Abstract
Sarcoidosis is a multisystem granulomatous disorder of unknown cause which may affect any organ or system but primarily involve the lungs and the lymphatic system. Extrapulmonary sarcoidosis represents approximately 30-50% of patients. We report the case of a 51-year-old female who presented with increasing complaints of a cough, weakness, weight loss, and chest pain and who was found to have a suspicious lesion on thorax computed tomography(CT). Fluorodeoxyglucose (FDG) positron emission tomography/CT performed for diagnostic purposes demonstrated increased FDG accumulation at the bilateral enlarged parotid and lacrimal gland and in the reticulonodular infiltration area located in the left lung as well as multiple lymphadenopathies with increased FDG accumulation. There were also hepatosplenomegaly and splenic uptake. Skin biopsy showed noncaseating granulomas, and the patient was diagnosed as stage 2 sarcoidosis.
Keywords: Fluorodeoxyglucose positron emission tomography/computed tomography, FDG, sarcoidosis
INTRODUCTION
Sarcoidosis is a multisystem granulomatous disorder of unknown cause which is mostly seen between the ages of 20 and 39 years, but it can also occur in the pediatric population and the elderly.[1] Although sarcoidosis primarily involves the lungs and the lymphatic system, it may affect any organ or system. Extrapulmonary sarcoidosis is common representing approximately 30–50% of patients, and mostly associated with concomitant thoracic involvement. The diagnosis of sarcoidosis is based on compatible clinical-radiological findings, the histological finding of noncaseating granulomas, and the exclusion of other conditions with similar histological or clinical features.[2] Patients most commonly presented with cough and dyspnea because of involvement of the lungs. Systemic symptoms including weight loss, fatigue, night sweats, and fever are also commonly seen in these patients. Chest radiography and multidetector computed tomography (CT) are commonly used routine diagnostic procedures for detecting pulmonary sarcoidosis, but these are unable to show active inflammation.[3,4]
The nuclear medicine techniques, such as 67-gallium scintigraphy and somatostatin receptor scintigraphy, can be used for diagnosis and evaluation of the extent of disease, but their roles are limited because of poor spatial resolution.[5,6] Recently, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT has been used for the evaluation of inflammatory and infectious diseases including sarcoidosis.[7] The sensitivity of FDG PET/CT is superior to gallium scintigraphy and is associated with a higher spatial resolution, a faster uptake of the radiotracer providing development of images within 2 h, and favorable dosimetry. In addition, FDG PET/CT better assess the extrapulmonary involvement of the disease.[8] There have been numerous case series highlighted the usefulness of FDG PET/CT in the diagnosis of sarcoidosis. Some reports proposed that FDG PET/CT can be used as a tool for the differential diagnosis of solid lesions of the chest and for evaluating inflammation and response to corticosteroids in patients with sarcoidosis.[8,9,10,11,12] Here, we report a case of sarcoidosis diagnosed by PET/CT when searching a possible primary tumor.
CASE REPORT
A 51-year-old female with a history of cough, weakness, weight loss, and chest pain for the last 2 years got the diagnosis of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She presented to the department of chest diseases with increasing complaints and losing 15 kg in 2 months. Chest X-ray showed bilateral hilar lymphadenopathies. Then, CT was performed and revealed that there were reticular and reticulonodular infiltrations and septal thickening in the right upper and left lower lobes as well as bilateral hilar lymphadenopathies which cause concentric narrowing at the left lower lobe bronchus. Transbronchial biopsy was performed from this region with suspicion of the tumoral lesion and showed alveolar macrophages and inflammatory cells. FDG PET/CT performed for diagnostic purposes demonstrated increased FDG accumulation at the bilateral enlarged parotid and lacrimal gland [Figure 1] and in the reticulonodular infiltration area located at left lung [Figure 2] as well as multiple lymphadenopathies with increased FDG accumulation [Figure 3]. There were also hepatosplenomegaly and splenic uptake which all consistent with either lymphoma or sarcoidosis involvement. On this physical examination, maculopapular rashes were noticed at the bilateral dorsum of hand [Figure 4], and histopathological examination showed noncaseating granulomas. Her serum angiotensin converting enzyme (ACE) level was 176 (normal range: 8–52), and serum calcium level was 9.77 (8.6–10) mg/dl. The patient was diagnosed as stage 2 sarcoidosis.
