Abstract
Background
Osteosarcoma is one of the most common malignant bone cancers worldwide. Although the traditional chemotherapies have made some progression in the past decades, the mortality of osteosarcoma in children and adolescent is very high. Herein, the role of actein in osteosarcoma was explored.
Material/Methods
Cell viability assay was performed in osteosarcoma cell lines 143B and U2OS. Colony formation analysis was included when cells were treated with different doses of actin. Cell cycle assay was conducted to further examine the role of actein. Cell apoptotic rate and the relative activities of caspase-3, caspase-8, and caspase-9 were detected in 143B and U2OS osteosarcoma cells. Moreover, transwell assays were used to explore the effects of actein on cell metastasis.
Results
Actein significantly inhibited osteosarcoma cell viability in a time- and dose-dependent manner. Actein also dramatically suppressed the colony formation ability in osteosarcoma143B and U2OS cells. It was revealed that osteosarcoma cells were arrested in G0/G1 phase in the cell cycle progression and induced to apoptosis by administration of actein. The activities of pro-apoptotic factors such as caspase-3 and caspase-9 were significantly increased by actein. Furthermore, administration of actein decreased cell migrated and invasive abilities in both 143B and U2OS cell lines.
Conclusions
Actein inhibits tumor growth by inducing cell apoptosis in osteosarcoma. The inhibitive roles of actein in cell proliferation, migration and invasion suggest that actein may serve as a potential therapeutic agent in the treatment of osteosarcoma.
MeSH Keywords: Apoptosis, Cell Cycle, Cell Proliferation, Osteosarcoma
Background
Osteosarcoma is among the most common malignant bone cancers, especially in adolescents and children. The annual incidence of osteosarcoma worldwide is 1/10000, with the majority of cases occurring in people under 20 years old [1]. The 5-year survival rate for primary osteosarcoma is between 20% and 70% with traditional chemotherapy; however, it was dramatically decreased to less than 30% when the tumor metastasizes into other organs, in most cases, the lungs [2,3]. Furthermore, patients with metastatic osteosarcoma show poor response to conventional chemotherapy [4,5], which makes it a priority to find novel therapeutic methods to improve the treatments of patients with metastatic osteosarcoma.
Black cohosh is a North American perennial plant that has been used for centuries by Native Americans. Evidence showed that it has been involved in multiple processes, including anti-inflammation, anti-rheumatism, pain killers, and anti-dysmenorrhoea [6,7]. In recent years it has become a common alternative in hormone replacement therapy for females to relieve menopausal symptoms. Millions of women turn to black cohosh as a more natural agent in the belief that it has more advantage without the risks of estrogen therapies [8]. The main components of the roots and rhizomes of black cohosh are triterpene glycosides, sugars, phenyl propanoids, tannins, and long-chain fatty acids [9]. The effects and mechanisms of these compounds remain largely unknown. However, many studies have shown that the extracts of black cohosh suppressed the in vitro growth of human breast cancer cells. Isopropyl alcohol extracts inhibited MCF7 cell proliferation and increased the inhibitory effects of tamoxifen in breast cancer therapy [10,11]. The ethanolic extract restrained the expression of cyclin D1 and increased the activity of the P21 protein in ER− human breast cancer cell lines [12]. In addition, extracts of black cohosh were also verified to enhance the efficacy (toxic adverse effects) of Adriamycin or Taxotere on mouse breast cancer cells EMT6, the mechanism of which are still unknown [8].
Furthermore, purified aglycones and triterpene glycosides have been demonstrated to selectively suppress the growth of various cancer cells, including human breast cancer cells MCF7 and MDA-MB-453 [13], human oral squamous carcinoma cells [14], and human liver cancer cells HepG2 [15] compared with the effects on non-malignant counterparts. Triterpene glycosides extracted from black cohosh induced cell cycle arrest at G1 phase in breast cancer cells, of which actein was the most active component in the plant. Actein was shown to decrease the expression of cell cycle regulators, including cyclin D1, CDK4, and phosphorylated EGFR. It also upregulated the activity of the CDK inhibitory protein P21 in ER− MCF7 breast cancer cells. Both processes contribute to actein-mediated arrest of cell cycle at G1 phase.
