Abstract
Although retinal vasculitis is common in multiple sclerosis (MS), it is not known if MS is associated with quantitative abnormalities in retinal blood vessels (BVs). Optical coherence tomography (OCT) is suitable for examining the integrity of the anterior visual pathways in MS. In this paper we have compared the size and number of retinal blood vessels in patients with MS, with and without a history of optic neuritis (ON), and control subjects from the cross-sectional retinal images from OCT. Blood vessel diameter (BVD), blood vessel number (BVN), and retinal nerve fiber layer thickness (RNFLT) were extracted from OCT images collected from around the optic nerves of 129 eyes (24 control, 24 MS + ON, 81 MS-ON) of 71 subjects. Associations between blood vessel metrics, MS diagnosis, MS disability, ON, and RNFLT were evaluated using generalized estimating equation (GEE) models. MS eyes had a lower total BVD and BVN than control eyes. The effect was more pronounced with increased MS disability, and persisted in multivariate models adjusting for RNFLT and ON history. Twenty-nine percent (29%) of MS subjects had fewer retinal blood vessels than all control subjects. MS diagnosis, disability, and ON history were not associated with average blood vessel size. The relationship between MS and lower total BVD/BVN is not accounted for by RNFLT or ON. Further study is needed to determine the relationship between OCT blood vessel metrics and qualitative retinal blood vessel abnormalities in MS.
OCIS codes: (110.4500) Optical coherence tomography; (170.5755) Retina scanning; (330.4300) Vision system - noninvasive assessment; (330.7329) Visual optics, pathology; (100.2960) Image analysis
1. Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated disease of the central nervous system (CNS) [1, 2] that frequently results in pathological changes in the visual pathways with resulting vision morbidity. The anterior visual pathway, particularly the retinal ganglion cells (RGCs) that comprise the optic nerve, is commonly affected in MS, both acutely through optic neuritis (ON) and chronically with retinal ganglion cell atrophy, which can occur independently from ON [3]. Imaging the anterior visual pathway provides a structural correlate of the clinical and functional consequences of axonal and neuronal loss in MS [4]. Optical coherence tomography (OCT) [5], a widely used non-invasive retinal imaging technique, generates high resolution cross-sectional images of the retina based on the interference patterns produced by low coherence light reflected from retinal tissues [6]. Structural retinal defects, including thinning of the peripapillary retinal nerve fiber layer (RNFL) [7–11] and thinning of the macular ganglion cell component [12, 13], have been demonstrated in MS eyes using OCT. Both of these measurements are a consequence of RGC atrophy [14] and accordingly are associated with history of ON and MS progression [15]. Non-RGC associated retinal changes have also been demonstrated in MS, including qualitative involvement of retinal blood vessels (BVs) in MS. Published data include histopathologic evidence for vascular inflammation in 20% of subjects in a post mortem series [16] and clinical evidence of retinal phlebitis in 16% of patients [17]. These changes, as well as other structural retinal changes such as microcystic macular edema of the retinal inner nuclear layer [18], and thinning of non-ganglion cell retinal layers [19], seem to be independent from MS effects on the RGCs.
Quantitative retinal vessel structural measurements are widely studied as a marker of neurological disease due to their ease of measurement and anatomical connections to the neuro-vascular system. These have been demonstrated to be associated with risk of cerebral vascular disease even after adjusting for systemic vascular risk factors [20]. Quantitative retinal blood vessel metrics are potential markers of MS based on the known clinical relationships between MS and retinal vessel inflammation [17, 21, 22], and between MS and ganglion cell loss, which potentially decreases metabolic demand [23, 24]. However, these relationships have not been thoroughly explored using OCT. There is a single report of a decrease in relative blood flow to the optic nerve head in MS eyes, measured using OCT angiography [25]. However, this technique is not yet widely available in the clinical setting, compared to quantitative static retinal blood vessel metrics which can be extracted from OCT scans that are acquired using currently available clinical instrumentation [26–28]. We sought to investigate quantitative retinal blood vessel metrics as a marker of MS using a cross sectional study of retinal blood vessel diameters in individuals with and without MS, and with and without a history of ON. We hypothesized that the measured blood vessel metrics from OCT images of patients with MS would be significantly different from those of healthy controls. This study is an important initial step in evaluating OCT measures of retinal vessel structure as a quantifiable in vivo biomarker of MS and its severity, and represents a novel assessment of retina blood vessel metrics in MS patients.
