Abstract
Deletion of the Plasmodium falciparum histidine-rich protein 2 (pfhrp2) gene may affect the performance of PfHRP2-based rapid diagnostic tests (RDTs). Here we investigated the genetic diversity of the pfhrp2 gene in clinical parasite isolates collected in recent years from the China-Myanmar border area. Deletion of pfhrp2 has been identified in 4 out of 97 parasite isolates. Sequencing of the pfhrp2 exon 2 from 67 isolates revealed a high level of genetic diversity in pfhrp2, which is reflected in the presence of many repeat types and their variants, as well as variable copy numbers and different arrangements of these repeats in parasite isolates. In addition, we observed pfhrp3 deletion in three of the four parasites harboring pfhrp2 deletion, suggesting of double deletions of both genes in these three isolates. Analysis of two cases, which were P. falciparum-positive by microscopy and PCR but failed by two PfHRP2-based RDTs, did not find pfhrp2 deletion. Further correlational studies of pfhrp2 polymorphisms with detection sensitivity are needed to identify factors influencing the performance of RDTs in malaria-endemic areas.
Keywords: malaria, rapid diagnostic tests, pfhrp2, deletion, variation
1. Introduction
Malaria remains endemic in 104 countries and estimated 3.4 billion people are at risk of malaria globally (WHO, 2013). Case management and treatment of malaria is highly dependent on accurate early diagnosis. Rapid diagnostic tests (RDTs) for malaria have the potential to improve case management and thereby reduce morbidity and mortality (Maltha et al., 2013; Mouatcho and Goldring, 2013). At least four target antigens are captured by various available RDTs: Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and lactate dehydrogenase (LDH); the pan-plasmodial aldolase and LDH (Abba et al., 2011). PfHRP2 is an abundant antigen produced during the blood stages of P. falciparum. It consists of a number of Ala- and His-rich amino acid repeats (Wellems and Howard, 1986) and shares epitopes with another histidine-rich protein, PfHRP3. Thus, antibodies specific for PfHRP2 have been found to also across react with PfHRP3 (Lee et al., 2006).
The performance of malaria RDTs is generally associated with the product quality, storage conditions, parasite or operator factors and parasite/antigen concentrations. One important factor is the variability within the parasite antigens detected by the RDTs, which includes presence or absence of the target epitopes and variations in the number of epitopes in a particular parasite isolate (Lee et al., 2006; Lee et al., 2012; Maltha et al., 2014; Talman et al., 2007). Genetic diversity may be particularly important for PfHRP2-based RDTs, since most of the RDTs are based on this antigen (Mouatcho and Goldring, 2013). Studies to date have documented extensive size variations between parasite strains and a high degree of genetic diversity within this antigen (Baker et al., 2010b; Baker et al., 2005; Deme et al., 2014; Kumar et al., 2012; Lee et al., 2006; Rock et al., 1987). An extreme situation is that certain parasite isolates even harbor a deletion of the pfhrp2 gene, leading to false negative results in diagnosis with PfHRP2-based RDTs. This phenomenon was first discovered in the Peruvian Amazon in 2010 (Gamboa et al., 2010). Since then, pfhrp2 deletion-associated poor performance of RDTs has been reported in many malaria-endemic regions (Cheng et al., 2014; Koita et al., 2012; Maltha et al., 2012; Pava et al., 2010; Wurtz et al., 2013). To date, unequivocal evidence for pfhrp2 and pfhrp3 gene deletions in P. falciparum isolates has been obtained in Peru (Gamboa et al., 2010; Maltha et al., 2012), Brazil (Houze et al., 2011), Senegal (Wurtz et al., 2013), and India (Kumar et al., 2012). Apart from gene deletion, PfHRP2 sequences in different regions showed differences in the number of amino acid repeats and even some rare amino acid variants (Baker et al., 2010b; Baker et al., 2005; Deme et al., 2014; Kumar et al., 2012; Lee et al., 2006). Given the significance of PfHRP2-based RDTs in malaria diagnosis, systematic mapping of PfHRP2 diversity in global malaria endemic regions is highly demanded, especially in areas where poor performance or failure of PfHRP2-based RDTs have been reported (Cheng et al., 2014).
