Abstract
Toll-like receptor 4 (TLR4) is considered to have a critical role in the occurrence and development of atherosclerosis in atherosclerosis-prone mice; however, it remains uncertain whether treatment with a TLR4 inhibitor may attenuate atherosclerosis. The present study aimed to determine the vascular protective effects of the TLR4 inhibitor CLI-095 on apolipoprotein E-deficient (ApoE−/−) mice. ApoE−/− mice were fed either chow or a high-fat diet, and were treated with or without CLI-095 for 10 weeks. The mean atherosclerotic plaque area in the aortic sections of CLI-095-treated mice was 54.3% smaller than in the vehicle-treated mice (P=0.0051). In vitro, murine peritoneal macrophages were treated with or without CLI-095, and were subsequently stimulated with oxidized low-density lipoprotein. Treatment with CLI-095 markedly reduced the expression levels of lectin-like oxidized low-density lipoprotein receptor-1 and acyl-coenzyme A:cholesterol acyltransferase-1, and significantly upregulated the expression levels of ATP-binding cassette transporter A1, predominantly via suppressing activation of the TLR4/nuclear factor-κB signaling pathway. The results of the present study indicated that the TLR4 inhibitor CLI-095 has the ability to suppress the progression of atherosclerosis in an in vivo model by reducing macrophage foam cell formation.
Keywords: toll-like receptor 4, CLI-095, foam cell, atherosclerosis, macrophage
Introduction
Atherosclerotic cardiovascular disease is one of the most common causes of mortality, and is considered a major burden on the healthcare systems of developed countries (1,2). Inflammation has a central role in the progression of atherogenesis (3,4). Previous studies have reported an association between inflammation and the early stages of atherosclerosis, including foam cell formation, monocyte adhesion and migration (5–7). Toll-like receptor 4 (TLR4) is a type of pattern recognition receptor, which elicits inflammation when activated by endogenous risk factors with pathogen-associated molecular patterns, including minimally modified low-density lipoprotein and lipopolysaccharide (LPS). TLR4 is able to induce activation and nuclear translocation of the transcription factor nuclear factor-κB (NF-κB), resulting in the expression of interleukin (IL)-1β, IL-6, IL-18, tumor necrosis factor-α (TNF-α) and other inflammatory mediators (8). Numerous studies have confirmed the importance of TLR4 and NF-κB in the suppression of atherosclerosis (9,10); therefore, regulation of the TLR4/NF-κB signaling pathway may have considerable potential in the treatment of atherogenesis. CLI-095, also known as resatorvid or TAK-242, is a small-molecule inhibitor of TLR4 signaling that is used for the treatment of septic shock, which acts by binding to the intracellular domain of TLR4. CLI-095 potently suppresses both ligand-dependent and -independent signaling of TLR4 (11). To determine the efficacy of CLI-095 for suppressing atherogenesis, the effects and mechanisms of CLI-095 were investigated in vitro and in vivo in the present study. The results demonstrated that CLI-095 was able to alleviate atherosclerotic plaque development in apolipoprotein E-deficient (ApoE−/−) mice by reducing foam cell formation.
Materials and methods
Chemicals and antibodies
The TLR4-specific inhibitor CLI-095 was purchased from InvivoGen (San Diego, CA, USA) and was dissolved in dimethyl sulfoxide (DMSO) to obtain a stock solution of 100 μg/ml. The following antibodies: Rabbit polyclonal anti-CD68 (cat. no. ab125212), rat monoclonal anti-α-anti-smooth muscle actin (SMA; cat. no. ab7817), rabbit polyclonal anti-lectin-like oxidized low-density lipoprotein receptor-1 (Lox-1; cat. no. ab60178), rabbit polyclonal anti-ATP-binding cassette transporter A1 (ABCA1; cat. no. ab7360), rabbit polyclonal anti-acyl-coenzyme A:cholesterol acyltransferase-1 (ACAT-1; cat. no. ab71407), rabbit polyclonal anti-NF-κB P65 (cat. no. ab16502) and mouse monoclonal anti-TLR4 (cat. no. ab30667) were purchased from Abcam (Cambridge, UK). Rabbit monoclonal anti-phosphorylated-NF-κB P65 (cat. no. 3033) and rabbit monoclonal anti-GAPDH (cat. no. 5174) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cholesterol, triglyceride and cholesteryl ester (CE) measurements
Total cholesterol and triglyceride commercial kits were obtained from the Jiancheng Bioengineering Institute (Nanjing, China). Cholesteryl ester (CE) enzyme-linked immunosorbent assay kit was obtained from Nanjing Anpei Electro-Mechanics Equipment Co., Ltd. (Nanjing, China). To measure total cholesterol and triglyceride, serum was separated from hemocytes by centrifugation at 13,523 × g at 4°C for 5 min and commercial kits were used according to the manufacturer's protocol. To conduct the CE ELISA, cells were harvested and washed in cold phosphate-buffered saline (PBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and lipids were extracted by resuspending the sample in 200 μl chloroform (Sigma-Aldrich, St. Louis, MO, USA), isopropanol (Amresco, LLC, Solon, Ohio) and NP-40 (Beyotime Institute of Biotechnology, Inc., Haimen, China) at a ratio of 7:11:0.1 in a Bullet Blender Storm microhomogenizer (Midwest Scientific, Valley Park, MO, USA) at room temperature. The extract was spun for 5–10 min at 15,000 × g and the ELISA kit was used according to the manufacturer's instructions.