2. Material and methods
2.1 Subjects
Flyers were distributed to participants within the North American Research Committee on Multiple Sclerosis registry and to participants from previous studies from our laboratory who had expressed interest in future research opportunities. All patients with MS had their diagnosis confirmed by their treating neurologist, based on revised McDonald criteria [29]. Participant recruitment occurred between February 2013 and September 2013. Criteria for inclusion were: physician-confirmed diagnosis of MS; age range 18-64 years; expanded disability status scale (EDSS) [30] score <8.0; ability to visit our laboratory for OCT imaging; and physician approval for study participation. Participants with MS (n = 58) and healthy controls without any history of ocular or neurologic disease (n = 13), were recruited for the study. We excluded some of the eyes (2 control, 2 MS-mild, and 5 MS-moderate) due to missing data (peripapillary OCT scan was not done), poor OCT image quality (where extensive manual correction would have been required) or centration, which may artificially decrease or increase the RNFL thickness (RNFLT), but might also modify the diameter of blood vessels [31]. Patients with ophthalmologic or neurologic disorders (other than MS) including glaucoma, diabetic and hypertensive retinopathy, and/or a refractive error greater than 6 diopters, were excluded from the study. ON and other eye health history were determined by self-report using a Biophotonics Imaging Lab questionnaire. The protocol was reviewed and approved by the Institutional Review Board at the University of Illinois at Urbana-Champaign and all participants signed an informed consent prior to participating in the study. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study.
The participants were divided into three groups according to MS disability level: mild (EDSS 1.0-3.5), moderate (EDSS 4.0-5.5), and severe (EDSS 6.0-7.5). Twenty-four (24) eyes from 13 healthy control subjects, 38 eyes from 20 MS-Mild patients, 31 eyes from 19 MS-Moderate patients, and 36 eyes from 19 MS-Severe patients were included in the analysis. Twenty-four (24) eyes, all in MS patients, had a history of ON.
2.2 Retinal imaging and image processing
Retinal imaging was performed using a commercial OCT system (Spectralis HRA-OCT, Heidelberg Engineering, Heidelberg, Germany). The OCT scanning protocol consisted of a 3.9 mm diameter circumferential scan centered on the ONH (1536 A-scans). Differences in retinal magnification were small as highly myopic or hyperopic subjects (refractive error greater than 6 diopters) were excluded from this study. Each circumferential scan was subsequently unwrapped and displayed as a rectangular B-scan OCT image. En face IR-SLO images were also obtained at the same time. All the OCT scans were performed by same group of people, all with the same level of training, and with 6 µm/pixel lateral and 3.5 µm/pixel axial digital resolution [32], respectively. After converting the images from the proprietary E2E format to tiff using the commercial viewing software (Heidelberg Eye Explorer, version 1.7.1.0), OCT images were flattened by manually fitting a line to the Bruch's membrane (BM) in order to make all OCT scans uniform.
Blood and blood flow within retinal vessels attenuates the OCT signal underneath the vessel and produces shadows which appear as vertically elongated “black bars” that transverse multiple layers in the OCT images. Using these shadows, blood vessels in the flattened OCT images were automatically segmented using freely available open source code (Optical Coherence Tomography Segmentation and Evaluation GUI (OCTSEG), Pattern Recognition Lab, Friedrich-Alexander University, Erlangen-Nuremberg, Germany) developed based on research work on OCT [33]. All segmentations were performed by the same person, who was blinded to the study groupings. We manually corrected the automatically segmented images after comparing each OCT image with the corresponding flattened IR-SLO image to identify and exclude features of the retina, other than the blood vessels, that caused optical shadowing. In a few cases, when the blood vessels were appearing oblique, we manually corrected the diameter to avoid adding extra diameter to the blood vessel measurement. This was done by measuring from the IR-SLO image the angular deviation of the vessel from the ideal orientation orthogonal to the image plane, and multiplying the measured diameter by the sine of the angle. We also corrected any segmentation artifacts, such as when multiple blood vessels were erroneously paired together to appear as a single blood vessel in the OCT image. We discarded all automatically segmented blood vessels that were 2 pixels or less in size (less than 12 µm in diameter) from the analysis, which could erroneously have appeared due to image noise. Similar methodologies for measuring retinal blood vessels from OCT scans have been shown to be reliable and reproducible [28, 34]. The inner and outer nerve fiber layer boundaries (INFL and ONFL, respectively) in the same OCT images were also segmented using the same software, initially automatically, and then occasionally corrected manually for any anomalies. The OCTSEG software generates a metadata containing the diameter of the blood vessels and the RNFLT in pixels, which was then read and processed in MatlabTM.