The main purpose of this study was to elucidate the effects of actein on human osteosarcoma growth and metastasis. To this end, we chose 2 osteosarcoma cell lines with distinct levels of aggressiveness: the highly aggressive 143B cell line and the less aggressive U2OS cell line. Cell proliferation, migration, and invasion were assessed after cells were exposed to actein. Cell cycle progression and cell apoptosis were also determined after actein treatment in osteosarcoma cells.
Material and Methods
Reagents
Actein was commercially purchased from ChromaDex (Laguna Hills, CA, catalog number 01355-101), which was purified by high-performance liquid chromatography (HPLC). Actein was preserved as a stock solution at a concentration of 80 μM. The stocking solution was diluted later based on experimental design. Cell Counting Kit-8 (CCK-8) was obtained from Boster Biology Inc. (Wuhan, China). All cell culture supplies were commercially obtained from Corning Co. (Singapore).
Cell culture
Osteosarcoma cell lines 143B and U2OS were from the American Type Culture Collection (ATCC, VA) and cultured in the recommended medium supplied with 10% fetal bovine serum (FBS, Gibco, USA) at 37°C and 5% CO2 in an incubator. For the administration of actein, cells were co-incubated with various concentrations of actein prior to tests. For all of the in vitro assays, 143B and U2OS cells were cultured for 2 days before treatment with actein.
Cell viability assay
Cell viability was determined by the CCK-8 assay. Briefly, 143B and U2OS cells were cultured in a 96-well plate (2×104 cells/well). Cells were administrated with indicated doses of actein for 36 h, or exposed to a fixed concentration (30 μM) of actein for different time durations (0, 12, 24, 36, and 48 h). At each time point, a 10-μL solution of CCK-8 was mixed into each well and 143B and U2OS cells were further incubated for 10 min at 37°C before proceeding to the absorbance detection. Air bubbles in the solution were strictly avoided during the whole process. The absorbance of each experimental group was read and calculated at a wavelength of 450 nm. Each treatment was repeated in triplicate.
Colony formation assay
After exposure to different doses of actein (0, 5, 10, 20, 40, and 80 μM), 143B and U2OS cells were spread into 12-well plates (100 cells per well). A colony that assembled more than 50 cells was considered as being formed successfully and was later counted. The number of colonies was calculated on the 10th day after seeding, when the plates were stained with crystal violet (5%). The colony formation rate was calculated with the following formula: colony formation rate=(number of colonies/number of seeded cells) ×100%.
Cell cycle assay
Each group of 143B and U2OS cells was seeded in 6-well plates at a concentration of 3×105 cells per well and pre-treated with actein at the indicated doses when a confluence of 85% was reached for both cell lines. The culture medium was refreshed every 2 days. After washing with pre-iced PBS 3 times, cells were then collected with low-speed centrifugation (1000 rpm for 5 min). Cell pellets were re-suspended in 1 ml of cold PBS solution, fixed with 75% ethanol, and stored at −20°C for additional 2 days. Before flow cytometry (FCM) analysis, cell solution was lysed, centrifuged, washed, and re-suspended in propidium iodide (PI) staining buffer containing 50 μl/ml PI and 250 μl/ml RNase A. Afterwards, the cell mixture was sustained at 4°C for 30 min in the dark and examined by fluorescence-activated cell-sorting (FACS) technique (Beckman, Germany).
Cell apoptosis
Morphological analysis of cell apoptosis was performed to reveal the apoptotic cells by Hoechst-33258 staining. 143B and U2OS cells were administrated with different doses of actein for 36 h. Thereafter, cells were washed with pre-cold PBS and fixed with iced methanol/acetic acid (3:1) for 15 min. Fixed cells were stained with Hoechst-33258 at a concentration of 5 μg/ml for 10 min at 37°C. The morphological changes in the cell nuclei were imaged and calculated with fluorescence microscope (Nikon, Germany).