Animal protocol
Male ApoE−/− mice (n=15; age, 6 weeks) were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China) and maintained under a specific pathogen-free environment in micro-isolator cages. They were kept at 18–23°C at humidity 40–60% under a 12-h light/dark cycle. The mice were separated into the control, vehicle and treatment groups, with six mice in each group. The mice in the control group were fed a chow diet, whereas the mice in the vehicle and treatment groups were fed a high-fat diet (21% fat, 0.15% cholesterol; Mediscience Diets Co., Ltd., Yangzhou, China). In addition, the mice in the treatment group received a daily intraperitoneal injection of CLI-095 at a dose of 3 mg/kg/day (mouse body weight) for 10 weeks. The mice in the vehicle group were administered a daily intraperitoneal injection of 20% DMSO/PBS (volume, 0.1 ml) Mice were sacrificed at 16 weeks of age. Following sacrifice by inhalation of CO2 (Sigma-Aldrich), blood and heart tissue samples were collected. All animal procedures were approved by the Animal Ethics Committee of the Dalian Medical University (Dalian, China). All in vivo experiments were performed in accordance with national legislation and institutional guidelines.
Atherosclerotic lesion analysis
The lesion area in the aortic root sections was measured following Oil-red-O and hematoxylin-eosin (H&E) staining, using computer-assisted image quantification with Image Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Images were captured using an Olympus fluorescent microscope (DP80; Olympus Corp., Tokyo, Japan). Collagen fibers were stained with Masson's trichrome stain. All staining solutions were obtained from BASO Precision Optics Ltd. (Taiwan, China).
Immunohistochemistry and immunocytochemistry
Frozen sections of the aortic root were fixed in methanol (Sigma-Aldrich), incubated with 3% H2O2 (ZSGB-BIO, Beijing, China), air-dried and incubated with 10% goat serum (ZSGB-BIO), for 15–30 min. The frozen sections were incubated with anti-CD68, anti-α-SMA and anti-Lox-1 antibodies overnight at 4°C. Fluorophore-conjugated secondary antibodies were used for immunofluorescence. Macrophages were extracted from the rat by cutting the outer skin of the peritoneum and exposing the inner skin lining the peritoneal cavity, 5 ml PBS [with 3% fetal bovine serum (FBS)] was injected into the peritoneal cavity using a 27 g needle. Following injection, the peritoneum was gently massaged to dislodge attached cells and the fluid was collected with a 25 g needle. The collected cell suspension was centrifuged at 211 × g for 8 min, the supernatant was discarded and the cells harvested. Macrophages extracted from the mice were fixed and stained with anti-Lox-1 antibody and 4′,6-diamidino-2-phenylindole.
Cell culture
Thioglycolate-elicited peritoneal macrophages were maintained in RPMI 1640 media (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing 5% CO2.
Lipoprotein uptake assay
Thioglycolate-elicited peritoneal macrophages were seeded in serum-free medium. Following overnight fasting, the macrophages were washed with PBS and cultured in medium with or without oxidized low-density lipoprotein (Ox-LDL; 100 μg/ml; Guangzhou Yiyuan Biotechnology Co., Ltd., Guangzhou, China). Subsequently, the cells were treated with CLI-095 (1 μM) for 36 h at 37°C in a CO2 incubator. Macrophages were fixed and stained with Oil-red-O and observed under a DP80 fluorescent microscope. Experiments were repeated in triplicate in each group.
Western blot analysis
Cell total proteins were extracted using a total protein extraction kit from Nanjing KeyGen Biotech. Co., Ltd. (Nanjing, China), which contains a lysis buffer, proteinase inhibitor, phosphorylase inhibitor and phenylmethylsulfonyl fluoride. The protein concentration was determined using a Pierce™ BCA Protein assay kit (Thermo Fisher Scientific, Inc.). Proteins (30–50 μg) were separated by 12% sodium-dodecyl sulfate-polyacrylamide gel electrophoresis for 1.5 h at 120 V in electrophoretic buffer solution. The proteins were then transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Following blocking with 5% milk/Tris-buffered saline (w/v; Sigma-Aldrich) at 25°C for 1 h, the membranes were incubated with primary antibodies at 37°C for 4 h, including anti-Lox-1 (dilution, 1:1,000), anti-GAPDH (1:1,000), anti-TLR4 (1:500), anti-T-P65 (1:1,000), anti-ABCA1 (1:1,000) and anti-ACAT1 (1:1,000). The membranes were then washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (cat. no. sc-2004; dilution, 1:10,000) and horseradish peroxidase-conjugated goat anti-mouse IgM (cat. no. sc-2064; dilution, 10,000) secondary antibodies obtained from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and Texas Red-X conjugated goat anti-rat IgG secondary antibody (dilution, 1:200; Thermo Fisher Scientific, Inc.; cat. no. T-6392) for 1 h at room temperature. The blots were treated with WesternBright ECL kit (Advansta Inc., Menlo Park, CA, USA) visualized using Bio-Rad imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and analyzed using Image Pro Plus 6.0.