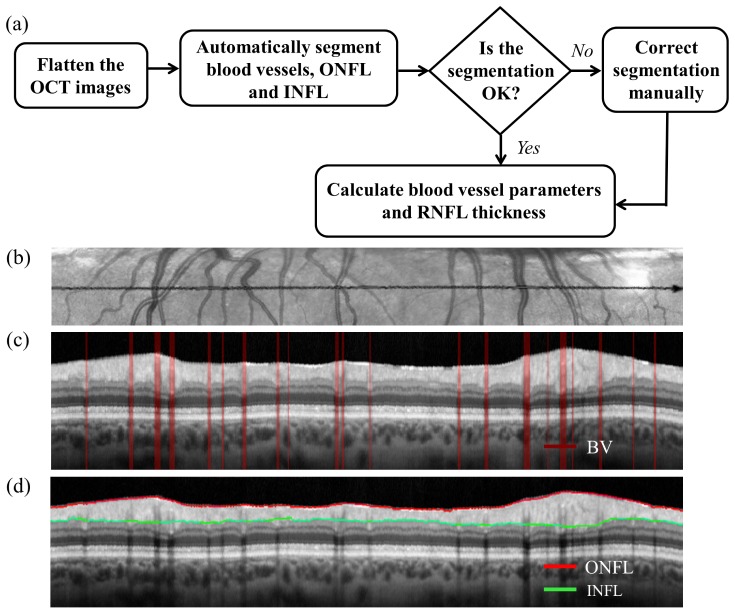
Figure 1(a) shows the flowchart of the segmentation and data analysis method while Fig. 1(b) shows a representative flattened IR-SLO image. Figure 1(c) shows the segmented blood vessels, and Fig. 1(d) shows the segmented inner and outer RNFL boundaries in a representative OCT image. The red lines in Fig. 1(c) represent the blood vessels. The separation between the outer and inner NFL is the RNFLT, which is averaged over the entire scan length. Different blood vessel metrics: total blood vessel diameter, total number of blood vessels, and average blood vessel diameter, were calculated from the segmented data.
Fig. 1.
a) Flowchart of the presented method, b) flattened IR-SLO image, c) segmented blood vessels, and d) segmented INFL and ONFL in the OCT image. The red lines in (c) represent the blood vessels.
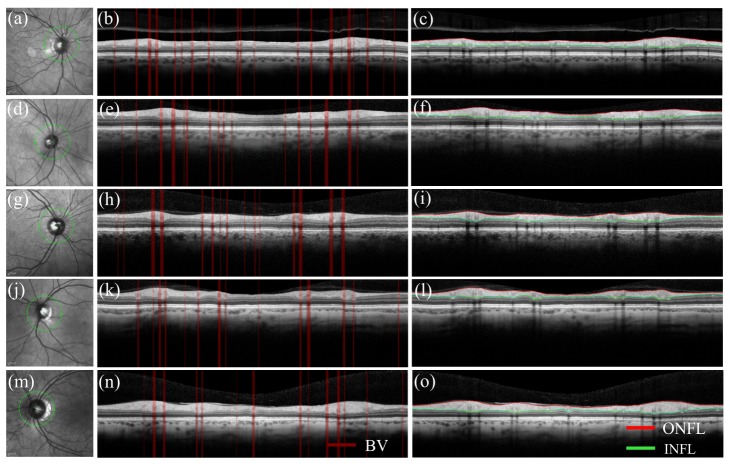
Figure 2 shows en face IR-SLO images, segmented blood vessels, and inner - outer nerve fiber layers of circumferential peripapillary OCT images, respectively, for: a)-c) healthy control; d)-f) mild MS; g)-i) moderate MS; j)-l) severe MS; and m)-o) ON subjects. The green circles on the IR-SLO images show the locations of the circumferential cross-sectional scans and the green arrows show the direction of the scan.
Fig. 2.
En-face IR-SLO images, and segmented blood vessels and inner - outer nerve fiber layers of circumferential peripapillary OCT images, respectively for: a)-c) healthy control; d)-f) mild MS; g)-i) moderate MS; j)-l) severe MS; and m)-o) ON subjects. The green circles show the scan locations and the green arrows show the scan directions.
2.3 Statistical analysis
All the statistical analyses were performed using SPSS (Version 23.0, IBM, Chicago, IL). At the eye level, each outcome variable (RNFLT, total blood vessel diameter, blood vessel number and average blood vessel diameter) was examined using generalized estimating equation (GEE) models [35], which account for within-subject correlation. Main effects of interest were MS diagnosis, MS disability (i.e., EDSS), and history of ON. Studied covariates were age, sex, and RNFLT (for blood vessel metrics). Exploratory analysis revealed all outcomes to be approximately normally distributed and therefore they were modeled as such. All outcomes were linearly related to MS disability, with control eyes considered to have no disability. Therefore, MS and disability were modeled as a single ordinal variable (0 = control, 1 = mild MS, 2 = moderate MS, 3 = severe MS). Univariate GEE models between each main variable and covariate were constructed for each outcome. Forward and backward methodologies were applied to construct a final multivariate model for each outcome with p<0.2 at the univariate level being the criterion for being introduced (forward modeling) or retained (backward modeling) in the final multivariate model. At the subject level, the proportion of participants with MS with outcome values outside the range of control subject values for both eyes was calculated.
