Introduction
Biomaterials used in scaffold fabrication may be made of ceramics, biodegradable metals, synthetic and natural polymers. They generally promote cell attachment and proliferation and are biodegradable, usually generating non-toxic degradation products1.
Silk fibroin (SF), one of the main proteins of silk worm Bombyx mori filaments, is a natural material that has been extensively investigated for its potential in textile, biomedical, photonic and electronic applications2. Moreover, SF is a biocompatible and biodegradable protein material that has been widely used as surgical suture for centuries. In recent years, new biomedical applications have been envisaged given the environmental stability, remarkable mechanical properties, controlled proteolytic biodegradability and morphological flexibility of this material thanks to the possibility of controlling its molecular structure and morphology through material processing and chemical modification3. As a naturally derived biomaterial, SF possesses outstanding mechanical properties, such as flexibility and tensile strength, and has demonstrated good biocompatibility both in vitro and in vivo4,5. SF is considered to be a useful material that facilitates collagen synthesis and re-epithelialisation in the treatment of skin injuries5,6.
Platelet gel is a blood component obtained through a process of gelification of a fresh or frozen/thawed platelet concentrate, which has been traditionally prepared from platelet-rich plasma derived from peripheral blood7. More recently, a procedure to obtain platelet gel from human umbilical cord blood has been developed and patented (US patent 8,501,170). Platelet gel has been found to be an interesting and useful product, rich in growth factors, able to support tissue regeneration in dentistry, oral-maxillofacial and bone surgery, wound healing and soft tissue injuries5,8,9.
The therapeutic effects of platelet gel are attributed primarily to the release of growth factors (such as platelet derived growth factor-BB, transforming growth factor-beta1, fibroblast growth factor-basic), which exhibit chemotactic and mitogenic properties. However, other authors hypothesise that the fibronectin content that comes with the platelet gel preparation may exert an extra bioactive effect on the cells and tissues, such as matrix metalloproteinase activation10,11.
Human mesenchymal stem cells derived from adipose tissue (ADMSC) are an attractive source of cell therapy for the regeneration of damaged tissues, because of these cells’ capacity for self-renewal and differentiation into various cell lineages12.
The relatively easy protocols for ADMSC isolation and expansion in culture, and the fact that these cells seem to be immunologically well tolerated, make them an attractive candidate for new studies in translational clinical research13. Accordingly, ADMSC have already been clinically tested for the reconstruction of bone defects with promising results14. Hence, bone tissue engineering approaches should focus on the stimulation and optimisation of the osteogenic differentiation process.
This study was aimed at testing the hypothesis that a SF scaffold associated with human cord blood platelet gel (CBPG) may represent an innovative treatment to improve bone regeneration. Although the advantages of using platelet gel from adult blood15 and a SF scaffold in bone regeneration have been independently reported, for the first time this study investigated the effects of’ combined use of CBPG and SF on osteogenic differentiation of ADMSC16.
Materials and methods
Patients
Between November 2013 and April 2014, a sample of periumbilical adipose tissue was harvested from six patients (four males and two females; mean age 56±17 years) for ADMSC isolation. The operations were performed according to all modern surgical and anaesthesiological standards. No peri-operative adverse events occurred. Written informed consent was obtained from all patients.
ADMSC isolation
Harvested human periumbilical adipose tissue was digested in 0.1% collagenase at 37 °C for 1 hour. Cells were collected by centrifugation for 10 minutes at 300 g and the supernatant was discarded. The pellet was suspended in a 75-cm2 flask (Nunc, Sigma-Aldrich S.r.l, Milan, Italy) and incubated at 37 °C under 5% CO2. Non-adherent cells were removed by replacing the medium. Cells were fed every 3 days with 10 mL of complete Dulbecco’s modified Eagle’s medium (DMEM) containing 10% foetal bovine serum (FBS; Gibco, Milan, Italy). When this primary culture of ADMSC reached 80% confluence, cells were harvested using 0.25% Trypsin-EDTA and washed in 10 mL of phosphate-buffered saline.
