Abstract
Background
Water-soluble components in mesenteric lymph have been implicated in the pathophysiology of acute lung injury and distal organ failure following trauma and haemorrhagic shock. Proteomics analyses have recently shown similarities and specificities of post-trauma/haemorrhagic shock lymph and plasma. We hypothesise that the metabolic phenotype of post-trauma/haemorrhagic shock mesenteric lymph and plasma share common metabolites, but are also characterised by unique features that differentiate these two fluids.
Materials and methods
Matched samples were collected from 5 brain-dead organ donors who had suffered extreme trauma/haemorrhagic shock. Metabolomics analyses were performed through ultra-high performance liquid chromatography mass spectrometry.
Results
Overall, 269 metabolites were identified in either fluid. Despite significant overlapping, metabolic phenotypes of matched lymph or plasma from the same patients could be used to discriminate sample fluid or biological patient/traumatic-injury origin. Metabolites showing relatively high levels in both fluids included markers of haemolysis and cell lysis secondary to tissue injury.
Discussion
High positive correlations were observed between the quantitative levels of markers of systemic metabolic derangement following traumatic/haemorrhagic hypoxaemia, such as succinate, oxoproline, urate and fatty acids. These metabolites might contribute to coagulopathies of trauma and neutrophil priming driving acute lung injury. Future studies will investigate whether the observed compositional specificities mirror functional or pathological adaptations after trauma and haemorrhage.
Keywords: small molecule, mass spectrometry, correlation, quantitative changes, trauma
Introduction
Despite the decrease in mortality due to multiple organ failure (MOF), morbidity and prolonged hospitalisation after trauma/haemorrhagic shock (T/HS) remain high (approximately 44% of survivors)1. Lung inflammation and dysfunction typically precede hepatic or renal insufficiency1. During the past decades, intestinal barrier dysfunction upon warm ischaemia and gut microbiome translocation have been implicated in the so-called “gut origin of sepsis”, a concept that describes the role of the intestine in the development of sepsis and post-operative MOF2. Besides ischaemia reperfusion (IR) subsequent to trauma/haemorrhagic shock (T/HS)3, fluid specificity of mediators to MOF has been demonstrated through lymphatic diversion experiments. Mesenteric lymph diversion prior to T/HS inhibits acute lung injury and attenuates MOF4, as it mitigates the accumulation of pro-inflammatory cytokines and arachidonic acid catabolites, thereby protecting from neutrophil activation5. Animal models of T/HS have shown that systemic lymph6,7 and, in particular, mesenteric lymph (ML) are enriched with protein components derived from tissue injury and cell lysis (especially haemolysis). These protein components drive neutrophil activation, vasculature remodelling through matrix metalloproteinases (e.g. MMP2), and might play hitherto under-investigated moonlighting functions8. These animal studies have been further supported by evidence in humans9, showing that the mesenteric lymph is characterised by compositional changes in comparison to plasma. This is in spite of the suggestion given by the Starling principle that lymph should be regarded as a mere plasma ultra-filtrate10. Recent advances in mass spectrometry-based proteomics have helped show that the lymphatic system collects extravasated fluid, proteins, and lipids from plasma and parenchymal organs through a filtration process at the interstitial space6. Most extravasated fluid is drained into the lymphatic system, while molecular components and bacteria are either captured in the lymph nodes (if >100 kDa) or processed in the conduit system (if <80–100 kDa)6. Low molecular weight components (<100 kDa) have been believed to play a key role in mediating inflammatory response and neutrophil priming leading to distal organ injury in response to T/HS5. Arachidonic acid, leukotriene catabolites and related enzymes were initially associated with neutrophil priming driving lung injury and MOF. More recently, the focus has shifted to hydrophilic components including proteins8,9 and bioactive peptides derived from extracellular matrix remodelling7, a category of molecules constituting an expanded self-antigen repertoire in the lymph7. However, the metabolic phenotype of post-shock mesenteric lymph in humans has not yet been explored owing to the difficulty of accessing these samples and the low sample volumes that can be harvested in the emergency unit or operating theatres. Innovations in the field of metabolomics have fostered a wide series of studies on metabolic adaptations to T/HS in plasma11–13. Metabolic markers of T/HS have been identified, suggesting potential mechanisms of systemic adaptation to haemorrhagic shock-induced hypoxaemia, such as accumulation of lactate, succinate3,12,14, polyamines and urate12. Therefore, plasma accumulation of these and other metabolites have been interpreted in the light of well-established metabolic sequelae to T/HS, involving lipolysis and fatty acid mobilisation, proteolysis, and hyperglycaemia, promoting the insurgence of an insulin-resistance-like phenotype15,16. These metabolic adaptations may play biologically-relevant functions, such as mediating neutrophil activation through succinate/HIF1α/IL1β signalling cascades17,18 and platelet activation19,20. This is important in the trauma population in that these metabolites contribute to two key factors in the so-called “lethal triad” of trauma, namely metabolic acidosis, a much debated prognostic factor of survival in trauma patients21, and coagulopathies of trauma22, a more recently revised concept23 that will benefit from an improved understanding of the molecular mechanisms following T/HS.
