Abstract
Background
Circulating tumor cells (CTCs) are tumor cells that leave the primary tumor site and enter the bloodstream, where they can spread to other organs; they are very important in the diagnosis, treatment, and prognosis of malignant tumors. However, few studies have investigated CTCs in esophageal squamous cell carcinoma (ESCC). The aim of this study was to investigate the CTCs in blood of ESCC patients and its potential relevance to clinicopathological features and prognosis.
Material/Methods
CTCs were acquired by a negative enrichment method that used magnetic activated cell sorting (MACSTM). Fluorescent immunohistochemistry (IHC) was used to identify the CTCs. Then, the positive CTC patients with ESCC were analyzed, after which the relationship between CTCs and clinicopathologic features was evaluated.
Results
In the present study, 62 out of 140 (44.3%) patients with ESCC were positive for CTCs. The positive rate of CTCs was significantly related with stage of ESCC patients (P=0.013). However, there was no relationship between CTC status and age, sex, smoking tumor history, tumor location, differentiation of tumor, lymphatic invasion, or lymph venous invasion (P>0.05). Kaplan-Meier analysis showed that patients positive for CTCs had significantly shorter survival time than patients negative for CTCs. Multivariate analysis demonstrated that stage and CTC status were significant prognostic factors for patients with ESCC.
Conclusions
CTCs positivity is an independent prognostic biomarker that indicates a worse prognosis for patients with ESCC.
MeSH Keywords: Esophageal Neoplasms; Neoplastic Cells, Circulating; Prognosis
Background
Esophageal carcinoma is a malignant tumor originating from the epithelium of the esophagus, and it has the characteristics of strong invasiveness and high mortality [1]. Around 300 000 people die of esophageal cancer every year around the world, but its incidence and mortality varies greatly in different countries. China has one of the highest incidences of esophageal cancer, at around 150 000 deaths per year [2]. Approximately 90% of these are cases of esophageal squamous cell carcinoma (ESCC). Distant metastasis and tumor recurrence are the main causes of death in patients with ESCC.
Circulating tumor cells (CTCs) are tumor cells that leave the primary tumor site and enter the bloodstream, where they can spread to other organs [3]. CTCs are very important in the diagnosis, treatment, and prognosis of malignant tumors. Abnormal proliferation of tumor cells results in decreased adhesion between cells, and between cells and the surrounding matrix. This loss of adhesion allows tumor cells to escape from the primary tumor into the circulatory system and become CTCs with the capacity for invasion and metastasis [4,5]. Compared with lung cancer, gastric cancer, breast cancer, and colorectal cancer [6–9], there has been less research into CTCs from esophageal cancer.
There are many methods for detecting CTCs. In this study, the CTCs were acquired by a negative enrichment method that used magnetic activated cell sorting (MACSTM). Fluorescent immunohistochemistry (IHC) was used to identify the CTCs. Then, the proportion of patients with ESCC who were positive for CTCs was analyzed, after which the relationship between CTCs and clinical pathological features and the prognosis of patients with ESCC was evaluated.
Material and Methods
Patients and samples
All patients were treated at the First Hospital of Lanzhou University for ESCC and those with complete clinical data were enrolled. All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration and its later amendments or comparable ethical standards. All 140 patients underwent surgical resection, and 16 of them were underwent esophageal cancer palliative resection. Among the 140 patients, 124 with ESCCs received chemotherapy or radiotherapy after surgery. A total of 140 patients were included in the present study and they were diagnosed with primary ESCC based on pathological findings. The patients were 117 men and 23 women, ranging in age from 36 to 78 years (mean ±SD: 62.8±8.5 years). The tumor-node-metastasis (TNM) stage of the ESCC patients was defined according to the 7th edition of the TNM classification of the International Union Against Cancer [10]. In addition, 25 healthy volunteers without cancer were randomly selected as a control group. Informed consent was obtained from all individual participants included in the study. The clinical and pathological features of the 140 patients with ESCC are summarized in Table 1.