Figure 1.
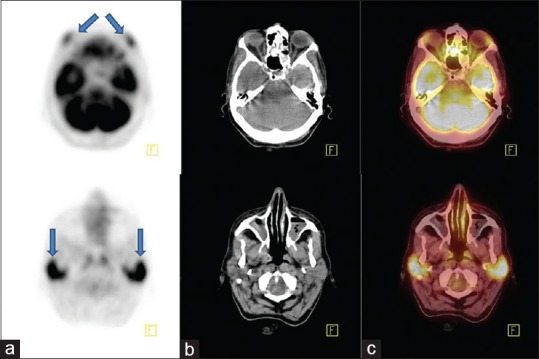
Axial positron emission tomography (a), computed tomography (b), and fusion (c) images of fluorodeoxyglucose positron emission tomography/computed tomography scintigraphy showed increased fluorodeoxyglucose accumulation at the bilateral enlarged lacrimal (upper row, arrow) and parotid gland (lower row, arrow)
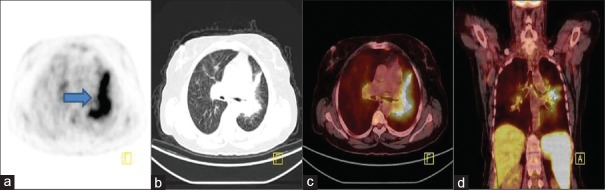
Figure 2.
Axial positron emission tomography (a), computed tomography (b), fusion (c), and coronal positron emission tomography/computed tomography fusion (d) images demonstrated hypermetabolism at the left parahilar region as well as bilateral hilar lymphadenopathies which cause concentric narrowing at the left lower lobe bronchus
Figure 3.
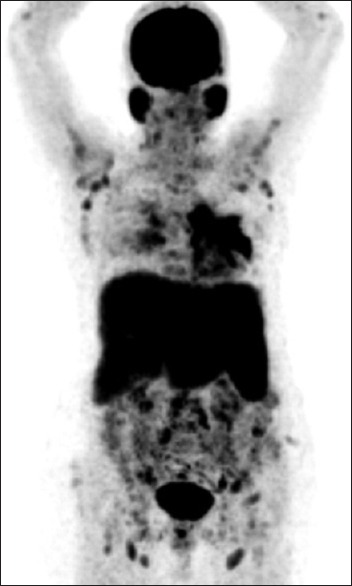
Maximum intensity projection image revealed multiple lesions of the supraclavicular, axillary and hilar lymph nodes, lungs, abdominal-pelvic lymph nodes, liver, and spleen
Figure 4.
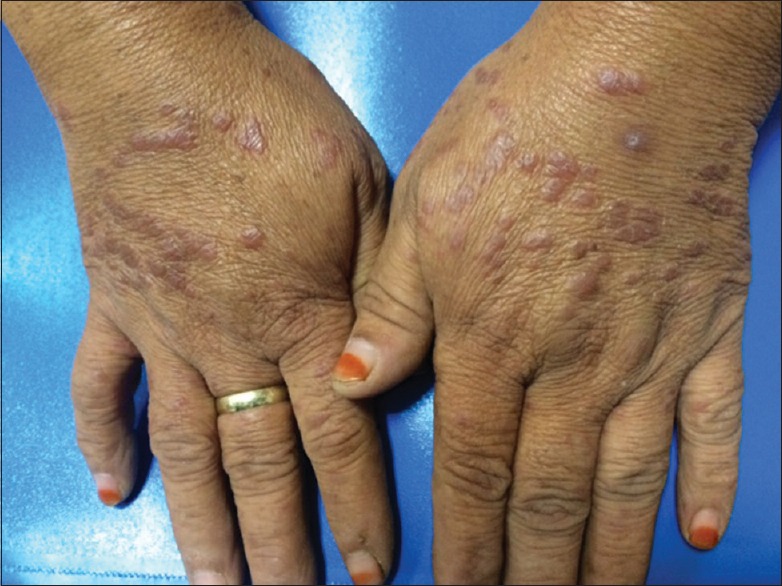
There were maculopapular rashes at the bilateral dorsum of hand
DISCUSSION
Extrathoracic manifestation is seen in >30% of sarcoidosis cases and typically in combination with the thoracic involvement. Being a whole body imaging method, FDG PET and PET/CT have an important role in the assessment of extrathoracic involvement and in determination of active disease extent. Sarcoidosis may involve any organ including the nervous system, heart, skin subcutaneous tissue, liver, spleen, retroperitoneal lymph nodes, gastrointestinal system, muscles, and bones.[11] In our case, PET/CT demonstrated bilateral lacrimal-parotid gland uptake and liver–spleen uptake associated with numerous sites of cervical, mediastinal, abdominopelvic, and inguinal lymph node uptake.