In the remote border regions of Southeast Asian countries, malaria remains prevalent (Cui et al., 2012) and RDTs have been widely used for malaria diagnosis as a case management practice. Recently, we compared two different RDTs in malaria diagnosis at the China-Myanmar border (Yan et al., 2013). From a limited analysis, we identified two P. falciparum cases, which were positive by both microscopy and PCR but consistently failed two PfHRP2-based RDTs. This has prompted us to examine pfhrp2 genetic diversity in greater detail and determine whether the two false-negative cases were due to pfhrp2 gene deletions.
2. Materials and Methods
2.1. Parasite isolates and DNA extraction
A total of 97 P. falciparum parasite isolates from the China-Myanmar border and western Thailand were analyzed in this study. Among them, 87 were obtained from malaria patients in the China-Myanmar border area between May 2011 and December 2012, of which 36 were also evaluated by two PfHRP2-based RDTs (Wondfo, China and Tycolpharm Co. Limited, United Kingdom) (Yan et al., 2013). Based on microscopy of Giemsa stained thick smears, parasite densities ranged from 40 to 105,920 parasites/μL of blood, assuming 8000 leukocytes/μL. The remaining ten parasite isolates were collected in Tak Province, western Thailand during mass blood surveys conducted between August 2011 and May 2012 (Li et al., 2014). Parasite DNA was extracted from dried blood spots on Whatman 3 M filter paper using the Qiagen DNA Mini Kit (Qiagen, Valencia, CA, USA). DNA was eluted in 50 μL of distilled water and stored under −20°C.
2.2. Amplification of the pfhrp2 and pfhrp3 genes
P. falciparum infections in 97 samples were verified by a nested PCR method (Yan et al., 2013). Previously established protocols were used for amplifications of pfhrp2 and pfhrp3 (Table S1). Specifically, the A262/A264 primers were used in the primary PCR reaction for pfhrp2 (Trouvay et al., 2013). For nest PCR, two sets of primers were used to amplify the exon 1/intron 1 part (+29 nt – +155 nt) and the exon 2 (+135 nt – 7 nt after the stop codon) of the pfhrp2 gene, respectively (Baker et al., 2005; Trouvay et al., 2013). All amplification conditions used were the same as the reference articles. The PCR products of the exon 2 were cloned in the T-vector (Takara Biotechnology, Dalian, China) for sequencing. For amplification of pfhrp3, primers targeting exon 2 (+77 nt – +180 nt) were used (Baker et al., 2005; Mariette et al., 2008; Trouvay et al., 2013). Samples were considered pfhrp2-negtive or pfhrp3-negtive if amplifications were not successful after two attempts.
2.3. Flanking genes and genotyping of pfhrp2-negative samples
To determine whether pfhrp2-negative samples by PCR were due to lack of parasite DNA in the samples, three polymorphic genes pfmsp1, pfmsp2 and pfglurp were amplified using established protocols (Meng et al., 2010). DNA from the reference strains 3D7, 7G8 and HB3 were used as positive controls. Two genes flanking pfhrp2, PF3D7_0831900 and PF3D7_0831700, were further detected by the nested-PCR using modified primers and conditions as described earlier (Akinyi et al., 2013).
2.4. DNA sequencing and analysis
The PCR products were purified using the Qiagen PCR purification kit (Qiagen, Valencia) and cloned into the pMD18-T vector. Cloned PCR products were sequenced on an ABI 3730XL DNA Analyzer with primers M13-47 (CGCCAGGGTTTTCCCAGTCA CGAC) and RV-M (GAGCGGATAACAATTTCACACAGG) (Takara, Dalian). The nucleotide sequences of pfhrp2 exon 2 were assembled using DNASTAR (Madison, WI, USA). Different types of amino acid repeats within exon 2 were identified and given a numeric code (1–18) as described earlier (Baker et al., 2005). These repeat types were aligned to visualize the similarity between parasite isolates and sequence from 3D7 was used as the reference. Pfhrp2 sequences were deposited in GenBank under accession numbers KP712709 – KP712775.
