Abstract
Mahonia bealei is a Chinese folk medicine used to treat various ailments, in particular gastrointestinal inflammation-related illnesses, and palmatine is one of its active constituents. In this study, ApcMin/+ mice, a genetically engineered model, were used to investigate the effects of palmatine on the initiation and progression of gut inflammation and tumorigenesis enhanced by a high-fat diet. The in vitro antiproliferation and anti-inflammation effects of palmatine were evaluated on HT-29 and SW-480 human colorectal cancer cell lines. The concentration-related antiproliferative effects of palmatine on both cell lines (P<0.01) were observed. Palmatine significantly inhibited lipopolysaccharide-induced increase in cytokine interleukin (IL)-8 levels in the HT-29 cells (P<0.01). In the in vivo studies with ApcMin/+ mice, after 10 or 20 mg/kg/day oral palmatine treatment, tumor numbers were significantly reduced in the small intestine and colon in a dose-dependent manner (P<0.01 compared with the model group). The results were supported by tumor distribution data, body weight changes and organ index. The effect on survival was also dose-dependent. Both the low- and high-dose palmatine treatments significantly increased the life span of the mice (P<0.01). The gut histology from the model group showed a prominent adenomatous change along with inflammatory lesions. With palmatine treatment, however, the dysplastic changes were greatly reduced in the small intestine and colon tissue. Reverse transcription-quantitative polymerase chain reaction analysis of interleukin (IL)-1α, IL1-β, IL-8, granulocyte-colony stimulating factor and granulocyte macrophage colony-stimulating factor in the gut tissue showed that these inflammatory cytokines were reduced significantly following treatment (all P<0.01); serum cytokine levels were also decreased. Data suggests that palmatine has a clinical value in colorectal cancer therapeutics, and this action is likely linked to the inhibition of inflammatory cytokines.
Keywords: Mahonia bealei, palmatine, inflammation, cytokine, small intestine, colon, colorectal cancer, ApcMin/+ mice
Introduction
Colorectal cancer is a leading cause of morbidity and mortality in patients worldwide. A significant number of men and women are at risk of developing invasive colon cancer in their lifetime (1). Inflammatory bowel disease is a group of inflammatory conditions in the human large and small intestines. It is recognized that chronic inflammation is a risk factor for tumor development, including colorectal cancer (2). Thus, targeting inflammatory pathways has been shown to be effective in preventing the formation of colon tumors and their malignant progression in animal and human studies (3–7).
There is growing evidence to suggest that certain natural products possess anti-inflammatory and anticancer potential. Mahonia bealei is an evergreen shrub of the Berberidaceae family, native to East Asia, and North and Central America. In Chinese folk medicine, the root, stem and leaf of M. bealei have been used for thousands of years as herbal medicines to treat various ailments, in particular gastrointestinal inflammation-related illnesses, such as diarrhea and dysentery (8). Modern phytochemistry studies have identified a number of alkaloids from M. bealei, such as berberine, palmatine and jatrorrhizine, the latter two being protoberberine alkaloids. These compounds have been shown to exhibit antioxidant, anti-inflammatory, and anticancer effects (9).
Compared with the extensive research on berberine (9), the investigation of palmatine is limited. Notably, several recently published studies have demonstrated that palmatine not only has anticancer potential, but is also an anti-inflammatory agent (10,11). Thus the aim of this study explore the effect of palmitine on colon cancer.