Statistical analysis
All data are presented as the mean ± standard deviation. Comparisons between different groups were analyzed using two-tailed Student's t-test. Statistical analysis was performed using SPSS version 13.0 statistical software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
CLI-095 potently attenuates the development of atherosclerosis
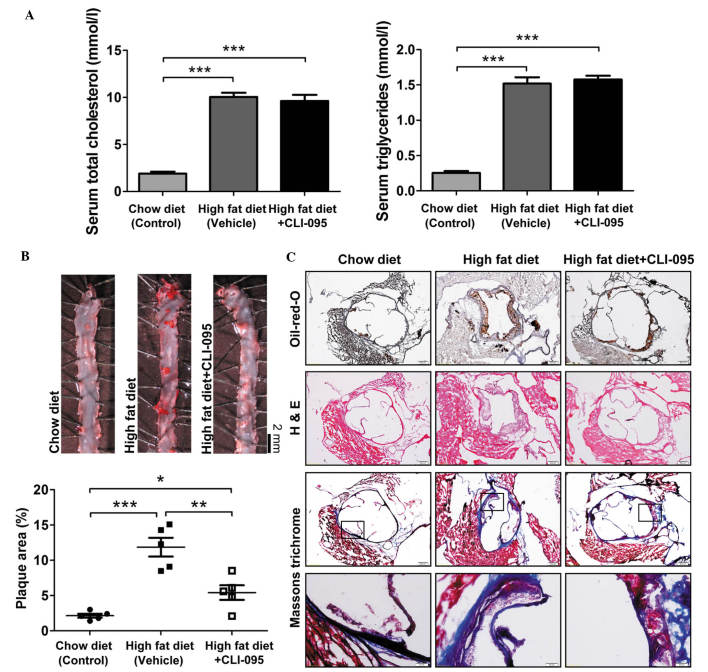
To address the role of TLR4 in the development of atherosclerosis, ApoE−/− mice were fed an atherogenic high-fat diet and were treated with or without CLI-095 via a daily intraperitoneal injection for a period of 10 weeks (dose, 3 mg/kg/day). As expected, ApoE−/− mice fed an atherogenic high-fat diet exhibited a significant increase in cholesterol and triglyceride levels, as compared with the chow diet-fed mice. In addition, ApoE−/− mice fed an atherogenic diet and treated with CLI-095 did not exhibit any variation in lipid metabolism, as compared with the vehicle-treated group (Fig. 1A). Subsequently, en face Oil-red-O staining was performed on the thoracic aorta. Mice treated with the TLR4 inhibitor CLI-095 exhibited markedly reduced atherosclerotic plaque size (Fig. 1B), suggesting that CLI-095 exerts a protective effect on atherosclerosis. Oil-red-O and H&E staining of serial cross sections of aortic roots also revealed fewer atherosclerotic lesions in the CLI-095-treated mice, as compared with the vehicle-treated mice; however, Masson's trichrome staining revealed that the total collagen content did not differ between the CLI-095- and vehicle-treated groups (Fig. 1C). These results indicate that CLI-095 may protect against atherosclerosis in ApoE−/− mice.
Figure 1.
CLI-095 attenuates the development of atherosclerotic lesions in ApoE−/− mice. (A) Serum total cholesterol and triglyceride levels were measured. (B) En face Oil-red-O staining of the thoracic aorta, and quantification of plaque area. ●, Chow diet (n=5); ■, high-fat diet (n=5); □, high-fat diet + CLI-095 (n=5). Magnification, ×4. (C) Representative photomicrographs of Oil-red-O, H&E and Masson's trichrome staining from mice with comparable lesion sizes. Results are presented as the mean ± standard deviation. Magnification, ×10 and ×40 (bottom row). *P≤0.05; **P≤0.01; ***P≤0.001. ApoE−/−, apolipoprotein E-deficient; H&E, hematoxylin and eosin.