3. Results
3.1. Pfhrp2 gene size variation and deletion
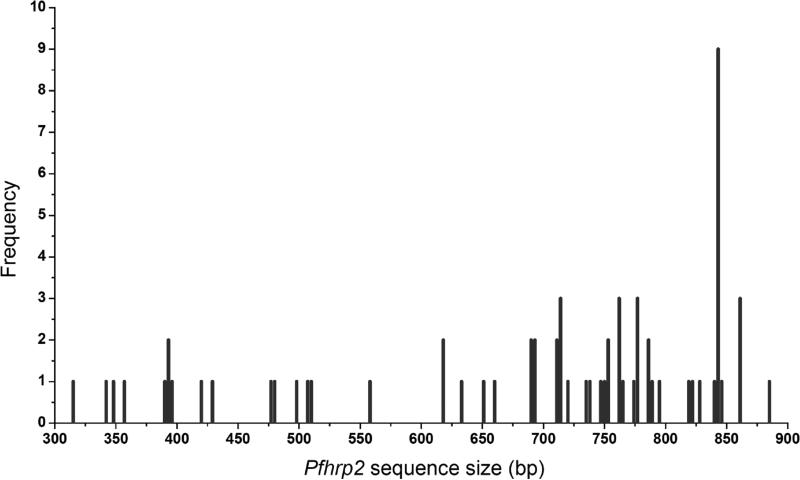
Of the 97 P. falciparum isolates, amplification of pfhrp2 exon 1/intron 1 and exon 2 were successful in 93 isolates; 4 samples (M0100441, M0N00199, M0N00529, and M0N00302) failed to amplify any pfhrp2 fragments. Sixty seven pfhrp2 PCR fragments were cloned and sequenced. The results showed that the amplified exon 2 fragments ranged from 319 to 902 bp, with most of them (~79%) being 500-900 bp (Fig. 1). Sequencing analysis revealed that 65.7% (44/67) isolates had unique sequences, whereas 23 (34.3%) sequences were present in >1 parasite isolate. It is noteworthy that nine identical sequences were shared among nine parasite isolates collected in a single village, an indication of the same clone in circulation.
Fig. 1.
Sizes and frequencies of pfhrp2 PCR fragments from different parasite isolates.
For the four parasite isolates that failed to amplify any pfhrp2 fragments, three (M0100441, M0N00199, and M0N00302) also failed to yield a PCR product for pfhrp3 exon 2, suggesting possible deletions of both pfhrp2 and pfhrp3 genes in these samples. The presence of parasite DNA in these four samples was confirmed by successful amplifications of three polymorphic genes pfmsp1, pfmsp2 and pfglurp (data not shown). In addition, two genes (PF3D7_0831700 and PF3D7_0831900) flanking pfhrp2 were also successfully amplified from these four samples.
3.2. Correlation between pfhrp2 gene deletion and RDT results
Thirty-six of the samples from the China-Myanmar border area have been evaluated by two PfHRP2-based RDTs (Yan et al., 2013). Two samples (M0100304 and M0500035) were slide-positive for P. falciparum infections but were negative by both RDTs. Based on microscopy, parasite densities of these two samples were estimated at 480 (M0100304) and 120 parasites/μL (M0500035), respectively. P. falciparum infections in these two samples were further confirmed by PCR. PCR and sequencing of pfhrp2 exon 2 from these two samples yielded pfhrp2 products of 633 and 690 bp, respectively. This result indicated that these two RDT-negative samples were not due to pfhrp2 gene deletion. Further comparison revealed that M0500035 shared the same pfhrp2 sequence with another sample M0100418, which was P. falciparum-positive by the two RDTs. In addition, the pfhrp2 exon 2 sequence fromM0100304 was very similar to those from three additional samples. These results suggest that other reasons (low parasitemia, mishandling of the RDTs, etc.) may be accountable for the false-negative results by the two RDTs.
Of the four samples with potential pfhrp2 deletion, one sample (M0100441) was positive by the two RDTs. However, since RDTs were not performed on the remaining three samples, a direct correlation between pfhrp2 gene deletion and RDT results could not be firmly established for these samples.
