Abstract
Background
Hepatitis B virus (HBV) is a hepatotropic virus that can infect extrahepatic tissue. Whether hematopoietic stem cells (HSCs) can be infected by HBV and serve as a potential virus reservoir is still unknown. In this study, the susceptibility of CD34+ HSCs to HBV was investigated.
Material/Methods
Cord blood–derived CD34+ HSCs were exposed to HBV in vitro, and immunocytochemistry, transmission electron microscopy, and RT-PCR were used to identify viral-related proteins and specific viral genomic sequences. Then, CD34+ HSCs were challenged by different titers of HBV, and intracellular and supernatant HBV DNA, and hepatitis B surface antigen (HBsAg) levels, were examined. In addition, CD34+ peripheral blood stem cells (PBSCs) from chronic HBV carriers were isolated and cultured, and HBV DNA levels were measured.
Results
HBV-infected CD34+ cells showed positive signals for HBsAg by DAB staining and TRITC staining, and HBV particles were identified. RT-PCR results showed that the 403 bp PCR products corresponding to the amplified hepatitis B S gene fragment were observed in CD34+ HSCs infected by HBV. In addition, supernatant and intracellular HBV DNA increased with the proliferation of CD34+ HSCs. Similar results were obtained from intracellular HBsAg quantification tests. In addition, HBV DNA levels both in cells and in supernatants of CD34+ PBSCs increased proportionally, and the increments of HBV DNA in the supernatants paralleled those found in cells.
Conclusions
HBV can replicate in CD34+ HSCs in cord blood or peripheral blood of chronic HBV carriers.
MeSH Keywords: Hematopoietic Stem Cells, Hepatitis B virus, Virus Replication
Background
Chronic hepatitis B virus (HBV) infection has afflicted more than 240 million people worldwide [1]. Chronic HBV patients are at higher risk for developing serious liver diseases, such as liver fibrosis, cirrhosis, and hepatocellular carcinoma, which have high morbidity and mortality rates, and which are thought to be due to long-lasting interactions between HBV and the host immune system [2,3]. The exact immune mechanism by which HBV escapes the host immune clearance process, and why HBV-infected hosts fail to eliminate HBV, are still unanswered questions. A number of studies [4–6] have found that HBV can persist in the host and avoid antiviral immune response by a number of strategies, including: exhausting T cells, cytokine imbalance, and impairing dendritic cell maturation and function. Although the liver is the main site of HBV replication, research indicates that the virus also replicates in extrahepatic tissues [7–10], especially in the lymphatic system and in bone marrow cells. As an extrahepatic virus reservoir, peripheral blood mononuclear cells (PBMCs) can be infected by HBV, a finding confirmed in a number of studies [11–13]. However, it is not clear if hematopoietic stem cells (HSCs), which are regarded as a source of immune cells, can be infected by HBV. Some studies [14] have suggested that HBV can infect and inhibit bone marrow cells, and hepatitis B surface antigen (HBsAg) can be cleared after bone marrow transplantation [15]. It would be of interest to know whether CD34+ HSCs, which are associated with immune cell production and differentiation, are susceptible to HBV infection, as this may help explain the mechanism of HBV persistent infection, as well as mother-to-child transmission. Thus, our study aimed to explore the susceptibility of human CD34+ HSCs to HBV infection and the possible clinical significance. In the present study, we first looked to see if CD34+ HSCs derived from umbilical cord blood could be infected by HBV in vitro, and then we explored the pattern of infection and replication of HBV in CD34+ peripheral blood stem cells (PBSCs) of chronic HBV carriers.
Material and Methods
Cord blood
Umbilical cord blood (UCB) was obtained after scheduled cesarean section procedures. UCB donors were healthy volunteers with no history of HBV exposure (negative markers for HBsAg and hepatitis B core antibody) and were anti-HCV negative. The study was approved by the ethical committee of the First Affiliated Hospital of Harbin Medical University. Written informed consent was obtained from all volunteers prior to participation.
Isolation HBV-positive serum samples from HBV-infected patients
HBV-positive serum samples were isolated from HBsAg and HBV DNA-positive plasma of four patients with chronic HBV infection. The general information (age, sex) and related clinical indicators of the patients are summarized in Table 1. As a negative control, HBV DNA-negative samples were isolated from healthy volunteers who were HBsAg and anti-HCV negative. Sera were filtered through a 0.22 μm pore size filter unit and stored at −20°C.
Table 1.
