Abstract
The aim of the present study was to investigate the potential neuroprotective effects of electroacupuncture (EA) in the treatment of cerebral ischemia/reperfusion (I/R) injury, and to elucidate the association between this neuroprotective effect and brain ultrastructure and expression of matrix metalloproteinase (MMP)-2 and 9. Rats underwent focal cerebral I/R injury by arterial ligation and received in vivo therapeutic EA at the Baihui (DU20) and Shenting (DU24) acupoints. The therapeutic efficacy was then evaluated following the surgery. The results of the current study demonstrated that EA treatment significantly ameliorated neurological deficits and reduced cerebral infarct volume compared with I/R injured rats. Furthermore, EA improved the learning and memory ability of rats following I/R injury, inhibited blood brain barrier breakdown and reduced neuronal damage in the ischemic penumbra. Furthermore, EA attenuated ultrastructural changes in the brain tissue following ischemia and inhibited MMP-2/MMP-9 expression in cerebral I/R injured rats. The results suggest that EA ameliorates anatomical deterioration, and learning and memory deficits in rats with cerebral I/R injury.
Keywords: electroacupuncture, cerebral ischemia-reperfusion, learning and memory, ultrastructure, matrix metalloproteinase-2, matrix metalloproteinase-9
Introduction
Stroke is the second leading cause of mortality worldwide, and often results in physical and cognitive dysfunction, and various other types of functional disorders (1,2). Cognitive dysfunction is common following stroke, with an incidence of up to 64% (3). Frequently observed forms of cognitive impairment include learning and memory disorders, attention deficits and other reductions in cognitive ability that seriously restrict the functional rehabilitation of stroke patients. Currently, the effect of cognitive impairment on the quality of life and daily living of patients is greater than the effect of physical dysfunction alone (4), and cognitive impairment increases the economic burden of patients (5). Stroke patients that develop cognitive disorders are more likely to develop dementia (6). Thus, timely treatment is critical for physical and mental function, and comprehensive recovery and prevention of dementia for patients following stroke.
Acupuncture is a popular treatment strategy in traditional Chinese medicine (TCM), and has been accepted and recognized as a therapy in China and Western countries (7). Acupuncture has a long history of use in the treatment of mental disorders and diseases of the brain (8). Acupuncture has been widely used to clinically treat cognitive disorders in China and has provided therapeutic benefits, and gained recognition from professionals and the general public (9–11). In TCM, two acupoints located on the Du meridian, Baihui (DU20; on the anterior midline in front of boundary of the frontal and parietal bones) and Shenting (DU24; on the anterior midline and the central parietal bone) may be important in the nervous system. The Shenting (DU24) acupoint is considered to be involved in the improvement of human health, and Baihui (DU20) in the adjustment of memory function. Thus, the Baihui (DU20) and Shenting (DU24) acupoints have been commonly focused on to treat cognitive disorders in China (12–14). Previous research by this group has demonstrated that electroacupuncture (EA) at Baihui (DU20) and Shenting (DU24) improves cognitive disorders following ischemic stroke (15,16), however, the underlying mechanisms remain to be elucidated. The present study aimed to further clarify the mechanism by which acupuncture achieves therapeutic benefits to cognitive dysfunction following ischemia/reperfusion (IR)-induced stroke.
Brain tissue damage progresses due to a series of complex pathophysiological changes following cerebral I/R injury. Disruption of the blood brain barrier (BBB) (17) and formation of brain edema (18) are considered important pathological changes that occur following I/R injury. The BBB is an important protective structure of the brain and facilitates exchange of material between the blood and the brain tissue (19). Alterations in the tight junction organization in the BBB results in blood vessel permeability and BBB breakdown following hypoxic ischemia (20), which allows white blood cells, plasminogen plasma protein, intravascular fluid and other substances to enter the brain (21). Inflammatory responses, brain edema formation and disruption of brain tissue structure subsequently occur, and eventually promote neuronal death or apoptosis (22,23). The integrity of the structure and function of neurons is the morphological basis of learning, memory and other cognitive activities (24). Thus, neuronal cell death or apoptosis may impair learning and memory. Matrix metalloproteinase (MMP)-2/MMP-9 are important collagenases of the MMP family and have previously been widely studied in acute cerebral ischemia. MMPs are important in the breakdown of the BBB, cerebral edema and inflammation, and pathophysiologic processes involving angiogenesis following cerebral ischemia (25,26). The present study hypothesized that MMP-2 and MMP-9 are also closely associated with neural cell apoptosis, neuron regeneration and the incidence of neural functional defects following cerebral I/R injury (27).
The present study used a rat model of embolic middle cerebral artery occlusion (MCAO) of focal cerebral I/R injury to observe the effect of EA at the Baihui (DU20) and Shenting (DU24) acupoints on learning and memory, and investigated the underlying molecular mechanisms of I/R injury.
Materials and methods
Materials and reagents
MMP-2, MMP-9 and β-actin primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). 2,3,5-Triphenyl tetrazolium chloride (TTC) and other chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise stated.
Animals
Healthy adult male Sprague-Dawley rats (weight, 250–280 g; age, 3–4 months) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China), housed under pathogen-free conditions at 22°C with a 12 h light/dark cycle, and received ad libitum access to food and water. All animal procedures were conducted in accordance with international ethical guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all experiments were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (Fuzhou, China).