Despite our growing understanding of the metabolome of T/HS plasma11–13, and the recent publication of thorough proteomics profiling of both fluids9, to the best of our knowledge no comparative study of the plasma and lymph metabolome has yet been reported. Here we present an observational UHPLC-MS metabolomics study of matched plasma and lymph samples from trauma patients who went on to organ donation due to brain death; an extremely rare set of samples from patients suffering lethal injuries. Consistent with proteomics observations9, we hypothesise that the metabolic phenotypes post-T/HS plasma and lymph are comparable, and that metabolite levels in both fluids are strongly correlated. We also anticipate that this approach will allow compositional specificity to be detected.
Materials and methods
Study population and data collection
Patients undergoing brain-dead organ donation at Denver Health Medical Center (DHMC) were evaluated for inclusion in the study which was carried out from 2008 through 2010, as previously reported9. The study was approved by the Combined Multiple Institution Review Board. Inclusion criteria were: trauma/haemorrhagic shock, brain death, mesenteric lymphatic injury, and all data were collected according to Health Insurance Portability and Accountability Act (HIPAA) regulations. Causes of death were heterogeneous and included death by self-inflicted gun wound, head trauma, motorcycle accident, and hanging.
Mesenteric lymph and plasma collection
Fluid was collected as previously reported9. Briefly, the cisterna chyli was visualised between the aorta and spine, and lymph was aspirated using a 27-gauge needle. During the donor operation, before cold preservation, a right medial visceral rotation was performed to expose the vena cava and the aorta just inferior to the take off of the superior mesenteric artery. At this level, the left renal vein was easily identified anteriorly crossing the aorta. Running along the retroperitoneal small bowel mesentery and crossing anterior and perpendicular to the left renal vein are typically large distended lymphatic vessels. These were cannulated with a 21-gauge angiocatheter to obtain the mesenteric lymph. Consent for organ donation and lymph collection was obtained by the organ procurement organisation, from the deceased (based on a document of gift: donor card, living will, or driver’s license) or from the next of kin.
Mesenteric lymph (100 μL-1 mL) was collected and placed in an EDTA-containing tube. Samples were centrifuged at 3,500 g for 10 minutes to remove cellular components, and stored in a freezer at −80 °C. Patients were categorised according to predominant illness or mechanism of injury.
Blood samples were collected in an EDTA vacutainer from each subject. The blood samples were stored upright at 4 °C until they were spun at 1,500 g at 4 °C for 15 minutes. The separated plasma was aliquoted and stored at −80°C for further analysis.
Sample extraction
Metabolomcs extraction
All the samples were processed as a batch. Twenty microlitres of plasma and lymph were extracted at 1:25 dilutions (20 μL in 480 μL) in ice-cold (−20 °C) lysis/extraction buffer (methanol:acetonitrile:water 5:3:2) to precipitate proteins. Samples were then agitated at 4 °C for 30 minutes and then centrifuged at 10,000 g for 15 minutes at 4 °C. Precipitated proteins and non-methanol soluble lipid pellets were discarded, while supernatants were stored at −80°C prior to metabolomics analysis.
Metabolomics analysis
Metabolomics analysis was performed as previously reported12,24,25, with minor modifications. Ten μl of sample extracts were injected into an UHPLC system (Ultimate 3000; Thermo, San Jose, CA, USA) and run on a Kinetex C18 column (150×2.1 mm i.d., 1.7 μm particle size; Phenomenex, Torrance, CA, USA) using either a 3-minute isocratic flow at 250 μL/minute (mobile phase: 5% acetonitrile, 95% double distilled H2O, 0.1% formic acid) or a 9-minute gradient from 5% to 95% organic solvent B (mobile phases: A=double distilled H2O, 0.1% formic acid; B=methanol, 0.1% formic acid). The UHPLC system was coupled online with a QExactive quadrupole Orbitrap instrument (Thermo), scanning in Full MS mode (3-minute method) or performing acquisition independent fragmentation (AIF-MS/MS analysis; 9-minute method) at 70,000 resolution in the 60–900 m/z range, 4kV spray voltage, 15 sheath gas and 5 auxiliary gas, operated in negative and then positive ion mode (separate runs). Calibration was performed before each analysis against positive or negative ion mode calibration mixes (Piercenet; Thermo Fisher, Rockford, IL, USA) to ensure sub ppm error of the intact mass. Technical variability was assessed running technical mixes at the beginning and the end of each batch. Coefficient of variations (standard deviation divided by the mean) were tested for all metabolites in technical mixes and assessed to be below 20% for metabolites to be considered in the interpretation of the data24. Metabolite assignments were performed using the software Maven (Princeton, NJ, USA), upon conversion of .raw files into .mzXML format through MassMatrix (Cleveland, OH, USA). The software allows for peak picking, feature detection and metabolite assignment against the KEGG pathway database. Assignments were further confirmed against chemical formula determination (as seen from isotopic patterns and accurate intact mass), and retention times against over 650 standards, including commercially available glycolytic and Krebs cycle intermediates, amino acids, glutathione homeostasis and nucleoside phosphates (SIGMA Aldrich, St. Louis, MO, USA; IROATech, Bolton, MA, USA). Structural isomer ambiguous assignments were validated by comparing MS/MS data against available small molecule fragmentation databases (Metlin, HMDB) or available standards.