3. Results
Table 1 shows the subject demographics and group characteristics. MS and control subjects did not differ by age (p = 0.59, t-test). However, there was a positive relationship between MS disability and age (p<0.05, linear regression). A higher proportion of MS subjects were male compared with normal subjects (p<0.05, Fishers exact).
Table 1. Subject demographics and group characteristics.
| Control | Mild MS | Moderate MS | Severe MS | |
|---|---|---|---|---|
| Eyes (Patients) | 24 (13) | 38 (20) | 31 (19) | 36 (19) |
|
Age (Years) Mean (SD) |
51.21 (10.6) | 50.31 (9.9) | 54.61 (6) | 54.86 (5.8) |
| Female Gender, N (%) | 22 (92) | 23 (61) | 24 (77) | 28 (78) |
| Optic Neuritis Eyes, N (%) | 0 (0) | 4 (11) | 13 (42) | 7 (19) |
|
Disease Duration (Years) Mean (SD) |
N/A | 7.88 (5.7) | 16.39 (9.7) | 14.2 (9.6) |
3.1 Retinal nerve fiber layer thickness
Peripapillary RNFLT ranged from 49.5 to 122.4 µm and was associated with age (p<0.05) and inversely associated with MS disability (p<0.05) and history of optic neuritis (p<0.05), but not sex in univariate analysis (Table 2). These remained significant in the full multivariate model, which adjusted for all covariates (Table 3). All of these remained significant in the final multivariate model with a change of 0.25 µm/year of age, −3.2 µm per stage of MS disability (control = 0) and −12.0 µm for history of optic neuritis (p<0.05, GEE).
Table 2. Univariate relationships between each retinal outcome variable and covariates in control (MS disability = 0) and MS eyes with and without optic neuritis. Each cell contains the beta coefficient (95% confidence interval) for the outcome in that row as a function of the covariate in that column adjusted for within subject correlation using GEE models. * p < 0.05; ** p < 0.005.
| Age (Years) | Male Gender |
MS disability (Control = 0) |
Optic Neuritis | RNFLT (μm) | |
|---|---|---|---|---|---|
| RNFL thickness (μm) | 0.23 (0.001, 0.470)* |
1.66 (−4.21, 7.52) |
−3.75 (−5.42, −2.07)** |
−14.45 (−23.45, −5.45)** |
N/A |
| Total blood vessel diameter (μm) | −0.72 (−4.25, 2.82) |
46.55 (−24.06, 117.17) |
−61.48 (−89.08, −33.87)** |
−60.97 (−127.18, 5.24) |
2.97 (0.62, 5.33)* |
| Total blood vessel number | −0.06 (−0.11, 0.00) |
0.75 (−0.56, 2.06) |
−1.36 (−1.80, −0.92)** |
−0.74 (−1.92, 0.44) |
0.03 (−0.01, 0.07) |
| Average blood vessel diameter (μm) | 0.22 (0.03, 0.42)* |
0.76 (−3.14, 4.66) |
1.42 (0.00, 2.83)* |
1.81 (−1.78, 5.40) |
0.08 (−0.04, 1.61) |
Table 3. Multivariate relationships between each retinal outcome variable and covariates, in control (MS disability = 0) and MS eyes with and without optic neuritis. Each cell contains the beta coefficient (95% confidence interval) for the outcome in that row as a function of the covariate in that column adjusted for all other covariates and within subject correlation using GEE models. * p < 0.05; ** p < 0.005.