Establishing the cerebral I/R injury rat model
MCAO was used to establish the rat model of cerebral I/R injury. Rats were fasted for 24 h prior to surgery, and the surgical procedures were performed as previously described by Longa et al (28), with slight modifications. Briefly, the rats were anesthetized by intraperitoneal injection with 10% chloral hydrate (300 mg/kg). The left common carotid artery (CCA), the left external carotid artery (ECA) and the internal carotid artery (ICA) were carefully exposed and isolated via a midline cervical incision. Nylon surgical thread (~18–22 mm) was inserted into the ICA to block the left middle cerebral artery (MCA) when the blunted distal end met resistance. Reperfusion was achieved when the thread was removed after 2 h of occlusion to restore blood supply to the MCA area. For rats in the sham group, the left CCA, ECA and ICA were exposed, but no ligations and occlusions were performed. The rectal temperature of rats was monitored and the body temperature was maintained at 37°C throughout the surgical procedures.
Upon recovery, the neurological deficit scores of the rats were assessed and they were randomly divided into 2 groups (n=20/group) as follows: Ischemia (MCAO) control group; and MCAO + EA group. Following surgery, the rats recovered in pre-warmed cages.
EA treatment
Following recovery from surgery (2 h after I/R), the rats in the EA group received EA treatment for 7 days. Acupuncture needles (0.3 mm in diameter) were inserted at a depth of 2 to 3 mm into the skin at the Baihui (DU20) and Shenting (DU24) acupoints. Stimulation was then generated using G6805 EA apparatus [Shanghai Huayi (Group) Company Ltd., Shanghai, China] and the stimulation parameters were set as follows: Waves of 1 and 20 Hz, and 1–3 mA were delivered for 30 min once per day.
Neurological assessment
The neurological deficit score was assessed in a single-blind manner, as previously described by Longa et al (28). A score of 0 indicated no neurological deficit was observed; score of 1 represented by a failure to fully extend the right forepaw, indicated a mild deficit; a score of 2 represented by circling to the right and a score of 3 represented by falling to the right, indicated moderate deficits; and a score of 4 was represented by failure to walk and indicated a severe deficit. Rats scoring 0 or 4, exhibiting either no or severe deficits, respectively, were excluded from the current study.
Morris water maze
At day 3 following surgery, the spatial learning and memory of rats was tested via the Morris water maze. The water maze apparatus (Chinese Academy of Sciences, Beijing, China) consisted of a tank (diameter, 120 cm; height, 50 cm) filled with water (depth, 30 cm; temperature, 25±2°C). A circular escape platform, measuring 6 cm in diameter and 28 cm in height, was submerged 2 cm below the surface of the water. The tank was divided into 4 equal quadrants. A video camera attached to a computer was placed above the center of the tank for recording and analysis of the rats. These points served as the starting positions at which each rat was lowered gently into the water, its head facing the wall of the water maze. Morris water maze tasks include orientation, navigation and space exploration trials. In the initial set of trials, each rat was placed in the water at 4 locations equidistant from the platform. If the rat arrived at the platform within the 90 sec time restriction and remained on it for 3 sec, it was considered to have successfully found the platform and was scored by the time taken. When the rat was unable to find the platform within 90 sec, it was placed on the platform for 10 sec and the time scored was 90 sec. The computer recorded the time taken for each rat to identify the safe platform, and each day the mean result of the time taken for the 4 quadrants was assessed for each rat. The initial set of trials was conducted over 5 days, with the experiment performed on each rat once per day.
The second part of the experiment was performed on day 7. This part assessed the ability of each rat to remember the position of the platform by examining the time in which the rat located the platform within the 90-sec time restriction. Following all trials, the rats were dried thoroughly with a hair dryer and returned to their cages.
Evaluation of infarct volume
At the end of experiments, rats were deeply anesthetized using 10% chloral hydrate and euthanized transcardially with 0.9% NaCl. The brains of all rats were removed rapidly and dissected into six coronal blocks at a thickness of 2 mm/section and stained with 2% solution of TTC in phosphate-buffered saline (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C for 20 min. Subsequently, the brain sections were fixed with 4% paraformaldehyde as previously described (29). Normal tissue stained deep red while infarct area exhibited a pale gray color due to lack of stain uptake. Images of the stained slices were captured with a Canon SX20 high-resolution digital camera (Canon, Inc., Tokyo, Japan), and the infarct volume was quantified with the Motic Med 6.0 system (Motic China Group Co., Ltd., Xiamen, China). The infarct volume was expressed as a percentage of the uninjured contralateral hemisphere volume.
Transmission electron microscopy (TEM)
Rats (n=6) were sacrificed via intraperitoneal injection of chloral hydrate (300 mg/kg) and the left ventricle was perfused with 200 ml of saline followed by 400 ml 4% paraformaldehyde (pH 7.4). The brain was then post-fixed in paraformaldehyde with 1% lanthanum nitrate tracer (LNT; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) for ~24–48 h follow by fixation in 3% glutaraldehyde-1.5% paraformaldehyde-1% LNT solution for 24 h and conventional embedding for electron microscopy. Fixed brains were dehydrated using a graded series of ethanol-1% LNT solution of increasing concentrations, embedded in epoxy resin, and cut into ultrathin sections (90 nm). The sections were mounted on copper grids, stained in uranyl acetate and lead citrate and then observed under TEM (H-7650; Hitachi Ltd, Tokyo, Japan). Comprehensive observation at low magnification was performed, followed by detailed observation of cell morphology, nuclei and cellular organelles.
Western blotting
Total proteins were extracted from the left cerebral hippocampal tissues and protein concentrations were determined by bicinchoninic acid assay. Protein samples (50 µg) were separated by electrophoresis on 12% SDS-PAGE gels, then transferred onto polyvinylidene difluoride membranes in a Tris-glycine transfer buffer. Following transfer, membranes were blocked for 2 h in 5% nonfat dry milk at room temperature. Following blocking, protein blots were detected with rabbit anti-MMP-2 (cat. no. 13132), anti-MMP-9 (cat. no. 3852), and anti-β-actin (cat. no. 3700S) antibodies (dilution, 1:1,000; Cell Signaling Technology, Inc.) at 4°C overnight followed by incubation with the appropriate HRP-conjugated secondary antibody (cat. no. 14C10; Cell Signaling Technology, Inc.) for 1 h at room temperature. Detected bands were visualized using enhanced chemiluminescence and images were captured using a ChemiDoc system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Western blots were repeated three times.