Our previous study observed the antitumor effects of different botanicals. For example, using a colon tumor-xenograft nude mouse model, significant antitumor activities of American ginseng and Oplopanax horridus were identified (12,13). However, the nude mouse is not a gut disease-specific animal model. Thus, in this study, to ascertain the anticancer effects of palmatine, in vitro evaluation was used to demonstrate that palmatine significantly inhibited colorectal cancer cell proliferation and the expression of inflammatory cytokine interleukin (IL)-8. Since it is desirable to use specific gut inflammatory and tumorigenesis animal models for in vivo studies ApcMin/+ mice, a model with a mutant Apc gene, were then used for investigation. This Min (multiple intestinal neoplasia) mouse is characterized by early lethality, colon tumors, and development of a number of polyps in the small intestine (14,15). To increase the possibility of gut diseases, the mice were fed high-fat rodent chow to mimic a Western diet (16), as previous research has shown that high-fat diet intake is able to promote colon cancer by increasing fatty acids and secondary bile acids in the colonic lumen (17). Data from the present study showed that palmatine significantly reduced the progression of high-fat diet-enhanced gut inflammation and tumorigenesis in ApcMin/+ mice, supported by the inflammatory cytokine level reductions. In addition, mice displayed increased survival rates, which was consistent with the pharmacological observations. These results suggest that palmatine has a potential clinical utility as a colon cancer therapeutic.
Materials and methods
Chemicals
Palmatine (98% purity; Fig. 1) was isolated from Mahonia bealei as previously described (18). McCoy's 5A and Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin and streptomycin were obtained from Mediatech, Inc. (Herndon, VA, USA). Dimethylsulfoxide and lipopolysaccharide were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Figure 1.

Chemical structure of palmatine.
Cell culture and proliferation assay
HT-29 and SW-480 human colorectal cancer cell lines were obtained from the American Type Tissue Collection (Rockville, MD, USA), with HT-29 maintained in McCoy's 5A and SW-480 in DMEM. All medium were supplemented with 10% FBS, penicillin (100 IU/ml) and streptomycin (100 µg/ml). The colon cancer cells were subcultured twice a week and incubated in a humidified atmosphere with 5% CO2 at 37°C.
Young adult mouse colon (YAMC) cells were obtained from the Digestive Disease Research Core Center at the University of Chicago (Chicago, IL, USA), and were grown in RPMI-1640 (Mediatech, Inc., Herndon, VA, USA) medium supplemented with 5% neonatal calf serum (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) ITS+ (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA; 6.25 mg/ml insulin, 6.25 mg/ml transferrin, 6.25 ng/ml selenous acid, 5.35 mg/ml linoleic acid, and 1.25 mg/ml bovine serum albumin), 5 IU/ml murine interferon-γ (PeproTech, Inc., Rocky Hill, NJ, USA), penicillin and streptomycin. The YAMC cells were cultured in a humidified atmosphere with 5% CO2 at 33°C.
Palmatine was dissolved in DMSO and stored in small aliquots at −20°C prior to use. The cancer cells were seeded in 96-well plates at a density of 5,000 cells/well, allowed to attach overnight and then treated with different concentrations of palmatine. Cell proliferation was measured at 48 h using the Cell Titer 96 Aqueous MTS Reagent (Promega Corporation, Madison, WI, USA) according to the manufacturer's instructions. The absorbance was read by an automated microplate reader (Epoch; Bio-Tek Instruments, Winooski, VT, USA) set to a wavelength of 490 nm. Data are expressed as the percentage of treated cells vs. control (vehicle set at 100%) (19).
IL-8 secretion analysis
The HT-29 and SW-480 cells were seeded in 24-well plates and cultured for 72 h. Cell monolayers were washed with phosphate-buffered saline and fresh medium was added in the presence of 100 ng/ml lipopolysaccharide (LPS) or different concentrations of palmatine plus 100 ng/ml LPS. After incubation for 6 h, the culture medium was collected, the secreted IL-8 was quantified by enzyme-linked immunosorbent assay (ELISA; Thermo Fisher Scientific, Inc.), and anti-inflammatory activities were calculated.
Animals and experimental protocol
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Chicago. Male C57BL/6J-ApcMin/J (n=5) and female C57BL/6J (n=5) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) for breeding. Mice were caged under controlled room temperature, humidity and light (12-h light:dark cycle) and allowed free access to rodent chow and tap water. After weaning, genotyping was conducted via tail biopsy using polymerase chain reaction (PCR)-based assays to identify ApcMin/+ mice (14).