Orbital sarcoidosis occurs in 10–50% of the patients with sarcoidosis.[2] FDG PET/CT and magnetic resonance imaging are helpful for evaluation of the orbital content, especially the lacrimal glands which presents as an enlargement of the gland that is commonly bilateral.[14] Parotid gland enlargement is rarely seen in sarcoidosis with an incidence of 6% and is often associated with widespread disease as in our case.[13] FDG PET/CT imaging is helpful to show parotid gland involvement. The finding of increased symmetric gallium activity in the lacrimal, parotid, and salivary glands combined with normal accumulation in the nasopharynx, giving the impression of the mottled coloring of the giant panda is termed as “panda” sign.[15] This finding has also previously been reported in FDG PET/CT imaging.[16]
Liver and spleen involvement of sarcoidosis is usually clinically silent and observed in 50–80% of autopsy specimens. It may cause hepatomegaly, cholestasis, and portal hypertension. Nodular hepatosplenic sarcoidosis is more common during the first 5 years of disease, which may be associated with abdominal or systemic symptoms and elevated serum ACE levels. Hepatic and splenic nodules are observed in 5% and 15% of patients in CT studies, respectively.[17] Hepatosplenic involvement may demonstrate diffuse or multinodular uptake on FDG PET/CT. However, diffuse and moderate FDG uptake in the spleen can be related to nonspecific inflammatory conditions.[7]
On FDG PET/CT, sarcoid lesions can demonstrate high standardized uptake values (SUVs) mimicking other pathological processes, including lymphomas and diffuse metastatic disease. Values of up to 15 have been reported in sarcoidosis previously.[18] There is no reported cut-off value for differentiating benign from malignant lesions by SUVmax value. Because FDG uptake is not specific and may mimic other pathologies, it is important for radiologists and nuclear medicine specialists to be aware of the many possible presentations of sarcoidosis. Furthermore, sarcoidosis may present with a malignancy at the same time in 4–13% of cancer patients which may cause interpretation difficulty.[19]
FDG PET/CT may also be helpful in detecting potential biopsy sites because the biopsy of metabolically active lesions is more likely led to a diagnostic yield. In the study by Teirstein et al., FDG PET indicated occult diagnostic sites that were not detected by physical examination, standard thoracic radiography, or CT in 15% of 139 patients.[18]
CONCLUSION
18F-FDG PET/CT can be effectively used in showing thoracic sites of sarcoidosis and also in identifying extrapulmonary involvement in a single imaging session, and to guide biopsy sampling in cases needing pathological confirmation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–73. [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 3.Malaisamy S, Dalal B, Bimenyuy C, Soubani AO. The clinical and radiologic features of nodular pulmonary sarcoidosis. Lung. 2009;187:9–15. doi: 10.1007/s00408-008-9118-2. [DOI] [PubMed] [Google Scholar]
- 4.Akira M, Kozuka T, Inoue Y, Sakatani M. Long-term follow-up CT scan evaluation in patients with pulmonary sarcoidosis. Chest. 2005;127:185–91. doi: 10.1378/chest.127.1.185. [DOI] [PubMed] [Google Scholar]