Relative quantitation was performed by exporting integrated peak areas values into Excel (Microsoft, Redmond, CA, USA) for statistical analysis including two-tailed t-test (p<0.05 was considered significant), and partially supervised partial least squares discriminant analysis (PLS-DA). PLS-DA, the loading and variable screening plots were calculated through the macro MultiBase (freely available at www.NumericalDynamics.com). Correlation analysis (Pearson’s correlation) was performed with Graphpad Prism 5.0 software (Prism, La Jolla, CA, USA) after confirming that data were normally distributed (Kolmogorov-Smirnov). Only significant results (p<0.05) were plotted.
Results
Metabolomics analyses were performed on 5 matched plasma vs lymph samples from brain-dead organ donors who had suffered severe T/HS, following gun wound, head trauma, motor cycle accident, and hanging. Although patients (n=5) were characterised by heterogeneous lethal traumatic injuries, this study represents a first metabolomics comparison of these clinically relevant biofluids in an extreme T/HS population.
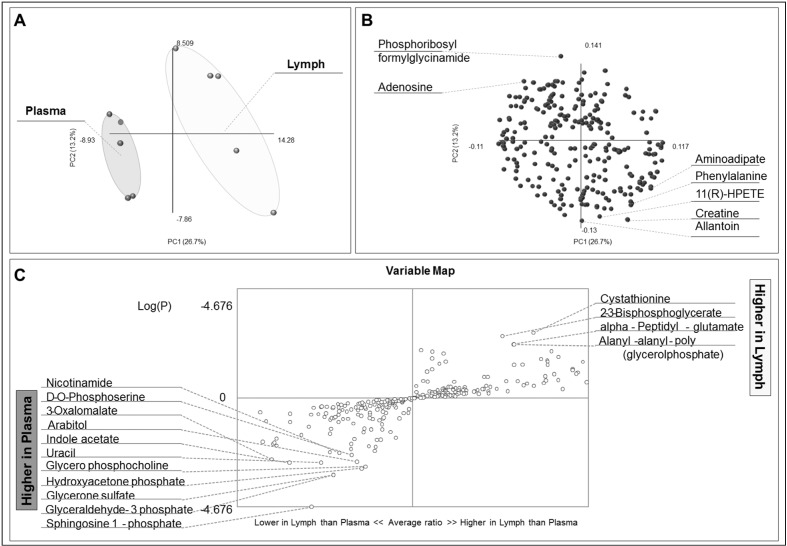
A total of 269 metabolites were confidently identified, as reported in Supplementary Table I, also including mass to charge ratios, experimental retention times, integrated peak areas, compound classes, polarity in which each metabolite has been detected, fold-change of matched lymph vs plasma ratios, and t-test p-values. Results were used to inform PLS-DA (Figure 1A), loading plot representation (Figure 1B), and variable screening analysis (Figure 1C), indicating that metabolic profiles could be used to discriminate between samples from either fluid.
Figure 1.
Partial least square discriminant analysis (PLS-DA) was performed on the basis of metabolomics profiles of lymph vs plasma samples.
(A) Sample clustering across principal components (PCs) 1 and 2 indicating the metabolic profiles are sufficiently powered to discriminate sample biofluid origin. (B) Loading plot shows the distribution of metabolites (loading variables) across the same PCs as in (A). Seven variables with the highest loadings are highlighted. (C) Fold changes and p-values are used to discriminate metabolites increasing or decreasing in lymph samples in comparison to matched plasma, plotted in the top right and bottom left quadrants, respectively.
Metabolomics results were used to plot heat maps and perform hierarchical clustering analysis (HCA) (Online Supplementary Figure 1). A subset of metabolites showed higher levels in plasma or lymph samples, respectively (Figure 2, left column), including nucleotides, purine catabolites and metabolic co-factors (pyridoxal, uracil, nicotinamide, NADH, folate, urate), glycerophospholipids and sphingolipids (sphingosine 1-phosphate), glycolysis and sugar metabolites (2/3-phosphoglycerate, glyceraldehyde 3-phosphate, arabitol, maltotriose, biphosphoglycerate), sterols and one carbon metabolites, or metabolites involved in arachidonic acid metabolism (prostaglandin G2).
