Abstract
Axon extension during development is guided by many factors, but the signaling mechanisms responsible for its regulation remain largely unknown. We have now investigated the role of the transmembrane protein CD47 in this process in N1E-115 neuroblastoma cells. Forced expression of CD47 induced the formation of neurites and filopodia. Furthermore, an Fc fusion protein containing the extracellular region of the CD47 ligand SHPS-1 induced filopodium formation, and this effect was enhanced by CD47 overexpression. SHPS-1–Fc also promoted neurite and filopodium formation triggered by serum deprivation. Inhibition of Rac or Cdc42 preferentially blocked CD47-induced formation of neurites and filopodia, respectively. Overexpression of CD47 resulted in the activation of both Rac and Cdc42. The extracellular region of CD47 was sufficient for the induction of neurite formation by forced expression, but the entire structure of CD47 was required for enhancement of filopodium formation by SHPS-1–Fc. Neurite formation induced by CD47 was also inhibited by a mAb to the integrin β3 subunit. These results indicate that the interaction of SHPS-1 with CD47 promotes neurite and filopodium formation through the activation of Rac and Cdc42, and that integrins containing the β3 subunit participate in the effect of CD47 on neurite formation.
INTRODUCTION
The extension of axons from neurons to their target cells during development of the nervous system is guided by a variety of environmental cues, which include diffusible chemoattractants and chemorepellents, extracellular matrix proteins, and cell adhesion molecules (Tessier-Lavigne and Goodman, 1996; Dickson, 2002). In response to these guidance cues, the growth cone of the axon produces and retracts filopodia and lamellipodia, processes that require the temporal and spatial regulation of the actin cytoskeleton. Members of the Rho family of small G proteins, including Rho, Rac, and Cdc42, are implicated as key mediators that link guidance signals to rearrangement of the actin cytoskeleton (Ridley et al., 1992; Nobes and Hall, 1995; Kozma et al., 1997; Luo et al., 1997; Hirose et al., 1998; Takai et al., 2001; Arakawa et al., 2003). Although Rac and Cdc42 promote neurite extension, Rho inhibits it or induces growth cone collapse. These proteins are also implicated in dendritic development (Threadgill et al., 1997). Although certain guidance factors, including slit, semaphorin, and ephrin, have been shown to regulate Rho family proteins (Wahl et al., 2000; Whitford and Ghosh, 2001; Wong et al., 2001), it remains unclear how other extracellular cues control neurite extension through these small G proteins.
CD47, also known as integrin-associated protein (IAP), was originally identified in association with the integrin αvβ3 (Brown et al., 1990). It is a member of the immunoglobulin (Ig) superfamily, possessing an Ig-V–like extracellular region, five putative transmembrane domains, and a short cytoplasmic tail (Brown and Frazier, 2001). CD47 is implicated in the regulation of multiple cellular processes including neutrophil migration (Cooper et al., 1995; Parkos et al., 1996), T-cell activation (Reinhold et al., 1997; Waclavicek et al., 1997), T- and B-cell apoptosis (Mateo et al., 1999; Pettersen et al., 1999), and platelet activation (Chung et al., 1997, 1999). The extracellular region of CD47 is responsible for its association with the integrin β3 subunit (Lindberg et al., 1996b). Although most CD47-mediated cellular responses likely involve the activation of integrins, in particular that of αvβ3 or αIIbβ3 (Brown and Frazier, 2001), the molecular mechanism of such activation is not fully understood. The extracellular region of CD47 also binds the putative ligands thrombospondin-1 (TSP1; Gao et al., 1996; Chung et al., 1997; Brown and Frazier, 2001) and SHP substrate-1 (SHPS-1; Jiang et al., 1999; Seiffert et al., 1999).
SHPS-1, also known as BIT or SIRPα, is a receptor-like transmembrane protein that contains three Ig-like domains in its extracellular region as well as putative tyrosine phosphorylation sites and binding sites for the SH2 domains of the protein tyrosine phosphatases, SHP-2 and SHP-1, in its cytoplasmic region (Fujioka et al., 1996; Ohnishi et al., 1996; Kharitonenkov et al., 1997). Through its formation of a complex with SHP-2, SHPS-1 promotes cell migration by regulating reorganization of the actin cytoskeleton (Inagaki et al., 2000). We and others have recently shown that CD47 and SHPS-1 constitute a cell-cell communication system (the CD47–SHPS-1 system) that plays an important role in a variety of cell functions. The engagement of SHPS-1 by CD47 inhibits formation of the SHPS-1–SHP-2 complex and thus contributes to inhibition of cell migration by cell-cell contact (Motegi et al., 2003). The binding of CD47 on red blood cells to SHPS-1 on macrophages also inhibits the phagocytosis of red blood cells by macrophages, a process that requires the interaction of SHP-1 with SHPS-1 (Oldenborg et al., 2000, 2001). Monoclonal antibodies to CD47 inhibit neutrophil transmigration (Liu et al., 2001), suggesting that the CD47-SHPS–1 system might mediate bidirectional inhibitory regulation of cell migration. The signaling pathway activated by the binding of SHPS-1 to CD47 remains largely unknown, however. In contrast, certain cellular responses triggered by the binding of TSP1 to CD47 appear to be mediated by the pertussis toxin (PTX)-sensitive heterotrimeric G protein Gi (Frazier et al., 1999; Brown and Frazier, 2001).
In the CNS, both CD47 and SHPS-1 are localized in synapse-rich regions such as the hippocampus and cerebellum (Jiang et al., 1999; H. Ohnishi and T. Matozaki, unpublished observation). In the retina, both molecules are localized at presynaptic or postsynaptic sites (or both); however, SHPS-1 fails to associate with synaptic sites in CD47 knockout mice, suggesting that the interaction of SHPS-1 with CD47 is necessary for its synaptic localization (Mi et al., 2000). In addition, the abundance of CD47 in the hippocampus has been associated with memory retention in rats (Huang et al., 1998), and long-term potentiation (LTP) is impaired in CD47 knockout mice (Chang et al., 1999), suggesting a role for CD47 in synaptic plasticity and memory formation in the hippocampus. Together, these observations suggest that, through its interaction with SHPS-1, CD47 might mediate neuronal cell-cell communication in a bidirectional manner and thereby modulate synaptic transmission. Four alternatively spliced isoforms of mouse CD47 mRNA have been identified, one of which, designated form 4, is most abundant in the brain (Reinhold et al., 1995).
Despite these various observations, however, the physiological functions of CD47 in neuronal cells and the mode of action of this protein remain mostly unknown. We have now examined the role of CD47 in the regulation of neurite remodeling in N1E-115 neuroblastoma cells.
MATERIALS AND METHODS
Primary Antibodies and Reagents
A rat mAb to mouse CD47 (miap 301) and a hamster mAb to mouse integrin β1 subunit were obtained from PharMingen (San Diego, CA). A hamster mAb to mouse integrin β3 subunit and a mouse mAb to human CD8 were from eBioscience (San Diego, CA). Mouse mAbs to Cdc42 and to Rac were from Santa Cruz Biotechnology (Santa Cruz, CA). A TSP1-derived synthetic peptide, 4N1K (KRFYVVMWKK), and its control peptides (KVFRWKYVMK or KRFYGGMWKK) were synthesized by BioSynthesis (Lewisville, TX) (Fujimoto et al., 2003) and Peptide Institute (Osaka, Japan). PTX was kindly provided by F. Okajima (Gunma University, Gunma, Japan). Wortmannin and echistatin were from Sigma (St. Louis, MO), and rhodamine-conjugated phalloidin was from Molecular Probes (Eugene, OR). A cDNA encoding the CRIB domain of rat p21PAKα was kindly provided by E. Manser (Institute of Molecular and Cell Biology, Singapore, Singapore).
Plasmids
Mouse CD47 form 2 cDNA was kindly provided by F. P. Lindberg (Washington University, St. Louis, MO). Mouse CD47 form 4 cDNA was obtained by PCR with mouse brain cDNA as template. Both CD47 cDNAs were subcloned in the pCAGGS vector (Niwa et al., 1991), which was kindly provided by J. Miyazaki (Osaka University, Osaka, Japan). Plasmids encoding green fluorescent protein (GFP)-N17Rac1 and GFP-NWASP–CRIB were kindly provided by S. Narumiya (Kyoto University, Kyoto, Japan) and Y. Takai (Osaka University, Osaka, Japan), respectively. Plasmid encoding GFP-actin was kindly provided by M. Takahashi (Kitasato University, Kanagawa, Japan), The GFP expression vector pGFP-N3 was from Clontech (Palo Alto, CA).