CLI-095 reduces macrophage recruitment to atherosclerotic plaques
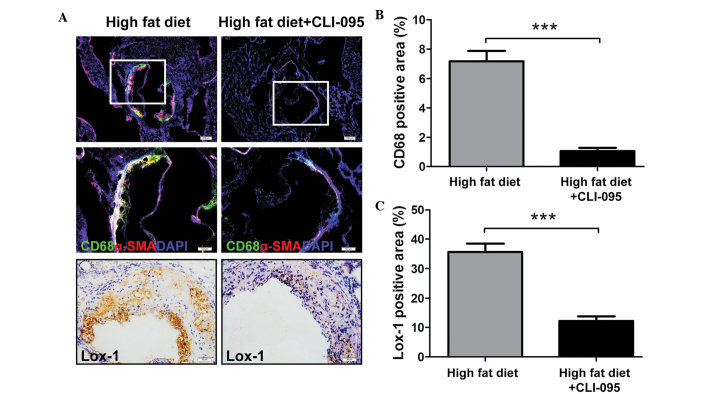
The recruitment of macrophages to the subintimal space is considered to have a key role in the progression of atherosclerosis (12). The present study revealed that the vehicle-treated ApoE−/− mice displayed clear macrophage recruitment to the atherosclerotic plaques, whereas only a limited number of macrophages were retained in the CLI-095-treated mice, as determined by immunofluorescent staining (Fig. 2A). The relative CD68-positive areas in the CLI-095-treated mice (n=5) were significantly smaller than those in the vehicle-treated mice (n=5) (Fig. 2B). In addition, α-SMA positive areas were markedly increased in the atherosclerotic plaques of the vehicle-treated group (Fig. 2A).
Figure 2.
CLI-095 decreases macrophage accumulation and Lox-1 expresssion in plaques. (A) Representative photomicrographs of lesion macrophage accumulation and Lox-1 expression following anti-CD68 and anti-Lox-1 antibody staining. Magnification (from top to bottom), ×10, ×20 and ×40. (B) Quantitative analysis of lesion macrophage accumulation (n=5) and (C) Lox-1 expression in the lesion (n=5). Results are presented as the mean ± standard deviation. ***P≤0.001. Lox-1, lectin-like oxidized low-density lipoprotein receptor-1; SMA, smooth muscle actin; DAPI, 4′6-diamidino-2-phenylindole.
A previous study demonstrated that overexpression of Lox-1, a macrophage and endotheliocyte receptor for Ox-LDL (13), in ApoE−/− mice upregulated the endothelial expression of vascular cell adhesion molecule-1 and increased the accumulation of CD68-positive cells in the plaques (14). In order to investigate whether CLI-095 modulates Lox-1 expression in plaque formation, the expression of Lox-1 was examined. Immunohistochemical staining of cross sections revealed a decreased expression of Lox-1 in CLI-095-treated mice, as compared with the vehicle-treated controls (Fig. 2A and C). These results suggest that CLI-095 is sufficient to reduce atherosclerotic plaque formation, possibly via the modulation of Lox-1 expression.
CLI-095 is sufficient to reduce murine peritoneal macrophage (MPM) foam cell formation in vitro
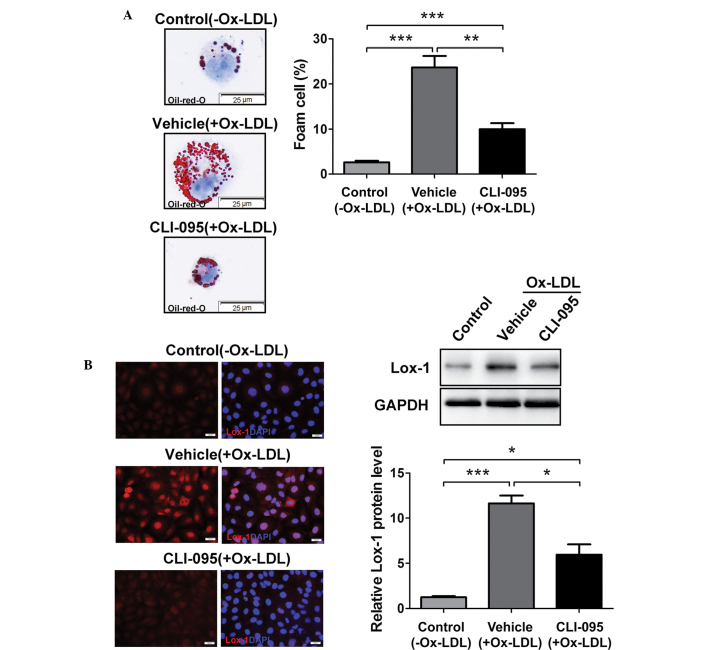
Lipoprotein uptake by macrophages promotes the formation of foam cells, which, in turn, contribute to the progression of atherosclerosis (15). To determine whether TLR4 inhibition is sufficient to regulate foam cell formation in thioglycolate-elicited MPMs in vitro, MPMs were treated with or without CLI-095, and foam cell formation was induced by Ox-LDL. Notably, the accumulation of cytoplasmic lipid droplets decreased in the CLI-095-treated MPMs, as compared with the vehicle-treated MPMs, as determined by Oil-red-O staining, and CLI-095 treatment resulted in a significant suppression of MPM foam cell formation (Fig. 3A). The upregulation of Lox-1 has been shown to promote lipoprotein uptake in macrophages (16), and the present study demonstrated that CLI-095 could suppress Lox-1 expression in cross sections from ApoE−/− mice (Fig. 2A). Subsequently, the expression of Lox-1 was determined using immunofluorescent staining and western blot analysis. The data revealed that stimulation of MPMs with Ox-LDL resulted in a significant increase in Lox-1 protein expression (vehicle vs. control), whereas CLI-095 treatment significantly decreased Lox-1 expression (Fig. 3B). These results suggest that CLI-095 exerts its protective effects on lipoprotein uptake by downregulating the expression of Lox-1.