Statistical analysis
All data were analyzed using SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA). Quantitative data were presented as mean ± standard deviation. Rank-sum testing was performed on the neurological deficit score results. One-way analysis of variance were used to assess statistical differences between multiple groups. The homogeneity of variance was analyzed using the least significant difference method and missing variance using the Games-Howell method. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of EA at Baihui (DU20) and Shenting (DU24) acupoints on neurological deficits in I/R injured rats
To investigate whether EA at the Baihui (DU20) and Shenting (DU24) acupoints attenuate ischemic brain injury, neurological scores were determined in rats at different time points following stroke. Supporting the hypothesis of the current study, rats in the sham group exhibited no manifestations of neurological deficits (Fig. 1), whereas all rats in the MCAO and MCAO + EA groups demonstrated clear symptoms of cerebral injury compared with the sham rats. However, EA significantly improved neurological deficit scores compared with the MCAO group (P<0.05; Fig. 1). These results suggested that EA at the Baihui (DU20) and Shenting (DU24) acupoints provided neuroprotective effects and promoted functional recovery in cerebral I/R injured rats.
Figure 1.
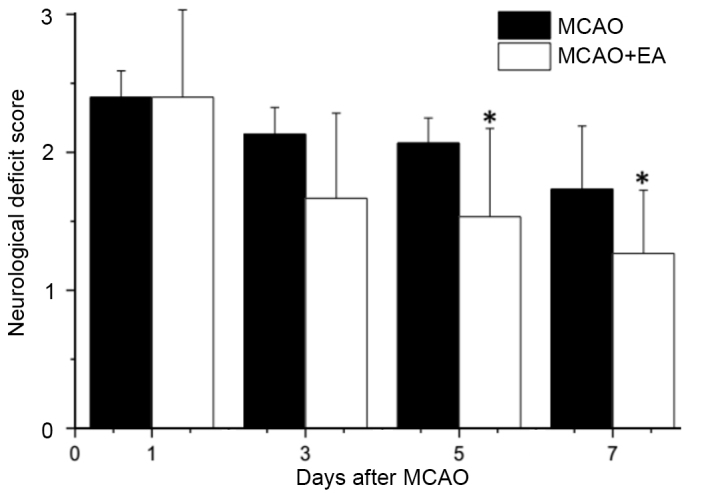
Effects of EA at the Baihui (DU20) and Shenting (DU24) acupoints on neurological deficits in cerebral ischemia reperfusion injury rats. Rats were assessed and the neurological deficit scores were determined. Data are presented as the mean ± standard deviation from 15 individual rats in the MCAO + EA group or MCAO group. *P<0.05 vs. the MCAO group. EA, electroacupuncture; MCAO, middle cerebral artery occlusion.
EA ameliorates cognitive impairment in cerebral I/R injured rats
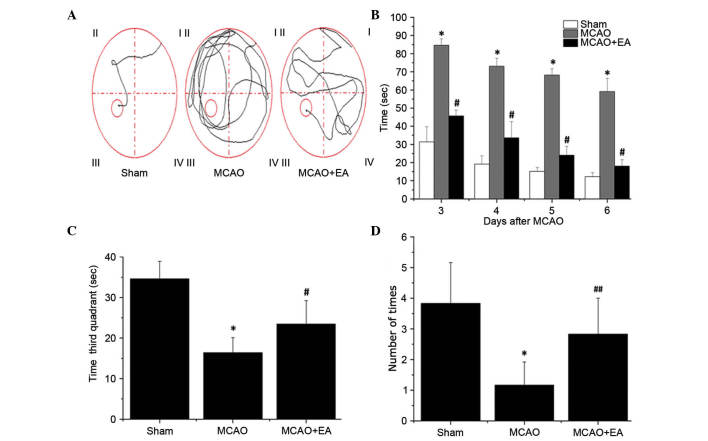
All rats were assessed in a Morris water maze on days 3–7 following MCAO surgery. As shown in Fig. 2, the latency to reach the hidden platform in the maze was significantly increased in the MCAO group compared with sham rats (P<0.01), whereas the time taken in the third quadrant for the rats to find the platform (within 90 sec) on day 7 and the number of times that rats crossed the platform's location was significantly decreased compared with rats in the sham group (P<0.01; Fig. 2). This indicates that cerebral I/R injury resulted in cognitive impairment. However, EA significantly decreased the latency and time taken in the third quadrant on day 7 (P<0.01), and increased the number of platform crossings in the Morris water maze (P<0.05) compared with the MCAO group (Fig. 2). These findings suggested that EA at the Baihui (DU20) and Shenting (DU24) acupoints may ameliorate cognitive impairment in cerebral I/R injured rats.
Figure 2.
Effects of EA on cognitive impairment in cerebral I/R injury rats. The learning and memory funtion of rats was determined using a Morris water maze test on days 3–7 following I/R injury. (A) Tracing images from the Morris water maze test. (B) Time taken for the rats to find the platform (within 90 sec). (C) Time taken in the third quadrant for the rats to find the platform (within 90 sec). (D) The number of times the rats passed through the area in which the platform was located on day 7 following I/R injury. Data are presented as the mean ± standard deviation from 15 individual rats in each group. *P<0.01 vs. sham group; #P<0.01 vs. MCAO group; ##P<0.05 vs. the MCAO group. EA, electroacupuncture; I/R, ischemia/reperfusion; MCAO, middle cerebral artery occlusion.