- 5.Lebtahi R, Crestani B, Belmatoug N, Daou D, Genin R, Dombret MC, et al. Somatostatin receptor scintigraphy and gallium scintigraphy in patients with sarcoidosis. J Nucl Med. 2001;42:21–6. [PubMed] [Google Scholar]
- 6.Schuster DM, Alazraki N. Gallium and other agents in diseases of the lung. Semin Nucl Med. 2002;32:193–211. doi: 10.1053/snuc.2002.124178. [DOI] [PubMed] [Google Scholar]
- 7.Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–68. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, et al. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–6. [PubMed] [Google Scholar]
- 9.Hashimoto M, Watanabe O, Sato K, Endo K, Heianna J, Itoh I, et al. The CT findings of pulmonary sarcoidosis. Tohoku J Exp Med. 1996;179:259–66. doi: 10.1620/tjem.179.259. [DOI] [PubMed] [Google Scholar]
- 10.Abehsera M, Valeyre D, Grenier P, Jaillet H, Battesti JP, Brauner MW. Sarcoidosis with pulmonary fibrosis: CT patterns and correlation with pulmonary function. AJR Am J Roentgenol. 2000;174:1751–7. doi: 10.2214/ajr.174.6.1741751. [DOI] [PubMed] [Google Scholar]
- 11.Koyama T, Ueda H, Togashi K, Umeoka S, Kataoka M, Nagai S. Radiologic manifestations of sarcoidosis in various organs. Radiographics. 2004;24:87–104. doi: 10.1148/rg.241035076. [DOI] [PubMed] [Google Scholar]
- 12.Basu S, Saboury B, Werner T, Alavi A. Clinical utility of FDG-PET and PET/CT in non-malignant thoracic disorders. Mol Imaging Biol. 2011;13:1051–60. doi: 10.1007/s11307-010-0459-x. [DOI] [PubMed] [Google Scholar]
- 13.Vardhanabhuti V, Venkatanarasimha N, Bhatnagar G, Maviki M, Iyengar S, Adams WM, et al. Extra-pulmonary manifestations of sarcoidosis. Clin Radiol. 2012;67:263–76. doi: 10.1016/j.crad.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Demirci H, Christianson MD. Orbital and adnexal involvement in sarcoidosis: Analysis of clinical features and systemic disease in 30 cases. Am J Ophthalmol. 2011;151:1074–80.e1. doi: 10.1016/j.ajo.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Kurdziel KA. The panda sign. Radiology. 2000;215:884–5. doi: 10.1148/radiology.215.3.r00jn31884. [DOI] [PubMed] [Google Scholar]
- 16.Oksüz MO, Werner MK, Aschoff P, Pfannenberg C. 18F-FDG PET/CT for the diagnosis of sarcoidosis in a patient with bilateral inflammatory involvement of the parotid and lacrimal glands (panda sign) and bilateral hilar and mediastinal lymphadenopathy (lambda sign) Eur J Nucl Med Mol Imaging. 2011;38:603. doi: 10.1007/s00259-010-1679-7. [DOI] [PubMed] [Google Scholar]
- 17.Warshauer DM, Dumbleton SA, Molina PL, Yankaskas BC, Parker LA, Woosley JT. Abdominal CT findings in sarcoidosis: Radiologic and clinical correlation. Radiology. 1994;192:93–8. doi: 10.1148/radiology.192.1.8208972. [DOI] [PubMed] [Google Scholar]
- 18.Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007;132:1949–53. doi: 10.1378/chest.07-1178. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury FU, Sheerin F, Bradley KM, Gleeson FV. Sarcoid-like reaction to malignancy on whole-body integrated 18F-FDG PET/CT: Prevalence and disease pattern. Clin Radiol. 2009;64:675–81. doi: 10.1016/j.crad.2009.03.005. [DOI] [PubMed] [Google Scholar]