Cell apoptosis was evaluated by an annexin V-fluorescein isothiocyanate (FITC) kit (Beyotime, Nantong, China). A total of 5×105 143B and U2OS cells were exposed to different doses of actein for 36 h. Afterwards, cells were centrifuged at 1000 rpm for 5 min and re-suspended with 500 μl of 1× binding buffer. A volume of 5 μl Annexin V-FITC and 5 μl of PI were, respectively, mixed with the cells. After incubation for 5 min in the dark, cell percentage in each well was detected by FACS (BD Bioscience, USA). Both 143B and U2OS cells, which were annexin-V-FITC-positive and PI-negative, were considered as early apoptosis. Cells that were positive for annexin-V-FITC and PI were considered to be in the late stage of apoptosis. The percentages of both cell lines in each condition were analyzed from 3 independent assays.
Caspase activity analysis
The relative activities of caspase-3, caspase-8, and caspase-9 in 143B and U2OS cells upon actein administration were determined with the caspase activity kits (Beyotime, Nantong, China), following the manufacturer’s protocol. Briefly, both cell lines were treated with actein (0, 20, 40, and 80μM) for 36 h and cell lysates were collected by low-speed centrifuge (1000 rpm). Analysis was performed in a 96-well plate by co-incubating proteins from cell lysates (143B and U2OS) and reaction buffers with substrates for caspase-3, caspase-8, and caspase-9, respectively. After being stably cultivated for 4 h, the absorbances of 405 nm of each well were read and calculated by a TECAN reader (NY, USA).
Transwell assay
Cell migration and invasion abilities were detected with Transwell chambers with pore size 8 μm (Corning, NY). Before each assay, 143B and U2OS cells were treated with actein at indicated concentrations. For cell migration assay, 100μL of 143B or U2OS cells (5×104) in serum-free culture media were spread into the upper chamber, which was fixed into 24-well plates. A total of 600 μL culture medium supplemented with 10% FBS was then added into the lower chamber. Cells were incubated at 37°C for 24 h, washed with PBS twice, fixed with cold methanol, and stained with crystal violet (5%) for 5 min at room temperature. Cells that adhered to the upper surface of the chamber were carefully removed using cotton swabs, and those on the bottom surface of the membrane were photographed and counted under a light microscope (Nikon) with 5 random fields. For the invasion analysis, the membranes of each upper chamber were pre-coated with Matrigel (BD Biosciences, USA) for 6 h at 37°C in an incubator. Each experiment was repeated in triplicate.
Statically analysis
All results are shown as the means ±SD unless otherwise indicated in the study. Student’s t-test was employed to evaluate the statistical significance between variables. All of the statistical analyses were performed with SPSS Statistics 18.0 software (Chicago, IL) and any value of p< 0.05 was considered as significant. Each experiment was repeated in triplicate.
Results
Actein inhibited cell proliferation in human osteosarcoma in a dose- and time-dependent manner
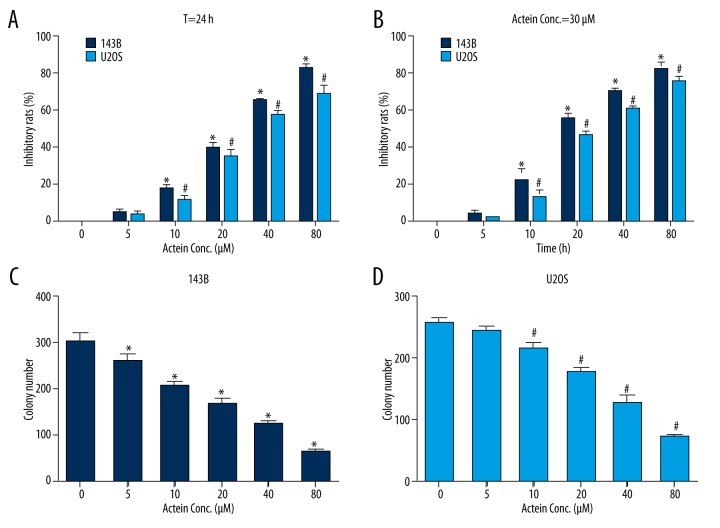
To explore the effects of actein administration on osteosarcoma cell proliferation, osteosarcoma cell lines 143B and U2OS were cultured and co-incubated with different concentrations of actein at different time intervals prior to cell viability assays. Our data showed that in 143B and U2OS cells, treatment with various doses of actein for 24 h dramatically decreased cell proliferation rates (Figure 1A). Moreover, the inhibitory rates increased as the concentration of actein increased. The lethal dose 50 value (LD50) was about 30 μM for both cell lines. Afterwards, 143B and U2OS cells were stimulated with 30 μM of actein for different time intervals. Figure 1B shows that longer exposure time led to higher inhibitory rates of cell proliferation in both cell lines, and the inhibitory rate for both cell lines was increased by up to 80% at 48 h. These data reveal that actein decreased cell proliferation rate in osteosarcoma cell lines in a dose- and time-dependent manner.