3.3. Repeat types of pfhrp2
The PfHRP2 protein from exon 2 is complex and composed of varying numbers of different types of amino acid repeats (Baker et al., 2010b; Baker et al., 2005). In this study, the protein sequences predicted from PfHRP2 exon 2 ranged from 104 to 286 amino acids. Analysis of the 67 sequences identified 33 repeat variants, which for convenience we assigned them to 13 repeat types that have been previously reported (Table 1) (Baker et al., 2010b; Kumar et al., 2013). In most cases, the PfHRP2 sequences harbor many types of these repeat motifs with each motif having variable copies, which give rise to large variation in the size of PCR fragments. Almost all sequences (66/67) begin with 1-4 copies of the Type 1 motif (AHHAHHVAD), whereas only one begins with a slight variation of this repeat motif (AHHAHHVAY). Similarly, 63 sequences end with a Type 12 motif (AHHAAAHHEAATH), four with minor variations of the Type 12 motif (AHHAAAHHEAATQ, AHHAAAHREAATH and AHHAAAHHGAATH). Type 2 (AHHAHHAAD) and 7 (AHHAAD) motifs, which were considered to correlate with the sensitivity of PfHRP2-based RDTs, were the most prominent in our PfHRP2 sequences with 100% and 92.5% prevalence, respectively. In particular, Type 2 motif is present in multiple copies in most isolates. Type 6 motif (AHHATD) was also prevalent in our study region and observed in 95.5% samples. In comparison, Type 3 (AHHAHHAAY), 5 (AHHAHHASD), 8 (AHHAAY) and 10 (AHHAAAHHATD) motifs were relatively abundant and found in more than half of the study populations, occurring at 76.1%, 61.2%, 77.6% and 58.2% , respectively. Type 13 repeat was observed in 4 isolates (6.0%), 3 of which were from Thailand. The type 14 (AHHAHHATD) repeat was present in 6 samples (9.0%), all of which were from China-Myanmar border area. One sequence contained a Type 15 motif (AHHAHHAAN), a motif most often detected in PfHRP3 (Baker et al., 2010a) (Table 1). Type 9 (AAY) and 11 (AHN) repeats were not found in the parasites of this study.
Table 1.
PfHRP2 repeat types and frequencies in two Southeast Asian sites*
| Types | Repeat sequences | Frequency in parasite isolates [n (%)] |
||
|---|---|---|---|---|
| Total (n=67) | China-Myanmar border (n=62) | Thailand (n=5) | ||
| 1 | AHHAHHVAD | 67 (100%) | 62 (100%) | 5 (100%) |
| 2 | AHHAHHAAD | 67 (100%) | 62 (100%) | 5 (100%) |
| 3 | AHHAHHAAY | 51 (76.1%) | 49 (79.0%) | 2 (40.0%) |
| 4 | AHH | 25 (37.3%) | 21 (33.9%) | 4 (80.0%) |
| 5 | AHHAHHASD | 41 (61.2%) | 40 (64.5%) | 1 (20.0%) |
| 6 | AHHATD | 64 (95.5%) | 59 (95.2%) | 5 (100%) |
| 7 | AHHAAD | 62 (92.5%) | 57 (91.9%) | 5 (100%) |
| 8 | AHHAAY | 52 (77.6%) | 48 (77.4%) | 4 (80.0%) |
| 9 | AAY | 0 | 0 | 0 |
| 10 | AHHAAAHHATD | 39 (58.2%) | 35 (56.5%) | 4 (80.0%) |
| 11 | AHN | 0 | 0 | 0 |
| 12 | AHHAAAHHEAATH | 67 (100%) | 62 (100%) | 5 (100%) |
| 13 | AHHASD | 4 (6.0%) | 1 (1.6%) | 3 (60.0%) |
| 14 | AHHAHHATD | 6 (9.0%) | 6 (9.7%) | 0 |
| 15 | AHHAHHAAN | 1 (1.5%) | 1 (1.6%) | 0 |
Designation of repeat types was based on an earlier publication
3.4. Variations in repeats
For eight of the repeat types described earlier (Type 1, 2, 4, 5, 6, 7, 10 and 12), there were variants that differed from the original type sequences by a single amino acid (Table 2). For convenience, the typical sequence and its variants were grouped into the same repeat type. For example, we identified 9 variants of the Type 2 repeat, which differed from the original Type 2 sequence AHHAHHAAD by a single amino acid (Table 2). These Type 2 variants were present in the parasite population as minor alleles. For a total of 734 Type 2 repeats identified in PfHRP2 sequences from the 67 parasite isolates, there were only 14 variant Type 2 repeats (1.9%). Altogether, 25 of the 67 isolates contained variant(s) of the eight repeat types; 18 isolates contained only one variant type. Whereas the majority of the mutations did not change the hydrophilic/hydrophobic property of the amino acids, it is not known whether these changes lead to changes in the antigenicity of the protein.
Table 2.