Table 1.
Clinicopathologic features and tumor characteristics.
| Characteristic | Number | % | |
|---|---|---|---|
| Age (years) | Mean±SD | 62.8±8.5 | |
| Range | 36–78 | ||
| Sex | Male | 117 | 83.6 |
| Female | 23 | 16.4 | |
| Smoking history | No | 16 | 11.4 |
| Yes | 124 | 88.6 | |
| Tumor location | Upper | 3 | 2.1 |
| Middle | 65 | 46.4 | |
| Lower | 72 | 51.4 | |
| Differentiation | Well | 27 | 19.3 |
| Moderate | 83 | 59.3 | |
| Poor | 30 | 21.4 | |
| pT | T1 | 54 | 38.5 |
| T2 | 12 | 8.6 | |
| T3 | 60 | 42.9 | |
| T4 | 14 | 10.0 | |
| pN | N0 | 63 | 45.0 |
| N1–3 | 77 | 55.0 | |
| pM | M0 | 124 | 88.6 |
| M1 | 16 | 11.4 | |
| TNM staging | I | 28 | 20.0 |
| II | 43 | 30.7 | |
| III–IV | 69 | 49.3 | |
| Lymphatic invasion | Negative | 63 | 45.0 |
| Positive | 77 | 55.0 | |
| Venous invasion | Negative | 48 | 34.3 |
| Positive | 92 | 65.7 |
Enrichment of CTCs
Blood (5 ml) was collected into citrate anticoagulant blood collection tubes from the patients with ESCC after fasting. Four ml of whole blood was mixed with a buffer to lyse the red cells. Then, the labelled magnetic particles (Lyle Bio Pharmaceutical Technology Co., Ltd., Jiangsu, China) was added and centrifuged for 20 min. A mixed solution of 3 ml of metal salts and phosphate-buffered saline (PBS) was added, and it was centrifuged for 5 min at room temperature (300 r/min). There were 3 layers in the solution after centrifugation. The upper 2 layers of solution was collected and placed in a 15-ml centrifugal tube, then we added concentrated (10 times to 14 ml) PBS and bovine serum albumin (BSA) buffer and centrifuged it for 5 min at a speed of 950 r/min at room temperature. The supernatant was removed to make a volume of 300 μl after being mixed well. The specimen was transferred to a new centrifuge tube and put on a magnetic frame (Promega Corporation, Madison, WI) for 2–3 min. The eluted liquid was removed into 1.5-ml centrifuge tubes separately, placed into the top of a 15-ml centrifuge tube, and centrifuged for 5 min at a speed of 2000 r/min for 3 min. The supernatant was removed to make a volume of 100 μl, then we added fixing liquid to it and allowed it to dry naturally at room temperature for 24 h.
IHC
The dried specimen was fixed, aged, dehydrated, covered with a coverslip, and sealed without bubbles. The specimens were placed in an automatic hybridizer for 1.5 h. The specimen then had the coverslips removed and the samples were washed with 0.2% BSA solution. After removal of the BSA solution from the specimen, the rabbit antihuman CK19 polyclonal antibody and mouse antihuman CD45 monoclonal antibody (Lyle Biological Medicine Technology Co., Ltd., Jiangsu, China) was added to the specimen. After incubating it for 1 h in the dark at room temperature, the specimens were washed with BSA solution twice to rinse out the remaining liquid. We added 10 μl of 4, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO) counterstain into the area of the specimen, applied a coverslip, and read the specimen under a fluorescence microscope.
The criteria of CTCs
The complete morphology and nucleus of the cells could be observed under the light microscope. The interpretation of the results of immunofluorescence staining was CK19(+), CD45(−), and DAPI(+). Cells with a signal >2 and without blood-borne leukocyte surface antigen (red circle or a red plate) were counted as positive CTCs. Cells positive for the presence of blood-borne cell surface antigen (red circle or sheet) or the signal ≤2 were counted as negative CTCs.