Effect of EA the Baihui (DU20) and Shenting (DU24) acupoints on infarct volumes in cerebral I/R injured rats
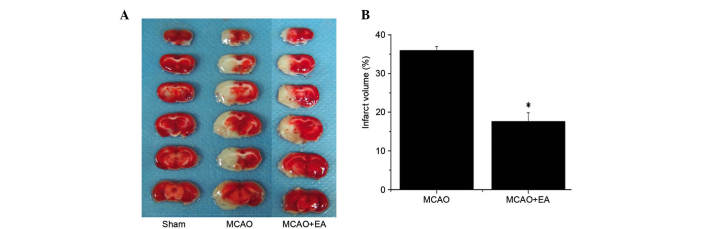
To evaluate the effect of EA on pathological damage, the effect of EA on cerebral infarction was determined. Infarct volume was measured using TTC staining. As demonstrated in Fig. 3, normal tissue stained deep red, whereas the infarct area was stained pale white indicating a lack of stain uptake. EA treatment significantly reduced cerebral infarct volumes in cerebral I/R injured rats compared with the MCAO group (P<0.01; Fig. 3). This result indicates that EA at the Baihui (DU20) and Shenting (DU24) acupoints may have therapeutic efficacy against cerebral I/R injury by reducing secondary infarct expansion.
Figure 3.
Effect of EA on cerebral infarction in cerebral ischemia/reperfusion injury rats. (A) Triphenyl tetrazolium chloride staining was used to measure cerebral infarct volume in the brain of sham, MCAO and MCAO + EA animals. Images were captured using a high-resolution digital camera. (B) Infarct volume was quantified using Motic Med 6.0 as a percentage of the total brain volume. Data are presented as the mean ± standard deviation from 6 individual rats in each group. *P<0.01 vs. the MCAO group. EA, electroacupuncture; MCAO, middle cerebral artery occlusion.
EA attenuates ultrastructural characteristics of neuronal impairment in cerebral I/R injured rats
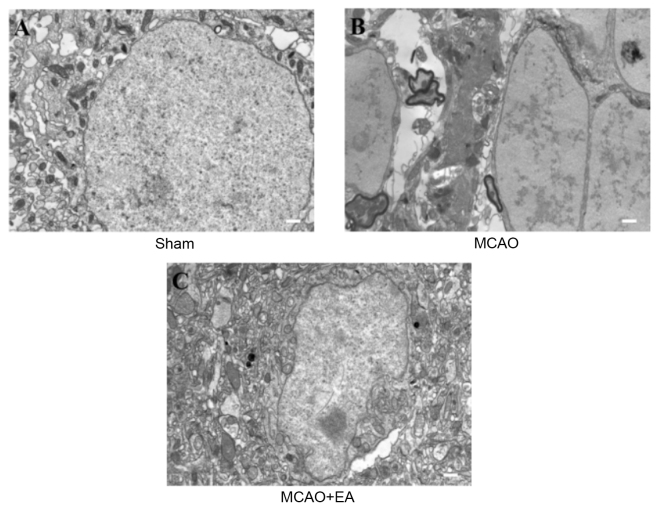
To evaluate whether EA at the Baihui (DU20) and Shenting (DU24) acupoints attenuates ultrastructural changes that are characteristic of neuronal impairment, TEM was performed as demonstrated in Fig. 4. In sham rats, the neuronal cells were normal in appearance, with intact cell membranes, normal nuclei, and uniform euchromatin distribution, and nucleoli were observed in the nuclei. Abundant mitochondria and rough endoplasmic reticulum were observed and parts of the endoplasmic reticulum expanded the intracellular pool shape (Fig. 4A). In the MCAO group, neuronal vacuolar changes, rupture of cell membranes and oncotic nuclei were observed. Extensive chromatin and organelle loss, and mitochondrial swelling, mitochondrial cristae disappearance and rough endoplasmic reticulum (Fig. 4B) were also present. In the EA group, cell and nuclear membrane integrity was improved compared with the MCAO group. The number of mitochondrial vacuoles was markedly reduced and there was evidence of rough endoplasmic reticulum expansion and degranulation (Fig. 4C). These results indicate that EA at the Baihui (DU20) and Shenting (DU24) acupoints may attenuate ultrastructural alterations that contribute to neuronal impairment.
Figure 4.
Effects of EA on the ultrastructural characteristics of neuronal impairment in cerebral ischemia/reperfusion injury rats. Following completion of the experiment, cerebral tissues from each group (n=4) were processed using transmission electron microscopy. (A) Normal structure in the sham group, rich in organelles. (B) Organelles were damaged by MCAO. (C) The damaging effect was reduced by EA. Scale bar =1 µm MCAO, middle cerebral artery occlusion; EA, electroacupuncture.