The data of HBV-positive serum donors.
| Patients | Age/sex | Diagnosis | Serological markers | Serum HBV DNA (IU/mL) | HBV genotypes | ||||
|---|---|---|---|---|---|---|---|---|---|
| HBsAg | Anti-HBs | Anti-HBc | HBeAg | Anti-HBe | |||||
| 1 | 37/M | CHB | + | − | + | + | − | 4660000* | C |
| 2 | 33/M | CHB | + | − | + | + | − | 14900000* | C |
| 3 | 23/M | Carrier | + | − | + | + | − | 78800000 | C |
| 4 | 35/M | CHB | + | − | + | + | − | 15000000 | B |
Serum HBV DNA levels were measured by 100 times dilution.
Cell lines
HepG2.2.15 cells were cultured with high-glucose DMEM (Gibco, USA), supplemented with 10% FBS and 380 ng/mL G418 in an incubator with 95% humidity and 5% CO2 at 37°C. HepG2.2.15 cells were used as positive controls.
CD34+ HSCs isolation and culture
UCB mononuclear cells were separated by density gradient centrifugation with lymphoprep (Boster Bio, China), and then CD34+ HSCs were purified from the mononuclear cell fraction by using a human CD34-positive selection kit (Stem Cell Technology, Vancouver, Canada) according to the manufacturer’s protocol. For expansion of CD34+ cells, hematopoietic growth factors were added as follows: IL-3 10 ng/mL, SCF 100 ng/mL, TPO 100 ng/mL, Flt-3 ligand 100 ng/mL (PeproTech, USA). About 4×104 cells were seeded in each plate well. The cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and harvested on Day 0, 6, and 12. Cell pellets and supernatants were stored at −80°C for further study.
Exposure of CD34+ HSCs to HBV
Primary CD34+ HSCs from UCB of healthy volunteers were divided into two groups: the HBV-infected group and the control group. The CD34+ cells of the HBV-infected group were inoculated with HBV positive sera (1.0×106 IU/mL) for 24 hours at 37°C and then washed. The cells were cultured in 24-well plates for 12 days and collected for HBV DNA detection at various time points from Day 0 to Day 12. In the subsequent experiments, HBV-infected CD34+ cells were collected on Day 12 after being co-cultured with HBV positive sera, and intracellular HBV was examined by immunohistochemistry and transmission electron microscopy. In addition, the cells were challenged by different titers of HBV. They were divided into three subgroups and inoculated into 24-well plates, and then the cells were cultured for 12 days. Then we added 50 μL of HBV-positive sera with different viral titers to cells in three groups: Group A titer: 1.0×104 IU/mL; Group B titer: 1.0×106 IU/mL; Group C titer, 1.0×108 IU/mL. The levels of HBV DNA and HBsAg in the cultures were measured.
Immunohistochemistry and immunofluorescence
After harvesting on Day 12, CD34+ cells were washed twice by PBS and dried onto glass cover slips. The HepG2.2.1.5 cells were used as a positive control. Both CD34+ cells and control cells were fixed with 4% paraformaldehyde for 30 minutes and incubated with 0.1% Triton X-100 in PBS for four minutes at room temperature, then rinsed in PBS three times. We added 3% H2O2 and incubated for 30 minutes, rinsed in PBS three times, and then the reaction was blocked with 5% goat serum in PBS at room temperature for 30 minutes. The slides were then incubated with mouse anti-HBsAg (1:200 dilution) and rabbit anti-CD34 (1:100 dilution) (Beijing Biosynthesis Biotechnology, China) at 4°C overnight. Then they were incubated with horseradish peroxidase-labeled goat anti-mouse IgG and alkaline phosphatase-conjugated goat anti-rabbit IgG (both 1:200 dilution) (Beijing Biosynthesis Biotechnology, China) for 30 minutes at room temperature, respectively. Finally, sections were stained with DAB and a BICP/NBT alkaline phosphatase color development kit (Beijing Zhongshan Biotechnology, China) according to the manufacturer’s protocol.
For immunofluorescent double-staining, the sections were incubated with rabbit anti-CD34 and mouse anti-HBsAg overnight at 4°C. Sections were then incubated with TRITC-conjugated goat anti-mouse IgG and FITC-conjugated goat anti-rabbit IgG (both 1:100 dilution) (Beijing Zhongshan Biotechnology, China) at room temperature for 30 minutes. After extensive washing, the sections were mounted and visualized using fluorescent microscope (OLYMPUS FV300, Japan).
Transmission electron microscopy
HBV-infected CD34+ HSCs were fixed in 2.5% glutaraldehyde for three hours, followed by 1% osmium tetroxide in 100 mM phosphate buffer for an additional 30 minutes. After dehydration, cells were embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and were visualized under transmission electron microscope (Hitachi, Japan). HepG2.2.15 cells were used as positive controls and healthy CD34+ HSCs as negative controls.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted with Trizol reagent and DNase I treated by the RNeasy mini-kit (QIAGEN, GER); mRNA was reverse transcribed to cDNA by using the Long-Range 2Step RT-PCR Kit (QIAGEN, GER) and readied for PCR. For the measurement of HB S gene mRNA, the specific primers were used according to Huang et al. [16]. GAPDH was used as an internal control gene. This experiment was repeated at least three times under the same conditions.