Figure 3.
CLI-095 reduces macrophage foam cell formation. (A) Decreased uptake of Ox-LDL in CLI-095-treated MPMs (1 μM) compared with vehicle-treated MPMs. Right graph shows quantification of foam cells. (B) Expression of Lox-1 in MPMs was assessed by confocal immunofluorescence and immunoblotting. The lower-right panel shows densitometric quantification. Magnification, ×100. Results are presented as the mean ± standard deviation (n=3). *P≤0.05; **P≤0.01; ***P≤0.001. MPMs, murine peritoneal macrophages; Ox-LDL, oxidized low-density lipoprotein; Lox-1, lectin-like oxidized low-density lipoprotein receptor-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; DAPI, 4′6-diamidino-2-phenylindole.
CLI-095 decreases the quantity of CE in MPMs by differentially regulating the expression of ABCA1 and ACAT-1
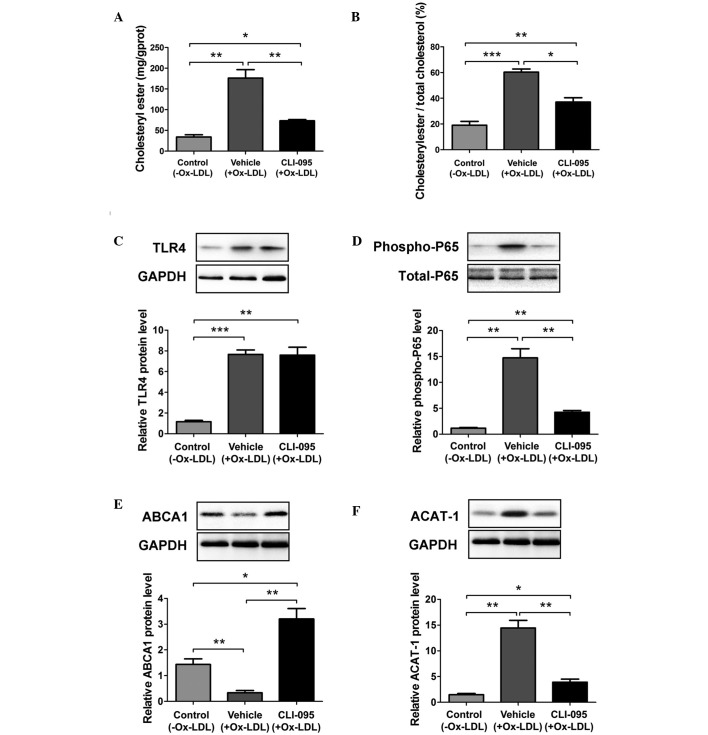
The accumulation of CE in macrophages has a critical role in foam cell formation. The possible effects of CLI-095 on macrophage CE accumulation in vitro were examined in the present study. Treatment with CLI-095 resulted in a marked decrease in the abundance of cellular CE in MPMs, as compared with the vehicle group (Fig. 4A). Furthermore, CLI-095 resulted in a marked reduction in the ratio of cellular CE to total cholesterol in MPMs (Fig. 4B). During the process of foam cell formation, excess cellular free cholesterol is converted to CE by the enzyme ACAT-1, or is removed from the cell by ABCA1-dependent cholesterol efflux (17–19). In addition, activation of NF-κB can suppress ABCA1 and enhance ACAT-1 expression to promote CE-laden cell formation (20,21). In the present study, Ox-LDL stimulation resulted in enhanced TLR4 expression as previously reported (22,23); however, the expression of TLR4 was not altered in the CLI-095-treated MPMs, as compared with the vehicle-treated MPMs (Fig. 4C). Notably, treatment with TLR4 inhibitor CLI-095 significantly reduced Ox-LDL-induced phosphorylation of NF-κB P65 (Fig. 4D), suggesting that CLI-095 may inhibit TLR4 signaling by affecting its adaptor proteins but without downregulating its expression. Furthermore, it was observed that CLI-095 markedly promoted ABCA1 expression and attenuated ACAT-1 expression (Fig. 4E and F). These data strongly indicate that CLI-095 may exert its vascular protective function by restricting CE synthesis and enhancing cholesterol efflux in macrophages.