Preparation of the SF/CBPG scaffold
Sheets of electrospun SF were produced according to the method of Alessandrino et al.17 Five-mm-diameter SF patches were cut by using a sterile 5-mm punch biopsy tool (KLS Martin cod. 28-240-05-07, Umkirch, Germany), placed into Petri dishes, washed with 70% ethanol solution for 1 hour, sterilised under ultraviolet light for 6 hours and then stored at 4 °C until use.
Immediately before use, each SF patch was placed into a well of a 96-multiwell plate (Corning Incorporated, Corning, NY, USA) and SF/CBPG scaffolds were prepared freshly by gelling a platelet concentrate derived from human umbilical cord blood over a 5-mm SF patch with a thickness of 0.5 mm.
CBPG was prepared as described in detail by Parazzi et al.7, with modifications. Briefly, cord blood was collected from healthy neonates and centrifuged at low speed to obtain platelet-rich plasma. The latter was centrifuged at high speed to remove excess plasma and concentrate the platelets to a count of about 1×106/μL. The platelet concentrate was frozen at −80 °C and thawed at the time of use. Gel formation was obtained by the addition of batroxobin in 10% calcium gluconate.
Osteogenic differentiation of ADMSC in a co-culture system with a SF/CBPG scaffold
A 12-well co-culture system (Nunc) was used. ADMSC were placed on the bottom layer at a density of 10,000 cells/well, while SF/CBPG or CBPG alone was placed on the upper layer. The cells were incubated for 15 days in DMEM plus 0.2% bovine serum albumin. A control where ADMSCs were cultured in standard condition (DMEM plus 10% FBS) was also set. After 4 days ADMSC cultured in the presence of a SF/CBPG scaffold showed the formation of three-dimensional (3D) multicellular spheroids.
Reverse transcriptase polymerase chain reaction analysis
Total RNA was prepared using RNeasy Mini Kit (Qiagen, Instrumentation Laboratory, Milan, Italy), and converted into double-stranded cDNA using a RT2 First Strand Kit (Bioscience Corporation, Frederick, MD, USA) according to the manufacturer’s protocol. Quantitative reverse transcriptase polymerase chain reaction (RT-PCR) was performed the using manufacturer’s protocols for the RT2 Profiler™ PCR Array (The Human Notch Signaling Pathway RT2 Profiler™ PCR Array Superarray, Gaithersburg, MD, USA). The analysis was performed on RNA extracted from ADMSC after 3, 7 and 10 days of co-culture with the SF/CBPG scaffold and compared to the experimental control. The GeneGlobe Data Analysis Center (Qiagen) was used to analyse real-time PCR data based on the ΔΔCt method with normalisation of the raw data to HPRT1 and GAPDH housekeeping genes as controls and a criterion of fold-change ≤1.5 considered as indicating up- or down-regulated genes.
For the quantitative evaluation of Osteopontin, Osteocalcin, Col1α1, and RUNX2 cDNA, the cDNA were amplified for 30 or 35 cycles using the following primers:
Osteopontin:5′-ccatctcagaagcagaatctcc-3′;5′-atggtcatcatcgtcgtcc-3′
Osteocalcin:5′-tctctctgacctcacagatccc-3′;5′-taccttattgccctcctgcttg-3′
Col1α1:5′-cccgaccctaagacaaagag-3′;5′-gctacttactgtcctcttctcc-3′
RUNX2:5′-ctccgctgtgaaaaacc-3′;5′-tgaaactcttgcctcgtcc-3′.