Original repeat types and their variants identified in the study parasite populations
| Type | Original type sequence | Variants * | # isolates with variant§ | # (%) of variant¶ |
|---|---|---|---|---|
| 1 | AHHAHHVAD | AHHAHHVAY | 1 | 1/191 (0.5%) |
| 2 | AHHAHHAAD | AHHAYHAAD | 3 | 14/734(1.9%) |
| AHHAHYAAD | 2 | |||
| AYHAHHAAD | 1 | |||
| AHHAHRAAD | 1 | |||
| AHHARHAAD | 1 | |||
| AHHAHHTAD | 1 | |||
| AHHAHTAAD | 1 | |||
| AHHAHHAVD | 1 | |||
| AHHAHHAAG | 3 | |||
| 4 | AHH | THH | 1 | 5/39 (12.8%) |
| ARH | 1 | |||
| AHR | 1 | |||
| AQH | 1 | |||
| AHY | 1 | |||
| 5 | AHHAHHASD | AHHAQHASD | 1 | 1/44 (2.3%) |
| 6 | AHHATD | AHLATD | 1 | 1/185 (0.5%) |
| 7 | AHHAAD | ARHAAD | 1 | 5/270 (1.9%) |
| AHRAAD | 2 | |||
| AHHAPD | 1 | |||
| AHHAAE | 1 | |||
| 10 | AHHAAAHHATD | AHYAAAHHATD | 1 | 1/59 (1.7%) |
| 12 | AHHAAAHHEAATH | AHHAAAHREAATH | 1 | 4/67 (6.0%) |
| AHHAAAHHGAATH | 1 | |||
| AHHAAAHHEAATQ | 2 | |||
Variants differing from the original type sequences by a single amino acid (highlighted in bold and underlined) are grouped into the same repeat type.
Number of parasites carrying the variant repeat type (from a total of 67 parasite isolates).
Indicates the number (%) of the variant type repeat within the total number of the corresponding type repeat identified in the 67 parasite isolates.
3.5. Variations in repeat type organization
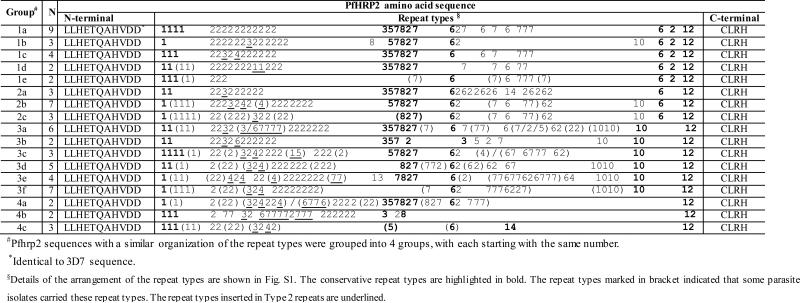
We have shown that PfHRP2 in parasites from the Greater Mekong Subregion displayed a high level of genetic diversity with 52 different pfhrp2 sequences identified from 67 P. falciparum isolates. We have attempted to assign them into groups based the recognition of more or less conserved arrangement of the repeat types within a sequence (Table 3, Fig. S1). Generally, Type 2 motifs always followed the Type 1 motif. In the middle of the sequences, repeat types were often arranged as 3-5-7-8-2-7-6. Before the ending Type 12, there was either a Type 2, Type 6 or Type 10 repeat in most cases. According to the similarities in domain organization, the 67 PfHRP2 sequences were divided into four groups: sequences in Group 1, 2, and 3 ended with repeat types 6-2-12, 6-12, and 10-12, respectively, while the remaining isolates were classified into Group 4. Detailed organization of repeats is illustrated in Fig. S1.
Table 3.
Organization of hrp2 exon 2 repeat types
4. Discussion
This study, as the first report of pfhrp2 genetic diversity from the China-Myanmar border area, was motivated from an earlier finding of false-negative cases of two PfHRP2-based RDTs (Yan et al., 2013). In search of a potential cause, we identified 4 out of 87 (4.6%) isolates in Myanmar as potentially having pfhrp2 gene deletions. In addition, three of these four isolates may also harbor pfhrp3 deletions. To date, hrp2 deletion has been unequivocally identified in several malaria endemic regions of the world, though the proportions of parasites carrying the deletion varied greatly. In Peru, P. falciparum isolates lacking pfhrp2 had reached a staggering 41% (Gamboa et al., 2010), whereas in Senegal the pfhrp2 deletion rate was still low (2.4%) (Wurtz et al., 2013). The pfhrp2 deletion rate in our study population is close to the rate (4.2%) reported in India (Kumar et al., 2013).