EA improves vascular ultrastructure in cerebral I/R injured rats
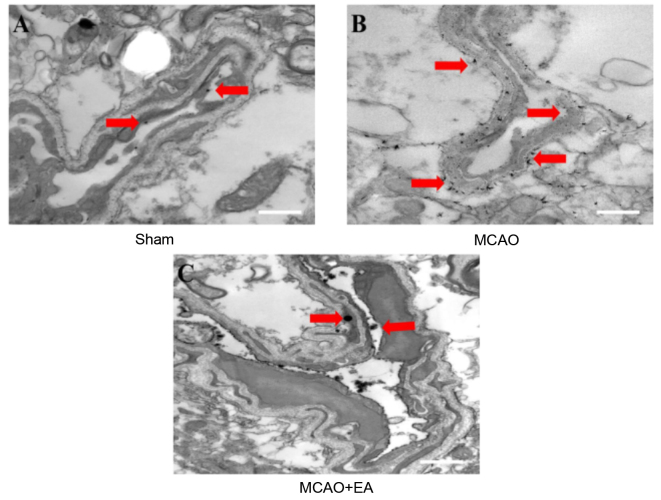
LNT was utilized to evaluate whether EA at the Baihui (DU20) and Shenting (DU24) acupoints improves vascular ultrastructure in cerebral I/R injured rats, as demonstrated in Fig. 5. Lanthanum particles were observed within the lumen, in the sham-operated group (Fig. 5A). In the MCAO group, however, lanthanum particles were dispersed inside and outside the vasculature and clearly localized to the tight junctions (Fig. 5B). Compared with the MCAO group, the number of lanthanum particles localized to the lumen was reduced in the EA group (Fig. 5C). This result suggests that EA at the Baihui (DU20) and Shenting (DU24) acupoints improves vascular ultrastructure in the brain tissues of cerebral I/R injured rats.
Figure 5.
Effects of EA on ultrastructural characteristics of vascular disruption in cerebral ischemia/reperfusion injury rats. Following the experiment, cerebral tissues from each group (n=4) were processed using transmission electron microscopy. Distribution of lanthanum ions are demonstrated in the representative images (red arrows). (A) Lanthanum nitrate remains within the blood vessels in the sham group whereas (B) the ions were scattered throughout the brain tissue in the MCAO group. (C) Lanthanum nitrate within the blood vessels in the MCAO + EA group. Scale bar =3 µm. EA, electroacupuncture; MCAO, middle cerebral artery occlusion.
EA inhibits the expression of MMP-2 and MMP-9 in the hippocampus following I/R injury
MMP-2 and MMP-9 are associated with cerebrovascular disruption and neuronal damage, therefore, the effects of EA on the protein expression levels of MMP-2 and MMP-9 in the hippocampus of ischemic cerebral tissues were determined by western blotting. The protein expression levels of MMP-2 (Fig. 6) and MMP-9 (Fig. 7) in the hippocampus were significantly increased in the MCAO group compared with the sham rats (P<0.01). Hippocampal expression levels of MMP-2 and MMP-9 were significantly decreased by EA treatment compared with the MCAO group (P<0.01; Figs. 6 and 7), demonstrating that EA significantly inhibited MMP-2 and MMP-9 expression following cerebral I/R injury.
Figure 6.
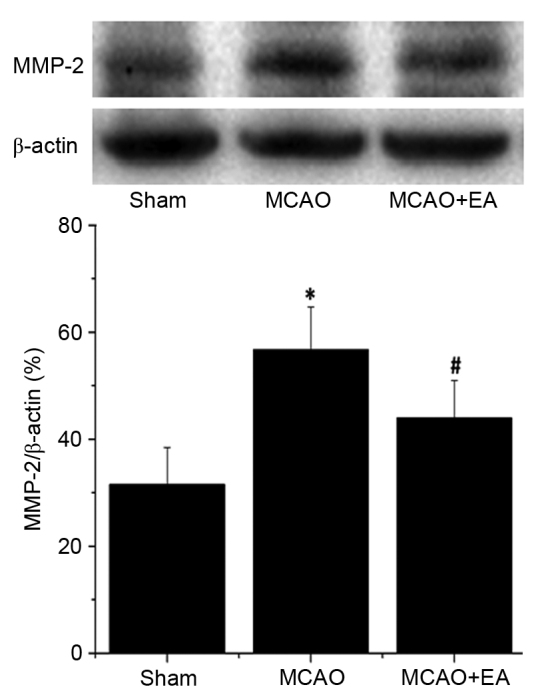
Effect of EA on the expression of MMP-2 in cerebral ischemia/reperfusion injury rats. Protein expression level of MMP-2 were determined by western blot analysis. β-actin served as an the internal control in the analyses. Data are presented as the mean ± standard deviation of 6 individual rats per group. *P<0.01 vs. the sham group, #P<0.01 vs. MCAO group. EA, electroacupuncture; MMP-2, matrix metalloproteinase-2; MCAO, middle cerebral artery occlusion.
Figure 7.
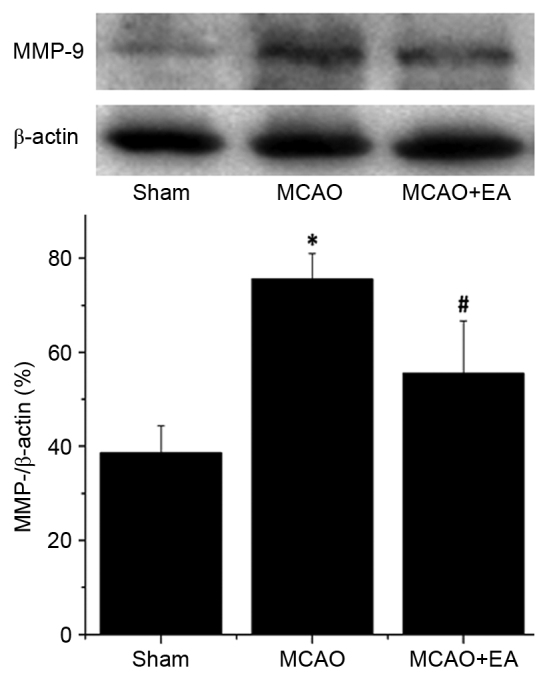
Effect of electroacupuncture on the expression of MMP-9 in cerebral ischemia/reperfusion injury rats. Protein expression level of MMP-9 was determined by western blot analysis. β-actin served as an internal control. Data are presented as the mean ± standard deviationf 6 individual rats per group. *P<0.01 vs. the sham group, #P<0.01 vs. the MCAO group. EA, electroacupuncture; MMP-2, matrix metalloproteinase-2; MCAO, middle cerebral artery occlusion.
