. Cardiovasc Toxicol. 2014 Mar;14(1):52–63. doi: 10.1007/s12012-013-9228-9. [DOI] [PubMed] [Google Scholar]
- 39.Brand G, Jacquot L. Sensitization and desensitization to allyl isothiocyanate (mustard oil) in the nasal cavity. Chem Senses. 2002 Sep;27(7):593–8. doi: 10.1093/chemse/27.7.593. [DOI] [PubMed] [Google Scholar]
- 40.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007 Jul;47(7):1026–36. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyer N, Dallel R, Artola A, Monconduit L. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain. 2014 Jul;155(7):1196–205. doi: 10.1016/j.pain.2014.03.001. [DOI] [PubMed] [Google Scholar]