Figure 2.
Hierarchical clustering analyses of quantitative dynamic changes of mesenteric lymph and plasma metabolites.
Each row represents a metabolite in each one of the 5 lymph- or plasma-matched samples. Relative quantitation has been performed upon intra-row Z-score normalisation using GENE E software (Broad Institute). Colour codes range from white to black (low to normal to high values, respectively). In the left column, fluid-dependent changes are plotted, indicating metabolites increasing either in plasma or lymph (from top to bottom). (Right column) Metabolites are highlighted that increase in matched plasma-matched and lymph-matched biological samples, indicating either a donor or trauma specificity. An extended full colour version of this figure, also including metabolite names and hierarchical clustering classifications, is provided in the Online Supplementary Figure 1.
A subset of metabolites increased selectively only in one of the matched plasma and lymph from the same patient, as highlighted in Figure 2 (right column). Owing to the small sample size, it is not possible to form any conclusions here as to whether the observed phenomenon is dependent upon inter-patient biological variability or to the severity of the injury.
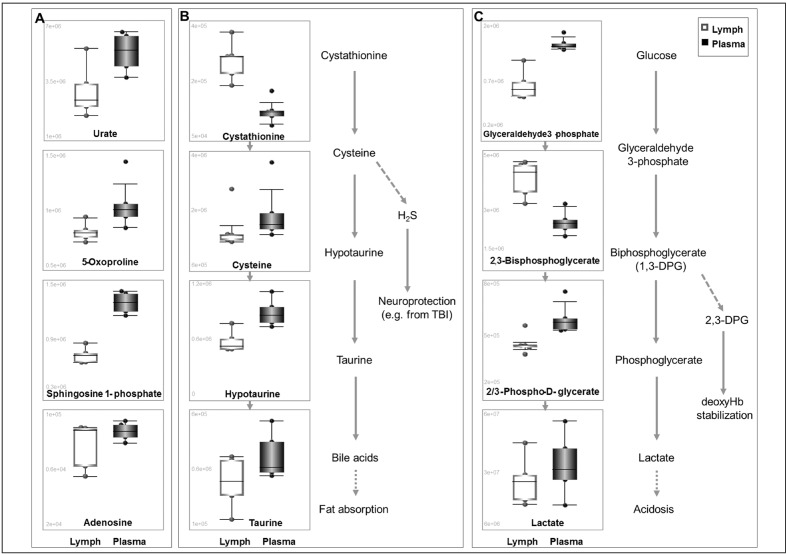
We therefore focused on a subset of the metabolites that showed statistically significant quantitative changes in matched plasma vs lymph samples. Since lymph from trauma patients has been previously reported to be enriched in proteins involved in energy metabolism as a byproduct of injury-driven cell lysis6,9, lymph was evaluated for metabolite enrichment associated with systemic responses to trauma and haemorrhage12,13. Of note, the levels of urate, 5-oxoproline, sphingosine 1-phosphate and adenosine, previously associated with early responses to haemorrhage and ischaemic hypoxia3,12,13 were significantly higher in plasma than in lymph (Figure 3A). Fluid-specific relative abundances of metabolites involved in specific pathways, such as sulphur metabolism and fat absorption (Figure 3B) or glycolysis (Figure 3C) might be indicative of functional systemic adaptations to trauma and haemorrhage that could contribute to vascular homeostasis26 and metabolic acidosis21.
Figure 3.
Box and whisker plots of metabolites from lymph (white) or plasma (black) that change in a statistically significant fashion (p<0.01, t-test) in matched samples from either fluid.
Highlighted are key metabolites that had been previously shown to change in human and rat plasma upon trauma and haemorrhage (A)12,13, sulphur metabolism (B) and glycolysis (C), a proxy for metabolic acidosis and a prognostic marker of survival upon trauma/haemorrhagic shock21.