Quantitative detection of HBV DNA by using real-time PCR
Intracellular and supernatant HBV DNA levels were measured by using COBAS TaqMan HBV test kits (Roche, Switzerland) according to the manufacturer’s instructions.
Analysis of HBsAg expression
CD34+ cells were collected on Day 0, 6, and 12 after being co-cultured with HBV. After the cells were lysed, the levels of HBsAg were detected by chemiluminescent microparticle immunoassay (Abbott, USA) according to the manufacturer’s instructions.
CD34+ PBSCs derived from HBV chronic carriers
To determine whether HBV can replicate in CD34+ HSCs in vivo, five patients chronically infected with HBV and treated at the Infectious Department of the First Affiliated Hospital of Harbin Medical University were consecutively recruited from September 2012 to August 2013. The inclusion criteria included: HBsAg was positive for six months or longer, and anti-HAV, HCV, HDV and HEV were negative. Serum HBV DNA levels of the five patients were detected using COBAS TaqMan HBV test kit (Roche, Switzerland); the detection limit of HBV DNA was 20 IU/mL. Of the five patients, serum HBV DNA levels of two patients were above the detection limit (above-detection limit group) and the other three were below the limit (below-detection limit group). The former were HBV carriers, the latter had liver cirrhosis. The main clinical and virological parameters of the patients are shown in Table 2. CD34+ cells were isolated from 60 mL of heparinized peripheral blood by using the magnetic cell sorting system, then cells were washed twice in PBS and cultured in 24-well plates, about 2×104 cells in each well. HBV DNA levels of the cells and supernatant were measured on Day 0, 6, and 12.
Table 2.
The clinical and virological parameters of HBV patients.
| Patients | Age/sex | Diagnosis | Previous antiviral therapy | Liver function test | Serological markers | Serum HBV DNA (IU/ml) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HBsAg | Anti-HBs | Anti-HBc | HBeAg | Anti-HBe | ||||||
| 1 | 40/F | Carrier | No | Normal | + | − | + | + | − | 10000000 |
| 2 | 40/M | Carrier | No | Normal | + | − | + | + | − | 4540000 |
| 3 | 41/M | Cirrhosis | No | ALT: 45U/L | + | − | + | − | + | <20 |
| 4 | 35/M | Cirrhosis | Adefovir Dipivoxil | ALT: 43.8U/L | + | − | + | + | − | <20 |
| 5 | 42/M | Cirrhosis | No | ALT: Normal; AST: 48.5U/L | + | − | + | − | + | <20 |
HBV DNA was measured by using COBAS TaqMan HBV test kits. The detection limit of HBV DNA was 20 IU/mL.
Statistical analysis
Statistical evaluations were performed using the SPSS17.0 software package. All experimental data were expressed as the mean ±SD. Comparisons among multiple groups were analyzed using one-way ANOVA, followed by two-group comparisons with LSD or Dunnett T3. A p value less than 0.05 was considered statistically significant.
Results
Infection of HBV to CD34+ HSCs derived from cord blood
After co-incubation with HBV-infected serum for 12 days, the CD34+ cells showed positive signals for HBsAg by DAB staining and positive signals for CD34 antigen by AP staining. The yellow signals of HBsAg was located in the cytoplasm, and the red signals of the CD34 antigen were found mostly on the membrane (Figure 1A). Immunofluorescence staining showed the same results (Figure 1B). The positive signals of HBsAg with red fluorescence were located in the membrane and cytoplasm. The positive signals of CD34 antigen with green fluorescence were found mostly on the membrane. These experiments showed that HBsAg was expressed in CD34+ HSCs.