To construct a cDNA for the chimeric protein CD8-CD47MMS, we first performed PCR with the sense primer 5′-CCGAATTCGTCTTCATCGGTGTGGGCGTG-3′, the antisense primer 5′-TAGAAGGCACAGTCGAGGCTGATC-3′, and the full-length mouse CD47 form 2 cDNA as template in order to obtain a cDNA encoding the multiple membrane-spanning segments and short cytoplasmic tail of CD47 (amino acids 161–324). The PCR product was digested with EcoRI and NotI and then subcloned into the corresponding sites of pTracer-CMV (Invitrogen, Carlsbad, CA). The extracellular domain of human CD8 (amino acids 1–194) was amplified from the corresponding full-length cDNA by PCR with the sense primer 5′-CCGGTACCGCCCTTCCAAGCTTATGGCC-3′ and the antisense primer 5′-CCGAATTCCCCACAAGTCCCGGCCAAGGG-3′. Both the PCR product and the plasmid containing CD47-MMS cDNA were then digested with EcoRI and KpnI, and the resulting fragments were ligated.
To construct a cDNA for the chimeric protein CD47EX-TM, we first performed PCR with the sense primer 5′-GGGAATTCGTCTTCATCGGTGTGGGCGTG-3′, the antisense primer 5′-TAGAAGGCACAGTCGAGGCTGATC-3′, and a cDNA encoding mouse SHPS-1ΔCyto (Sato et al., 2003) as template in order to obtain a cDNA encoding the transmembrane domain and a short portion of the cytoplasmic domain of SHPS-1 (amino acids 374–404). The PCR product was digested with EcoRI and NotI and then subcloned into the corresponding sites of pTracer-CMV. A cDNA for the extracellular domain of CD47 (amino acids 1–140) was also amplified from full-length mouse CD47 form 4 cDNA by PCR with the sense primer 5′-CCGGTACCTGATCCAGACACCTGCGGCG-3′ and the antisense primer 5′-CCGAATTCCTTTTCATTTGGAGAAAACCA-3′. Both the PCR product and the plasmid containing SHPS-1-TM cDNA were then digested with EcoRI and KpnI and the resulting fragments were ligated.
To generate a cDNA for the CD47 deletion mutant CD47ΔCterm (lacking amino acids 290–321), we performed PCR with mouse CD47 form 4 cDNA as template, the sense primer 5′-TGGGCAACGTGCTGGTTGTTGTGC-3′, and the antisense primer 5′-CCGAATTCTCACAGGTCCTCCTCGGAGATCAGCTTCTGTTCCTTCATATAAACTAA-3′. The PCR product was then digested with EcoRI and subcloned into the corresponding site of pCAGGS.
Cell Culture and Transfection
All cells were maintained at 37°C under a humidified atmosphere of 5% CO2 in air. Mouse N1E-115 neuroblastoma cells (kindly provided by S. Narumiya) and COS-7 cells were cultured in DMEM (Invitrogen, Rockville, MD) supplemented with 10% fetal bovine serum (FBS; Invitrogen). H-Ras–transformed CHO (CHO-Ras) cells overexpressing mouse CD47 (kindly provided by N. Honma, Kirin Brewery Co. Ltd., Gunma, Japan) were cultured in αMEM (Sigma) supplemented with 2 mM l-glutamine, 10 mM HEPES-NaOH (pH 7.4), 10% FBS, and geneticin (500 μg/ml; Invitrogen). Mouse hippocampal neurons were isolated from mouse embryos at gestational day 17–18 as described previously (Hayashi et al., 2002). The cells were plated on culture dishes at a density of 4 × 104 cells/cm2. The hippocampal neurons were maintained in serum-free medium (Neurobasal A medium [Invitrogen] supplemented with 0.5 mM glutamine and B27 supplement [Invitrogen]) at 37°C in a humidified 10% CO2 atmosphere. Cells were transfected with expression plasmids by the use of LipofectAMINE2000 (Invitrogen).
Assay for Neurite Formation and Immunocytofluorescence Analysis
For determination of neurite and filopodium formation, N1E-115 cells or primary cultured neurons were cultured on cover glasses or in culture dishes coated with SHPS-1–Fc; the latter were prepared by incubating 35-mm dishes overnight at 4°C with SHPS-1–Fc (25 μg/ml) in PBS and then washing twice with PBS. For cultured neurons, culture dishes were subsequently coated with poly-d-lysine (25 μg/ml). Cells were transfected with the indicated plasmids, fixed for 20–30 min at room temperature with PBS containing 4% paraformaldehyde and 0.1% glutaraldehyde, and then permeabilized for 60 min at room temperature with PBS containing 0.1% Triton X-100 and 5% goat serum. They were incubated for 1 h at room temperature or overnight at 4°C with primary antibodies diluted in the permeabilization solution, washed with PBS, and then incubated for 1 h at room temperature with Cy3- or Alexa488-conjugated secondary antibodies (Molecular Probes). For costaining of F-actin, cells were incubated with rhodamine-conjugated phalloidin together with the secondary antibodies. The cells were finally washed with PBS and mounted. Fluorescence signals were acquired with an Olympus AX-70 microscope (Tokyo, Japan). Digital images were obtained with a cooled CCD camera, PXL (Photometrics, Tucson, AZ) and IPLab Image analysis software (Scanalytics, Billerica, MA), or confocal laser scanning microscope, LSM 5 Pascal (Zeiss, Oberkochen, Germany). For quantitation of neurite formation, >100 cells were identified in randomly chosen fields of view; a neurite was defined as a process whose length was at least equal to that of the cell body, and the percentage of cells with neurites was calculated.
Preparation of SHPS-1–Fc Fusion Proteins
Mouse and human SHPS-1–Fc fusion proteins were prepared as described previously (Motegi et al., 2003). In brief, cDNAs encoding the extracellular domain of mouse SHPS-1 (amino acids 1–371) or that of human SHPS-1 (amino acids 1–368) were amplified by PCR from the corresponding full-length cDNAs, digested with EcoRI and SpeI, and ligated into the corresponding sites of pEFneoFc76, an Fc fusion protein expression vector encoding a cDNA of the Fc portion of human IgG1. The resulting constructs were digested with PstI and NotI, and the released fragments were subcloned into the corresponding sites of pTracer-CMV. CHO-Ras cells were transfected with the resulting plasmids and subjected to selection with Zeocin (Invitrogen). Several cell lines producing each SHPS-1–Fc protein were identified by immunoblot analysis of culture supernatants with polyclonal antibodies to human IgG. The SHPS-1–Fc fusion proteins were then purified from such culture supernatants by column chromatography on protein A-Sepharose 4FF (Amersham Pharmacia Biotech, Piscataway, NJ).
Immunoblot Analysis
N1E-115 cells (∼1 × 106) were washed with ice-cold PBS and then lysed on ice in 1 ml of lysis buffer (20 mM Tris-HCl, pH 7.6, 140 mM NaCl, 1 mM EDTA, 1% NP-40) containing 1 mM PMSF, aprotinin (10 μg/ml), and 1 mM sodium vanadate. The lysates were centrifuged at 21,000 × g for 15 min at 4°C, and the resulting supernatants were subjected to immunoblot analysis as described previously (Motegi et al., 2003). For immunoblot analysis with the mAb to CD47, however, cells were solubilized in SDS sample buffer under nonreducing conditions.
Assay of Activated Rac and Cdc42
Activated Rac and Cdc42 were assayed as described previously (Inagaki et al., 2000; Kawakatsu et al., 2002). In brief, COS-7 cells in 60-mm dishes were transfected with 2 μg of pCAGGS containing full-length mouse CD47 form 4 cDNA and, after 24 h, lysed in a solution containing either 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 10 mM NaF, 1 mM EDTA, and 1 mM EGTA for the Rac assay or 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1% Triton X-100, 10 mM MgCl2, 0.2% sodium deoxycholate, and 1 mM DTT for the Cdc42 assay; both solutions also contained 1 mM PMSF, leupeptin (10 μg/ml), and aprotinin (10 μg/ml). The cell lysates were then incubated for 45 min at 4°C with a glutathione S-transferase (GST) fusion protein that contained the CRIB domain (amino acids 70–106) of rat p21PAKα and was bound to glutathione-Sepharose beads (Amersham Pharmacia Biotech). Lysate proteins that bound to the beads were subjected to immunoblot analysis with mAbs to Rac or to Cdc42. The total abundance of each small G protein was also determined by immunoblot analysis of cell lysates.