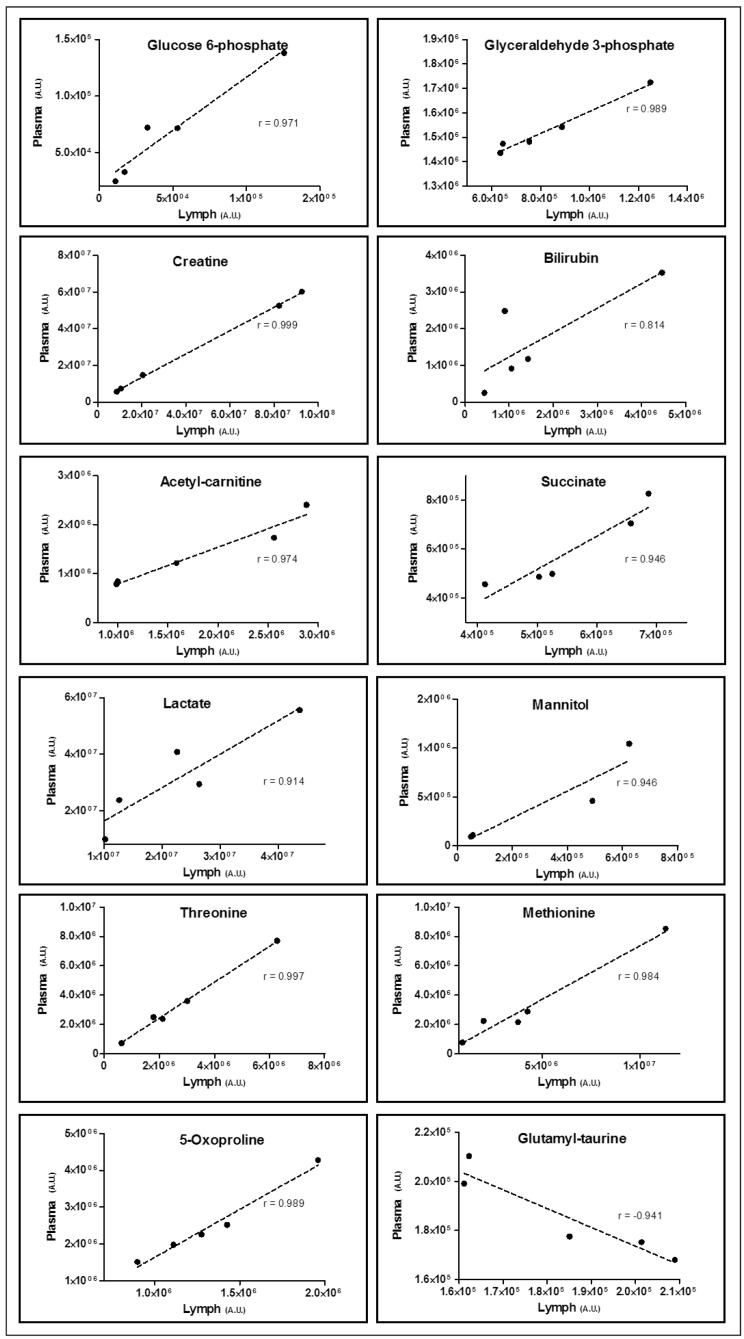
Correlative analyses of metabolite levels in matched plasma and lymph showed that approximately 150 metabolites out of 269 had correlation more than /0.75/ (Supplementary Table I). The most significant correlations are plotted in Figure 4, including glycolytic metabolites (glucose 6-phosphate, glyceraldehyde 3-phosphate, lactate), transfusion markers (mannitol), nitrogen metabolism (creatine) and haemolysis markers (bilirubin), amino acids (threonine, methionine), fatty acid metabolism (acetyl-carnitine), and previously documented markers of trauma and haemorrhage (succinate, 5-oxoproline) (Figure 4). Gamma-glutamyl taurine was the top metabolite showing negative correlations between plasma and lymph, suggesting a selective exchange of this compound between the fluids.
Figure 4.
Pearson’s linear correlation coefficients were calculated for all the metabolites in lymph- and plasma-matched samples from the same patient (n=5).
Though preliminary, these results indicate that more than 150 metabolites showed correlations more than /0.75/ (p<0.05) (Online Supplementary Table I), suggesting that the two fluids are in metabolic equilibrium after trauma and haemorrhage in the tested samples. Only a subset of metabolites, including γ-glutamyl taurine (gamma-glutamyl cycle), show opposite trends in matched samples, indicating that this metabolite is selectively exchanged in either fluid after trauma/haemorrhagic shock.
Discussion
The direct comparison of the two fluids made in the present study helped determine semi-quantitative alterations in the levels of the metabolites involved in homeostatic responses after severe T/HS in patients sustaining lethal trauma. Despite the limited number of biological replicates assayed in this study (n=5), collection of these samples was not an easy task. Previous omics studies comparing molecular phenotype of plasma vs lymph (e.g. proteomics) were performed on pooled samples owing to technical constraints such as lengthy sample preparation protocols (including immune-affinity column-based depletion of the most abundant 14 plasma proteins) and instrument time9. Owing to the advances in analytical technologies in the field of metabolomics, here we adopted a high throughput 3-minute method to assay hydrophilic metabolites, and a more extensive longer UHPLC gradient to cover also methanol-soluble lipid components through independent runs for each of the plasma and lymph samples.