| Age (Years) | Male Gender |
MS Disability (Control = 0) |
Optic Neuritis | RNFLT (μm) | |
|---|---|---|---|---|---|
| RNFL thickness (μm) | 0.25 (0.04, 0.46)* |
1.39 (−3.62, 6.40) |
−3.16 (−4.72, −1.60)** |
−12.04 (−21.80, −2.28)* |
N/A |
| Total blood vessel diameter (μm) | −0.04 (−3.14, 3.05) |
41.69 (−28.32, 111.71) |
−54.46 (−84.82, −24.11)** |
−5.69 (−91.56, 80.19) |
1.65 (−0.86, 4.15) |
| Total blood vessel number | −0.03 (−0.08, 0.02) |
0.76 (−0.53, 2.04) |
−1.28 (−1.79, −0.77)** |
0.04 (−1.27, 1.35) |
0.01 (−0.04, 0.05) |
| Average blood vessel diameter (μm) | 0.15 (−0.05, 0.35) |
0.35 (−3.46, 4.17) |
1.59 (0.1, 3.09)* |
1.29 (−2.76, 5.34) |
0.08 (−0.05, 0.21) |
3.2 Total blood vessel diameter
Total peripapillary blood vessel diameter ranged from 348 to 1386 µm and was associated with MS disability (p<0.05) and RNFLT (p<0.05), but not history of ON, age, or sex in univariate models (Table 2). In the full multivariate model, which adjusted for all covariates (Table 3), only MS disability was significant (Table 3). Only MS-disability remained in the final model with a decrease of −59.7 µm of blood vessel diameter per stage of MS-disability (control = 0) (p<0.05).
3.3 Total blood vessel number
Number of peripapillary blood vessels ranged from 5 to 17 and was associated only with MS disability (p<0.05), but not age, sex, ON, or RNFLT in univariate models (Table 2). In the full multivariate model, accounting for all covariates, MS-disability remained significant (Table 3). Only MS-disability remained in the final model with a decrease of 1.35 blood vessels per stage of MS disability (control = 0) (p<0.05). In 17/58 (29%) of MS subjects, all tested eyes had a total blood vessel number less than 14, the lowest recorded blood vessel number across control eyes.
3.4 Average blood vessel diameter
Average blood vessel diameter ranged from 42.6 to 87.2 µm and was associated with age (p<0.05) and MS-disability (p<0.05), but not sex, RNFLT, or history of ON in univariate models (Table 2). In the full multivariate model, accounting for all covariates, MS-disability remained significant (Table 3).
4. Discussion
Retinal changes including reduction of peripapillary RNFLT and macular ganglion cell layer [7, 8,11], microcystic macular edema of the retinal inner nuclear layer [18], and thinning of non-ganglion cell retinal layers [19], have been well documented in MS using static OCT imaging. This study contributes evidence for additional, previously uncharacterized, changes on cross-sectional OCT in MS patients, namely reduction in total diameter and number of peripapillary retinal vessels. We demonstrate these parameters to be reduced compared to healthy control subjects and find that the degree of reduction correlates with the degree of MS disability. Though average blood vessel diameter is minimally larger in MS eyes in univariate and full multivariate models, it was not significantly different in the final multivariate model. Therefore, we attribute the decrease in total vessel diameter associated with MS to a decrease in number of vessels.
In exploratory analysis, control eyes differed from MS eyes, and MS eyes differed by severity. Rather than present two distinct analyses (control vs. MS, and MS mild vs. moderate vs. severe), a single analysis was presented. This increases statistical efficiency. Further, the reported change in RNFLT with advancing age was an increase which may reflect the narrow age range in our sample (3 decades) as well as possible spurious associations. Note that the point estimate corresponds to a RNFLT increase of 6 microns/decade of age, which is near the detection limit of OCT. Excluding age from the multivariate RNFL model does not impact the results.
One potential reason for a smaller number of retinal vessels in MS subjects may be ganglion cell loss, leading to lower metabolic demands on the retinal circulation. Wang, et. al., showed lower optic nerve head blood flow in MS patients with a history of ON, compared with both MS patients without a history of ON, and control subjects, which supports such a mechanism in the optic nerve head circulation [25]. They also demonstrated no difference in parafoveal blood flow between controls, MS-ON, and MS + ON, arguing against such a mechanism in the retinal circulation. Similarly, our results demonstrate no association between retinal blood vessel metrics and clinical (history of ON) or structural (RNFL) measures of RGC injury. Though a direct comparison is challenging due to the different anatomical regions measured, our results contrast the parafoveal blood flow measures in Wang, et. al. as we find a difference between MS and control subjects. Further investigation is necessary to directly compare dynamic (i.e. blood flow) with structural (i.e. blood vessel size and number) measures. Additional insight would be gained by comparing retinal vessels in MS subjects with those in patients with optic neuropathies of other causes.