As found in other studies, sequence variations do not seem to accurately foretell the performance of the RDTs. PfHRP3 also shares antigenic epitopes with PfHRP2 and thus plays a role in the performance of PfHRP2-based RDTs (Baker et al., 2005). In our study, the two false-negative P. falciparum cases by PfHRP2-based RDTs contained 633 and 690 bp of pfhrp2 exon 2 sequences, respectively. Moreover, one even shared the same pfhrp2 sequence with another sample detected by the PfHRP2-based RDTs. Such a discrepancy could be resulted from relatively low parasite densities of the two samples, or errors made during the RDT testing (e.g., insufficient blood transferred to the RDT, wrong buffer volume or wrong reading time). Conversely, one parasite isolate failed to amplify any pfhrp2 gene fragments but was detected as P. falciparum positive by the RDTs. We do not know the reason for this puzzling result, which could be due to sequence variations in the primer regions of pfhrp2 or methodological problem that led to amplification failure. A more thorough comparison between the RDTs and other diagnostic methods (microscopy and PCR) is needed to resolve these discrepancies.
Analysis of pfhrp2 genetic diversity also revealed striking geographical differences, which might also be linked to the variability of RDT performance. First, the average length of our pfhrp2 amplicons was 687 bp, which was shorter than what was reported in Mozambique and Tanzania (Ramutton et al., 2012), but longer than in Vanuatu (Baker et al., 2010b). Our parasite population lacked repeat types 9 and 11. Whereas Type 11 repeat was absent in Senegal (Wurtz et al., 2013), it was detected in all the sequenced isolates from India (Kumar et al., 2013). In addition, Type 4 repeat was present less frequently in <40% of our samples. As Type 4 and 7 were thought to be the targets of RDTs (Deme et al., 2014), such a difference at the population level could potentially result in varied performance of RDTs. Furthermore, we have detected multiple minor variants of eight typical repeat sequences. It is unknown whether they also affect the sensitivity of the RDTs.
In summary, sequencing analysis revealed deletion as well as high-level genetic diversity of the pfhrp2 gene in the parasite population from the China-Myanmar border. Genetic diversity is reflected in the presence of different types of repeats, as well as variations in sequence, copy number, and arrangement of these repeats. In addition, we also detected deletion of pfhrp3 gene in some parasite samples. Future studies should be directed to determine what antigen epitopes are detected by the pfhrp2 antibodies used in the RDTs in order to improve the detection sensitivity. Discovery of new antigens suitable for RDT development should also be encouraged.
Supplementary Material
Acknowledgement
This study was supported by grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH U19AI089672).
Footnotes
Authors’ contributions
PL and HX carried out the experimental work and data analysis. ZZ, ZY, YC, GY and JS participated in data analysis. PL performed manuscript writing. QF and LC conceived the study and participated in writing. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, Donegan S, Garner P. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. The Cochrane database of systematic reviews. 2011:CD008122. doi: 10.1002/14651858.CD008122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyi S, Hayden T, Gamboa D, Torres K, Bendezu J, Abdallah JF, Griffing SM, Quezada WM, Arrospide N, De Oliveira AM, Lucas C, Magill AJ, Bacon DJ, Barnwell JW, Udhayakumar V. Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium falciparum parasites from Peru. Scientific reports. 2013;3:2797. doi: 10.1038/srep02797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Ho M-F, Pelecanos A, Gatton M, Chen N, Abdullah S, Albertini A, Ariey F, Barnwell J, Bell D, Cunningham J, Djalle D, Echeverry DF, Gamboa D, Hii J, Kyaw MP, Luchavez J, Membi C, Menard D, Murillo C, Nhem S, Ogutu B, Onyor P, Oyibo W, Wang SQ, McCarthy J, Cheng Q. Global sequence variation in the histidine-rich proteins 2 and 3 of Plasmodium falciparum: implications for the performance of malaria rapid diagnostic tests. Malaria Journal. 