Correlative evidence was provided at the small molecule level as to the interconnectedness of plasma and lymph phenotype, substantiating well established observations made over the last 100 years corroborating the Starling principle10. However, the metabolic profiles of plasma and lymph samples are sufficiently different to allow discrimination of samples from either fluid through PLS-DA, and identify compounds and compound classes specifically enriched in either plasma or lymph samples. It is worthy of note that some of these metabolites are involved in glycolysis, and mirror cell lysis events (especially haemolysis) occurring after traumatic tissue injury, as previously reported at the protein level6,9. Interestingly, proteomics studies indicated that post-T/HS mesenteric lymph was enriched with the glycolytic enzyme bisphosphoglycerate mutase, a red blood cell protein that plays a major role in regulating haemoglobin oxygen affinity by controlling the levels of its allosteric effector 2,3-bisphosphoglycerate, as it mediates its interconversion to the glycolytic 1,3 isomer through the Rapoport-Luebering shunt9. Consistently, increased levels of 2,3-bisphosphoglycerate were found in lymph samples. Other markers of haemolysis were detected in both fluids, such as bilirubin and haemine (a product of haem catabolism increasing in post-T/HS plasma13), whose relative levels in plasma and lymph were highly positively correlated (r=0.814, p<0.01). Other metabolites, such as mannitol, reflect administration of transfusion of red blood cells stored in other additive solution than mannitol-free AS-325 to haemorrhaged patients (2 of 5 of the enrolled patients). Mannitol was detected in all patients although this was 5–10-fold higher in fluids from the 2 transfused patients. Since humans do not synthesise mannitol, such basal level of this metabolite might potentially mirror a bacterial presence, though further targeted studies may be necessary to follow up on this very preliminary evidence.
Previous studies have suggested that the accumulating metabolic enzymes in biological fluids in response to trauma can play unanticipated “moonlighting” functions8. In this context, it is worth noting that the levels of metabolic markers of sugar, protein and nucleotide hypercatabolism secondary to T/HS12,13,15,16 accumulating in plasma (e.g. succinate, 5-oxoproline, lactate) were significantly (p<0.01) positively correlated (r>0.95) with the relative levels in matched lymph samples. Succinate, which has been reported to accumulate under systemic hypoxaemia14, might play a key role in mediating neutrophil activation through the stabilisation of hypoxic responses through HIF1α and the induction of IL1β signalling cascades17,18. Further mechanistic studies will be necessary to understand whether succinate might be involved in acute lung injury and distal organ failure mediated by water-soluble components of post-T/HS plasm/lymph.
Trauma patients are frequently coagulopathic early after injury and become hypercoagulable within days. Organ3 and plasma12 accumulation of succinate has been reported during systemic ischaemic hypoxia and might affect platelet activation19,20, helping explain the underlying causes of hyperfibrinolysis and trauma-induced coagulopathy.
The metabolic response to trauma and sepsis is characterised by major increases in urinary excretion of nitrogen- and sulphur-containing compounds26. Given this, cystathionine accumulated in lymph while downstream sulphur containing metabolites, including cysteine, hypotaurine and taurine are higher in matched plasma samples. Cystathionine is a key substrate for the generation of hydrogen sulfide, a molecule mediating cardio- and neuro-protective effects upon traumatic brain injury27,28.
Impaired nitrogen metabolism after T/HS has been associated with activation of the purinergic signalling through the stimulation of adenosine receptors, mitigating immune system activation and lung injury29. Indeed, time-course studies have shown that 2-fold adenosine increases can be detected within minutes from early mild haemorrhage in rats12. Prolonged adenosine oxidation and catabolism during T/HS results in the accumulation of urate in rats12, a contributor to redox homeostasis that can scavenge reactive oxygen species and be converted into allantoin30. Since humans and higher primates do not have a functional uricase, the enzyme responsible for conversion of urate to allantoin, it is relevant that here we observed both adenosine and allantoin as two of the top ten metabolites with the highest loading weights in the PLS-DA. Interestingly, another key contributor to sample clustering into lymph and plasma groups in PLS-DA was sphingosine 1-phosphate, a potential mediator of shock-induced acute lung injury, as demonstrated through sphingosine kinase inhibition experiments in rats31. Nitrogen metabolism is also a consequence of extensive proteolysis13, arginine consumption12,32 and extracellular matrix remodelling by proteases that are specifically enhanced upon trauma and haemorrhage in mesenteric lymph, such as matrix metalloproteinase 28. Byproducts of protein catabolism and protease activities are enriched in lymph in the form of self-antigen peptides7 or, as observed here, as modified amino acid residues deriving from collagen remodelling, such as trans-hydroxyproline. Polyamines, a sink for amine group accumulation, have been previously associated with adaptive metabolic responses to compensate metabolic acidosis12, as well as extracellular matrix remodelling (as they serve as substrates for transglutaminase enzymes) and they have been shown to influence platelet activation19. The levels of polyamines (cadaverine, spermidine and spermine) increased significantly in matched lymph vs plasma samples.