RESULTS
Effects of Forced Expression of CD47 on the Formation of Neurites and Filopodia in N1E-115 Cells
We first examined the endogenous expression of CD47 in mouse N1E-115 neuroblastoma cells. Immunoblot analysis of N1E-115 cell lysates with a mAb to mouse CD47 revealed an immunoreactive protein of ∼50 kDa, similar to that apparent in CHO-Ras cells overexpressing CD47 form 2 (Figure 1A). Of the four alternatively spliced isoforms (forms 1–4) of CD47 mRNA identified, form 2 is present ubiquitously whereas form 4 is the predominant isoform in the brain (Reinhold et al., 1995). Compared with the protein encoded by the form 2 mRNA, that encoded by the form 4 mRNA lacks 21 amino acids in the extracellular juxtamembrane region and contains an additional 18 amino acids in the COOH-terminal tail (Reinhold et al., 1995; Jiang et al., 1999). We prepared expression plasmids containing either form 2 or form 4 mouse CD47 cDNA. Transient transfection of N1E-115 cells with either of these plasmids resulted in the production of an ∼50-kDa protein immunoreactive with the mAb to CD47 (Figure 1B).
Figure 1.
Expression of CD47 and effects of serum deprivation on neurite formation in N1E-115 cells. (A) Lysates of N1E-115 cells, of CHO-Ras cells, or of CHO-Ras cells stably expressing mouse CD47 form 2 (CHO-Ras-CD47) were subjected to immunoblot analysis with a mAb to CD47 (αCD47). (B) N1E-115 cells transfected with a vector for GFP (Mock) or with a vector containing mouse CD47 form 2 or form 4 cDNA were lysed 24 h after transfection and subjected to immunoblot analysis with a mAb to CD47. (C) N1E-115 cells were cultured in the absence (Serum (-); a and c) or presence (Serum (+); b and d) of 10% FBS for 24 h, fixed, and stained with a mAb to CD47 (c and d); phase-contrast images of the same cells are also shown (Phase; a and b). Scale bar, 50 μm. All results shown are representative of three separate experiments.
In the absence of serum, most (∼80%) N1E-115 cells became flattened and extended neurites with prominent growth cones (Figure 1C), consistent with previous observations (Hirose et al., 1998). A neurite was defined as a process with a length at least equal to that of the cell body. We also confirmed the previous observation (Suidan et al., 1992; Jalink et al., 1993; Hirose et al., 1998) that the addition of 10% FBS to these cells induced growth cone collapse and neurite retraction (Figure 1C). Thus, only ∼20% of cells possessed neurites in the presence of serum. Immunostaining of N1E-115 cells with the mAb to CD47 revealed a low level of CD47 expression in the absence or presence of FBS (Figure 1C), consistent with the results of immunoblot analysis (Figure 1A).
We then examined the effect of forced expression of CD47 on the morphology of N1E-115 cells, as revealed by staining of F-actin with rhodamine-conjugated phalloidin. The expression of CD47 was detected by immunostaining of cells with the mAb to CD47. Even in the presence of 10% FBS, forced expression of CD47 form 2 or form 4, but not that of GFP (control), induced marked neurite formation and the adoption of a flattened cell morphology within 24 h after transfection (Figure 2A). Quantitative analysis revealed that ∼40% of cells transfected with CD47 form 2 or form 4 cDNA exhibited neurite formation; this effect of CD47 overexpression was therefore smaller than that of serum deprivation (Figure 2B). Serum deprivation induced the formation of prominent filopodia at growth cone-like structures apparent at the distal end of neurites (Figure 2C). Forced expression of CD47 form 4, but not that of GFP, promoted the formation of filopodia at growth cones as well as at the periphery of the cell body (Figure 2C). Forced expression of CD47 form 2 also promoted formation of filopodia at growth cones (our unpublished results). Given that expression of each form of CD47 yielded similar phenotypes, we examined the effects only of CD47 form 4 in subsequent experiments.
Figure 2.
Neurite and filopodium formation induced in N1E-115 cells by overexpression of CD47. (A) N1E-115 cells were transfected with expression vectors for CD47 form 2 (a and d), CD47 form 4 (b and e), or GFP (c and f). After incubation for 24 h in the presence of 10% FBS, the cells were stained with rhodamine-conjugated phalloidin (Phalloidin; a-c) or a mAb to CD47 (d and e); GFP fluorescence (GFP) is shown in f. Arrows indicate cells expressing recombinant CD47 or GFP. Scale bar, 50 μm. (B) N1E-115 cells were cotransfected with vectors for GFP and CD47 form 2 or 4 (or the corresponding empty vector [Vector]). After incubation for 24 h in the presence of 10% FBS, the cells were stained with a mAb to CD47. The percentage of cells expressing both CD47 and GFP that exhibited neurites at least as long as the cell body was determined. The effect of serum deprivation on neurite formation was also quantified for N1E-115 cells transfected with the GFP vector alone (Serum (-)). Data are means ± SE of values from three separate experiments. (C) N1E-115 cells were transfected with an expression vector for GFP (a and c) or for CD47 form 4 (b). After culture for 24 h in the presence (a and b) or absence (c) of 10% FBS, the cells were stained with rhodamine-conjugated phalloidin (red). Expression of GFP or CD47 was detected by GFP fluorescence and immunostaining with a mAb to CD47, respectively (green). Scale bar, 10 μm. Results in A and C are representative of three separate experiments.
Effects of an SHPS-1–Fc Fusion Protein and Forced Expression of CD47 on Formation of Filopodia in N1E-115 Cells
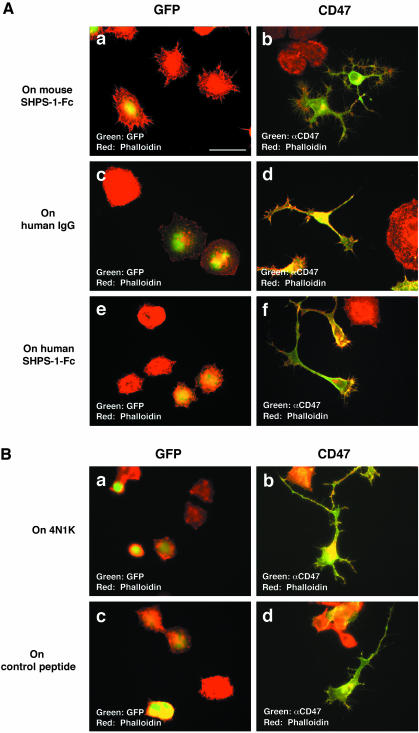
SHPS-1 (Jiang et al., 1999; Seiffert et al., 1999) and TSP1 (Gao et al., 1996; Chung et al., 1997) are ligands for CD47. It is also shown that plating of postnatal mouse cerebellar neurons on recombinant SHPS-1 induces neurite formation in vitro (Jiang et al., 1999). We therefore next examined the effect of a recombinant fusion protein containing the extracellular region of mouse SHPS-1 and the Fc portion of human Ig on the morphology of N1E-115 cells in the presence of 10% FBS. Plating of the cells on a dish coated with the SHPS-1–Fc protein induced the formation of filopodia at the cell periphery (Figure 3A). It also induced the generation of small spike-like neurites, but the length of these processes did not exceed that of the cell body. These effects were not observed with cells plated on dishes coated with either control human IgG or a human SHPS-1–Fc fusion protein (Figure 3A). Consistent with these observations, mouse SHPS-1–Fc binds to mouse CD47 expressed on the surface of CHO-Ras cells (Sato et al., 2003), whereas human SHPS-1–Fc does not (our unpublished results). Mouse SHPS-1–Fc also enhanced filopodium formation at growth cones as well as at the trunks of neurites and the periphery of the cell body in cells overexpressing CD47 (Figure 3A). The number of filopodium (per 10 μm-length of neurite) in CD47-overexpressing cells plated on SHPS-1–Fc (13.0 ± 0.9, n = 5 cells) was significantly larger than that of CD47-overexpressing cells plated on human IgG (2.9 ± 0.3, n = 5 cells, p < 0.0001). Moreover, these effects were not observed in cells plated on human SHPS-1–Fc. Mouse SHPS-1–Fc did not affect the neurite elongation or the number of neurite per cell in cells overexpressing CD47 (our unpublished results).
Figure 3.