Discussion
Cognitive impairment following stroke is common and negatively affects the life of patients (30). Acupuncture is one of most frequently used treatment in TCM as it is simple and has limited side effects, and has been used extensively in the treatment of post-stroke onset of cognitive disorders (9–11). Baihui (DU20) and Shenting (DU24) are important acupoints of the Du meridian, which is often targeted to treat cognitive disorders in China. Our previous research demonstrated that EA at the Baihui (DU20) and Shenting (DU24) acupoints improves cognitive impairment following ischemic stroke (15,16).
In the present study, a commonly used MCAO animal model was used to accurately simulate pathological processes in focal cerebral ischemia (31). When performed successfully, development of cognitive dysfunction is characteristic of this experimental model (32). After 7 days of EA at the Baihui (DU20) and Shenting (DU24) acupoints, the neurological deficit scores of the EA group were significantly decreased compared with the MCAO model group, indicating that EA exerts a neuroprotective effect.
To further investigate the neuroprotective effects of EA, the Morris water maze test was used to determine learning and memory abilities, and deficits in cerebral I/R injured rats. The test is widely used in the study of animal cognition behavior, and was designed to detect spatial learning and memory ability in animal behavioral experiments (33–36). The results of the directional navigation and spatial probe tests demonstrated that rat behavior in the EA group was improved compared with the MCAO group. Thus, this evidence indicated that EA at the Baihui (DU20) and Shenting (DU24) acupoints improve the learning and memory ability of MCAO rats, a result that was consistent with previous research (15).
However, learning and memory disorders are complex. Acupuncture has been previously demonstrated to improve learning and memory dysfunction, and current research suggests that the effect of acupuncture treatment following cerebral ischemia may be mediated by promotion of cholinergic neural transmission, facilitating dopaminergic synaptic transmission and enhancing neurotrophin signaling (37,38). However, the specific mechanisms remain to be elucidated. The present research considers that behavioral change occurs as a result of the pathological effects that occur following ischemic insult, and used TTC staining to measure the cerebral infarction volume of rats to measure this pathological effect. The findings of the current study demonstrated that cerebral infarction volume was decreased in the MCAO group, indicating that EA at the Baihui (DU20) and Shenting (DU24) acupoints affects pathological progression in brain tissue following cerebral ischemia. The macrostructural changes result from smaller tissue alterations that occur at the microstructural level. Based on results from LNT experiments, increased lanthanum particles were visible inside and outside of the blood vessels in MCAO rats, which illustrates that vascular permeability has been modified and the BBB has been compromised. However, cerebral ultrastructural morphology was improved in animals treated with EA compared with MCAO group. These results indicate that EA at the Baihui (DU20) and Shenting (DU24) acupoints may improve the outcome of pathological progression following cerebral ischemia.
The BBB provides a protective barrier between the periphery and the central nervous system (CNS), and as such, is important for the protection of CNS tissue (17). Following cerebral ischemia, vascular permeability is altered and the BBB damaged, which results in brain edema, inflammation and harmful pathological changes within affected brain areas (39). Extensive CNS tissue damage, neuronal necrosis and apoptosis subsequently occur over time (22,40). The brain is comprised of neurons and glial cells (41), and the structural and functional integrity of neurons is considered the morphological basis of learning and memory (24), thus, neuronal damage leads to learning and memory dysfunction. The TEM results of the current study demonstrate that the morphology and extent of neuronal damage in the EA group was markedly improved compared with the MCAO group. These findings indicate that EA at the Baihui (DU20) and Shenting (DU24) acupoints may reduce the effects on learning and memory following cerebral ischemia via improving neuronal ultrastructure and morphology.
MMP-2 and MMP-9 are important collagenases in the MMP family that degrade extracellular matrix, are involved in the breakdown of the BBB, promote expansion of cerebral infarct volume and other pathological processes that follow cerebral I/R injury (42). In the present study, MMP-2 and MMP-9 expression levels were consistent with TTC and LNT results, which is indicative of an association between the integrity of the BBB and cerebral ischemia, and cerebral infarction volume and MMP-2 and MMP-9 expression. In addition, evidence from previous studies suggests that MMP-2 and MMP-9 also involved in axon growth and regeneration, myelin formation during neurogenesis, and neuronal apoptosis (43,44). MMP-2 may participate in acute neuron injury and delayed repair mechanisms, and MMP-9 is associated with increased lesion volume expansion and a decline in neurological function (45). In the current study, the changes to protein expression levels of MMP-2 and MMP-9 in the hippocampus were consistent with reduced behavioral performance in rats, indicating that EA at the Baihui (DU20) and Shenting (DU24) acupoints may improve learning and memory function via inhibiting protein expression of MMP-2 and MMP-9.
The present study selected only a single time point to intervene, and only one test to assess learning and memory function. Certain previous research suggest that the water maze is not suitable for study of experimental stroke as the result of test may be closely associated with the learning and memory function of rats (46). TEM was performed to detect breakdown of the BBB, however, the presence and structure of tight junctions and other associated proteins was not assessed. Therefore, the interpretation of the data is restricted by these limitations in the study design. However, the overall pattern in the data remains clear and limitations will be addressed and improved in subsequent studies.
In conclusion, the results of the present study indicate that EA at the Baihui (DU20) and Shenting (DU24) acupoints may improve the learning and memory ability of rats following cerebral ischemia. The mechanism of the therapeutic effects of EA may be associated with improved brain ultrastructure and morphological integrity, and reduced expression of MMP-2 and MMP-9 proteins in the hippocampus, thus reducing brain tissue damage and improving function following cerebral I/R.