Statistical analyses
The relationships between CTCs and clinical pathological characteristics of patients with ESCC were analyzed with the chi-square test. The survival of patients with ESCC was analyzed by the Kaplan-Meier method. Cox proportional hazards models were used for the multifactor analysis. The primary outcomes were disease-free survival (DFS) and overall survival (OS). SPSS v. 17.0 statistical software (SPSS, Chicago, IL) was used for statistical analysis. Statistical significance was defined when P<0.05.
Results
CTCs analysis
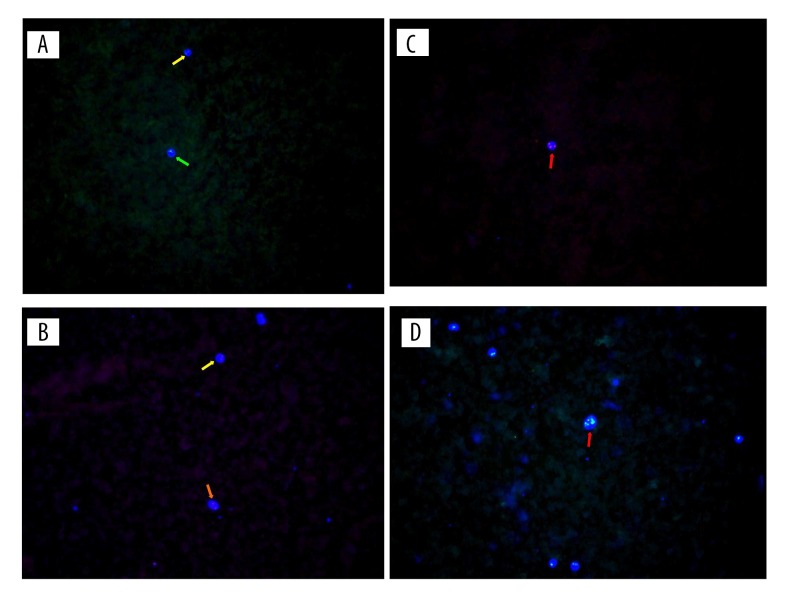
No positive of CTCs was identified in any blood specimens of the 25 healthy volunteers. Positive of CTCs were identified in 62 of 140 patients with ESCC (44.3%). Figure 1 shows the CTCs detected by fluorescent ICH.
Figure 1.
The CTCs of ESCC patients detected by the fluorescent immunohistochemistry (IHC). (A, B) Means negative CTCs; (C, D) Means positive CTCs; green arrows indicate cell with 1 signal point; yellow arrows indicate cells with 2 signal points; orange arrow indicate cell with red plate; red arrows indicate cells with more than 2 signal points.
Relationship between CTCs status and clinicopathological findings
Table 2 summarizes the clinicopathological features of the patients with ESCC. Of the 140 patients with ESCC, 62 were positive for CTCs according to the current criteria. In this study, CTCs positivity was found in 11.5% of patients with stage I, 21.0% with stage II, and 48.4% with stage III–IV. The positive rate of CTCs was significantly related with stage status of ESCC patients (P=0.013), meaning that stage was significantly more advanced among CTCs-negative patients compared with CTCs-positive patients. However, there was no relationship between CTC status and age, sex, smoking history tumor, tumor location, differentiation of tumor, lymphatic invasion, or lymph venous invasion (P>0.05).
Table 2.