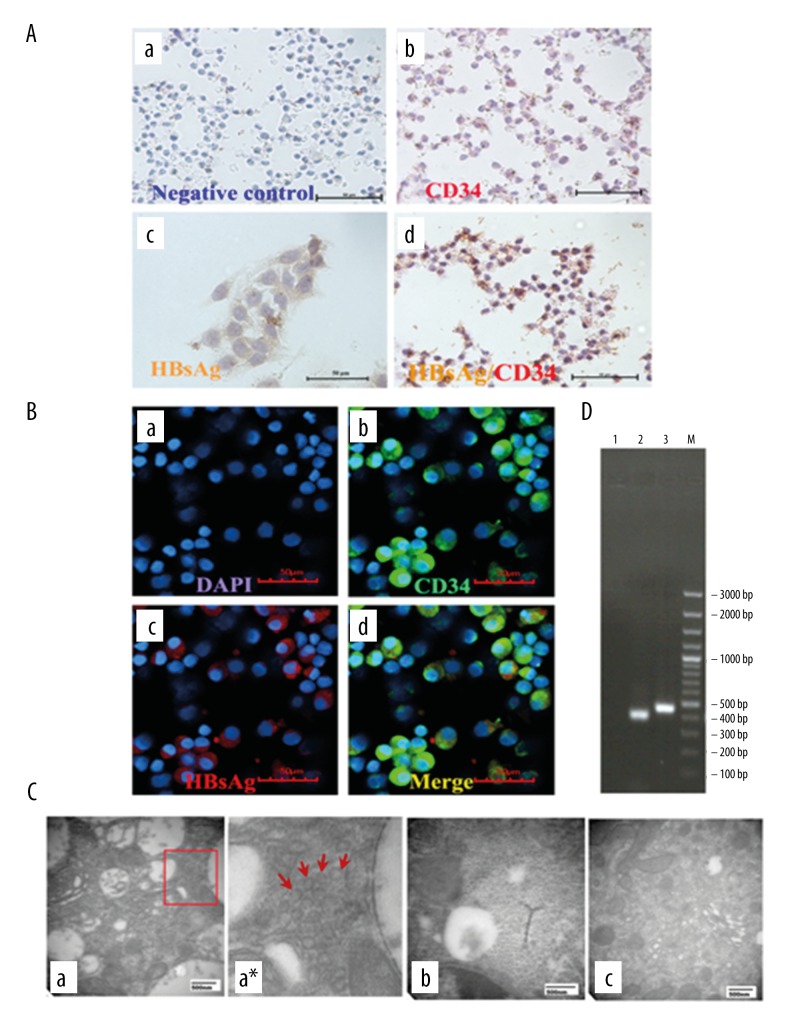
Figure 1.
The infection of HBV in CD34-positive cells from cord blood. (A) Immunohistochemistry. (a) Negative control without primary antibodies; (b) CD34+ cells (red only) without HBV infection; (c) Expression of HBsAg (yellow) in HepG2.2.15 as positive control; (d) Expression of HBsAg (yellow) indicated in CD34+ (red) cells by DAB staining (×200). (B) Immunofluorescence. (a) Cell nuclei were visualized with DAPI; (b) CD34+ antigens (green) were found in membrane; (c) HBsAg (red) were located in the cytoplasm; (d) b and c were merged (×400). (C) Transmission electron microscopy. (a) HBV-infected CD34+ HSCs: HBV particles were roughly spherical with diameter of about 40 nm and localized exclusively in cytoplasm; (a*) Clipped and amplified HBV particles from Figure A; (b) HepG2.2.15 cells were used as positive control; (c) healthy CD34+ HSCs as negative control. (D) Reverse transcription-polymerase chain reaction (RT-PCR) products. Lane 1, healthy CD34+ HSCs as negative control; Lane 2, the 403 bp PCR products corresponding to the amplified HB S gene fragment; Lane 3, GAPDH as loading control.
HBV particles could be found in HBV-infected CD34+ HSCs by applying transmission electron microscopic analysis, as shown in Figure 1C, in which HBV particles were roughly spherical with diameters of about 40 nm and localized exclusively in the cytoplasm.
In order to further confirm that HBV can replicate in HBV-infected CD34+ HSCs, analysis for hepatitis B (HB) S gene-specific RNA was performed. The results showed that the 403 bp PCR products corresponding to the amplified HB S gene fragments were observed in CD34+ HSCs infected by HBV, and no expression of these gene fragments were observed in the HSCs of healthy controls (Figure 1D). These results demonstrated that HB S encoding mRNAs are expressed in the HBV-infected CD34+ HSCs, and they provide evidence that HBV could replicate actively in infected CD34+ HSCs.