Effects of an SHPS-1–Fc fusion protein and forced expression of CD47 on filopodium formation. (A) N1E-115 cells plated on dishes coated with mouse SHPS-1–Fc (a and b), control human IgG (c and d), or human SHPS-1–Fc (e and f) were transfected with a vector for either GFP (a, c, and e) or CD47 form 4 (b, d, and f). After culture for 24 h in the presence of 10% FBS, the cells were stained with rhodamine-conjugated phalloidin (red); those transfected with the CD47 vector were also immunostained with a mAb to CD47 (green), whereas those transfected with the GFP vector were detected by GFP fluorescence (green). (B) N1E-115 cells plated on dishes coated with the 4N1K peptide (a and b) or a control peptide (c and d) were transfected with a vector for either GFP (a and c) or CD47 form 4 (b and d). After culture for 24 h in the presence of 10% FBS, the cells were stained as in A. Scale bar, 50 μm. All results are representative of three separate experiments.
A synthetic peptide (4N1K) based on the COOH-terminal region of TSP1 and containing the RFYVVM sequence is able to substitute for the intact protein in stimulation of integrin-dependent platelet activation, an effect mediated by the binding of the 4N1K peptide or intact TSP1 to CD47 (Chung et al., 1997; Dorahy et al., 1997). We have recently shown that the interaction of the 4N1K peptide with CD47 alters the binding affinity of integrin αIIbβ3 (Fujimoto et al., 2003). We thus examined the effect of the 4N1K peptide on filopodium formation in N1E-115 cells. However, plating of cells on dishes coated with the 4N1K peptide or a control peptide failed to induce filopodium formation in N1E-115 cells transfected with a vector for either GFP or CD47 (Figure 3B).
Role of CD47 in the Formation of Neurites and Filopodia in Response to Serum Deprivation
To evaluate the contribution of endogenous CD47 to the neurite and filopodium formation elicited by serum deprivation, we examined the effects of mouse SHPS-1–Fc on these processes. One hour after serum deprivation, ∼35% of N1E-115 cells plated on control human IgG exhibited neurite formation (Figure 4, A and C). In contrast, ∼55% of cells plated on SHPS-1–Fc manifested neurite formation 1 h after serum deprivation. Furthermore, the percentage of cells with long processes (those whose length was more than twice that of the cell body) was markedly greater for those plated on SHPS-1–Fc than for those plated on human IgG (Figure 4C). In addition, filopodium formation was more extensive in cells plated on SHPS-1–Fc than in those grown on human IgG (Figure 4, A and B). The number of cells extending neurites and the extent of filopodium formation were greater for cells plated on SHPS-1–Fc than for those plated on human IgG even at 3 or 15 h after serum deprivation (Figure 4, A and C), although the effects of SHPS-1–Fc were smaller than that observed at 1 h after serum deprivation (Figure 4C). These results suggest that the binding of SHPS-1 to endogenous CD47 expressed on N1E-115 cells potentially promotes the initiation of neurite and filopodium formation by serum deprivation. In contrast, SHPS-1–Fc did not affect the number of neurite per cell in serum-deprived cells (our unpublished results).
Figure 4.
Promotion by mouse SHPS-1–Fc of neurite and filopodium formation triggered in N1E-115 cells by serum deprivation. (A) N1E-115 cells were plated on dishes coated with control human IgG (a-c) or with mouse SHPS-1–Fc (d-f) and were cultured for 5 h in the presence of 10% FBS. After incubation in the absence of serum for an additional 1 h (a and d),3h(bande),or15h(candf),the cells were stained with rhodamine-phalloidin. Scale bar, 50 μm. (B) Cells were plated on dishes coated with control human IgG (a) or with mouse SHPS-1–Fc (b) and subjected to serum deprivation for 1 h as in A before staining with rhodamine-phalloidin. Scale bar, 10 μm. (C) Cells treated and analyzed as in A were evaluated for neurite extension. The percentages of cells with neurites whose length corresponded to 1× to 2× (open column), 2× to 3× (gray column), or >3× (closed column) that of the cell body were determined. Data are means ± SE of three separate experiments. All results are representative of three separate experiments.
Roles of Rac and Cdc42 in CD47-induced Neurite and Filopodium Formation
Activation of Rac and Cdc42 mediates neurite formation in response to serum deprivation (Kozma et al., 1997). We thus examined whether Rac or Cdc42 contributes to CD47-dependent neurite formation. Expression of a dominant negative mutant of Rac1 (N17Rac1) fused to GFP, but not of GFP alone, prevented the induction of neurite formation in N1E-115 cells by overexpression of CD47 (Figure 5, A and B). NWASP-CRIB specifically binds the GTP-bound (active) form of Cdc42 and thereby inhibits its activity (Takenawa and Miki, 2001). Expression of NWASP-CRIB fused to GFP, however, only partially inhibited neurite formation in response to forced expression of CD47 (Figure 5, A and B). Expression of GFP–N17Rac1, but not that of GFP, partly inhibited filopodium formation in N1E-115 cells overexpressing CD47 and plated on mouse SHPS-1–Fc (Figure 5C). In contrast, expression of GFP-NWASP–CRIB markedly inhibited filopodium formation under these conditions.
Figure 5.
Effects of a dominant negative mutant of Rac and of NWASP-CRIB on CD47-dependent neurite and filopodium formation. (A) N1E-115 cells were cotransfected with a vector for CD47 form 4 and a vector for either GFP (GFP + CD47; a and d), GFP-N17Rac1 (GFP-RacDN; GFP-RacDN + CD47; b and e), or GFP-NWASP–CRIB (GFP-NWASP-CRIB + CD47; c and f). After culture for 24 h in the presence of 10% FBS, the cells were immunostained with a mAb to CD47 (d-f) or monitored for GFP fluorescence (a-c). Scale bar, 50 μm. (B) N1E-115 cells were cotransfected with a vector for CD47 form 4 (closed column; or the corresponding empty vector; open column) and a vector for either GFP (GFP + vector, GFP + CD47), GFP-N17Rac1 (RacDN + vector, RacDN + CD47), or GFP-NWASP–CRIB (NWASP-CRIB + vector, NWASP-CRIB + CD47). After culture for 24 h in the presence of 10% FBS, the cells were analyzed as in A and neurite formation was quantitated as described in Figure 2B. Data are means ± SE of values from three separate experiments. (C) N1E-115 cells were plated on dishes coated with mouse SHPS-1–Fc and were transfected, cultured, and analyzed as in A. Scale bar, 50 μm. Results in A and C are representative of three separate experiments.
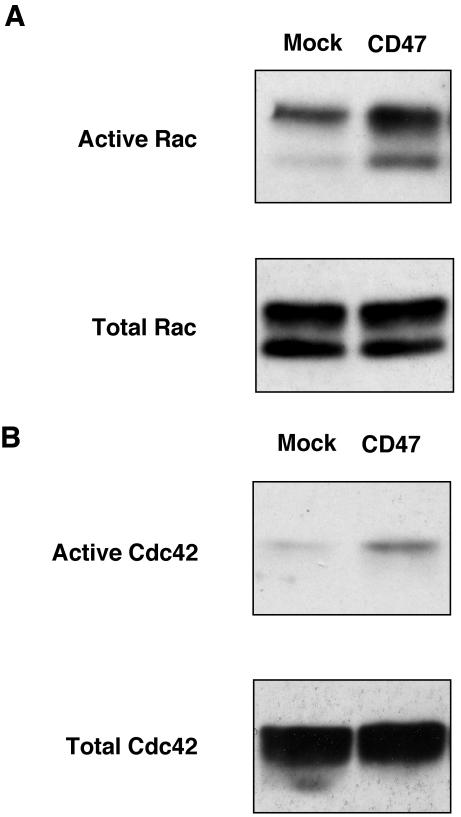
We next examined whether forced expression of CD47 indeed results in the activation of Rac or Cdc42. The activity of these small G proteins was monitored directly by precipitation of the GTP-bound form of each protein with a GST fusion protein containing the Cdc42/Rac binding domain of the effector protein p21PAKα (Glaven et al., 1999). Expression of CD47 induced a marked increase in the proportion of activated Rac (Figure 6A) and a smaller increase in that of activated Cdc42 (Figure 6B) in COS-7 cells. These results suggest that the promotion of neurite formation by CD47 is mediated by the activation of Rac and, to a lesser extent, by that of Cdc42, whereas the CD47-dependent formation of filopodia in cells plated on SHPS-1–Fc appears to be mediated predominantly through the activation of Cdc42.
Figure 6.