Correlation between clinicopathological findings and CTCs status.
| Characteristic | Total (%) | CTCs | P | ||
|---|---|---|---|---|---|
| Positive (%) n=62 |
Negative (%) n=78 |
||||
| Age (years) | 0.577 | ||||
| ≤60 | 62.8±8.5 | 63.3±9.8 | 62.4±9.2 | ||
| >60 | 36–78 | 36–77 | 39–78 | ||
| Sex | 0.708 | ||||
| Male | 117 (83.6) | 51 (82.3) | 66 (84.6) | ||
| Female | 23 (16.4) | 11 (17.7) | 12 (15.4) | ||
| Smoking history | 0.963 | ||||
| No | 16 (11.4) | 7 (11.3) | 9 (11.5) | ||
| Yes | 124 (88.6) | 55 (88.7) | 69 (88.5) | ||
| Tumor location | 0.869 | ||||
| Upper | 3 (2.1) | 1 (1.6) | 2 (2.6) | ||
| Middle | 65 (46.4) | 30 (48.4) | 35 (44.9) | ||
| Lower | 72 (51.4) | 31 (50.0) | 41 (52.6) | ||
| Differentiation | 0.993 | ||||
| Well | 27 (19.3) | 12 (19.4) | 15 (19.2) | ||
| Moderate | 83 (59.3) | 37 (59.7) | 46 (59.0) | ||
| Poor | 30 (21.4) | 13 (21.0) | 17 (21.8) | ||
| pT | 0.211 | ||||
| T1 | 54 (38.6) | 18 (29.0) | 36 (46.2) | ||
| T2 | 12 (8.6) | 6 (9.7) | 6 (7.7) | ||
| T3 | 60 (42.9) | 30 (48.4) | 30 (38.5) | ||
| T4 | 14 (10.0) | 8 (12.9) | 6 (7.7) | ||
| pN | 0.321 | ||||
| N0 | 63 | 25 (40.3) | 38 (48.7) | ||
| N1–3 | 77 | 37 (59.7) | 40 (51.3) | ||
| pM | 0.963 | ||||
| M0 | 124 (88.6) | 55 (88.7) | 69 (88.5) | ||
| M1 | 16 (11.4) | 7 (11.3) | 9 (11.5) | ||
| TNM staging | 0.013 | ||||
| I | 28 (20.0) | 9 (11.5) | 19 (30.6) | ||
| II | 43 (30.7) | 13 (21.0) | 30 (38.5) | ||
| III–IV | 69 (49.3) | 30 (48.4) | 39 (50.0) | ||
| Lymphatic invasion | 0.758 | ||||
| Negative | 63 (45.0) | 27 (43.5) | 36 (46.2) | ||
| Positive | 77 (55.0) | 35 (56.5) | 42 (53.8) | ||
| Venous invasion | 0.243 | ||||
| Negative | 48 (34.3) | 18 (29.0) | 30 (38.5) | ||
| Positive | 92 (65.7) | 44 (71.0) | 48 (61.5) | ||
Survival analysis
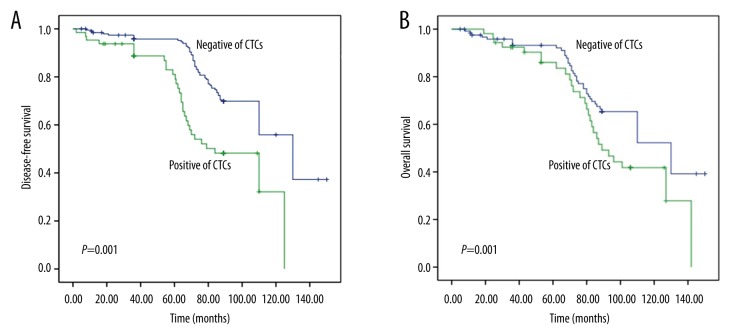
According to the detection result, 140 cases ESCC patients were divided into 2 groups by CTCs status (positive and negative for CTCs). Kaplan-Meier analysis showed that CTCs-positive patients had significantly shorter survival time (DFS and OS) than CTCs-negative patients (Figure 2). Results of univariate analysis of OS are summarized in Table 3. OS was significantly correlated with differentiation, pT, pN, stage, lymphatic invasion, and CTCs status, and was significantly longer in CTCs-negative patients than in CTCs-positive patients (P=0.013). Multivariate analysis demonstrated that stage and CTCs status were significant prognostic factors for OS and DFS of patients with ESCC (Table 4).
Figure 2.