The study protocol is shown in Fig. 2. Before they were 8-weeks old, all mice consumed standard rodent chow. Starting at week 8, the animals received a Western high-fat diet, and the four experimental groups (n=5 per group) are as follows: i) Wild-type mice as negative control; ii) ApcMin/+ mice in untreated model group as a positive control; iii) ApcMin/+ mice receiving a low-dose of palmatine in animal chow, equivalent to 10 mg/kg/day; and iv) ApcMin/+ mice receiving a high-dose of palmatine in animal chow, equivalent to 20 mg/kg/day. The two doses of palmatine were selected based on data obtained from a pilot study in which the effective oral dose of palmatine started at 6–8 mg/kg/day. No significant adverse events were observed in mice that were administered palmatine.
Figure 2.

Animal groups and experiment time line. Before they were 8-weeks old, all mice consumed standard rodent chow. Starting on week 8, all animals received a Western high-fat diet, and the four experimental groups (n=5 per group) are as follows: i) Wild-type, wild-type mice. ii) Model, ApcMin/+ mice model group. iii) Lo-dose, ApcMin/+mice low-dose of palmatine (10 mg/kg/day). iv) ApcMin/+mice Hi-dose, high-dose of palmatine (20 mg/kg/day).
The high-fat Western diet (Harlan Laboratories, Madison, WI, USA) contains 20% fat and includes beef tallow (35 g/kg), lard (30 g/kg) and corn oil (80 g/kg) (16). For the drug groups, palmatine was evenly mixed with the high-fat chow. The daily oral palmatine dose was calculated based on the amount of chow consumption. Animal body weight was measured at least once a week.
Animal survival data was obtained when a mouse was critically ill (as determined by Animal Care Facility staff, who assisted in the regular monitoring of the animals) and expected to die within the next 36 h. At that point, the animal was sacrificed by cervical dislocation after collecting blood from the heart, followed by the removal of the organs and subsequent measurements and analyses. The small intestine and colon were harvested, flushed immediately with ice-cold PBS and cut open longitudinally. Two independent investigators who were blinded with respect to the treatment groups counted tumor numbers and sizes under a dissection microscope (SZX7 Stereo Microscope; Olympus Corporation, Tokyo, Japan). Small intestine and colon tissue samples were fixed in 10% neutral-buffered formalin, embedded in paraffin blocks, and processed by routine histological staining. Gut tissue was collected for reverse transcription-quantitative PCR analysis of inflammatory cytokines. Plasma samples were collected to determine the levels of cytokines using the ELISA kits.
In addition, at the end of the experiment, the spleen, thymus and liver were removed and weighed. Organ index was calculated based on the following equation: Organ index (%) = organ weight (g) / body weight (g) x 100 (20,21).
Histological assessment
Colon and small intestine samples (2 mm2) were harvested from the APCmin/+ mice. Two wedge-shaped incisions were made with a scalpel (blade no. 15c; Swann Morton, Ltd., Sheffield, UK). Subsequent to harvesting, the material was placed in a sterile test tube filled with a 5% formalin solution.
Following fixation, the material was put through a Thermo Scientific Citadel Tissue Processor (Thermo Fisher Scientific, Inc.) using a standard processor program (rinsing in 50, 90 and 98% alcohol series in turns for 1 h each, rinsing three times with xylene and embedding in paraffin). Once embedded in paraffin blocks and cut with a semi-automatic Shandon Finesse 325 microtome (Thermo Fisher Scientific, Inc.) to a thickness of 7 µm, the material was stained with hematoxylin and eosin (H&E; Leica Microsystems, Inc., Buffalo Grove, IL, USA) solution using a Thermo Scientific Gemini AS Automated Slide Stainer (Thermo Fisher Scientific, Inc.). Histological examination was performed by descriptive analysis under light microscopy with an Olympus BX41 microscope (Olympus Corporation) and the stained sections were examined for histopathological changes by a gastrointestinal pathologist.