Activation of Rac and Cdc42 by forced expression of CD47. COS-7 cells were transfected with a vector for CD47 form 4 (CD47) or the corresponding empty vector (Mock). The cells were lysed 24 h after transfection, and the GTP-bound (active) forms of Rac (A) or Cdc42 (B) were precipitated with a GST fusion protein containing the Rac/Cdc42 binding domain of p21PAKα. The resulting precipitates were subjected to immunoblot analysis with mAbs to Rac or to Cdc42 (top panels). Whole cell lysates were also directly subjected to immunoblot analysis with the same mAbs to determine the total amounts of Rac or Cdc42 (bottom panels). Results are representative of three separate experiments.
Determination of the Regions of CD47 Required for Induction of Neurite and Filopodium Formation
CD47 possesses an Ig-V–like extracellular region, five putative transmembrane segments, and a short cytoplasmic tail (Brown and Frazier, 2001). We next determined which regions of CD47 are required for the induction of neurite and filopodium formation. For this purpose, we generated two cDNAs encoding chimeric proteins (Figure 7A): one containing the extracellular Ig region of mouse CD47 fused to the transmembrane region and proximal portion of the cytoplasmic tail of mouse SHPS-1 (CD47EX-TM), and the other comprising the extracellular Ig region of human CD8 fused to the multiple membrane-spanning domains and short cytoplasmic tail of mouse CD47 (CD8-CD47MMS). In addition, we generated a cDNA that encodes a mouse CD47 mutant lacking the short cytoplasmic tail at the COOH-terminus (CD47ΔCterm; Figure 7A). Expression of either CD47EX-TM or CD47ΔCterm in N1E-115 cells induced neurite formation to an extent similar to that observed with wild-type CD47 (Figure 7, B and C). In contrast, expression of CD8-CD47MMS failed to induce neurite formation (Figure 7, B and C), although it did promote filopodium formation at the cell periphery (Figure 7B).
Figure 7.
Identification of the regions of CD47 responsible for the promotion of neurite and filopodium formation. (A) Schematic representation of the CD47 mutants studied. CD47WT, wild-type CD47 form 4. (B) N1E-115 cells were transfected with a vector for CD47WT (a), CD47EX-TM (b), or CD8-CD47MMS (c), incubated for 24 h in the presence of 10% FBS, and stained with rhodamine-phalloidin (red; a-c) as well as with mAbs to either CD47 (green; a and b) or CD8 (green; c). Scale bar, 50 μm. (C) N1E-115 cells were cotransfected with a vector for GFP and the vectors for indicated mutants (or the corresponding empty vector [Vector]), incubated for 24 h in the presence of 10% FBS, and then stained with a mAb to CD47. Quantitative analysis of neurite formation was performed as in Figure 2B. Data are means ± SE of values from three separate experiments. (D) N1E-115 cells plated on dishes coated with mouse SHPS-1–Fc were transfected, incubated, and stained as in A. Scale bar, 50 μm. Results in B and D are representative of three separate experiments.
Expression of CD47EX-TM in N1E-115 cells plated on mouse SHPS-1–Fc promoted filopodium formation but to a markedly reduced extent compared with the effect of the wild-type CD47 (Figure 7D). However, the extent of SHPS-1–Fc binding to cell surface CD47EX-TM was markedly decreased, compared with that apparent with wild-type CD47 (our unpublished results), suggesting that the decreased ligand affinity of CD47EX-TM was attributable to the decreased filopodium formation by CD47EX-TM on SHPS-1–Fc. In addition, the expression of CD8-CD47MMS failed to enhance the filopodium formation, which was promoted by SHPS-1–Fc (Figure 7D). Expression of CD47ΔCterm also enhanced the filopodium formation, but the effect was slightly smaller than that of wild-type CD47 (our unpublished results). These results indicate that the extracellular region of CD47 is sufficient for promotion of neurite formation in N1E-115 cells, with the multiple transmembrane domains and short cytoplasmic tail being dispensable for this effect. The entire structure of CD47 appears to be required, however, for maximal enhancement of filopodium formation in cells plated on SHPS-1–Fc.
Participation of Integrins, But Not of PTX-sensitive Gi or Phosphoinositide 3-kinase, in CD47-mediated Neurite and Filopodium Formation
CD47 interacts with integrins such as αvβ3, αIIbβ3, and α2β1 through its extracellular region and participates in integrin-mediated biological responses including cell migration and platelet activation (Brown and Frazier, 2001). Laminin has been shown to promote neurite formation by binding to the integrin β1 subunit in serum-deprived N1E-115 cells (Sarner et al., 2000). In fact, we confirmed the surface expression of either integrin β1 or β3 subunit in N1E-115 cells by using FACS analysis and immunocytochemistry (our unpublished results). We therefore examined the effect on CD47-dependent neurite formation of the disintegrin echistatin, an RGD-containing peptide from viper venom that specifically inhibits the function of integrins containing β1 or β3 subunits (Pfaff et al., 1994). Echistatin markedly inhibited neurite formation induced by forced expression of CD47 in N1E-115 cells (Figure 8A). In contrast, this peptide inhibited only slightly the formation of filopodia promoted by CD47 expression in cells plated on mouse SHPS-1–Fc (Figure 8B); it also failed to affect filopodium formation in nontransfected cells plated on SHPS-1–Fc (our unpublished results). We also tested the effects of inhibitory mAbs to integrin β1 or β3 subunits (Pasterkamp et al., 2003). The mAb to β3, but not that to β1, exhibited a significant inhibitory effect on neurite formation induced by forced expression of CD47 (Figure 8A). Neither the mAb to β3 nor that to β1 inhibited the enhancement by CD47 of filopodium formation in cells plated on SHPS-1–Fc (Figure 8B). These results suggest that the β3 subunit of integrins contributes to CD47-dependent neurite formation but that it plays only a minor role, if any, in the enhancement by CD47 of filopodium formation in cells plated on SHPS-1–Fc. We also examined the effect of echistatin on neurite formation elicited by serum deprivation. Echistatin markedly inhibited the formation of neurites induced by serum deprivation in cells plated on either control human IgG or SHPS-1–Fc (Figure 8C). Furthermore, the percentage of cells with long processes (those whose length was more than twice that of the cell body) was markedly decreased in the echistatin-treated cells (Figure 8C). These results suggest that CD47, through its interaction with the β3 subunit of integrins, participates in the integrin-mediated neurite formation and elongation induced by serum deprivation.
Figure 8.
Effects of echistatin, inhibitory mAbs to integrin subunits, PTX, or wortmannin on CD47-induced neurite and filopodium formation. (A) N1E-115 cells were cotransfected with a vector for CD47 (closed column; or the corresponding empty vector; open column) and a vector for GFP and were then cultured for 24 h with 10% FBS in the absence (Control) or presence of echistatin (0.05 μg/ml). Alternatively, the transfected cells were cultured for 24 h, detached from the dish, incubated for 5 min with normal mouse IgG (5 μg/ml; Mouse IgG) or with mAbs to the integrin β3 (5 μg/ml; β3-antibody) or β1 (5 μg/ml; β1-antibody) subunits, and then replated and cultured for 24 h. All cells were then visualized either by CD47 immunofluorescence or by GFP fluorescence, and neurite formation was quantified as in Figure 2B. Data are means ± SE of values from three separate experiments. (B) N1E-115 cells plated on dishes coated with mouse SHPS-1–Fc were transfected with a vector for CD47 and treated with echistatin or the mAbs to integrin β1 or β3 subunits as in A. They were then stained with rhodamine-phalloidin (red) and a mAb to CD47 (green). Scale bar, 50 μm. (C) N1E-115 cells were plated on dishes coated with control human IgG or with mouse SHPS-1–Fc and were cultured for 4 h in the presence of 10% FBS. Cells were subjected to serum deprivation in the absence or presence of echistatin (0.05 μg/ml). After incubation for 1 h, the percentages of cells with neurites whose length corresponded to 1× to 2× (open column), 2× to 3× (gray column), or >3× (closed column) that of the cell body were determined. Data are means ± SE of values obtained from 15 randomly chosen fields. (D) N1E-115 cells were cotransfected with a vector for CD47 (closed column; or the corresponding empty vector; open column) and a vector for GFP, cultured for 24 h with 10% FBS in the absence (Control) or presence of PTX (100 ng/ml) or wortmannin (100 nM), and then stained with a mAb to CD47. Quantitative analysis of neurite formation was performed as in Figure 2B. Data are means ± SE of values from three separate experiments. (E) N1E-115 cells plated on dishes coated with mouse SHPS-1–Fc were transfected with a vector for CD47, cultured for 24 h with 10% FBS in the absence (Control; a) or presence of PTX (100 ng/ml; b), or wortmannin (100 nM; c), and stained with rhodamine-phalloidin (red) and a mAb to CD47 (green). Scale bar, 50 μm. Results in B, C, and E are representative of three separate experiments.