Kaplan-Meier curves of ESCC patients according to CTCs status. (A) Disease-free survival (DFS); (B) Overall survival (OS).
Table 3.
Univariate survival analysis of clinicopathological findings.
| Characteristic | Number | 3-year overall survival rate (%) | P | |
|---|---|---|---|---|
| Age (years) | 0.127 | |||
| ≤60 | 45 | 62.2 | ||
| >60 | 95 | 48.4 | ||
| Sex | 0.221 | |||
| Male | 117 | 51.3 | ||
| Female | 23 | 62.5 | ||
| Tumor location | 0.614 | |||
| Upper | 3 | 66.7 | ||
| Middle/lower | 137 | 52 | ||
| Differentiation | 0.018 | |||
| Well/moderate | 110 | 60 | ||
| Poor | 30 | 83.3 | ||
| pT | 0.018 | |||
| pT1/pT2 | 66 | 69.7 | ||
| pT3/pT4 | 74 | 50 | ||
| pN | <0.001 | |||
| pN0 | 63 | 76.2 | ||
| pN1–3 | 77 | 46.8 | ||
| TNM staging | <0.001 | |||
| I–II | 71 | 78.9 | ||
| III–IV | 69 | 40.6 | ||
| Lymphatic invasion | 0.027 | |||
| Negative | 63 | 66.7 | ||
| Positive | 77 | 48.1 | ||
| Venous invasion | 0.58 | |||
| Negative | 48 | 64.6 | ||
| Positive | 92 | 59.8 | ||
| CTCs | 0.013 | |||
| Negative | 78 | 70.5 | ||
| Positive | 62 | 50 | ||
Table 4.
Multivariate survival analysis of clinicopathological findings.
| Variables | Hazard ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Overall survival | Lymphatic invasion-positive | 1.57 | 0.83–3.01 | 0.32 |
| Stage III/IV | 2.15 | 1.32–4.89 | 0.0035 | |
| CTCs-positive | 1.82 | 0.91–4.88 | 0.046 | |
| Disease-free survival | pN | 2.41 | 0.75–7.02 | 0.16 |
| Lymphatic invasion-positive | 2.64 | 1.18–5.34 | 0.039 | |
| Stage III/IV | 3.02 | 1.83–5.81 | 0.0021 | |
| CTCs-positive | 1.86 | 0.87–3.15 | 0.035 |
Discussion
CTCs are tumor cells that enter the peripheral blood circulation from the primary tumor or metastasis [11]. They are an early form of recurrence and metastasis of malignant tumors [11,12]. CTCs are also known as rare blood cells, tumor micro-metastasis, latent tumor cells, or circulating epithelial cells [3,13,14]. CTCs exist in many forms in the peripheral circulation, either independently or in the form of cell clumps. Some CTCs may also be combined with platelets, which form a shell on the CTCs surface. Under normal circumstances, the CTCs will be destroyed by natural killer cells in the peripheral circulation [15,16]. However, CTCs combined with platelets forming a shell can escape from the immune surveillance of natural killer cells and become closely associated with endothelial cells, which leads to the occurrence of tumor cell metastasis.
The detection of CTCs is mainly divided into 2 steps: enrichment and identification of CTCs. Enrichment involves sorting out tumor cells using some physical characteristics such as the size and density of tumor cells or by the specific binding of antigens and antibodies. Identification involves counting the number of cells and analyzing the characteristics of the tumor cells using the specific markers in the nucleus or on the surface of tumor cells. In this study, we used an immunomagnetic separation method to enrich for tumor cells, and used IHC to identify the CTCs. CK19 is a sensitive marker of epithelial cells and epithelial tumor cells, so CK19 was used as a marker in the identification of CTCs. In the enrichment process, a small number of white blood cells remained and CD45 may be expressed on the surface of normal white blood cells. Therefore, we identified CK19-positive/CD45-negative cells as CTCs.