Study approval
All patients gave informed consent to the investigations. The ethical committee of the IRCCS Foundation Neurological Institute “Carlo Besta” approved the protocols and investigations, which were carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Results
CBPG modulates the osteogenic differentiation of human ADMSC in an in vitro co-culture system
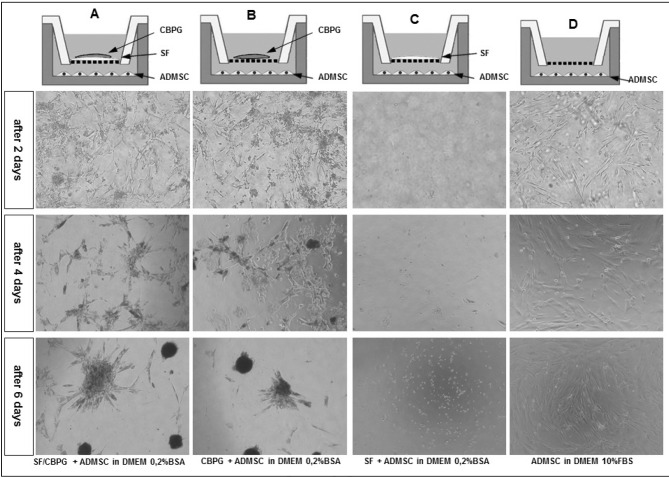
ADMSC (n=6) were cultured with SF/CBPG for 7 days in a cell co-culture trans-well system without contacts, as shown in Figure 1. SG/CBPG scaffolds were prepared freshly by gelling a platelet concentrate derived from human umbilical cord blood over a 0.5-mm SF patch prepared as described by Navone et al.5.
Figure 1.
Schematic representation of the ADMSC co-culture system in the presence of a SF/CBPG scaffold.
A 12-well co-culture system (Nunc) was used. ADMSC were placed on the bottom layer and cultured in DMEM plus 0.2% BSA. In the upper layer we placed the SF/CBPG scaffold (column A), CBPG (column B) and SF (column C). A control in which ADMSC were cultured in standard conditions (DMEM +10% FBS) was also prepared (column D). After the first 4 days we observed a reduction of cell growth and the formation of 3D multicellular spheroids both in cells cultured with the SF/CBPG scaffold and CBPG. The analysis was repeated three times on six different ADMSC lines.
ADMSC: adipose mesenchymal stem cells; SF/CBPG: silk fibroin/cord blood platelet gel; DMEM: standard culture medium.
To prevent ADMSC growth, FBS was replaced in the culture medium by 0.2% BSA. In this condition we observed no cell growth for 3 weeks in the samples without the SF/CBPG scaffold or without CBPG alone. In the samples with the SF/CBPG scaffold and in the one with CBPG alone we found that ADMSC cell growth was comparable to that obtained in standard conditions (10% FBS) in the first 3 days. Surprisingly, after the first 4 days we observed a reduction of cell growth and the formation of 3D multicellular spheroids (Figure 1).
Osteogenic differentiation of human ADMSC characterised by quantitative RT-PCR
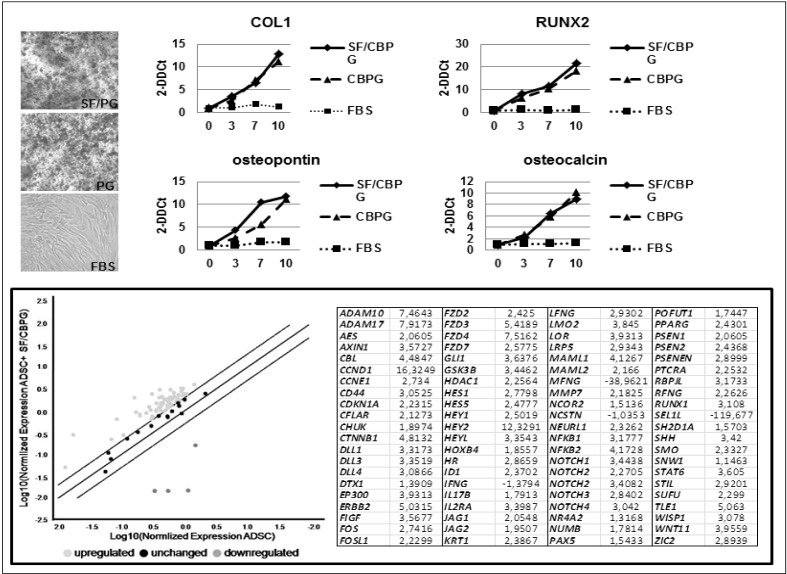
An analysis of alizarin red staining (Figure 2) was performed to confirm the robust osteogenic differentiation of ADMSC cultured in the presence of SF/CBPG and CBPG alone compared to the standard conditions. The osteogenic differentiation was confirmed by quantitative RT-PCR.