Figure 1.
Actein inhibited cell proliferation in human osteosarcoma cells. (A) Cell viability was detected when both 143B and U2OS osteosarcoma cells were treated with different concentrations of actein (0, 5, 10, 20, 40, and 80 μM) for 24 h. (B) Cell viability was examined when 143B and U2OS cells were administrated with 30 μM actein at various time intervals (0, 6, 12, 24, 36, and 48 h). (C) Colony formation assay for 143B cells when cells were treated with different concentrations of actein (0, 5, 10, 20, 40, and 80 μM) for 24 h. (D) Colony formation assay for U2OS cells when cells were treated with different concentrations of actein for 24 h. * P<0.05 vs. control 143B cells, # P<0.05 vs. control U2OS cells.
Actein inhibited colony formation in both 143B and U2OS cells
To further evaluate the inhibitory effects of actein in osteosarcoma cell proliferation, colony formation assay was performed with 143B and U2OS cell lines. Similarly, we administered cells with different concentrations of actein (0, 5, 10, 20, 40, and 80 μM) for 36 h at a 37°C incubator. It was shown that approximately 300 colonies were visualized in control 143B cells; however, only 200 and 80 colonies were observed when cells were treated with 10 μM and 80 μM of actein, respectively (Figure 1C). In U2OS cells, colony number dramatically decreased, from 250 in the non-treated group to 70 in the 80 μM actein-stimulated counterpart (Figure 1D). These results suggest that actein inhibited colony formation in osteosarcoma cells, conjointly supporting the conclusion that actein suppressed cell proliferation in osteosarcoma cell lines 143B and U2OS.
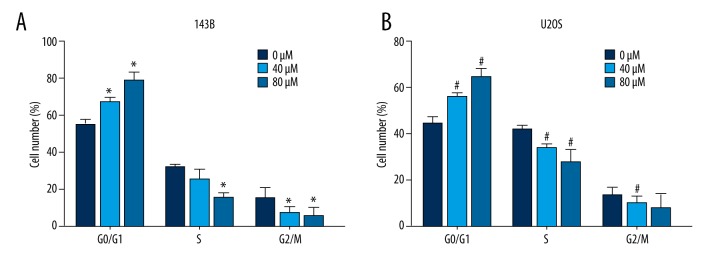
Actein caused cell cycle arrest in G0/G1 phase in osteosarcoma cells
Next, we detected the cell cycle progression in 143B and U2OS cells after exposure to actein. Both cell lines were pre-stimulated with actein for 36 h at the concentration of 0, 40, and 80 μM. In 143B cells, we observed that cells were increasingly accumulated in G0/G1 phase, with the percentage of cells in this phase approaching 80% with 80 μM actein administration. In contrast, cells in S phase and G2/M phase decreased dramatically (Figure 2A). Similarly, in U2OS cells, the cell percentages in G0/G1 phase increased by 20% and 35% upon 40 μM and 80 μM actein treatment, respectively. The U2OS cells in S phase were dramatically decreased accordingly (Figure 2B). All of the results suggest that actein shifted cell cycle from S phase and G2/M phase to G0/G1 phase for osteosarcoma cell lines in a dose-dependent manner. These observations indicate that actein inhibited cell proliferation, possibly by affecting cell cycle progression in osteosarcoma.
Figure 2.