A PTX-sensitive Gi is thought to mediate CD47-dependent cellular responses such as cell spreading and platelet activation (Gao et al., 1996; Frazier et al., 1999; Brown and Frazier, 2001). However, PTX failed to inhibit the promotion of neurite formation by forced expression of CD47 in N1E-115 cells (Figure 8D). Phosphoinositide (PI) 3-kinase is also implicated in neurite formation induced by serum deprivation (Sarner et al., 2000). In addition, the spreading of platelets induced by TSP1-CD47 interaction and the polarization of B cells induced by SHPS-1–CD47 interaction are sensitive to the PI 3-kinase inhibitor wortmannin (Gao et al., 1996; Yoshida et al., 2002). However, treatment of N1E-115 cells with wortmannin failed to inhibit neurite formation induced by CD47 (Figure 8D). PTX or wortmannin also had no effect on the enhancement by CD47 of filopodium formation in N1E-115 cells plated on mouse SHPS-1–Fc (Figure 8E). These results indicate that neither Gi nor PI 3-kinase participates in the promotion of neurite formation by CD47 or in the enhancement by CD47 of filopodium formation in cells plated on SHPS-1–Fc.
Effects of an SHPS-1–Fc Fusion Protein and Forced Expression of CD47 on Formation of Filopodia in Cultured Hippocampal Neurons
To investigate the physiological importance of CD47 function, we examined the effect of SHPS-1–Fc on the cell morphology using primary cultured neurons. Mouse hippocampal neurons were plated on a dish coated with the SHPS-1–Fc protein and then cotransfected with vectors for GFP-actin and CD47. Plating of cells overexpressing CD47 on mouse SHPS-1–Fc markedly promoted filopodium formation, compared with the effect of plating of cells overexpressing CD47 on control human IgG (Figure 9).
Figure 9.
Effects of an SHPS-1–Fc fusion protein and forced expression of CD47 on filopodium formation in cultured hippocampal neurons. Mouse hippocampal neurons were cultured on dishes coated with control human IgG (A and B) or mouse SHPS-1-Fc (C and D) for 3 days. Neurons were then cotransfected with the vectors for GFP-actin and CD47 (A-D). Four days after transfection, neurons were immunostained with a mAb to CD47 (red). The cell morphology was examined by fluorescence of GFP-actin (green). Magnification: (A and C) ×630; (B and D) ×1000. Scale bar for A, 20 μm; for B, 10 μm. All results are representative of three separate experiments.
DISCUSSION
We have shown that forced expression of CD47 promoted the formation of neurites and filopodia in N1E-115 neuroblastoma cells even in the presence of serum. The formation of both neurites and filopodia requires rearrangement of the actin cytoskeleton. CD47 is also implicated in the regulation of platelet spreading (Chung et al., 1997), of migration and phagocytosis in neutrophils (Gresham et al., 1989; Parkos et al., 1996; Lindberg et al., 1996a), and of the promotion of polarity in B cells (Yoshida et al., 2000), all processes that also require rearrangement of the actin cytoskeleton. Forced expression of CD47 may thus promote the formation of neurites and filopodia through regulation of the actin cytoskeleton. We also found that plating of nontransfected N1E-115 cells on mouse SHPS-1–Fc induced filopodium formation in the presence of serum. Human SHPS-1–Fc, which does not bind to mouse CD47, was unable to induce this response. In addition, the promotion of filopodium formation by forced expression of CD47 was enhanced by mouse SHPS-1–Fc. Together, these results suggest that the binding of SHPS-1 to endogenous or recombinant CD47 expressed on the surface of N1E-115 cells promotes filopodium formation. Furthermore, SHPS-1–Fc markedly enhanced both neurite and filopodium formation induced by serum deprivation in non-transfected N1E-115 cells.
In contrast to mouse SHPS-1–Fc, a synthetic peptide (4N1K) based on the COOH-terminal domain of TSP1 failed to induce filopodium formation in nontransfected N1E-115 cells and did not enhance this response in cells overexpressing CD47. The binding of the 4N1K peptide or intact TSP1 to CD47 stimulates integrin-mediated platelet activation, spreading, and aggregation (Chung et al., 1997; Dorahy et al., 1997; Fujimoto et al., 2003). Our present results suggest that the binding of TSP1 to CD47 does not stimulate the formation of neurites and filopodia in N1E-115 cells. However, TSP1 might be present in the serum and it could participate in neurite formation induced by CD47.
We also investigated the molecular mechanisms by which forced expression of CD47 promotes the formation of neurites and filopodia. In cultured fibroblasts, Rac and Cdc42 mediate the growth factor-induced formation of lamellipodia and filopodia, respectively (Nobes and Hall, 1995; Takai et al., 2001). In N1E-115 cells, dominant active mutants of Rac and of Cdc42 also induced lamellipodium and filopodium formation, respectively (Kozma et al., 1997); however, neurite formation induced by serum deprivation was inhibited by expression of dominant negative mutants of Rac or of Cdc42 (Kozma et al., 1997). Thus, both Rac and Cdc42 are required for neurite formation in N1E-115 cells, with the former participating predominantly in lamellipodium formation and the latter in filopodium formation. Expression of a dominant negative mutant of Rac markedly inhibited neurite formation induced by forced expression of CD47, whereas expression of NWASP-CRIB partly inhibited this response. In contrast, expression of NWASP-CRIB markedly inhibited SHPS-1–Fc-induced filopodium formation in CD47-overexpressing cells, whereas the dominant negative mutant of Rac exhibited only a small such inhibitory effect. Forced expression of CD47 indeed induced the activation of both Rac and Cdc42, although its effect on Rac activity was greater. Overexpression of CD47 may therefore promote neurite fomation predominantly through the activation of Rac, whereas SHPS-1–Fc-induced filopodium formation in CD47-overexpressing cells might be mediated mostly by Cdc42.
We also showed that the extracellular region of CD47 was sufficient for the induction of neurite fomation by forced expression. CD47 associates with the integrins αIIbβ3, αVβ3, and α2β1 (Brown and Frazier, 2001). Echistatin, a disintegrin for the integrin β1 and β3 subunits, and an mAb to the β3 subunit (but not an mAb to β1) each inhibited neurite formation induced by forced expression of CD47, implicating the β3 subunit in this response. The extracellular region of CD47 interacts with the integrin β3 subunit and is sufficient for CD47-dependent integrin function (Lindberg et al., 1996b), consistent with our observation that the extracellular region of CD47 was sufficient for neurite formation induced by overexpression of CD47. In addition, integrins mediate the activation of Rac and Cdc42, thereby regulating cell polarity, cell spreading, and cell adhesion (Price et al., 1998; Etienne-Manneville and Hall, 2001; Del Pozo et al., 2002). Forced expression of CD47 may thus activate Rac and Cdc42 and thereby promote neurite formation through the interaction of its extracellular region with the integrin β3 subunit in N1E-115 cells. In contrast, CD47EX-TM failed to enhance filopodium formation induced by SHPS-1–Fc, suggesting that the extracellular region of CD47 is insufficient for the enhancement by CD47 of filopodium formation induced by SHPS-1–Fc. However, we also found that the binding of SHPS-1–Fc to CD47EX-TM expressed on N1E-115 cells was markedly reduced, compared with that apparent with wild-type CD47 (our unpublished results). This might be due to the lack of a long-range disulfide bond (between the extracellular region and the multiple membrane-spanning domains), which is shown to be required for the maximal affinity of CD47 for SHPS-1 (Rebres et al., 2001), in CD47EX-TM. Thus, the reduced affinity of CD47EX-TM for SHPS-1 may be attributable to its minimal effect on the enhancement of filopodium formation by SHPS-1–Fc. In either case, our present results suggest that the entire structure of CD47 is required for the enhancement by CD47 of filopodium formation induced by SHPS-1–Fc. Furthermore, neither echistatin nor the mAb to the integrin β3 subunit inhibited the enhancement by CD47 of filopodium formation induced by SHPS-1–Fc. Integrins might therefore not contribute to this effect of CD47.