Figure 2.
Analysis of alizarin red staining.
Top left: alizarin red staining was performed to confirm the presence of mineral matrix deposition in ADMSC cultured in the presence of SF/CBPG or CBPG. Top right: equal amounts of cDNA isolated from ADMSC co-cultured for 6 days with SF/CBPG or CBPG were amplified for RUNX2, COL1, Osteopontin and Osteocalcin and compared to that obtained from ADMSC cultured in standard conditions. Interestingly, gene expression was significantly higher in SF/CBPG-cultured cells. Bottom: a real-time PCR assay showed the Notch cascade upregulation involved in the osteogenic differentiation of ADMSC cultured in the presence of SF/CBPG or CBPG. ADMSC: adipose mesenchymal stem cells; SF/CBPG: silk fibroin/cord blood platelet gel; DMEM: standard culture medium.
The expression of the typical osteogenic genes COL1, RUNX2, Osteopontin and Osteocalcin18 was tested in the cells cultured in the presence of SF/CBPG and CBPG alone and compared to standard culture conditions (10% FBS). Interestingly, the expression of COL1, RUNX2, Osteopontin and Osteocalcin was significantly higher in SF/CBPG-cultured cells. Of note, in SF/CBPG the analysis was stopped at 10 days because of inadequate quality of RNA, probably due to the small number of viable cells observed and to an abundant amount of mineral matrix. Moreover, mesenchymal stem cells usually need about 2–3 weeks’ stimulation before the endpoint osteogenic assays become positive. Using the SF/CBPG scaffold the same result was reached after 7 days.
We made a further analysis of the osteogenic differentiation pathway with special regard to the Notch signalling cascade. Notch genes are known to play a key role in the osteogenic differentiation of mesenchymal stem cells19.
To this aim, we performed a real-time polymerase chain reaction assay, which showed the Notch cascade upregulation involved in the osteogenic differentiation of ADMSC cultured in the presence of the SF/CBPG scaffold and CBPG alone (Figure 2).
Comparative analysis of ADMSC cultured in platelet lysate of foetal bovine serum
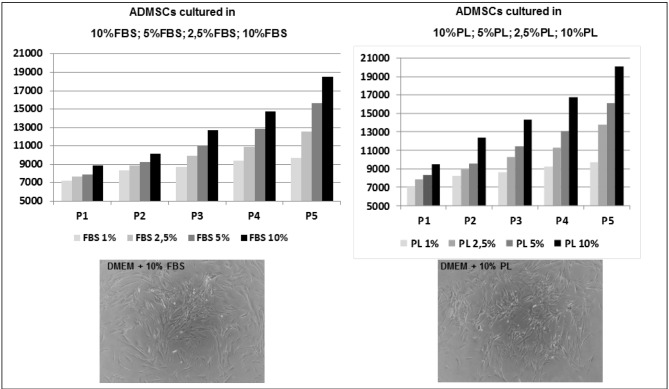
Lastly, we compared the isolation and culture of ADMSC using either FBS or platelet lysate as a medium to evaluate both the growth rate and the presence of osteogenic differentiation as in the presence of SF/CBPG.
Figure 3 shows ADMSC cultures grown in FBS or platelet lysate (PL) medium at passage 3.
Figure 3.
Evaluation of the osteogenic potential of the PL.