Alternatively, the decrease in blood vessels could be related to known qualitative inflammatory retinal vascular pathology in MS. The proportion of our subjects with abnormal retinal blood vessel number (29%) is similar to the proportion reported to have retinal blood vessel changes at autopsy (20%) [16] and observed clinically (16%) [36]. Furthermore, retinal phlebitis has been reported to correlate with MS activity, but not RGC loss, which is similar in pattern to the associations we found for retinal blood vessel number and total diameter. We are not proposing that there is active vasculitis in the peripapillary vessels that were measured. Rather, that it is possible that the peripapillary changes represent adaptive or pathological changes related to current or prior phlebitis in the peripheral retina. Though this evidence is circumstantial, and the location of the qualitative pathology (in the retinal periphery) is distant from the location of our blood vessel measurements (around the optic nerve), the connected nature of blood vessels makes it conceivable that there are changes in proximal vessels associated with distal pathology. Future study is needed to compare clinical and imaging findings in persons with MS in order to determine if blood vessel metrics are associated with current or past retinal phlebitis.
Our study has several limitations. The cross-sectional nature of our study precludes commenting on the longitudinal nature of these changes within individuals. We are also unable to comment on correlations with retinal phlebitis within individuals since the peripapillary nature of the imaging acquired is not sufficient to assess this. We were unable to differentiate arteries from veins, and an important area for future study is to investigate each type of vessel separately, perhaps using comparisons to fundus photography to make the distinction. Further, wide field retinal exams were not available for this group of subjects to facilitate a direct comparison with active qualitative retinal vascular changes. This is an important area of future research.
5. Conclusions
Our results demonstrate quantitative changes in the total diameter and number of retinal blood vessels in MS eyes. Though the reasons for this are not certain, comparison with RGC measures suggest that this is not a secondary change in response to RGC loss. Further studies are needed to confirm our findings, and explore the implications and biological basis of decreased blood vessel diameter and number in MS. Finally, additional studies are needed to determine whether these changing blood vessel metrics can be used as early indicators for disease progression in MS.
Acknowledgments
We thank Eric Chaney for his assistance with managing our IRB protocol and Darold Spillman from the Beckman Institute for Advanced Science and Technology, and Imaging at Illinois, for providing logistical, operations, and information technology support for this project. We also thank Dr. Joelle Hallak, Director of the Ophthalmic Clinical Trials and Translational Center, Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, for her helpful comments and input. This research was supported in part by a grant from the National Institutes of Health (NIBIB R01 EB013723, S.A.B., NMSS IL 0003, R.W.M., NEI K23 EY024345, H.E.M.), the National Multiple Sclerosis Society (IL 0003), and an unrestricted grant from Research to Prevent Blindness to the UIC Department of Ophthalmology and Visual Sciences. Additional information can be found at http://biophotonics.illinois.edu.
References and links
- 1.Prineas J., “Pathology of multiple sclerosis,” in Handbook of Multiple Sclerosis, Cook S., ed. (Marcel Dekker, New York, 2011), pp. 289–324. [Google Scholar]
- 2.Motl R. W., Pilutti L. A., “The benefits of exercise training in multiple sclerosis,” Nat. Rev. Neurol. 8(9), 487–497 (2012). 10.1038/nrneurol.2012.136 [DOI] [PubMed] [Google Scholar]
- 3.Schmitt C., Strazielle N., Ghersi-Egea J. F., “Brain leukocyte infiltration initiated by peripheral inflammation or experimental autoimmune encephalomyelitis occurs through pathways connected to the CSF-filled compartments of the forebrain and midbrain,” J. Neuroinflammation 9(1), 187 (2012). 10.1186/1742-2094-9-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balantrapu S., Sandroff B. M., Pula J. H., Motl R. W., “Integrity of the anterior visual pathway and its association with ambulatory performance in multiple sclerosis,” Mult. Scler. Int. 2013, 481035 (2013). 10.1155/2013/481035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang D., Swanson E. A., Lin C. P., Schuman J. S., Stinson W. G., Chang W., Hee M. R., Flotte T., Gregory K., Puliafito C. A., et, “Optical coherence tomography,” Science 254(5035), 1178–1181 (1991). 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hee M. R., Izatt J. A., Swanson E. A., Huang D., Schuman J. S., Lin C. P., Puliafito C. A., Fujimoto J. G., “Optical coherence tomography of the human retina,” Arch. Ophthalmol. 113(3), 325–332 (1995). 10.1001/archopht.1995.01100030081025 [DOI] [PubMed] [Google Scholar]