Acknowledgments
The present study was sponsored by the International S&T Cooperation Program of China (grant no. 2011DFG33240) and the Mechanism of Acupuncture to Improve Cognitive Function (grant no. X2012004; collaborative). The authors would like to thank Clarity Manuscript Consultants for their help in editing this manuscript.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 3.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Kim BJ, Bae HJ, Lee J, Lee J, Han MK, O KY, Park SH, Kang Y, Yu KH, Lee BC. Impact of post-stroke cognitive impairment with no dementia on health-related quality of life. J Stroke. 2013;15:49–56. doi: 10.5853/jos.2013.15.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, et al. Forecasting the future of stroke in the United States: A policy statement from the American heart association and American stroke association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 6.Pendlebury ST, Rothwell PM. Prevalence, incidence and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 7.Kim SK, Bae H. Acupuncture and immune modulation. Auton Neurosci. 2010;157:38–41. doi: 10.1016/j.autneu.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Jittiwat J, Wattanathorn J. Ginger pharmacopuncture improves cognitive impairment and oxidative stress following cerebral ischemia. J Acupunct Meridian Stud. 2012;5:295–300. doi: 10.1016/j.jams.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhang GC, Fu WB, Xu NG, Liu JH, Zhu XP, Liang ZH, Huang YF, Chen YF. Meta analysis of the curative effect of acupuncture on post-stroke depression. J Tradit Chin Med. 2012;32:6–11. doi: 10.1016/S0254-6272(12)60024-7. [DOI] [PubMed] [Google Scholar]
- 10.Cao H, Wang Y, Chang D, Zhou L, Liu J. Acupuncture for vascular mild cognitive impairment: A systematic review of randomised controlled trials. Acupunct Med. 2013;31:368–374. doi: 10.1136/acupmed-2013-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Y, Bai L, Ren Y, Chen S, Wang H, Zhang W, Tian J. FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients. Magn Reson Imaging. 2012;30:672–682. doi: 10.1016/j.mri.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Zhao L, Yang S, Chen Z, Li Y, Peng X, Yang Y, Zhu M. Clinical observation on effect of scalp electroacupuncture for mild cognitive impairment. J Tradit Chin Med. 2013;33:46–50. doi: 10.1016/S0254-6272(13)60099-0. [DOI] [PubMed] [Google Scholar]
- 13.Lin YW, Hsieh CL. Electroacupuncture at baihui acupoint (GV20) reverses behavior deficit and long-term potentiation through N-methyl-d-aspartate and transient receptor potential vanilloid subtype 1 receptors in middle cerebral artery occlusion rats. J Integr Neurosci. 2010;9:269–282. doi: 10.1142/S0219635210002433. [DOI] [PubMed] [Google Scholar]
- 14.Wang WW, Xie CL, Lu L, Zheng GQ. A systematic review and meta-analysis of baihui (GV20)-based scalp acupuncture in experimental ischemic stroke. Sci Rep. 2014;4:3981. doi: 10.1038/srep03981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7:1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Ye H, Huang J, Tao J, Jiang C, Lin Z, Zheng G, Chen L. The synergistic effect of acupuncture and computer-based cognitive training on post-stroke cognitive dysfunction: A study protocol for a randomized controlled trial of 2×2 factorial design. BMC Complement Altern Med. 2014;14:290. doi: 10.1186/1472-6882-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin R, Song Z, Yu S, Piazza A, Nanda A, Penninger JM, Granger DN, Li G. Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke. 2011;42:2033–2044. doi: 10.1161/STROKEAHA.110.601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balami JS, Chen RL, Grunwald IQ, Buchan AM. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2011;10:357–371. doi: 10.1016/S1474-4422(10)70313-6. [DOI] [PubMed] [Google Scholar]
- 19.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Krueger M, Härtig W, Reichenbach A, Bechmann I, Michalski D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One. 2013;8:e56419. doi: 10.1371/journal.pone.0056419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao Dang Q, Lapergue B, Tran-Dinh A, Diallo D, Moreno JA, Mazighi M, Romero IA, Weksler B, Michel JB, Amarenco P, Meilhac O. High-density lipoproteins limit neutrophil-induced damage to the blood-brain barrier in vitro. J Cereb Blood Flow Metab. 2013;33:575–582. doi: 10.1038/jcbfm.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spatz M. Past and recent BBB studies with particular emphasis on changes in ischemic brain edema: Dedicated to the memory of Dr. Igor Klatzo. Acta Neurochir Suppl. 2010;106:21–27. doi: 10.1007/978-3-211-98811-4_3. [DOI] [PubMed] [Google Scholar]
- 23.Yang D, Li SY, Yeung CM, Chang RC, So KF, Wong D, Lo AC. Lycium barbarum extracts protect the brain from blood-brain barrier disruption and cerebral edema in experimental stroke. PLoS One. 2012;7:e33596. doi: 10.1371/journal.pone.0033596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, El-Zammar Z, Alam S, Hallenbeck JM, Kidwell CS, Warach S. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010;41:e123–e128. doi: 10.1161/STROKEAHA.109.570515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: Critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38:376–385. doi: 10.1016/j.nbd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Hartman R, Ayer R, Marcantonio S, Kamper J, Tang J, Zhang JH. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J Neurochem. 2009;111:726–736. doi: 10.1111/j.1471-4159.2009.06362.x. [DOI] [PubMed] [Google Scholar]