Actein caused cell cycle arrest in G0/G1 phase in osteosarcoma cells. (A) The cell percentage in each cell cycle phase was shown after 143B cells were exposed to actein (0, 40, and 80 μM). (B) After receiving the same treatment as 143B cells, U2OS cells were subject to cell cycle analysis. The percentage of cells in each cell cycle phase are also shown. * P<0.05 vs. control 143B cells, # P<0.05 vs. U2OS cells.
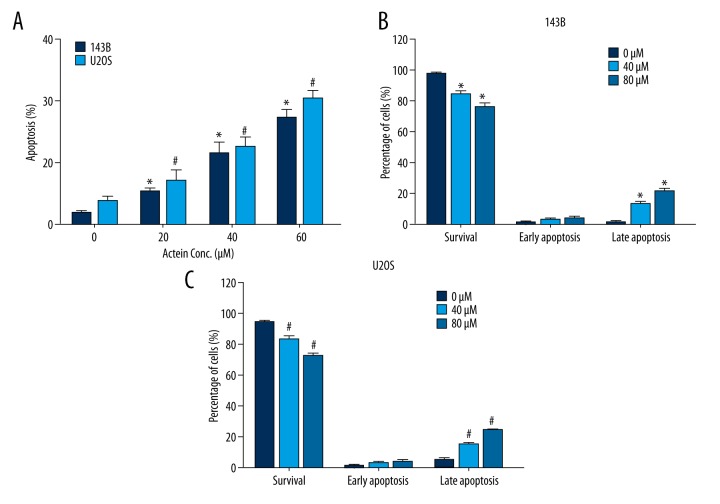
Actein induced cell apoptosis by upregulating the activities of caspase-3 and caspase-9
Actein was reported to induce cell apoptosis in breast cancer [16]. Thus, we examined the effects of actein on osteosarcoma cell survival by initially examining the nuclei morphology. After exposure to actein for 36 h at various doses (0, 20, 40, and 80 μM), nuclei of 143B and U2OS cells were crushed and fragmented, with the chromatin condensed, as revealed by the Hoechst-33258 staining (data not shown). By counting the abnormal nuclei, it was shown that, compared to control cells, fragmented nuclei in actein-treated 143B cells were increasingly upregulated by approximately 5%, 12%, and 18% with 20, 40 and 80 μM, respectively, actein treatment (Figure 3A). Similar results were also observed in U2OS cells, where cell apoptosis rates were dramatically increased from 5% in untreated cells to 27% in 80 μM actein-treated cells (Figure 3A). Furthermore, using flow cytometry, it was shown that the survival of 143B cells notably decreased from 90% to 75% upon actein administration, whereas the cell percentage in late-stage apoptosis increased by up to 20% (Figure 3B). U2OS cells were also shown to be apoptotic upon actein treatment in a dose-dependent manner (Figure 3C). These data jointly reveal that actein induced cell apoptosis in osteosarcoma cells.
Figure 3.
Actein increased cell apoptosis rates in both 143B and U2OS cell lines. (A) Hoechst-33258 staining showed that the number of fragmented nuclei was increased when cells were treated with various doses of actein for 36 h in both 143B and U2OS osteosarcoma cells. (B) FITC-PI staining showed the percentages of survival cells; cells in early apoptosis and late apoptosis were significantly different among distinct treatments of actein in 143B cells. (C) FITC-PI staining showed the percentage of survival cells was decreased, while cells in late apoptotic stage were significantly increased upon actein administration in U2OS cells. * P<0.05 vs. control 143B cells, # P<0.05 vs. U2OS cells.
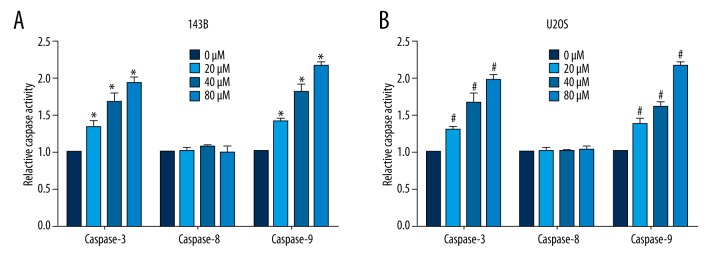
Thereafter, the relative caspase activity was also determined upon actein administration (0, 20, 40, and 80μM). It was shown that the activities of caspase-3 and caspase-9 in both 143B (Figure 4A) and U2OS cells (Figure 4B) were notably increased in a dose-dependent manner. However, the activity of caspase-8 remained unchanged in both osteosarcoma cell lines. The increased activities of caspase-3 and caspase-9 strongly support that actein induced cell apoptosis in osteosarcoma cells.