Most theories of tumor metastasis suggest that tumor cells fall off and migrate into the peripheral circulation during the primary stage of tumor metastasis. For some patients from whom tumor tissue cannot be easily obtained, the detection of CTCs in the peripheral circulation can provide a new way to determine their prognosis and may provide new methods for their treatment. Therefore, it is of clinical significance to detect CTCs in the peripheral circulation of patients with malignant tumors. The relationship between the prognosis of patients with breast cancer and CTCs has been demonstrated in many previous studies [17–19]. Compared with histological examination of tumor tissue, CTCs are easy to obtain, can be treated repeatedly, and are relatively noninvasive. This may become a new method for performance of “tumor biopsies in real-time.” In this study, the positive rate of CTCs was related with stage status of ESCC patients. This means that CTCs-positive ESCC patients are at a more advanced stage of disease compared with CTCs-negative ESCC patients. However, there was no relationship between CTCs status and age, sex, smoking, tumor history, tumor location, differentiation of tumor, lymphatic invasion, or lymph venous invasion.
Recently, good progress has been made in research on CTCs, particularly for patients with carcinoma. Gao et al. explored CTCs levels in pancreatic cancer by using a newly-developed platform-integrated subtraction enrichment and immunostaining-fluorescence in situ hybridization (SE-iFISH). Results showed that CTCs could be detected in pancreatic cancer patients in various stages, whether localized, locally advanced, or metastatic [20]. CTCs count could serve as a prognostic marker for metastatic malignant tumors, but the latest studies show that CTCs has positive significance in early recurrence of malignant tumors. Some researchers have examined the CTCs levels in patients with colorectal cancer by using a sensitive device. Eventually, they found that CTCs may be a simple, independent prognostic marker for non-metastatic colorectal cancer patients who are at high risk of early recurrence [21]. In the present study, we observed that CTCs positivity was associated with advanced tumor stage in ESCC patients. These results suggest that CTCs could be used to predict early recurrence of ESCC patients who received surgical treatment. This requires further study so that postoperative CTCs detection can improve patient survival.
The mechanism of formation of CTCs in patients with malignant tumors may be that the tumor cells located in their original position transform from epithelial to mesenchyme cells [22]. The adhesion between mesenchyme cells is decreased. These deciduous cells have a stronger propensity for movement and invasion. They can cross the surrounding matrix, invade blood vessels, and become tumor cells in the peripheral circulation [23,24]. CTCs can be combined with platelets, which form a layer or shell using tissue factors of the cell surface [15]. This prevents the immune killer cells from recognizing the surface antigens of tumor cells, so that CTCs can escape immune surveillance [25]. Some proteins are expressed abnormally, such as integrin proteins, and they are also involved in leading tumor cells into the circulatory system and forming CTCs [26]. For the above reasons, CTCs- positive ESCC patients are more likely to have regional or distant metastases in the early stages of the disease. The results of long-term survival analysis also confirmed this conclusion. The CTCs-positive patients had shorter survival than CTCs-negative ESCC patients. Multivariate analysis revealed that CTCs positivity is an independent prognostic biomarker that indicates a worse prognosis for patients with ESCC.
Conclusions
CTCs-positive ESCC patients tend to have worse prognosis. We predict that CTCs may be useful in early diagnosis of ESCC, timely monitoring of the recurrence and metastasis of cancers, accurate assessment of patient prognosis, and guidance of the clinical treatment.