2010a;9:129. doi: 10.1186/1475-2875-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Ho MF, Pelecanos A, Gatton M, Chen N, Abdullah S, Albertini A, Ariey F, Barnwell J, Bell D, Cunningham J, Djalle D, Echeverry DF, Gamboa D, Hii J, Kyaw MP, Luchavez J, Membi C, Menard D, Murillo C, Nhem S, Ogutu B, Onyor P, Oyibo W, Wang SQ, McCarthy J, Cheng Q. Global sequence variation in the histidine-rich proteins 2 and 3 of Plasmodium falciparum: implications for the performance of malaria rapid diagnostic tests. Malaria journal. 2010b;9:129. doi: 10.1186/1475-2875-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, McCarthy J, Gatton M, Kyle DE, Belizario V, Luchavez J, Bell D, Cheng Q. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. The Journal of infectious diseases. 2005;192:870–877. doi: 10.1086/432010. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Gatton ML, Barnwell J, Chiodini P, McCarthy J, Bell D, Cunningham J. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malaria journal. 2014;13:283. doi: 10.1186/1475-2875-13-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, Fan Q, Fang Q, Jongwutiwes S, Parker D, Sirichaisinthop J, Kyaw MP, Su XZ, Yang H, Yang Z, Wang B, Xu J, Zheng B, Zhong D, Zhou G. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta tropica. 2012;121:227–239. doi: 10.1016/j.actatropica.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deme AB, Park DJ, Bei AK, Sarr O, Badiane AS, Gueye Pel H, Ahouidi A, Ndir O, Mboup S, Wirth DF, Ndiaye D, Volkman SK. Analysis of pfhrp2 genetic diversity in Senegal and implications for use of rapid diagnostic tests. Malaria journal. 2014;13:34. doi: 10.1186/1475-2875-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, Incardona S, Perkins M, Bell D, McCarthy J, Cheng Q. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PloS one. 2010;5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houze S, Hubert V, Le Pessec G, Le Bras J, Clain J. Combined deletions of pfhrp2 and pfhrp3 genes result in Plasmodium falciparum malaria false-negative rapid diagnostic test. Journal of clinical microbiology. 2011;49:2694–2696. doi: 10.1128/JCM.00281-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koita OA, Doumbo OK, Ouattara A, Tall LK, Konare A, Diakite M, Diallo M, Sagara I, Masinde GL, Doumbo SN, Dolo A, Tounkara A, Traore I, Krogstad DJ. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. The American journal of tropical medicine and hygiene. 2012;86:194–198. doi: 10.4269/ajtmh.2012.10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Pande V, Bhatt RM, Shah NK, Mishra N, Srivastava B, Valecha N, Anvikar AR. Genetic deletion of HRP2 and HRP3 in Indian Plasmodium falciparum population and false negative malaria rapid diagnostic test. Acta tropica. 2013;125:119–121. doi: 10.1016/j.actatropica.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Kumar N, Singh JP, Pande V, Mishra N, Srivastava B, Kapoor R, Valecha N, Anvikar AR. Genetic variation in histidine rich proteins among Indian Plasmodium falciparum population: possible cause of variable sensitivity of malaria rapid diagnostic tests. Malaria journal. 2012;11:298. doi: 10.1186/1475-2875-11-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Baker J, Andrews KT, Gatton ML, Bell D, Cheng Q, McCarthy J. Effect of sequence variation in Plasmodium falciparum histidine- rich protein 2 on binding of specific monoclonal antibodies: Implications for rapid diagnostic tests for malaria. Journal of clinical microbiology. 2006;44:2773–2778. doi: 10.1128/JCM.02557-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Gatton ML, Pelecanos A, Bubb M, Gonzalez I, Bell D, Cheng Q, McCarthy JS. Identification of optimal epitopes for Plasmodium falciparum rapid diagnostic tests that target histidine-rich proteins 2 and 3. Journal of clinical microbiology. 2012;50:1397–1405. doi: 10.1128/JCM.06533-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhao Z, Wang Y, Xing H, Parker DM, Yang Z, Baum E, Li W, Sattabongkot J, Sirichaisinthop J, Li S, Yan G, Cui L, Fan Q. Nested PCR detection of malaria directly using blood filter paper samples from epidemiological surveys. Malaria journal. 2014;13:175. doi: 10.1186/1475-2875-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltha J, Gamboa D, Bendezu J, Sanchez L, Cnops L, Gillet P, Jacobs J. Rapid diagnostic tests for malaria diagnosis in the Peruvian Amazon: impact of pfhrp2 gene deletions and cross-reactions. PloS one. 2012;7:e43094. doi: 10.1371/journal.pone.0043094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in endemic settings. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19:399–407. doi: 10.1111/1469-0691.12151. [DOI] [PubMed] [Google Scholar]