Comparison of ADMSC cultured in standard conditions (left) and in the presence of PL (right). ADMSC had a comparable growth rate in the two media up to 12 passages. Different concentrations of FBS and PL were tested. Interestingly, there was no formation of any 3D multicellular spheroids as previously observed in SF/CBPG conditions. ADMSC: adipose mesenchymal stem cells; FBS: foetal bovine serum; PL: platelet lysate.
We observed a comparable growth rate in the two media up to 12 passages as already reported in the literature20. Interestingly, there was no formation of any 3D-multicellular spheroids as previously observed in SF/CBPG conditions. This result suggests that the PL medium is a good substitute of FBS in the expansion of ADMSC via ex vivo cell culture. The use of FBS is not highly recommended by European regulations because some ruminant materials have been implicated in prion transmission. In this context, serum substitutes are desirable for easier translation of mesenchymal stem cells to a clinical use and PL can be considered as a valid alternative. With the latter experiment we also showed that only ADMSC cultured in the presence of a SF/CBPG scaffold or CBPG alone can be committed to an osteogenic fate, while the presence of PL in culture medium has no effect on ADMSC differentiation.
Discussion
The applications of CBPG have spread into many fields of clinical medicine, especially in sports medicine and in orthopaedic surgery. CBPG has been considered a promising tool to treat cartilage or bone injuries when pharmacological treatments are ineffective. The mechanism of “platelet-therapy” is attributed to various growth factors released by platelets, which can stimulate cell growth, migration, mobilisation and differentiation. Through these biofactors, CBPG can exert its ability to promote tissue regeneration.
The aim of this work was to investigate the possibility of using a SF/CBPG scaffold in cell therapy protocols because of advantages related to biocompatibility and biodegradability. ADMSC were used to test the effects of SF/CBPG in culture on mesenchymal stem cell growth and function.
Previous studies7 have already found that, when placed in specific media, mesenchymal stem cells from different sources grown in PL show increased expression of genes underlying the osteogenic differentiation pathway. In this study we demonstrate for the first time by molecular analysis that an indirect contact between SF/CBPG and ADMSC leads to the formation of 3D spheres caused by an intense overexpression of COL1 and RUNX2, known as osteogenic genes.
These results suggest that a SF/CBPG patch can be a useful tool for bone regenerative medicine. Further studies are needed to assess the effectiveness of the SF/CBPG patch in preclinical experiments using animal models.
Conclusions
ADMSC are an attractive source of cell therapy for the regeneration of damaged tissues, because of these cells’ capacity for self-renewal and their ability to differentiate into various cell lineages. They are also relatively easy to isolate and expand in culture. Clinical studies on the use of ADMSC in the reconstruction of bone defects have given promising results. The process of osteogenic differentiation does, however, need to be optimised.
SF is a natural biocompatible and biodegradable protein with remarkable mechanical properties. It is considered to be a useful material for facilitating collagen synthesis and re-epithelialisation in certain settings. Platelet gel, rich in growth factors, is also able to support tissue regeneration.
This study investigated the effects of’ combined use of CBPG and SF on osteogenic differentiation of ADMSC, showing that a SF/CBPG scaffold can support both ADMSC growth and osteogenic differentiation, useful features for osteogenic tissue engineering applications.
Acknowledgements
This work was supported by IRCCS Foundation Neurological Institute “C. Besta”.
Footnotes
Authorship contributions
ALMF: manuscript writing, collection and assembly of data. NG: collection of CBPG. MPS: collection of adipose tissue. VT and VC: carried out RT-PCR tests. GA: manuscript writing. PR: study conception and design, manuscript review. EP: final approval of manuscript. All Authors read and approved the final manuscript.
Disclosure of conflicts of interest
PR and NG declare that they are co-inventors of US patent 8,501,170. The remaining Authors declare that they have no financial competing interests.