- 7.Akçam H. T., Capraz I. Y., Aktas Z., Batur Caglayan H. Z., Ozhan Oktar S., Hasanreisoglu M., Irkec C., “Multiple sclerosis and optic nerve: an analysis of retinal nerve fiber layer thickness and color Doppler imaging parameters,” Eye (Lond.) 28(10), 1206–1211 (2014). 10.1038/eye.2014.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher J. B., Jacobs D. A., Markowitz C. E., Galetta S. L., Volpe N. J., Nano-Schiavi M. L., Baier M. L., Frohman E. M., Winslow H., Frohman T. C., Calabresi P. A., Maguire M. G., Cutter G. R., Balcer L. J., “Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis,” Ophthalmology 113(2), 324–332 (2006). 10.1016/j.ophtha.2005.10.040 [DOI] [PubMed] [Google Scholar]
- 9.Gordon-Lipkin E., Chodkowski B., Reich D. S., Smith S. A., Pulicken M., Balcer L. J., Frohman E. M., Cutter G., Calabresi P. A., “Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis,” Neurology 69(16), 1603–1609 (2007). 10.1212/01.wnl.0000295995.46586.ae [DOI] [PubMed] [Google Scholar]
- 10.Pulicken M., Gordon-Lipkin E., Balcer L. J., Frohman E., Cutter G., Calabresi P. A., “Optical coherence tomography and disease subtype in multiple sclerosis,” Neurology 69(22), 2085–2092 (2007). 10.1212/01.wnl.0000294876.49861.dc [DOI] [PubMed] [Google Scholar]
- 11.Lamirel C., Newman N. J., Biousse V., “Optical coherence tomography (OCT) in optic neuritis and multiple sclerosis,” Rev. Neurol. (Paris) 166(12), 978–986 (2010). 10.1016/j.neurol.2010.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saidha S., Syc S. B., Durbin M. K., Eckstein C., Oakley J. D., Meyer S. A., Conger A., Frohman T. C., Newsome S., Ratchford J. N., Frohman E. M., Calabresi P. A., “Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness,” Mult. Scler. 17(12), 1449–1463 (2011). 10.1177/1352458511418630 [DOI] [PubMed] [Google Scholar]
- 13.Walter S. D., Ishikawa H., Galetta K. M., Sakai R. E., Feller D. J., Henderson S. B., Wilson J. A., Maguire M. G., Galetta S. L., Frohman E., Calabresi P. A., Schuman J. S., Balcer L. J., “Ganglion cell loss in relation to visual disability in multiple sclerosis,” Ophthalmology 119(6), 1250–1257 (2012). 10.1016/j.ophtha.2011.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cettomai D., Hiremath G., Ratchford J., Venkatesan A., Greenberg B. M., McGready J., Pardo C. A., Kerr D. A., Frohman E., Balcer L. J., McArthur J. C., Calabresi P. A., “Associations between retinal nerve fiber layer abnormalities and optic nerve examination,” Neurology 75(15), 1318–1325 (2010). 10.1212/WNL.0b013e3181f735bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talman L. S., Bisker E. R., Sackel D. J., Long D. A., Jr, Galetta K. M., Ratchford J. N., Lile D. J., Farrell S. K., Loguidice M. J., Remington G., Conger A., Frohman T. C., Jacobs D. A., Markowitz C. E., Cutter G. R., Ying G. S., Dai Y., Maguire M. G., Galetta S. L., Frohman E. M., Calabresi P. A., Balcer L. J., “Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis,” Ann. Neurol. 67(6), 749–760 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolinko Y., Krakorova K., Cendelin J., Tonar Z., Kralickova M., “Microcirculation of the brain: morphological assessment in degenerative diseases and restoration processes,” Rev. Neurosci. 26(1), 75–93 (2015). 10.1515/revneuro-2014-0049 [DOI] [PubMed] [Google Scholar]
- 17.Sepulcre J., Murie-Fernandez M., Salinas-Alaman A., García-Layana A., Bejarano B., Villoslada P., “Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS,” Neurology 68(18), 1488–1494 (2007). 10.1212/01.wnl.0000260612.51849.ed [DOI] [PubMed] [Google Scholar]
- 18.Saidha S., Sotirchos E. S., Ibrahim M. A., Crainiceanu C. M., Gelfand J. M., Sepah Y. J., Ratchford J. N., Oh J., Seigo M. A., Newsome S. D., Balcer L. J., Frohman E. M., Green A. J., Nguyen Q. D., Calabresi P. A., “Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study,” Lancet Neurol. 11(11), 963–972 (2012). 10.1016/S1474-4422(12)70213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saidha S., Syc S. B., Ibrahim M. A., Eckstein C., Warner C. V., Farrell S. K., Oakley J. D., Durbin M. K., Meyer S. A., Balcer L. J., Frohman E. M., Rosenzweig J. M., Newsome S. D., Ratchford J. N., Nguyen Q. D., Calabresi P. A., “Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography,” Brain 134(2), 518–533 (2011). 10.1093/brain/awq346 [DOI] [PubMed] [Google Scholar]