CD47 is physically coupled to a PTX-sensitive Gi protein (Frazier et al., 1999). In addition, PTX blocks platelet spreading, melanoma cell spreading, and chemotaxis of smooth muscle cells all in response to the 4N1K peptide (Gao et al., 1996; Chung et al., 1997; Wang et al., 1999), suggesting that the binding of TSP1 to CD47 activates downstream signaling via a PTX-sensitive Gi. However, PTX failed to inhibit neurite and filopodium formation induced by forced expression of CD47 in N1E-115 cells. Cell spreading induced by the 4N1K peptide is also blocked by wortmannin, suggesting a role for PI 3-kinase in this response (Gao et al., 1996). However, again, wortmannin did not inhibit neurite and filopodium formation induced by the forced expression of CD47 in N1E-115 cells. Thus, neither Gi nor PI 3-kinase appears to contribute to the neurite and filopodium formation induced by overexpression of CD47 in N1E-115 cells.
In summary, we propose the following model for the mode of CD47 action in the promotion of neurite and filopodium formation in neuronal cells. In response to its engagement by SHPS-1, CD47 promotes neurite and filopodium formation induced by extracellular stimuli such as serum deprivation. Its promotion of neurite formation is mediated presumably through interaction of its extracellular region with integrins containing the β3 subunit. In contrast, its promotion of filopodium formation requires an intact CD47 structure but not integrins. The promotion of neurite formation by CD47 is also mediated predominantly through the activation of Rac, whereas that of filopodium formation is mediated mainly through Cdc42. However, whether CD47 induces the activation of Rac through integrins as well as the mechanism by which it activates Cdc42 remain unknown.
We also found that forced expression of CD47, through its interaction with SHPS-1, promoted the formation of filopodia in cultured hippocampal neurons. During development, dendritic protrusions start out as filopodia, which search out contacts with synaptic terminals and then mature into adult spines (Ziv and Smith, 1996). In adults, modulation of the number and shape of spines is associated with synaptic plasticity (Lendvai et al., 2000). Our results suggest the possibility that, through its interaction with SHPS-1, CD47 expressed on the surface of neurons positively regulates filopodium formation and thereby contributes to the generation and reconstruction of neuronal networks. Ligand-receptor interactions involving several transmembrane proteins are implicated in learning and the formation of long-term memory (Murase and Schuman, 1999). For example, N-cadherin is thought to play an important role in the establishment of neuronal networks, with N-cadherin knockout mice exhibiting a marked defect in LTP (Manabe et al., 2000). CD47 knockout mice also exhibit impaired memory retention and defective LTP (Chang et al., 1999). Such phenotypes might be due to an impairment of neurite and filopodium formation that results from the loss of the promoting effect of CD47, through its interaction with SHPS-1, on this process.
Acknowledgments
We thank S. Narumiya, Y. Takai, J. Miyazaki, F. P. Lindberg, E. Manser, M. Takahashi, F. Okajima, and N. Honma for reagents and H. Kobayashi and K. Tomizawa for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas Cancer, a Grant-in-Aid for Scientific Research (B), and a grant of the 21st Century COE Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a grant from the Uehara Memorial Foundation; a grant from the Brain Science Foundation; a grant from the Nakajima Foundation; and a grant from the Japan Research Foundation for Clinical Pharmacology.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-01-0019. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-01-0019.
Abbreviations used: FBS, fetal bovine serum; GFP, green fluorescent protein; GST, glutathione S-transferase; Ig, immunoglobulin; LTP, long-term potentiation; PI, phosphoinositide; PTX, pertussis toxin; SHPS-1, SHP substrate-1; TSP1, thrombospondin-1.
References
- Arakawa, Y., Bito, H., Furuyashiki, T., Tsuji, T., Takemoto-Kimura, S., Kimura, K., Nozaki, K., Hashimoto, N., and Narumiya, S. (2003). Control of axon elongation via an SDF-1α/Rho/mDia pathway in cultured cerebellar granule neurons. J. Cell Biol. 161, 381-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, E.J., and Frazier, W.A. (2001). Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11, 130-135. [DOI] [PubMed] [Google Scholar]
- Brown, E.J., Hooper, L., Ho, T., and Gresham, H. (1990). Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol. 111, 2785-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H.P., Lindberg, F.P., Wang, H.L., Huang, A.M., and Lee, E.H. (1999). Impaired memory retention and decreased long-term potentiation in integrin-associated protein-deficient mice. Learn. Mem. 6, 448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, J., Gao, A.G., and Frazier, W.A. (1997). Thrombospondin acts via integrin-associated protein to activate the platelet integrin αIIbβ3. J. Biol. Chem. 272, 14740-14746. [DOI] [PubMed] [Google Scholar]
- Chung, J., Wang, X.Q., Lindberg, F.P., and Frazier, W.A. (1999). Thrombospondin-1 acts via IAP/CD47 to synergize with collagen in α2β1-mediated platelet activation. Blood 94, 642-648. [PubMed] [Google Scholar]
- Cooper, D., Lindberg, F.P., Gamble, J.R., Brown, E.J., and Vadas, M.A. (1995). Transendothelial migration of neutrophils involves integrin-associated protein (CD47). Proc. Natl. Acad. Sci. USA 92, 3978-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo, M.A., Kiosses, W.B., Alderson, N.B., Meller, N., Hahn, K.M., and Schwartz, M.A. (2002). Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4, 232-239. [DOI] [PubMed] [Google Scholar]
- Dickson, B.J. (2002). Molecular mechanisms of axon guidance. Science 298, 1959-1964. [DOI] [PubMed] [Google Scholar]
- Dorahy, D.J., Thorne, R.F., Fecondo, J.V., and Burns, G.F. (1997). Stimulation of platelet activation and aggregation by a carboxyl-terminal peptide from thrombospondin binding to the integrin-associated protein receptor. J. Biol. Chem. 272, 1323-1330. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2001). Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell 106, 489-498. [DOI] [PubMed] [Google Scholar]
- Frazier, W.A., Gao, A.G., Dimitry, J., Chung, J., Brown, E.J., Lindberg, F.P., and Linder, M.E. (1999). The thrombospondin receptor integrin-associated protein (CD47) functionally couples to heterotrimeric Gi. J. Biol. Chem. 274, 8554-8560. [DOI] [PubMed] [Google Scholar]
- Fujimoto, T.T., Katsutani, S., Shimomura, T., and Fujimura, K. (2003). Thrombospondin-bound integrin-associated protein (CD47) physically and functionally modifies integrin αIIbβ3 by its extracellular domain. J. Biol. Chem. 278, 26655-26665. [DOI] [PubMed] [Google Scholar]
- Fujioka, Y., Matozaki, T., Noguchi, T., Iwamatsu, A., Yamao, T., Takahashi, N., Tsuda, M., Takada, T., and Kasuga, M. (1996). A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 16, 6887-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, A.G., Lindberg, F.P., Dimitry, J.M., Brown, E.J., and Frazier, W.A. (1996). Thrombospondin modulates αvβ3 function through integrin-associated protein. J. Cell Biol. 135, 533-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaven, J.A., Whitehead, I., Bagrodia, S., Kay, R., and Cerione, R.A. (1999). The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J. Biol. Chem. 274, 2279-2285. [DOI] [PubMed] [Google Scholar]
- Gresham, H.D., Goodwin, J.L., Allen, P.M., Anderson, D.C., and Brown, E.J. (1989). A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J. Cell Biol. 108, 1935-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, K., Kawai-Hirai, R., Ishikawa, K., and Takata, K. (2002). Reversal of neuronal polarity characterized by conversion of dendrites into axons in neonatal rat cortical neurons in vitro. Neuroscience 110, 7-17. [DOI] [PubMed] [Google Scholar]
- Hirose, M. et al. (1998). Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J. Cell Biol. 141, 1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, A.M., Wang, H.L., Tang, Y.P., and Lee, E.H. (1998). Expression of integrin-associated protein gene associated with memory formation in rats. J. Neurosci. 18, 4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki, K., Yamao, T., Noguchi, T., Matozaki, T., Fukunaga, K., Takada, T., Hosooka, T., Akira, S., and Kasuga, M. (2000). SHPS-1 regulates integrin-mediated cytoskelet al reorganization and cell motility. EMBO J. 19, 6721-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink, K., Eichholtz, T., Postma, F.R., van Corven, E.J., and Moolenaar, W.H. (1993). Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 4, 247-255. [PubMed] [Google Scholar]