Figure 4.
CLI-095 decreases the level of cholesteryl ester in MPMs by regulating the expression of ABCA1 and ACAT-1. (A) Cholesteryl ester content in CLI-095- and vehicle-treated MPMs incubated with Ox-LDL (100 μg/ml) (n=3). (B) Ratio of cellular cholesteryl ester and total cholesterol in MPMs (n=3). (C–E) Western blot analysis of (C) TLR4 expression, (D) NF-κB P65 phosphorylation, (E) ABCA1 expression and (F) ACAT-1 expression in CLI-095- and vehicle-treated MPMs incubated with Ox-LDL (100 μg/ml) (n=3). Results are presented as the mean ± standard deviation (n=3). *P≤0.05; **P≤0.01; ***P≤0.001. MPMs, murine peritoneal macrophages; ABCA1, ATP-binding cassette transporter A1; ACAT-1, acyl-coenzyme A:cholesterol acyltransferase-1; NF-κB, nuclear factor-κB; TLR4, toll-like receptor 4; Ox-LDL, oxidized low-density lipoprotein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
TLR4 is a member of the TLR family (TLR1-TLR13), which regulates the innate immune response. The TLR4/NF-κB pathway is one of the most studied signaling pathways in atherosclerosis; it is well known that some atherogenic factors, including LPS and Ox-LDL, are important ligands of TLR4. Following ligand binding to TLR4, the intracellular toll/interleukin-1 receptor (TIR) domains of TLR4 recruit the following signaling adaptor proteins: TIR domain-containing adaptor protein, myeloid differentiation factor 88, TIR-domain-containing adaptor-inducing interferon-β (TRIF) and TRIF-related adaptor molecule. Subsequently, numerous kinases and ubiquitin ligases, such as IL-1 receptor-associated kinase (IRAK)-1, IRAK-4, tumor necrosis factor receptor-associated factor 6 and transforming growth factor-β-activated kinase 1, are recruited and activated, culminating in the nuclear translocation of NF-κB (8). The activated NF-κB can subsequently induce the production of inflammatory mediators (8). Over the past decade, several TLR4 inhibitors have been developed to treat inflammatory diseases in animals (24,25). CLI-095, a selective inhibitor of TLR4 signaling, has been shown to potently suppress the activation of NF-κB, as well as the production of TNF-α, which is induced by TLR4-specific ligands interfering with the interactions between TLR4 intracellular domain and its adaptors in macrophages (11,26). The present study demonstrated that decreased NF-κB activation in CLI-095-treated mouse macrophages was not dependent on TLR4 expression. Some of these findings are consistent with those of previous studies (27,28).
Atherosclerosis is a chronic inflammatory disease. TLR4 has been shown to promote atherosclerosis by increasing the secretion of inflammatory mediators, foam cell formation and monocyte adhesion in ApoE−/− mice fed a high-fat diet (9,22,29). In addition, NF-κB has been demonstrated to augment foam cell formation by interfering with cellular lipid metabolism (30–32). In the present study, the molecular mechanisms underlying CLI-095-induced reductions in atherosclerotic lesions were determined. The results suggested that CLI-095 was able to inhibit the TLR4/NF-κB signaling cascade, in order to promote cholesterol efflux, and suppress lipoprotein uptake and CE synthesis in macrophages.
Foam cell formation has a critical role in atherosclerosis and its mechanisms include uptake of atherogenic lipoproteins, impaired cellular cholesterol efflux and disturbed intracellular cholesterol processing. Notably, Lox-1, ABCA1 and ACAT-1 have an important role in these three mechanisms respectively. The present data suggested that CLI-095 may upregulate the expression of ABCA1 and downregulate that of Lox-1 and ACAT-1 in vitro. These findings were consistent with those from previous studies; both ABCA1 overexpression and Lox-1 deficiency in mice have been shown to lead to decreased atherosclerotic lesions (14,33–35). However, previous studies have demonstrated that loss of ACAT-1 expression may lead to severe atherosclerosis (36,37). A possible explanation for the controversy between previous studies and the present results is that global ACAT-1 knockout causes monocytosis in ApoE−/− mice during the development of atherosclerosis; however, there is no evidence to demonstrate that the inhibition of TLR4 signaling causes monocytosis.
Both the present and previous studies demonstrated that TLR4 regulates cholesterol biosynthesis in vitro; however, whether TLR4 is able to regulate lipid metabolism in vivo remains controversial. The results of the present study revealed that CLI-095 did not reduce increased serum cholesterol and triglyceride levels in mice receiving a high-fat diet. In addition, Higashimori et al (9) reported that TLR4 deficiency was not associated with reduced levels of cholesterol and triglycerides, whereas Aspichueta et al (38) reported that endotoxic rats exhibited increased levels of serum very low-density lipoprotein-apoB, -triglyceride, and -cholesterol. In addition, Lu et al (39) reported that Rs-LPS, a TLR4 antagonist, decreased the serum levels of cholesterol and triglycerides in non-diabetic mice; therefore, further research is required to determine whether and how TLR4 affects lipid metabolism in animals.