Lipid mobilisation is another distinctive trait of metabolic alterations subsequent to T/HS13. Proteomics studies showed that plasma lipoproteins were particularly enriched in post T/HS plasma in comparison to lymph9. Conversely, the accumulation of arachidonic acid and pro-inflammatory catabolites such as leukotrienes have been reported to mediate neutrophil priming and acute lung injury, and higher levels of these metabolites were detected in lymph33. It is also worth noting that the levels of some fatty acids, as well as of some amino acids, were found to increase in matched plasma and lymph from one of the biological replicates. Owing to the small sample size of the present study, the possibility cannot be ruled out that the observed phenomenon is due to inter-patient biological variability or trauma-specific metabolic signatures.
In the light of the “gut origin of sepsis” hypothesis, we specifically looked for bacterial metabolites in mesenteric lymph. Metabolites of potential bacterial origin like citramalate13 were present but not selectively enriched in lymph, consistent with previous studies showing absence of bacterial DNA and 16S rRNA in post-T/HS porcine lymph34. Future experiments aimed at identification of bacterial metabolites are required to provide definitive evidence. Definitive clues will be obtained from future experiments in animal models (e.g. enteral heavy labelled substrate administration and induction of T/HS in germ-free rats).
Conclusions
The present study compared the post T/HS plasma and mesenteric lymph metabolomes from brain-dead organ donor patients. Despite the observational nature of the study on this small patient population, and the heterogeneity of the causes of death, which limit the scope and generalisability of the reported results, we identified a list of 269 metabolites and documented semi-quantitative changes. Unfortunately, lymph samples are difficult to obtain from trauma patients, making it challenging to expand the cohort of patients enrolled and so widen the scope of this work. However, for the first time, through UHPLC-MS approaches we have been able to document metabolites specific to either fluid, independently from the lethal traumatic injury suffered from the patient, and identify small molecules that showed patient/trauma-dependent trends without pooling samples as in recent proteomics comparative studies on the two fluids9. Metabolic analyses were used to perform correlative analyses and the results indicated that metabolite levels in either fluid are strongly interdependent in the extreme trauma population investigated in this study, substantiating the Starling principle. Furthermore, these results complement and expand upon previous observations on proteomics specificities of post-T/HS plasma and lymph9, as well as improving the general understanding of systemic metabolic derangements after T/HS involving other fluids than plasma12,13. In the light of the documented role that small molecule mediators play in priming neutrophil activation and inflammatory responses that drive acute lung injury and, ultimately, MOF18, further studies will be mandatory to understand whether the observed correlations between metabotypes of post-T/HS lymph and plasma also mirror metabolic adaptation to trauma and haemorrhage in other clinically relevant fluids, such as brochoalveolar lavage fluid35.
Supplementary Information
Footnotes
Funding and resources
This work was supported in part by grants from the National Institutes of Health, National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM049222, National Center for Research Resources (Grant Number S10RR023015), and University of Colorado Comprehensive Cancer Center Core Support (P30 CA046934-17).
Authorship contributions
EEM, CCS, AB, and KCH designed the study. HBM, AS, and TN collected the samples. AD, TN, MW, and KCH performed metabolomics analyses. TN and MW prepared the supplementary report. AD prepared the figures and wrote the paper. All Authors contributed to the final version of the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Sauaia A, Moore EE, Johnson JL, et al. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg. 2014;76:582–92. doi: 10.1097/TA.0000000000000147. discussion 592–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinross J, von Roon AC, Penney N, et al. The gut microbiota as a target for improved surgical outcome and improved patient care. Curr Pharm Des. 2009;15:1537–45. doi: 10.2174/138161209788168119. [DOI] [PubMed] [Google Scholar]
- 3.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–5. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnotti LJ, Upperman JS, Xu DZ, et al. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–27. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zallen G, Moore EE, Johnson JL, et al. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83:83–8. doi: 10.1006/jsre.1999.5569. [DOI] [PubMed] [Google Scholar]
- 6.Hansen KC, D’Alessandro A, Clement CC, et al. Lymph formation, composition and circulation: a proteomics perspective. Int Immunol. 2015;27:219–27. doi: 10.1093/intimm/dxv012. [DOI] [PubMed] [Google Scholar]