- Jiang, P., Lagenaur, C.F., and Narayanan, V. (1999). Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem. 274, 559-562. [DOI] [PubMed] [Google Scholar]
- Kawakatsu, T., Shimizu, K., Honda, T., Fukuhara, T., Hoshino, T., and Takai, Y. (2002). Trans-interactions of nectins induce formation of filopodia and lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J. Biol. Chem. 277, 50749-50755. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov, A., Chen, Z., Sures, I., Wang, H., Schilling, J., and Ullrich, A. (1997). A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181-186. [DOI] [PubMed] [Google Scholar]
- Kozma, R., Sarner, S., Ahmed, S., and Lim, L. (1997). Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell. Biol. 17, 1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai, B., Stern, E.A., Chen, B., and Svoboda, K. (2000). Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876-881. [DOI] [PubMed] [Google Scholar]
- Lindberg, F.P., Bullard, D.C., Caver, T.E., Gresham, H.D., Beaudet, A.L., and Brown, E.J. (1996a). Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 274, 795-798. [DOI] [PubMed] [Google Scholar]
- Lindberg, F.P., Gresham, H.D., Reinhold, M.I., and Brown, E.J. (1996b). Integrin-associated protein immunoglobulin domain is necessary for efficient vitronectin bead binding. J. Cell Biol. 134, 1313-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Merlin, D., Burst, S.L., Pochet, M., Madara, J.L., and Parkos, C.A. (2001). The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J. Biol. Chem. 276, 40156-40166. [DOI] [PubMed] [Google Scholar]
- Luo, L., Jan, L.Y., and Jan, Y.N. (1997). Rho family GTP-binding proteins in growth cone signalling. Curr. Opin. Neurobiol. 7, 81-86. [DOI] [PubMed] [Google Scholar]
- Manabe, T. et al. (2000). Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol. Cell. Neurosci. 15, 534-546. [DOI] [PubMed] [Google Scholar]
- Mateo, V., Lagneaux, L., Bron, D., Biron, G., Armant, M., Delespesse, G., and Sarfati, M. (1999). CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat. Med. 5, 1277-1284. [DOI] [PubMed] [Google Scholar]
- Mi, Z.P., Jiang, P., Weng, W.L., Lindberg, F.P., Narayanan, V., and Lagenaur, C.F. (2000). Expression of a synapse-associated membrane protein, P84/SHPS-1, and its ligand, IAP/CD47, in mouse retina. J. Comp. Neurol. 416, 335-344. [PubMed] [Google Scholar]
- Motegi, S. et al. (2003). Role of the CD47-SHPS-1 system in regulation of cell migration. EMBO J. 22, 2634-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase, S., and Schuman, E.M. (1999). The role of cell adhesion molecules in synaptic plasticity and memory. Curr. Opin. Cell Biol. 11, 549-553. [DOI] [PubMed] [Google Scholar]
- Niwa, H., Yamamura, K., and Miyazaki, J. (1991). Efficient selection for highexpression transfectants with a novel eukaryotic vector. Gene 108, 193-199. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53-62. [DOI] [PubMed] [Google Scholar]
- Ohnishi, H., Kubota, M., Ohtake, A., Sato, K., and Sano, S. (1996). Activation of protein-tyrosine phosphatase SH-PTP2 by a tyrosine-based activation motif of a novel brain molecule. J. Biol. Chem. 271, 25569-25574. [DOI] [PubMed] [Google Scholar]
- Oldenborg, P.A., Zheleznyak, A., Fang, Y.F., Lagenaur, C.F., Gresham, H.D., and Lindberg, F.P. (2000). Role of CD47 as a marker of self on red blood cells. Science 288, 2051-2054. [DOI] [PubMed] [Google Scholar]
- Oldenborg, P.A., Gresham, H.D., and Lindberg, F.P. (2001). CD47-signal regulatory protein α (SIRPα) regulates Fcγ and complement receptor-mediated phagocytosis. J. Exp. Med. 193, 855-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos, C.A., Colgan, S.P., Liang, T.W., Nusrat, A., Bacarra, A.E., Carnes, D.K., and Madara, J.L. (1996). CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J. Cell Biol. 132, 437-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp, R.J., Peschon, J.J., Spriggs, M.K., and Kokodkin, A.L. (2003). Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424, 398-405. [DOI] [PubMed] [Google Scholar]
- Pettersen, R.D., Hestdal, K., Olafsen, M.K., Lie, S.O., and Lindberg, F.P. (1999). CD47 signals T cell death. J. Immunol. 162, 7031-7040. [PubMed] [Google Scholar]
- Pfaff, M., McLane, M.A., Beviglia, L., Niewiarowski, S., and Timpl, R. (1994). Comparison of disintegrins with limited variation in the RGD loop in their binding to purified integrins αIIbβ3, αVβ3 and α5β1 and in cell adhesion inhibition. Cell Adhes. Commun. 2, 491-501. [DOI] [PubMed] [Google Scholar]
- Price, L.S., Leng, J., Schwartz, M.A., and Bokoch, G.M. (1998). Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9, 1863-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebres, R.A., Vaz, L.E., Green, J.M., and Brown, E.J. (2001). Normal ligand binding and signaling by CD47 (Integrin-associated Protein) requires a long range disulfide bond between the extracellular and membrane-spanning domains. J. Biol. Chem. 276, 34607-34616. [DOI] [PubMed] [Google Scholar]
- Reinhold, M.I., Lindberg, F.P., Plas, D., Reynolds, S., Peters, M.G., and Brown, E.J. (1995). In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J. Cell Sci. 108, 3419-3425. [DOI] [PubMed] [Google Scholar]
- Reinhold, M.I., Lindberg, F.P., Kersh, G.J., Allen, P.M., and Brown, E.J. (1997). Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J. Exp. Med. 185, 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J., Paterson, H.F., Johnston, C.L., Diekmann, D., and Hall, A. (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401-410. [DOI] [PubMed] [Google Scholar]
- Sarner, S., Kozma, R., Ahmed, S., and Lim, L. (2000). Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells. Mol. Cell. Biol. 20, 158-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, R. et al. (2003). Regulation of multiple functions of SHPS-1, a transmembrane glycoprotein, by its cytoplasmic region. Biochem. Biophys. Res. Commun. 309, 584-590. [DOI] [PubMed] [Google Scholar]
- Seiffert, M., Cant, C., Chen, Z., Rappold, I., Brugger, W., Kanz, L., Brown, E.J., Ullrich, A., and Buhring, H.J. (1999). Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 94, 3633-3643. [PubMed] [Google Scholar]
- Suidan, H.S., Stone, S.R., Hemmings, B.A., and Monard, D. (1992). Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron 8, 363-375. [DOI] [PubMed] [Google Scholar]
- Takai, Y., Sasaki, T., and Matozaki, T. (2001). Small GTP-binding proteins. Physiol. Rev. 81, 153-208. [DOI] [PubMed] [Google Scholar]
- Takenawa, T., and Miki, H. (2001). WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 114, 1801-1809. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., and Goodman, C.S. (1996). The molecular biology of axon guidance. Science 274, 1123-1133. [DOI] [PubMed] [Google Scholar]
- Threadgill, R., Bobb, K., and Ghosh, A. (1997). Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19, 625-634. [DOI] [PubMed] [Google Scholar]
- Waclavicek, M., Majdic, O., Stulnig, T., Berger, M., Baumruker, T., Knapp, W., and Pickl, W.F. (1997). T cell stimulation via CD47: agonistic and antagonistic effects of CD47 monoclonal antibody 1/1A4. J. Immunol. 159, 5345-5354. [PubMed] [Google Scholar]
- Wahl, S., Barth, H., Ciossek, T., Aktories, K., and Mueller, B.K. (2000). Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 149, 263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.Q., Lindberg, F.P., and Frazier, W.A. (1999). Integrin-associated protein stimulates α2β1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. J. Cell Biol. 147, 389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford, K.L., and Ghosh, A. (2001). Plexin signaling via off-track and rho family GTPases. Neuron 32, 1-3. [DOI] [PubMed] [Google Scholar]
- Wong, K. et al. (2001). Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 107, 209-221. [DOI] [PubMed] [Google Scholar]
- Yoshida, H. et al. (2000). Integrin-associated protein/CD47 regulates motile activity in human B-cell lines through CDC42. Blood 96, 234-241. [PubMed] [Google Scholar]
- Yoshida, H. et al. (2002). Interaction between Src homology 2 domain bearing protein tyrosine phosphatase substrate-1 and CD47 mediates the adhesion of human B lymphocytes to nonactivated endothelial cells. J. Immunol. 168, 3213-3220. [DOI] [PubMed] [Google Scholar]
- Ziv, N.E., and Smith, S.J. (1996). Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17, 91-102.- [DOI] [PubMed] [Google Scholar]