In conclusion, the results of the present study demonstrated that the TLR4 inhibitor CLI-095 was able to effectively reduce atherosclerosis in ApoE−/− mice by suppressing foam cell formation. The present study also provides novel insights into the protective effects of TLR4 inhibition on enhancing cholesterol efflux by upregulating the expression of ABCA1, and reducing CE biosynthesis by downregulating the expression of ACAT-1, which is mediated by inhibiting the TLR4/NF-κB signaling pathway. These results suggested that TLR4 may be considered a potential therapeutic target for the prevention of atherosclerotic progression.
Acknowledgments
The authors of the present study would like to thank Dr Dan He and Dr Kuang Peng for their helpful discussions and technical assistance. This study was supported by funds from the Second Affiliated Hospital of Dalian Medical University and the National Natural Science Foundation of China (grant no. 81372853).
References
- 1.Yang Z, Hall AG. The financial burden of overweight and obesity among elderly Americans: The dynamics of weight, longevity, and health care cost. Health Serv Res. 2008;43:849–868. doi: 10.1111/j.1475-6773.2007.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang J, Peng W, Li H, Lu Y, Wang K, Fan F, Li S, Xu Y. Inhibitory effects of vinpocetine on the progression of atherosclerosis are mediated by Akt/NF-κB dependent mechanisms in apoE−/− mice. PLoS One. 2013;8:e82509. doi: 10.1371/journal.pone.0082509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S, Xu H, Peng J, Wang C, Jin Y, Liu K, Sun H, Qin J. Potent anti-inflammatory effect of dioscin mediated by suppression of TNF-α-induced VCAM-1, ICAM-1 and EL expression via the NF-κB pathway. Biochimie. 2015;110:62–72. doi: 10.1016/j.biochi.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Jehs T, Faber C, Juel HB, Bronkhorst IH, Jager MJ, Nissen MH. Inflammation-induced chemokine expression in uveal melanoma cell lines stimulates monocyte chemotaxis. Invest Ophthalmol Vis Sci. 2014;55:5169–5175. doi: 10.1167/iovs.14-14394. [DOI] [PubMed] [Google Scholar]
- 8.Lim KH, Staudt LM. Toll-like receptor signaling. Cold Spring Harb Perspect Biol. 2013;5:a011247. doi: 10.1101/cshperspect.a011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashimori M, Tatro JB, Moore KJ, Mendelsohn ME, Galper JB, Beasley D. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:50–57. doi: 10.1161/ATVBAHA.110.210971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 13.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 14.Akhmedov A, Rozenberg I, Paneni F, Camici GG, Shi Y, Doerries C, Sledzinska A, Mocharla P, Breitenstein A, Lohmann C, et al. Endothelial overexpression of LOX-1 increases plaque formation and promotes atherosclerosis in vivo. Eur Heart J. 2014;35:2839–2848. doi: 10.1093/eurheartj/eht532. [DOI] [PubMed] [Google Scholar]
- 15.Itabe H. Oxidized low-density lipoproteins: What is understood and what remains to be clarified. Biol Pharm Bull. 2003;26:1–9. doi: 10.1248/bpb.26.1. [DOI] [PubMed] [Google Scholar]
- 16.Hossain E, Ota A, Karnan S, Takahashi M, Mannan SB, Konishi H, Hosokawa Y. Lipopolysaccharide augments the uptake of oxidized LDL by up-regulating lectin-like oxidized LDL receptor-1 in macrophages. Mol Cell Biochem. 2015;400:29–40. doi: 10.1007/s11010-014-2259-0. [DOI] [PubMed] [Google Scholar]
- 17.Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- 18.Sekiya M, Osuga J, Igarashi M, Okazaki H, Ishibashi S. The role of neutral cholesterol ester hydrolysis in macrophage foam cells. J Atheroscler Thromb. 2011;18:359–364. doi: 10.5551/jat.7013. [DOI] [PubMed] [Google Scholar]
- 19.Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ, Heinecke JW. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei L, Xiong Y, Chen J, Yang JB, Wang Y, Yang XY, Chang CC, Song BL, Chang TY, Li BL. TNF-alpha stimulates the ACAT1 expression in differentiating monocytes to promote the CE-laden cell formation. J Lipid Res. 2009;50:1057–1067. doi: 10.1194/jlr.M800484-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao GJ, Tang SL, Lv YC, Ouyang XP, He PP, Yao F, Chen WJ, Lu Q, Tang YY, Zhang M, et al. Antagonism of betulinic acid on LPS-mediated inhibition of ABCA1 and cholesterol efflux through inhibiting nuclear factor-kappaB signaling pathway and miR-33 expression. PLoS One. 2013;8:e74782. doi: 10.1371/journal.pone.0074782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin YW, Liao SQ, Zhang MJ, Liu Y, Li BH, Zhou Y, Chen L, Gao CY, Li JC, Zhang LL. TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACAT1 expression. Cell Death Dis. 2014;5:e1574. doi: 10.1038/cddis.2014.