Figure 4.
Actein increased the activities of caspase-3 and caspase-9. (A) Relative activities of caspase-3, caspase-8, and caspase-9 were determined upon various doses of actein stimulation for 36h in 143B cells. (B) Relative activities of caspase-3, caspase-8, and caspase-9 were determined upon various doses of actein stimulation for 36 h in U2OS cells. * P<0.05 vs. 143B cells, # P<0.05 in U2OS cells.
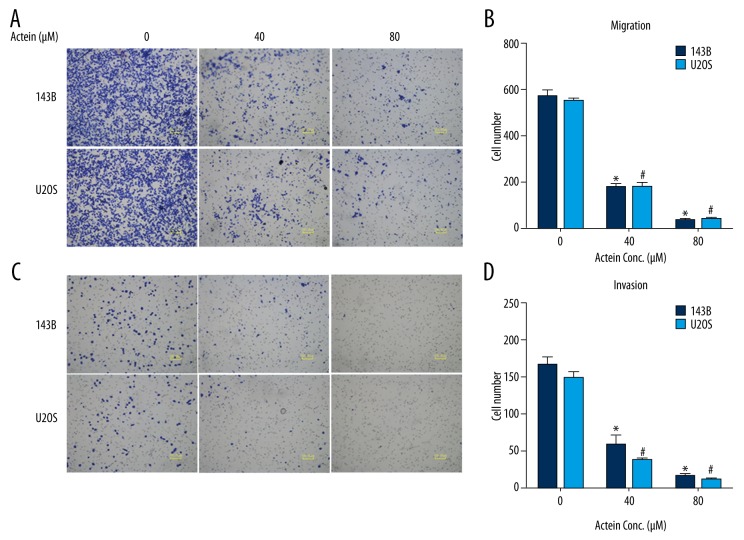
Actein suppressed cell migration and invasion in cultured osteosarcoma cell lines
Transwell assays were then conducted to examine the effects of actein on cell migration and invasion in human osteosarcoma. In the migration assay (Figure 5A), the number of 143B cells migrated to the lower surface of the membrane fell to 30% by 40 μM actein administration and even 8% by 80 μM actein treatment as compared with untreated control cells. Significant decreases in migrated cells were also observed in U2OS cells upon actein administration (Figure 5B). Likewise, after the upper surface of the membrane was pre-coated with Matrigel for 6 h (invasion assay, Figure 5C), cells invaded through the chamber were similarly significantly decreased with actein doses increasing in 143B cells, indicating the suppression of invasive ability in 143B cells. Furthermore, the invasive abilities of U2OS cells were also inhibited by 75% and 95%, after cells were stimulated with 40 μM and 80 μM, respectively, actein relative to controls (Figure 5D). All of these data reveal that actein suppressed cell migration and invasion in vitro in osteosarcoma.
Figure 5.
Actein inhibited cell migration and invasion in both 143B and U2OS cells. (A) Representative photographs of cell migration assay for both 143B and U2OS cells are shown. Five fields were randomly chosen for visualization and calculation. (B) Quantification of the migrated cells for both cell lines upon actein treatment. (C) Representative photographs of cell invasion assay for both 143B and U2OS cells. (D) Quantification of cells invaded through the Matrigel for osteosarcoma cell lines upon actein administration. * P<0.05 vs. 143B cells, # P<0.05 in U2OS cells.