RNA extraction and RT-qPCR
Total RNA was isolated from mouse small intestine and colon tissues using the miRNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions, and was used as a template to synthesize cDNA for RT-qPCR. First strand cDNA was synthesized the using Thermo Scientific Maxima First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). RT-qPCR was performed on a 7900HT real-time PCR system (Applied Biosystems, Foster City, CA, USA) with the following cycling conditions: 1 PCR cycle, 95°C, 10 min; 40 PCR cycles, 60°C, 1 min, 95°C, 15 sec. RT-qPCR with SYBR Green dye (Qiagen) was used to determine the expression of genes. Signals were analyzed by the ABI Prism Sequence Detection System (version 2.2; Thermo Fisher Scientific, Inc.). The 2−ΔΔCq method for relative quantification was used (22). Primers for RT-qPCR were purchased from Sigma-Aldrich and are listed in Table I. β-actin was used as an endogenous control. Each sample was run in triplicate.
Table I.
Primers used for RT-qPCR analysis of inflammatory cytokines.
| Gene | Primer sequence |
|---|---|
| IL1α | F: 5′-CGAAGACTACAGTTCTGCCATT-3′ |
| R: 5′-GACGTTTCAGAGGTTCTCAGAG-3′ | |
| IL1β | F: 5′-GCAACTGTTCCTGAACTCAACT-3′ |
| R: 5′-ATCTTTTGGGGTCCGTCAACT-3′ | |
| IL8 | F: 5′-ACTGAGAGTGATTGAGAGTGGAC-3′ |
| R: 5′-AACCCTCTGCACCCAGTTTTC-3′ | |
| G-CSF | F: 5′-ATGGCTCAACTTTCTGCCCAG-3′ |
| R: 5′-CTGACAGTGACCAGGGGAAC-3′ | |
| GM-CSF | F: 5′-GGCCTTGGAAGCATGTAGAGG-3′ |
| R: 5′-GGAGAACTCGTTAGAGACGACTT-3′ | |
| β-actin | F: 5′-GGCTGTATTCCCCTCCATCG-3′ |
| R: 5′-CCAGTTGGTAACAATGCCATGT-3′ |
F, forward; R, reverse; IL, interleukin; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-colony stimulating factor.
Statistical analysis
Data are presented as the mean ± standard derivation. Polyp data was analyzed using a one-way analysis of variance (ANOVA) with Newman-Keuls post-hoc analysis. All inflammatory mediator data were analyzed using a two-way ANOVA with Student Newman-Keuls post hoc analysis. GraphPad Prism software (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) was used for the analyses, including the Kaplan-Meier survival curve. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects on colorectal cancer cell proliferation and secretion of inflammatory cytokine IL-8
Fig. 3A shows the concentration-related inhibitory effects of palmatine on HT-29 and SW-480 colorectal cancer cell growth (P<0.01 compared with the control). The IC50 for HT-29 and SW-480 cells was 68.3 and 72.6 µM, respectively. Using the YAMC cells, it was also observed that palmatine did not significantly affect the growth of these non-cancer cells even at high concentrations (100–120 µM). These results suggested that palmatine possesses in vitro antiproliferative potential on human colorectal cancer cells.
Figure 3.