Footnotes
Conflicts of interest
The authors report no conflicts of interest in this work.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Source of support: The Science and Technology Project in Gansu Province China (145RJZA230)
References
- 1.Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol. 2012;30(18):2265–72. doi: 10.1200/JCO.2011.38.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JB, Dawsey SM, Fan JH, et al. Common genetic variants related to vitamin D status are not associated with esophageal squamous cell carcinoma risk in China. Cancer Epidemiol. 2015;39(2):157–59. doi: 10.1016/j.canep.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Wang X, He H, et al. Combination of circulating tumor cells with serum carcinoembryonic antigen enhances clinical prediction of non-small cell lung cancer. PLoS One. 2015;10(5):e0126276. doi: 10.1371/journal.pone.0126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marx V. Tracking metastasis and tricking cancer. Nature. 2013;494(7435):133–36. doi: 10.1038/494131a. [DOI] [PubMed] [Google Scholar]
- 5.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Shiratsuchi H, Lin J, et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget. 2014;5(23):12383–97. doi: 10.18632/oncotarget.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Lee J, Kim ST, et al. Circulating tumor cells are predictive of poor response to chemotherapy in metastatic gastric cancer. Int J Biol Markers. 2015;30(4):e382–86. doi: 10.5301/jbm.5000151. [DOI] [PubMed] [Google Scholar]
- 8.Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–22. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol. 2011;29(12):1547–55. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell; Oxford, UK: 2009. [Google Scholar]
- 11.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–39. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Z, Zhang Y, Yin Z. Therapeutic targeting of circulating tumor cells – a new strategy in treatment of liver cancer recurrence and metastasis after hepatectomy. Chin J Oncol. 2014;36(6):401–4. [PubMed] [Google Scholar]
- 13.Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2014;7(1):1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Braun S. Circulating tumor cells revisited. JAMA. 2010;303(11):1092–93. doi: 10.1001/jama.2010.292. [DOI] [PubMed] [Google Scholar]
- 15.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang JM, Casavant BP, Beebe DJ. Circulating tumor cells: getting more from less. Sci Transl Med. 2012;4(141):141ps13. doi: 10.1126/scitranslmed.3004261. [DOI] [PubMed] [Google Scholar]
- 17.Bidard FC. Circulating tumor cells, a tremendous prognostic factor in inflammatory breast cancer. J Natl Cancer Inst. 2015;107(11):djv281. doi: 10.1093/jnci/djv281. [DOI] [PubMed] [Google Scholar]
- 18.Lianidou ES, Markou A, Strati A. The role of CTCs as tumor biomarkers. Adv Exp Med Biol. 2015;867:341–67. doi: 10.1007/978-94-017-7215-0_21. [DOI] [PubMed] [Google Scholar]
- 19.Maltoni R, Fici P, Amadori D, et al. Circulating tumor cells in early breast cancer: A connection with vascular invasion. Cancer Lett. 2015;367(1):43–48. doi: 10.1016/j.canlet.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Zhu Y, Zhang Z, Zhang C, et al. Clinical significance of pancreatic circulating tumor cells using combined negative enrichment and immunostaining-fluorescence in situ hybridization. J Exp Clin Cancer Res. 2016;35(1):66. doi: 10.1186/s13046-016-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai WS, Chen JS, Shao HJ, et al. Circulating tumor cell count correlates with colorectal neoplasm progression and is a prognostic marker for distant metastasis in non-metastatic patients. Sci Rep. 2016;6:24517. doi: 10.1038/srep24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–84. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan D, Xia H, Zhang Y, et al. P-Akt/miR-200 signaling regulates epithelial-mesenchymal transition, migration and invasion in circulating gastric tumor cells. Int J Oncol. 2014;45(6):2430–38. doi: 10.3892/ijo.2014.2644. [DOI] [PubMed] [Google Scholar]
- 24.Steinert G, Schölch S, Niemietz T, et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014;74(6):1694–704. doi: 10.1158/0008-5472.CAN-13-1885. [DOI] [PubMed] [Google Scholar]
- 25.Santos MF, Mannam VK, Craft BS, et al. Comparative analysis of innate immune system function in metastatic breast, colorectal, and prostate cancer patients with circulating tumor cells. Exp Mol Pathol. 2014;96(3):367–74. doi: 10.1016/j.yexmp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Mishra DK, Scott KL, Wardwell-Ozgo JM, et al. Circulating tumor cells from 4D model have less integrin beta 4 expression. J Surg Res. 2015;193(2):745–53. doi: 10.1016/j.jss.2014.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]