The pattern of infection and replication of HBV to CD34+ cells from cord blood
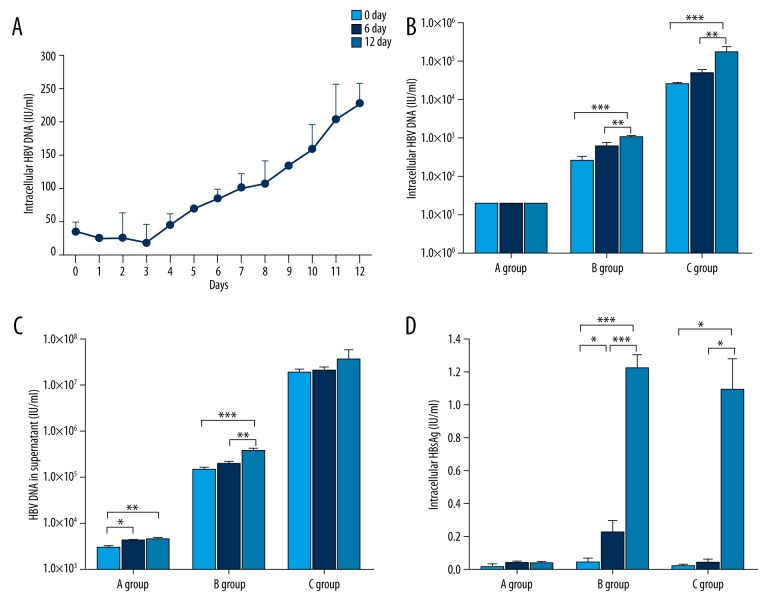
To gain insight into whether HBV might sustain a productive infection in CD34+ cells, we carried out a series of experiments. First, the CD34+ cells were incubated with HBV serum for 24 hours, and then the cells were washed and seeded in 24-well plates, and intracellular HBV DNA was tested for daily by using real-time PCR. Intracellular HBV DNA increased gradually from Day 0 to the Day 12, and ranged from 35.85±13.79 IU/mL to 227.50±3 0.41 IU/mL (Figure 2A). Then, CD34+ HSCs were challenged by a different load of HBV (Group A: 1.0×104 IU/mL; Group B: 1.0×106 IU/mL; Group C: 1.0×108 IU/mL). The intracellular HBV DNA was all under the detection limit at the different time points for Group A, whereas the level of HBV DNA in Group B and Group C was significantly increased on Day 12 as compared to Day 0 (p<0.001 and p<0.001, respectively. Figure 2B). A similar increasing trend of HBV DNA was found in the supernatant of cultures (Figure 2C). Compared with Day 0, supernatant HBV DNA levels on Day 12 in Group A and Group B were significantly increased (p<0.01 and p<0.001, respectively); Group C showed a growing trend (p>0.05). Spontaneous increases in viral titers indicated that virions were produced by HSCs infected with HBV. These experiments showed that HBV DNA can replicate in CD34+ HSCs.
Figure 2.
The pattern of infection and replication of HBV to CD34 positive cells in cord blood. (A) The CD34+ cells were incubated with HBV DNA-positive serum for 24 hours, and then washed and seeded. Intracellular HBV DNA increased gradually from Day 0 to Day 12, and ranged from 35.85±13.79 IU/mL (mean ± standard deviation) to 227.50±30.41 IU/mL. CD34+ HSCs were challenged by different load levels of HBV (HBV DNA of sera in Group A: 1.0×104 IU/mL; Group B: 1.0×106 IU/mL; Group C: 1.0×108 IU/mL). The results are shown in B-D. In (B) the intracellular HBV DNA were under the limit of detection at different time points for Group A, whereas in Group B (p<0.001) and Group C (p<0.001) the levels of HBV DNA were significantly increased on Day 12 compared to Day 0. In (C) we compared HBV DNA in the culture supernatant on Day 12 with Day 0; Group A (p<0.01) and Group B (p<0.001) had significantly increased levels, and Group C had no significant difference (p>0.05), but still showed a growing trend. In (D) the intracellular HBsAg expressions of the three groups were analyzed, and the HBsAg of both Group B (p<0.001) and Group C (p<0.05) were significantly higher on Day 12 than Day 0, and Group A HBsAg expressions were under the detection limit (0.05 IU/mL). (* p<0.05, ** p<0.01, *** p<0.001).
The intracellular HBsAg expressions of the three groups were analyzed. As shown in Figure 2D, the HBsAg levels of Group B and C were significantly higher on Day 12 than on Day 0 (p<0.001 and p<0.05, respectively). However, Group A levels were under the detection limit (0.05 IU/mL). These results suggest that the expression of HBsAg may be due to de novo synthesis rather than the original adhesion, and only the active replication of the HBV has HBsAg expression of continuous increase.