- 20.Moss H. E., “Retinal vascular changes are a marker for cerebral vascular diseases,” Curr. Neurol. Neurosci. Rep. 15(7), 40 (2015). 10.1007/s11910-015-0561-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engell T., “Neurological disease activity in multiple sclerosis patients with periphlebitis retinae,” Acta Neurol. Scand. 73(2), 168–172 (1986). 10.1111/j.1600-0404.1986.tb03259.x [DOI] [PubMed] [Google Scholar]
- 22.Kerrison J. B., Flynn T., Green W. R., “Retinal pathologic changes in multiple sclerosis,” Retina 14(5), 445–451 (1994). 10.1097/00006982-199414050-00010 [DOI] [PubMed] [Google Scholar]
- 23.Harris A., Ciulla T. A., Chung H. S., Martin B., “Regulation of retinal and optic nerve blood flow,” Arch. Ophthalmol. 116(11), 1491–1495 (1998). 10.1001/archopht.116.11.1491 [DOI] [PubMed] [Google Scholar]
- 24.Russo A., Costagliola C., Rizzoni D., Ghilardi N., Turano R., Semeraro F., “Arteriolar diameters in glaucomatous eyes with single-hemifield damage,” Optom. Vis. Sci. 93(5), 504–509 (2016). 10.1097/OPX.0000000000000822 [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Jia Y., Spain R., Potsaid B., Liu J. J., Baumann B., Hornegger J., Fujimoto J. G., Wu Q., Huang D., “Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis,” Br. J. Ophthalmol. 98(10), 1368–1373 (2014). 10.1136/bjophthalmol-2013-304547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortune B., Reynaud J., Cull G., Burgoyne C. F., Wang L., “The effect of age on optic nerve axon counts, SDOCT scan quality, and peripapillary retinal nerve fiber layer thickness measurements in Rhesus monkeys,” T Transl. Vis. Sci. Technol. 3(3), 2 (2014). 10.1167/tvst.3.3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu T. P., Tong Y. H., Zhan H. J., Ma J., “Update on retinal vessel structure measurement with spectral-domain optical coherence tomography,” Microvasc. Res. 95, 7–14 (2014). 10.1016/j.mvr.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 28.Rim T. H., Choi Y. S., Kim S. S., Kang M. J., Oh J., Park S., Byeon S. H., “Retinal vessel structure measurement using spectral-domain optical coherence tomography,” Eye (Lond.) 30(1), 111–119 (2016). 10.1038/eye.2015.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polman C. H., Reingold S. C., Banwell B., Clanet M., Cohen J. A., Filippi M., Fujihara K., Havrdova E., Hutchinson M., Kappos L., Lublin F. D., Montalban X., O’Connor P., Sandberg-Wollheim M., Thompson A. J., Waubant E., Weinshenker B., Wolinsky J. S., “Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria,” Ann. Neurol. 69(2), 292–302 (2011). 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtzke J. F., “Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS),” Neurology 33(11), 1444–1452 (1983). 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 31.Schippling S., Balk L. J., Costello F., Albrecht P., Balcer L., Calabresi P. A., Frederiksen J. L., Frohman E., Green A. J., Klistorner A., Outteryck O., Paul F., Plant G. T., Traber G., Vermersch P., Villoslada P., Wolf S., Petzold A., “Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria,” Mult. Scler. 21(2), 163–170 (2015). 10.1177/1352458514538110 [DOI] [PubMed] [Google Scholar]
- 32.Spectralis Hardware Operating Manual, (Heidelberg Engineering GmbH, 2007).
- 33.Mayer M. A., Hornegger J., Mardin C. Y., Tornow R. P., “Retinal nerve fiber layer segmentation on FD-OCT scans of normal subjects and glaucoma patients,” Biomed. Opt. Express 1(5), 1358–1383 (2010). 10.1364/BOE.1.001358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muraoka Y., Tsujikawa A., Kumagai K., Akiba M., Ogino K., Murakami T., Akagi-Kurashige Y., Miyamoto K., Yoshimura N., “Age- and hypertension-dependent changes in retinal vessel diameter and wall thickness: an optical coherence tomography study,” Am. J. Ophthalmol. 156(4), 706–714 (2013). 10.1016/j.ajo.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 35.Liang K.-Y., Zeger S. L., “Longitudinal data analysis using generalized linear models,” Biometrika 73(1), 13–22 (1986). 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 36.Parri R., Crunelli V., “An astrocyte bridge from synapse to blood flow,” Nat. Neurosci. 6(1), 5–6 (2003). 10.1038/nn0103-5 [DOI] [PubMed] [Google Scholar]