- Maltha J, Guiraud I, Lompo P, Kabore B, Gillet P, Van Geet C, Tinto H, Jacobs J. Accuracy of PfHRP2 versus Pf-pLDH antigen detection by malaria rapid diagnostic tests in hospitalized children in a seasonal hyperendemic malaria transmission area in Burkina Faso. Malaria journal. 2014;13:20. doi: 10.1186/1475-2875-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariette N, Barnadas C, Bouchier C, Tichit M, Menard D. Country-wide assessment of the genetic polymorphism in Plasmodium falciparum and Plasmodium vivax antigens detected with rapid diagnostic tests for malaria. Malaria journal. 2008;7:219. doi: 10.1186/1475-2875-7-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Zhang R, Yang H, Fan Q, Su X, Miao J, Cui L, Yang Z. In vitro sensitivity of Plasmodium falciparum clinical isolates from the China-Myanmar border area to quinine and association with polymorphism in the Na+/H+ exchanger. Antimicrobial agents and chemotherapy. 2010;54:4306–4313. doi: 10.1128/AAC.00321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatcho JC, Goldring JP. Malaria rapid diagnostic tests: challenges and prospects. Journal of medical microbiology. 2013;62:1491–1505. doi: 10.1099/jmm.0.052506-0. [DOI] [PubMed] [Google Scholar]
- Pava Z, Echeverry DF, Diaz G, Murillo C. Large variation in detection of histidine-rich protein 2 in Plasmodium falciparum isolates from Colombia. The American journal of tropical medicine and hygiene. 2010;83:834–837. doi: 10.4269/ajtmh.2010.10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramutton T, Hendriksen IC, Mwanga-Amumpaire J, Mtove G, Olaosebikan R, Tshefu AK, Onyamboko MA, Karema C, Maitland K, Gomes E, Gesase S, Reyburn H, Silamut K, Chotivanich K, Promnares K, Fanello CI, von Seidlein L, Day NP, White NJ, Dondorp AM, Imwong M, Woodrow CJ. Sequence variation does not confound the measurement of plasma PfHRP2 concentration in African children presenting with severe malaria. Malaria journal. 2012;11:276. doi: 10.1186/1475-2875-11-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EP, Marsh K, Saul AJ, Wellems TE, Taylor DW, Maloy WL, Howard RJ. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology. 1987;95(Pt 2):209–227. doi: 10.1017/s0031182000057681. [DOI] [PubMed] [Google Scholar]
- Talman AM, Duval L, Legrand E, Hubert V, Yen S, Bell D, Le Bras J, Ariey F, Houze S. Evaluation of the intra- and inter-specific genetic variability of Plasmodium lactate dehydrogenase. Malaria journal. 2007;6:140. doi: 10.1186/1475-2875-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouvay M, Palazon G, Berger F, Volney B, Blanchet D, Faway E, Donato D, Legrand E, Carme B, Musset L. High performance of histidine-rich protein 2 based rapid diagnostic tests in French Guiana are explained by the absence of pfhrp2 gene deletion in P. falciparum. PloS one. 2013;8:e74269. doi: 10.1371/journal.pone.0074269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems TE, Howard RJ. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World Malaria Report 2013. 2013 [Google Scholar]
- Wurtz N, Fall B, Bui K, Pascual A, Fall M, Camara C, Diatta B, Fall KB, Mbaye PS, Dieme Y, Bercion R, Wade B, Briolant S, Pradines B. Pfhrp2 and pfhrp3 polymorphisms in Plasmodium falciparum isolates from Dakar, Senegal: impact on rapid malaria diagnostic tests. Malaria journal. 2013;12:34. doi: 10.1186/1475-2875-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Li N, Wei X, Li P, Zhao Z, Wang L, Li S, Li X, Wang Y, Yang Z, Zheng B, Zhou G, Yan G, Cui L, Cao Y, Fan Q. Performance of two rapid diagnostic tests for malaria diagnosis at the China-Myanmar border area. Malaria journal. 2013;12:73. doi: 10.1186/1475-2875-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.