HBV infection and replication in CD34+ PBSCs from chronic HBV chronic
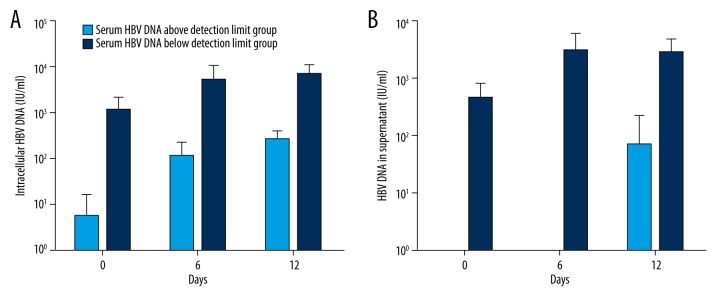
To determine whether HBV can replicate in CD34+ PBSCs of chronic HBV carriers, we detected HBV DNA levels in cells and supernatants of cultures. The results indicated that HBV DNA levels both in cells and supernatants increased progressively during the cell culture period; furthermore, the increments of HBV DNA in the supernatants paralleled those found in cells at corresponding time points. When comparing HBV DNA levels of cultures derived from patients with different HBV DNA detection limits (an above-detection limit group and a below-detection limit group), HBV DNA levels of cultures from patients with levels above the detection limit increased more obviously than those from patients with levels below detection limit (Figure 3).
Figure 3.
HBV infection and replication in CD34+ PBSCs from HBV chronic carriers. Comparison of HBV DNA levels in culture of CD34+ PBSCs derived from five patients with different serum HBV DNA levels (above-detection limit group and below-detection limit group); the results are shown as follows: (A) The intracellular HBV DNA of CD34+ PBSCs and (B) HBV DNA in culture supernatants of CD34+ PBSCs. The results indicate that HBV DNA levels both in cells and supernatants increased progressively during the culture period; furthermore, HBV DNA levels of cultures from patients of the above-detection limit group increased more obviously than that of the below-detection limit group.
Discussion
HSCs are pluripotent stem cells that are susceptible to hosting a number of viruses [17–19] such as HCV and HIV, because of their strong self-renewal ability. However, it has not been clear whether HSCs can be infected by HBV. Therefore, our conducted in vitro and in vivo experiments; the results indicated that HBV not only can infect hematopoietic stem cells but also can replicate in them. HSCs are pluripotent stem cells that can differentiate into all cell types in the hematolymphoid system, so this may be another important reservoir for HBV infection and might provide a continuous source of virally infected cells in vivo for self-renewing and differentiation of a large number of immune cells carrying HBV. Thus, our study provides experimental data for future research on the mechanism of persistent HBV infection, and proposes a path for intrauterine infection. Recently, there have been some clinical reports that the receptors can clear HBsAg after bone marrow transplantation [20–23], not only because of the adoptive transfer of immunity to HBV from donors, but also because of immune reconstitution. Our study can also partially explain this phenomenon, which provides the experimental basis for future treatment of chronic HBV infection by using bone marrow transplantation.
In this study, cord blood–derived CD34+ cells were exposed to HBV, and the direct evidence that HBV can infect CD34+ HSCs in vitro was provided as follows: detection of HBsAg expression by immunocytochemistry and immunofluorescence, and observation of the presence of HBV particles by transmission electron microscopy. These results showed that CD34+ HSCs are targets of HBV. In related research [24], HBV antigen and HBV DNA could be found in bone marrow cells after being exposed to HBV in vitro and in vivo. Other studies [25,26] have proved that HBV can infect and suppress the differentiation of hematopoietic cell lines, and display tropism in immature hematopoietic cells. However, these studies only focused on findings of the presence of HBV and the effect on bone marrow cells and hematopoietic cell lines, and it remains to be further demonstrated which subset of bone marrow cells can be infected and whether HBV can replicate in these infected cells. In the present study, we explored the pattern of infection and replication by using purified CD34+ HSCs co-cultured with HBV, and used this method to help study the mechanism of persistent infection.
To indicate the possibility of HBV replication in CD34+ HSCs, additional experiments were performed. All these results indicated that HBV not only can infect hematopoietic stem cells but also can replicate in them. First, HBV mRNA was detected by RT-PCR; the results showed that the HB S gene (403 bp) mRNA appeared positive due to the appearance of newly synthesized HBV transcripts. HBV DNA is a clear indicator of viral replication, and HBV mRNA is essential for viral replication and expression because it is not only a template for reverse transcription but also for translation. Therefore, as HBV mRNA appeared positive in the cultured cells, this proved that HBV could replicate actively in HBV-infected CD34+ HSCs. Second, CD34+ HSCs were challenged by different titers of HBV, and the results showed that supernatant and intracellular HBV DNA increased with the proliferation of CD34+ HSCs, and that similar results can be obtained from an intracellular HBsAg quantification test. This demonstration of increasing viral load and protein expression of HBV along with HSCs proliferation further demonstrated that the virus can infect and replicate in CD34+ HSCs. These findings showed that CD34+ HSCs not only can take up HBV, but also can release the complete virus particle out of the cells. This means that HBV can finish a complete cycle of infection in CD34+ HSCs, just like it does in hepatocytes. A major breakthrough, therefore, was the determination that CD34+ cells were the permissive cells of HBV, and this new understanding may contribute to further exploration of the mechanism of persistent HBV infection and maternal-infantile transmission. So far, how HBV infects hematopoietic stem cells has been unknown; it may be that they have the same receptors as hepatocytes that mediate the interactions. This will need to be researched in the future. Furthermore, this study indicated that there was a different likelihood of infection of HSCs when the cells were exposed to different HBV viral loads: the higher virus loaded, the greater amount of virus replicated. Other studies [27–29] have shown that the risk of intra-uterine and perinatal transmission of HBV was clearly related to the level of maternal viremia. Perhaps these phenomena can be partly explained by our research.