References
- 1.Farè S, Torricelli P, Giavaresi G, et al. In vitro study on silk fibroin textile structure for anterior cruciate ligament regeneration. Mater Sci Eng C Mater Biol Appl. 2013;33:3601–8. doi: 10.1016/j.msec.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Melke J, Midha S, Ghosh S, et al. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016;31:1–16. doi: 10.1016/j.actbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Taddei P, Chiono V, Anghileri A, et al. Silk fibroin/gelatin blend films crosslinked with enzymes for biomedical applications. Macromol Biosci. 2013;13:1492–510. doi: 10.1002/mabi.201300156. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Bella E, Lee CS, et al. The synergistic effects of 3-D porous silk fibroin matrix scaffold properties and hydrodynamic environment in cartilagine tissue regeneration. Biomaterials. 2010;31:4672–81. doi: 10.1016/j.biomaterials.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Navone SE, Pascucci L, Dossena M, et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res Ther. 2014;5:7. doi: 10.1186/scrt396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roh DH, Kang SY, Kim JY, et al. Wound healing effect of silk fibroin/alginate-blended sponge in full thickness skin defect of rat. J Mater Sci Mater Med. 2006;17:547–52. doi: 10.1007/s10856-006-8938-y. [DOI] [PubMed] [Google Scholar]
- 7.Parazzi V, Lavazza C, Boldrin V, et al. Extensive characterization of platelet gel releasate from cord blood in regenerative medicine. Cell Transplant. 2015;24:2573–84. doi: 10.3727/096368915X687471. [DOI] [PubMed] [Google Scholar]
- 8.Forni F, Marzagalli M, Tesei P, Grassi A. Platelet gel: applications in dental regenerative surgery. Blood Transfus. 2013;11:102–7. doi: 10.2450/2012.0007-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longo F, Guida A, Aversa C, et al. Platelet rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw: personal experience and review of the literature. Int J Dent. 2014:1–7. doi: 10.1155/2014/298945. Article ID 298945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jinnin M, Ihn H, Mimura Y, et al. Regulation of fibrogenic/fibrolytic genes by platelet-derived growth factor C, a novel growth factor, in human dermal fibroblasts. J Cell Physiol. 2005;202:510–7. doi: 10.1002/jcp.20154. [DOI] [PubMed] [Google Scholar]
- 11.Moroz A, Deffune E. Platelet-rich plasma and chronic wounds: remaining fibronectin may influence matrix remodeling and regeneration success. Cytotherapy. 2013;15:1436–9. doi: 10.1016/j.jcyt.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: implications in tissue regeneration. World J Stem Cells. 2014;6:312–21. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilar E, Bagó JR, Soler-Botija C, et al. Fast-proliferating adipose tissue mesenchymal-stromal-like cells for therapy. Stem Cells Dev. 2014;23:2908–20. doi: 10.1089/scd.2014.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesimaki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–9. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Civinini R, Macera A, Nistri L, et al. The use of autologous blood-derived growth factors in bone regeneration. Clin Cases Miner Bone Metab. 2011;8:25–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Mottaghitalab F, Hosseinkhani H, Shokrgozar MA, et al. Silk as a potential candidate for bone tissue engineering. J Control Release. 2015;215:112–28. doi: 10.1016/j.jconrel.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Alessandrino A, Marelli B, Arosio C, et al. Electrospun silk fibroin mats for tissue engineering. Eng Life Sci. 2008;8:219–225. [Google Scholar]
- 18.Xu FT, Li HM, Yin QS, et al. Effect of activated autologous platelet-rich plasma on proliferation and osteogenic differentiation of human adipose-derived stem cells in vitro. Am J Transl Res. 2015;7:257–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Dishowitz M, Zhu F, Sundararaghavan H, et al. Jagged1 immobilization to an osteoconductive polymer activates the Notch signaling pathway and induces osteogenesis. J Biomed Mater Res A. 2014;102:1558–67. doi: 10.1002/jbm.a.34825. [DOI] [PubMed] [Google Scholar]
- 20.Kinzebach S, Dietz L, Klüter H, et al. Functional and differential proteomic analyses to identify platelet derived factors affecting ex vivo expansion of mesenchymal stromal cells. BMC Cell Biol. 2013;14:48. doi: 10.1186/1471-2121-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]