Discussion
Osteosarcoma is responsible for approximate 20% of all primary bone sarcomas and is associated with a relatively high mortality rate, especially among children and adolescents [17]. Osteosarcoma arises within the bones and metastasizes mostly to the lungs. The conventional treatment strategy for this malignancy is chemotherapy with assistance of surgical resections [18]. However, the risk adaptation of chemotherapy for individuals varies and the satisfaction of postoperative clinical stratification differs due to the differences in tumor size and sensitivity of patients to chemotherapy [19]. Therefore, identification of novel treatment strategies for osteosarcoma remains urgent for doctors and researchers.
The present study aimed to investigate the roles of actein in human osteosarcoma growth and metastasis in vitro. It was shown in our results that actein could suppress osteosarcoma cells proliferation in a dose- and time-dependent manner. The colony formation assay also demonstrated that colony formation capacity of both cell lines were decreased after actein administration. Furthermore, cell migration and invasion were also restrained upon actein treatment in a dose-dependent manner. Last but not least, actein increased cell apoptosis in osteosarcoma cells by upregulating the activities of caspase-3 and caspase-9. All of these data suggest the inhibitory effects of actein in human osteosarcoma. Our findings indicate that actein might function as a therapeutic agent against human osteosarcoma due to its critical anti-cancer properties.
The homeostasis in the human body is maintained by the cycle of cell birth, growth, differentiation, and death. Apoptosis is one of the most effective pathways to eliminate the harmful or unnecessary cells, which is characterized by chromosomal DNA fragmentation, nuclear disintegration, and cell shrinkage, followed by translocation of the phosphatidyl serine moiety and membrane blebbing [20]. The main molecular mechanisms of apoptosis include dysregulation of the mitochondrial pathway, the inactivation of caspase pathway, and the deficiency of the death signal pathway [21,22]. To date, 14 mammalian caspases have been identified and can be categorized into 3 classes: initiator caspases (caspase-8 and caspase-9), effector caspases (caspase-3), and inflammatory caspases [23,24]. Initiator caspases interact with the motifs, including the death effector domain (DED) and the apoptotic process begins. In our study, we demonstrated that actein administration caused little effect on caspase-8 but it notably increased the activity of caspase-9 in a dose-dependent manner (Figure 5). Caspase-8 is always associated with the extrinsic apoptosis pathway, while caspase-9 is involved in the intrinsic pathway. Our data suggest that the intrinsic apoptosis pathway may be predominant in mediating the cell death caused by actein administration. Caspase-3, the most significant executioner caspase, can effectively cleave the inhibitor of caspase-activated DNase (ICAD). It was also shown to be upregulated by actein treatment in osteosarcoma cells. All these data jointly suggest that actein can induce osteosarcoma cells apoptosis via the intrinsic apoptosis pathway.
Conclusions
Our study results verified the inhibitory effects of actein in human osteosarcoma, which was achieved by inhibiting cell growth and metastasis and inducing cell apoptosis. Our data provide strong evidence that actein, one of the effective constituent of black cohosh, might serve as a potent therapeutic agent against human osteosarcoma.
Footnotes
Source of support: Departmental sources
References
- 1.Whelan J, McTiernan A, Cooper N, et al. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int J Cancer. 2012;131(4):E508–17. doi: 10.1002/ijc.26426. [DOI] [PubMed] [Google Scholar]
- 2.Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer. 2008;113(9):2597–604. doi: 10.1002/cncr.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielack SS, Carrle D, Hardes J, et al. Bone tumors in adolescents and young adults. Curr Treat Options Oncol. 2008;9(1):67–80. doi: 10.1007/s11864-008-0057-1. [DOI] [PubMed] [Google Scholar]
- 4.Hegyi M, Semsei AF, Jakab Z, et al. Good prognosis of localized osteosarcoma in young patients treated with limb-salvage surgery and chemotherapy. Pediatr Blood Cancer. 2011;57(3):415–22. doi: 10.1002/pbc.23172. [DOI] [PubMed] [Google Scholar]