In order to clarify the infectious status of CD34+ cells in vivo, we amplified CD34+ PBSCs derived from chronic HBV carriers; the results showed that HBV DNA levels in cells and supernatants increased in a time-dependent way, which suggested that CD34+ PBSCs, as one of the composition of PBMC, were susceptible to HBV infection and supported virus replication. Furthermore, CD34+ cells might function as viral reservoirs that play a role in HBV persistence and dissemination in vivo. One study [17] that looked at how HCV can replicate in CD34+ hematopoietic progenitor cells in chronic hepatitis C virus carriers had results that correspond with our study results. Moreover, we found that HBV DNA levels of cultures from patients who had HBV levels above the detection limit increased more obviously than those of patients with HBV levels below the detection limit. These results were consistent with our study’s in vitro experiments. That is to say, the viral load of sera can partly reflect the intracellular HBV DNA level of CD34+ cells. Thus, HBV DNA level of CD34+ PBSCs might be used to predict HBV levels in HBV carriers, which may be of benefit to the prediction, diagnosis, and treatment of HBV-related diseases. In addition, our previous experiments [30] proved that HBV DNA infected and integrated into the bone marrow (BM) of HSCs from patients with chronic HBV infection and the BM HSCs generated defective T cells. Although both HBV and HCV can result in persistent infection, chronic HBV infection is more difficult to clear than HCV, which might be due to defective immune cells differentiated from HBV-infected HSCs. Contrarily, HCV infection [17] has little or no effect on the differentiation of CD34+ cells from chronic HCV carriers. Thus, this study partially elucidates why patients with HBV and HIV infection could be cured by bone marrow transplantation.
Conclusions
Above all, we demonstrated that HBV can replicate in CD34+ HSCs no matter where it is, in cord blood or peripheral blood of chronic HBV carriers. CD34+ HSCs might be another possible reservoir of HBV and a source of immune cells that play an important role in the pathogenesis of persistent HBV infection. Further studies are needed to demonstrate how HBV works in HSCs and its effects on the differentiation of HSCs.
Acknowledgements
We thank professor Yang Bao-Feng, Li Lan-Juan, and Weng Xin-Hua for technical assistance and Luo Yun-ping and Xiang Rong for critical reading of the manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: This research was supported by the National Key Technologies Research and Development Program of China during the Eleventh Five-year Plan Period (2008ZX10002-006), the National Key Technologies Research and Development Program of China during the Twelfth Five-year Plan Period (2012ZX10002007), National Natural Science Foundation of China (30571638), China Postdoctoral Science Foundation Founded Project (20070420890) and Specialized Research Fund for the Doctoral Program of Higher Education of China (20092307110007)
References
- 1.World Health Organization. Hepatitis B fact sheet. 2014. Available online: http://www.who.int/mediacentre/factsheets/fs204/en/
- 2.Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 3.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 4.Ye B, Liu X, Li X, et al. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Op den Brouw ML, Binda RS, van Roosmalen MH, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: A possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280–89. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun C, Fu B, Gao Y, et al. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8:e1002594. doi: 10.1371/journal.ppat.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rong Q, Huang J, Su E, et al. Infection of hepatitis B virus in extrahepatic endothelial tissues mediated by endothelial progenitor cells. Virol J. 2007;4:36. doi: 10.1186/1743-422X-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamelin JP, Zoulim F, Trépo C. Lymphotropism of hepatitis B and C viruses: An update and a newcomer. Int J Clin Lab Res. 1995;25:1–6. doi: 10.1007/BF02592570. [DOI] [PubMed] [Google Scholar]
- 9.Ma R, Xing Q, Shao L, et al. Hepatitis B virus infection and replication in human bone marrow mesenchymal stem cells. Virol J. 2011;8:486. doi: 10.1186/1743-422X-8-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciesek S, Helfritz FA, Lehmann U, et al. Persistence of occult hepatitis B after removal of the hepatitis B virus-infected liver. J Infect Dis. 2008;197:355–60. doi: 10.1086/525286. [DOI] [PubMed] [Google Scholar]