- 28.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 29.Tao J, Chen B, Gao Y, Yang S, Huang J, Jiang X, Wu Y, Peng J, Hong Z, Chen L. Electroacupuncture enhances hippocampal NSCs proliferation in cerebral ischemia-reperfusion injured rats via activation of notch signaling pathway. Int J Neurosci. 2014;124:204–212. doi: 10.3109/00207454.2013.840781. [DOI] [PubMed] [Google Scholar]
- 30.Shim H. Vascular cognitive impairment and post-stroke cognitive deficits. Curr Neurol Neurosci Rep. 2014;14:418. doi: 10.1007/s11910-013-0418-4. [DOI] [PubMed] [Google Scholar]
- 31.Fu YK, Chang CJ, Chen KY, Hwang LC, Wu KH, Chang KW, Jan ML, Chen CC, Chang CH. Imaging of regional metabolic activity by (18) F-FDG/PET in rats with transient cerebral ischemia. Appl Radiat Isot. 2009;67:1743–1747. doi: 10.1016/j.apradiso.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Boyko M, Kutz R, Gruenbaum BF, Cohen H, Kozlovsky N, Gruenbaum SE, Shapira Y, Zlotnik A. The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion. Cogn Affect Behav Neurosci. 2013;13:847–859. doi: 10.3758/s13415-013-0177-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang ZZ, Zhang Y, Liu YQ, Zhao N, Zhang YZ, Yuan L, An L, Li J, Wang XY, Qin JJ, et al. RNA interference-mediated phosphodiesterase 4D splice variants knock-down in the prefrontal cortex produces antidepressant-like and cognition-enhancing effects. Br J Pharmacol. 2013;168:1001–1014. doi: 10.1111/j.1476-5381.2012.02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Souza Silva MA, Lenz B, Rotter A, Biermann T, Peters O, Ramirez A, Jessen F, Maier W, Hüll M, Schröder J, et al. Neurokinin3 receptor as a target to predict and improve learning and memory in the aged organism. Proc Natl Acad Sci USA. 2013;110:15097–15102. doi: 10.1073/pnas.1306884110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langdon KD, Granter-Button S, Harley CW, Moody-Corbett F, Peeling J, Corbett D. Cognitive rehabilitation reduces cognitive impairment and normalizes hippocampal CA1 architecture in a rat model of vascular dementia. J Cereb Blood Flow Metab. 2013;33:872–879. doi: 10.1038/jcbfm.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Intino G, Paradisi M, Fernandez M, Giuliani A, Aloe L, Giardino L, Calzà L. Cognitive deficit associated with cholinergic and nerve growth factor down-regulation in experimental allergic encephalomyelitis in rats. Proc Natl Acad Sci USA. 2005;102:3070–3075. doi: 10.1073/pnas.0500073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung MC, Yip KK, Ho YS, Siu FK, Li WC, Garner B. Mechanisms underlying the effect of acupuncture on cognitive improvement: A systematic review of animal studies. J Neuroimmune Pharmacol. 2014;9:492–507. doi: 10.1007/s11481-014-9550-4. [DOI] [PubMed] [Google Scholar]
- 38.Greggio S, de Paula S, de Oliveira IM, Trindade C, Rosa RM, Henriques JA, DaCosta JC. NAP prevents acute cerebral oxidative stress and protects against long-term brain injury and cognitive impairment in a model of neonatal hypoxia-ischemia. Neurobiol Dis. 2011;44:152–159. doi: 10.1016/j.nbd.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Pop V, Sorensen DW, Kamper JE, Ajao DO, Murphy MP, Head E, Hartman RE, Badaut J. Early brain injury alters the blood-brain barrier phenotype in parallel with β-amyloid and cognitive changes in adulthood. J Cereb Blood Flow Metab. 2013;33:205–214. doi: 10.1038/jcbfm.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pillai DR, Shanbhag NC, Dittmar MS, Bogdahn U, Schlachetzki F. Neurovascular protection by targeting early blood-brain barrier disruption with neurotrophic factors after ischemia-reperfusion in rats. J Cereb Blood Flow Metab. 2013;33:557–566. doi: 10.1038/jcbfm.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 42.Leonardo CC, Eakin AK, Ajmo JM, Collier LA, Pennypacker KR, Strongin AY, Gottschall PE. Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia-ischemia in the neonatal rat. J Neuroinflammation. 2008;5:34. doi: 10.1186/1742-2094-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manso H, Krug T, Sobral J, Albergaria I, Gaspar G, Ferro JM, Oliveira SA, Vicente AM. Variants of the matrix metallopro-teinase-2 but not the matrix metalloproteinase-9 genes significantly influence functional outcome after stroke. BMC Med Genet. 2010;11:40. doi: 10.1186/1471-2350-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei W, Zhang W, Huang Y, Li Y, Zhu G, Chen F, Li J. The therapeutic effect of (DL)-3-n-butylphthalide in rats with chronic cerebral hypoperfusion through downregulation of amyloid precursor protein and matrix metalloproteinase-2. J Int Med Res. 2012;40:967–975. doi: 10.1177/147323001204000315. [DOI] [PubMed] [Google Scholar]
- 45.Batra A, Latour LL, Ruetzler CA, Hallenbeck JM, Spatz M, Warach S, Henning EC. Increased plasma and tissue MMP levels are associated with BCSFB and BBB disruption evident on post-contrast flair after experimental stroke. J Cereb Blood Flow Metab. 2010;30:1188–1199. doi: 10.1038/jcbfm.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu HS, Shen H, Harvey BK, Castillo P, Lu H, Yang Y, Wang Y. Post-treatment with amphetamine enhances reinnervation of the ipsilateral side cortex in stroke rats. Neuroimage. 2011;56:280–289. doi: 10.1016/j.neuroimage.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]