- 7.Clement CC, Cannizzo ES, Nastke M-D, et al. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PloS One. 2010;5:e9863. doi: 10.1371/journal.pone.0009863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alessandro A, Dzieciatkowska M, Peltz ED, et al. Dynamic changes in rat mesenteric lymph proteins following trauma using label-free mass spectrometry. Shock Augusta Ga. 2014;42:509–17. doi: 10.1097/SHK.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzieciatkowska M, D’Alessandro A, Moore EE, et al. Lymph is not a plasma ultrafiltrate: a proteomic analysis of injured patients. Shock Augusta Ga. 2014;42:485–98. doi: 10.1097/SHK.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel C. Starling: the formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol. 1997;82:1–30. doi: 10.1113/expphysiol.1997.sp004000. [DOI] [PubMed] [Google Scholar]
- 11.Kinross JM, Alkhamesi N, Barton RH, et al. Global metabolic phenotyping in an experimental laparotomy model of surgical trauma. J Proteome Res. 2011;10:277–87. doi: 10.1021/pr1003278. [DOI] [PubMed] [Google Scholar]
- 12.D’Alessandro A, Moore HB, Moore EE, et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am J Physiol Regul Integr Comp Physiol. 2015 doi: 10.1152/ajpregu.00030.2015. DOI: ajpregu.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltz ED, D’Alessandro A, Moore EE, et al. Pathologic metabolism: an exploratory study of the plasma metabolome of critical injury. J Trauma Acute Care Surg. 2015;78:742–51. doi: 10.1097/TA.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinopoulos C. Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex. J Neurosci Res. 2013;91:1030–43. doi: 10.1002/jnr.23196. [DOI] [PubMed] [Google Scholar]
- 15.Cuthbertson DP. The metabolic response to injury and other related explorations in the field of protein metabolism: an autobiographical account. Scott Med J. 1982;27:158–71. doi: 10.1177/003693308202700210. [DOI] [PubMed] [Google Scholar]
- 16.Aller M-A, Arias J-I, Alonso-Poza A, et al. A Review of metabolic staging in severely injured patients. Scand J Trauma Resusc Emerg Med. 2010;18:27. doi: 10.1186/1757-7241-18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De la Peña NC, Sosa-Melgarejo JA, Ramos RR, et al. Inhibition of platelet aggregation by putrescine, spermidine, and spermine in hypercholesterolemic rabbits. Arch Med Res. 2000;31:546–50. doi: 10.1016/s0188-4409(00)00238-1. [DOI] [PubMed] [Google Scholar]
- 20.Spath B, Hansen A, Bokemeyer C, et al. Succinate reverses in-vitro platelet inhibition by acetylsalicylic acid and P2Y receptor antagonists. Platelets. 2012;23:60–8. doi: 10.3109/09537104.2011.590255. [DOI] [PubMed] [Google Scholar]
- 21.Mikhail J. The trauma triad of death: hypothermia, acidosis, and coagulopathy. AACN Clin Issues. 1999;10:85–94. [PubMed] [Google Scholar]
- 22.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 23.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–7. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemkov T, D’Alessandro A, Hansen KC. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids. 2015;47:2345–57. doi: 10.1007/s00726-015-2019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55:1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiteman M, Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J Cell Mol Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Li H, Ge J. A cardioprotective insight of the cystathionine γ-lyase/hydrogen sulfide pathway. IJC Heart Vasc. 2015;7:51–7. doi: 10.1016/j.ijcha.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Shan H, Wang T, et al. Dynamic change of hydrogen sulfide after traumatic brain injury and its effect in mice. Neurochem Res. 2013;38:714–25. doi: 10.1007/s11064-013-0969-4. [DOI] [PubMed] [Google Scholar]
- 29.Haskó G, Xu D-Z, Lu Q, et al. Adenosine A2A receptor activation reduces lung injury in trauma/hemorrhagic shock. Crit Care Med. 2006;34:1119–25. doi: 10.1097/01.CCM.0000206467.19509.C6. [DOI] [PubMed] [Google Scholar]
- 30.Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, Xu D-Z, Feketeova E, et al. Attenuation of shock-induced acute lung injury by sphingosine kinase inhibition. J Trauma. 2004;57:955–60. doi: 10.1097/01.ta.0000149495.44582.76. [DOI] [PubMed] [Google Scholar]
- 32.Luiking YC, Deutz NEP. Exogenous arginine in sepsis. Crit Care Med. 2007;35:S557–63. doi: 10.1097/01.CCM.0000279191.44730.A2. [DOI] [PubMed] [Google Scholar]
- 33.Morishita K, Aiboshi J, Kobayashi T, et al. Lipidomics analysis of mesenteric lymph after trauma and hemorrhagic shock. J Trauma Acute Care Surg. 2012;72:1541–7. doi: 10.1097/TA.0b013e318256df15. [DOI] [PubMed] [Google Scholar]
- 34.Reino DC, Pisarenko V, Palange D, et al. Trauma Hemorrhagic Shock-Induced Lung Injury Involves a Gut-Lymph-Induced TLR4. Pathway in Mice. PLoS ONE. 2011 doi: 10.1371/journal.pone.0014829. PMC3150139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Synenki L, Chandel NS, Budinger GRS, et al. Bronchoalveolar lavage fluid from patients with acute lung injury/acute respiratory distress syndrome induces myofibroblast differentiation. Crit Care Med. 2007;35:842–8. doi: 10.1097/01.CCM.0000257254.87984.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.