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang K, Zhang XJ, Cao LJ, Liu XH, Liu ZH, Wang XQ, Chen QJ, Lu L, Shen WF, Liu Y. Toll-like receptor 4 mediates inflammatory cytokine secretion in smooth muscle cells induced by oxidized low-density lipoprotein. PLoS One. 2014;9:e95935. doi: 10.1371/journal.pone.0095935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564) Invest Ophthalmol Vis Sci. 2009;50:1247–1254. doi: 10.1167/iovs.08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenhammar J, Rundgren M, Forestier J, Kalman S, Eriksson S, Frithiof R. Toll-like receptor 4 inhibitor TAK-242 attenuates acute kidney injury in endotoxemic sheep. Anesthesiology. 2011;114:1130–1137. doi: 10.1097/ALN.0b013e31820b8b44. [DOI] [PubMed] [Google Scholar]
- 26.Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 27.Gárate I, García-Bueno B1, Madrigal JL, Caso JR, Alou L, Gómez-Lus ML, Leza JC. Toll-like 4 receptor inhibitor TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress. J Neuroinflammation. 2014;11:8. doi: 10.1186/1742-2094-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun M, Deng B, Zhao X, Gao C, Yang L, Zhao H, Yu D, Zhang F, Xu L, Chen L, Sun X. Isoflurane preconditioning provides neuroprotection against stroke by regulating the expression of the TLR4 signalling pathway to alleviate microglial activation. Sci Rep. 2015;5:11445. doi: 10.1038/srep11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SJ, Choi EK, Seo KW, Bae JU, Park SY, Kim CD. TLR4-mediated expression of Mac-1 in monocytes plays a pivotal role in monocyte adhesion to vascular endothelium. PLoS One. 2014;9:e104588. doi: 10.1371/journal.pone.0104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira V, van Dijk KW, Groen AK, Vos RM, van der Kaa J, Gijbels MJ, Havekes LM, Pannekoek H. Macrophage-specific inhibition of NF-kappaB activation reduces foam-cell formation. Atherosclerosis. 2007;192:283–290. doi: 10.1016/j.atherosclerosis.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Wu JF, Tang YY, Zhang M, Li Y, Chen K, Zeng MY, Yao F, Xie W, Zheng XL, et al. Urotensin II increases foam cell formation by repressing ABCA1 expression through the ERK/NF-κB pathway in THP-1 macrophages. Biochem Biophys Res Commun. 2014;452:998–1003. doi: 10.1016/j.bbrc.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Lee HY, Kim SD, Baek SH, Choi JH, Cho KH, Zabel BA, Bae YS. Serum amyloid A stimulates macrophage foam cell formation via lectin-like oxidized low-density lipoprotein receptor 1 upregulation. Biochem Biophys Res Commun. 2013;433:18–23. doi: 10.1016/j.bbrc.2013.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, et al. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. [DOI] [PubMed] [Google Scholar]
- 34.Hu C, Dandapat A, Sun L, Chen J, Marwali MR, Romeo F, Sawamura T, Mehta JL. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovasc Res. 2008;79:287–293. doi: 10.1093/cvr/cvn110. [DOI] [PubMed] [Google Scholar]
- 35.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fazio S, Major AS, Swift LL, Gleaves LA, Accad M, Linton MF, Farese RV., Jr Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages. J Clin Invest. 2001;107:163–171. doi: 10.1172/JCI10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang LH, Gui J, Artinger E, Craig R, Berwin BL, Ernst PA, Chang CC, Chang TY. Acat1 gene ablation in mice increases hematopoietic progenitor cell proliferation in bone marrow and causes leukocytosis. Arterioscler Thromb Vasc Biol. 2013;33:2081–2087. doi: 10.1161/ATVBAHA.112.301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aspichueta P, Pérez-Agote B, Pérez S, Ochoa B, Fresnedo O. Impaired response of VLDL lipid and apoB secretion to endotoxin in the fasted rat liver. J Endotoxin Res. 2006;12:181–192. doi: 10.1179/096805106X102174. [DOI] [PubMed] [Google Scholar]
- 39.Lu Z, Zhang X, Li Y, Jin J, Huang Y. TLR4 antagonist reduces early-stage atherosclerosis in diabetic apolipoprotein E-deficient mice. J Endocrinol. 2013;216:61–71. doi: 10.1530/JOE-12-0338. [DOI] [PubMed] [Google Scholar]