- 5.Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome – the French pediatric experience. Cancer. 2005;104(5):1100–9. doi: 10.1002/cncr.21263. [DOI] [PubMed] [Google Scholar]
- 6.De Capite A, Lancaster T, Puthoff D. Salicylic acid treatment increases the levels of triterpene glycosides in black cohosh (Actaea Racemosa) rhizomes. J Chem Ecol. 2016;42(1):13–16. doi: 10.1007/s10886-015-0655-x. [DOI] [PubMed] [Google Scholar]
- 7.Nian Y, Yang J, Liu TY, et al. New anti-angiogenic leading structure discovered in the fruit of Cimicifuga yunnanensis. Sci Rep. 2015;5:9026. doi: 10.1038/srep09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233–39. doi: 10.1007/s10549-004-4260-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee YS, Choi EM. Actein isolated from black cohosh promotes the function of osteoblastic MC3T3-E1 cells. J Med Food. 2014;17(4):414–23. doi: 10.1089/jmf.2013.2841. [DOI] [PubMed] [Google Scholar]
- 10.Bodinet C, Freudenstein J. Influence of marketed herbal menopause preparations on MCF-7 cell proliferation. Menopause. 2004;11(3):281–89. doi: 10.1097/01.gme.0000094209.15096.2b. [DOI] [PubMed] [Google Scholar]
- 11.Gaowa S, Sun AJ, Jiang Y, et al. Ultrasonographic observation of the breast in early postmenopausal women during therapy with Cimicifuga foetida extract and sequential therapy with estrogen and progestin. Chin Med J (Engl) 2015;128(8):1000–4. doi: 10.4103/0366-6999.155052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garita-Hernandez M, Calzado MA, Caballero FJ, et al. The growth inhibitory activity of the Cimicifuga racemosa extract Ze 450 is mediated through estrogen and progesterone receptors-independent pathways. Planta Med. 2006;72(4):317–23. doi: 10.1055/s-2005-916233. [DOI] [PubMed] [Google Scholar]
- 13.Einbond LS, Shimizu M, Xiao D, et al. Growth inhibitory activity of extracts and purified components of black cohosh on human breast cancer cells. Breast Cancer Res Treat. 2004;83(3):221–31. doi: 10.1023/B:BREA.0000014043.56230.a3. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Mimaki Y, Sakagami H, Sashida Y. Cycloartane glycosides from the rhizomes of Cimicifuga racemosa and their cytotoxic activities. Chem Pharm Bull (Tokyo) 2002;50(1):121–25. doi: 10.1248/cpb.50.121. [DOI] [PubMed] [Google Scholar]
- 15.Tian Z, Si J, Chang Q, et al. Antitumor activity and mechanisms of action of total glycosides from aerial part of Cimicifuga dahurica targeted against hepatoma. BMC Cancer. 2007;7:237. doi: 10.1186/1471-2407-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einbond LS, Wen-Cai Y, He K, et al. Growth inhibitory activity of extracts and compounds from Cimicifuga species on human breast cancer cells. Phytomedicine. 2008;15(6–7):504–11. doi: 10.1016/j.phymed.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Shao Z, Xiong L, Yang S. Inhibition of autophagy enhances cisplatin-induced apoptosis in the MG63 human osteosarcoma cell line. Oncol Lett. 2015;10(5):2941–46. doi: 10.3892/ol.2015.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan J, Zhang X, Liu T, Zhang X. Strategies and developments of immunotherapies in osteosarcoma. Oncol Lett. 2016;11(1):511–20. doi: 10.3892/ol.2015.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baranski Z, de Jong Y, Ilkova T, et al. Pharmacological inhibition of Bcl-xL sensitizes osteosarcoma to doxorubicin. Oncotarget. 2015;6(34):36113–25. doi: 10.18632/oncotarget.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatzel K, Kuroki L, Dmitriev I, et al. Membrane-proximal TRAIL species are incapable of inducing short circuit apoptosis signaling: Implications for drug development and basic cytokine biology. Sci Rep. 2016;6:22661. doi: 10.1038/srep22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulda S. Exploiting mitochondrial apoptosis for the treatment of cancer. Mitochondrion. 2010;10(6):598–603. doi: 10.1016/j.mito.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Walczak H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb Perspect Biol. 2013;5(5):a008698. doi: 10.1101/cshperspect.a008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27(48):6194–206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 24.Pop C, Salvesen GS. Human caspases: Activation, specificity, and regulation. J Biol Chem. 2009;284(33):21777–81. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]