- 11.Pontisso P, Vidalino L, Quarta S, et al. Biological and clinical implications of HBV infection in peripheral blood mononuclear cells. Autoimmun Rev. 2008;8:13–17. doi: 10.1016/j.autrev.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Umeda M, Marusawa H, Seno H, et al. Hepatitis B virus infection in lymphatic tissues in inactive hepatitis B carriers. J Hepatol. 2005;42:806–12. doi: 10.1016/j.jhep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Coffin CS, Osiowy C, Gao S, et al. Hepatitis B virus (HBV) variants fluctuate in paired plasma and peripheral blood mononuclear cells among patient cohorts during different chronic hepatitis B (CHB) disease phases. J Viral Hepat. 2015;22:416–26. doi: 10.1111/jvh.12308. [DOI] [PubMed] [Google Scholar]
- 14.Zeldis JB, Mugishima H, Steinberg HN, et al. In vitro hepatitis B virus infection of human bone marrow cells. J Clin Invest. 1986;78:411–17. doi: 10.1172/JCI112591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiba T, Yokosuka O, Goto S, et al. Successful clearance of hepatitis B virus after allogeneic stem cell transplantation: Beneficial combination of adoptive immunity transfer and lamivudine. Eur J Haematol. 2003;71:220–23. doi: 10.1034/j.1600-0609.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Lu D, Ji G, et al. Hepatitis B virus (HBV) vaccine-induced escape mutants of HBV S gene among children from Qidong area, China. Virus Res. 2004;99:63–68. doi: 10.1016/j.virusres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Sansonno D, Lotesoriere C, Cornacchiulo V, et al. Hepatitis C virus infection involves CD34+ hematopoietic progenitor cells in hepatitis C virus chronic carriers. Blood. 1998;92:3328–37. [PubMed] [Google Scholar]
- 18.McNamara LA, Collins KL. Hematopoietic stem/precursor cells as HIV reservoirs. Curr Opin HIV AIDS. 2011;6:43–48. doi: 10.1097/COH.0b013e32834086b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee P, Crawford L, Samuelson E, et al. Hematopoietic stem cells and retroviral infection. Retrovirology. 2010;7:8. doi: 10.1186/1742-4690-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilan Y, Nagler A, Adler R, et al. Ablation of persistent hepatitis B by bone marrow transplantation from a hepatitis B-immune donor. Gastroenterology. 1993;104:1818–21. doi: 10.1016/0016-5085(93)90664-x. [DOI] [PubMed] [Google Scholar]
- 21.Hui CK, Lie A, Au WY, et al. A long-term follow-up study on hepatitis B surface antigen-positive patients undergoing allogeneic hematopoietic stem cell transplantation. Blood. 2005;106:464–69. doi: 10.1182/blood-2005-02-0698. [DOI] [PubMed] [Google Scholar]
- 22.Lau GK, Suri D, Liang R, et al. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity tohepatitis B core antigen. Gastroenterology. 2002;122:614–24. doi: 10.1053/gast.2002.31887. [DOI] [PubMed] [Google Scholar]
- 23.Lindemann M, Barsegian V, Runde V, et al. Transfer of humoral and cellular hepatitis B immunity by allogeneic hematopoietic cell transplantation. Transplantation. 2003;75:833–38. doi: 10.1097/01.TP.0000054841.42796.68. [DOI] [PubMed] [Google Scholar]
- 24.Elfassi E, Romet-Lemonne JL, Essex M, et al. Evidence of extrachromosomal forms of hepatitis B viral DNA in a bone marrow culture obtained from a patient recently infected with hepatitis B virus. Proc Natl Acad Sci USA. 1984;81:3526–28. doi: 10.1073/pnas.81.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg HS, Bouffard P, Trépo C, et al. In vitro inhibition of hemopoietic cell line growth by hepatitis B virus. J Virol. 1990;64:2577–81. doi: 10.1128/jvi.64.6.2577-2581.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sing GK, Prior S, Fernan A, et al. Hepatitis B virus differentially suppresses myelopoiesis and displays tropism for immature hematopoietic cells. J Virol. 1993;67:3454–60. doi: 10.1128/jvi.67.6.3454-3460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: A case-control study. J Med Virol. 2002;67:20–26. doi: 10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Gui XE, Teter C, et al. Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine. 2014;32:6091–97. doi: 10.1016/j.vaccine.2014.08.078. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Zhang J, Yang H, et al. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol. 2003;71:360–66. doi: 10.1002/jmv.10493. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Lan Y, Cao F, et al. Infected hematopoietic stem cells and with integrated HBV DNA generate defective T cells in chronic HBV infection patients. J Viral Hepat. 2014;21:39–47. doi: 10.1111/jvh.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]