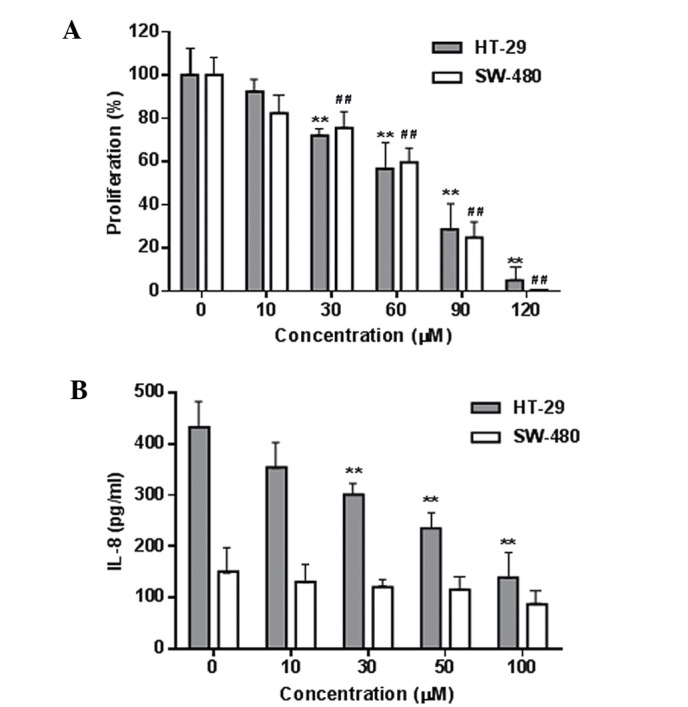
Effects of palmatine on cell proliferation and inflammatory cytokine IL-8 secretion in H-29 and SW-480 human colorectal cancer cells. (A) Concentration-related antiproliferative effects of palmatine on the two cell lines. (B) Palmatine inhibited IL-8 secretion in HT-29 cells. **P<0.01 compared with HT-29 control; ##P<0.01 compared with SW-480 control. IL, interleukin.
The LPS-induced production of the inflammatory cytokine IL-8 in these two cancer cells was determined, and the IL-8 level was higher in the HT-29 cells than that in the SW-480 cells (Fig. 3B). Palmatine significantly reduced the IL-8 production in HT-29 cells (P<0.01 compared with the control). This result was consistent with a previous study that demonstrated that the HT-29 cell line is a suitable in vitro model for the evaluation of anti-inflammation and anti-inflammatory responses (23).
Effects on body weight and organ index using ApcMin/+ mice
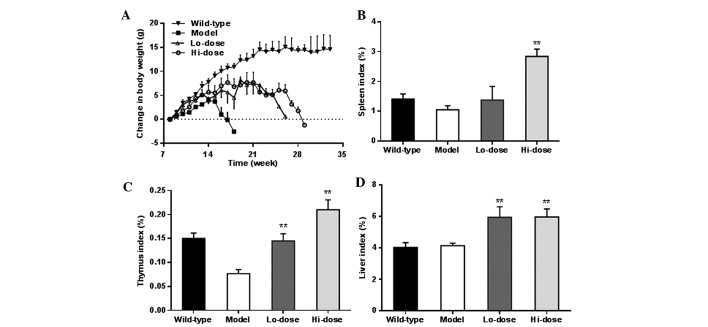
To further evaluate the effects of palmatine on colorectal cancer and inflammatory responses, the in vivo ApcMin/+ mouse model was employed. The weight changes of animals in the different experimental groups (Fig. 3) after week 8 are shown in Fig. 4A. As expected, wild-type mice fed with a Western high-fat diet had notable weight gain, approximately 13 g increase over the 14-week observation period. However, the ApcMin/+ mice in the model group did not respond to the high-fat diet well. The body weight increase was slower compared with that of the wild-type mice up to week 7. From week 8, the mice in the model group lost weight gradually until sacrifice. The ApcMin/+ mice treated with palmatine showed noticeably less body weight reduction compared with the model group, and their weight only gradually decreased from week 14.
Figure 4.
Effects of palmatine on body weight and organ index. (A) Changes in body weight. Compared with the control, ApcMin/+ mice treated with a low-dose and high-dose of palmatine showed less reduction of body weight. Changes in the organ index of the (B) spleen, (C) thymus and (D) liver, respectively. **P<0.01 compared with the model group. Lo-dose, low-dose of palmatine (10 mg/kg/day); Hi-dose, high-dose of palmatine (20 mg/kg/day).
Fig. 4B–D shows the changes in the organ weight index of the spleen, thymus and liver, respectively. In a dose-dependent manner, palmatine increased the spleen index and thymus index compared with the model group (P<0.01). The liver index was also significantly increased following palmatine treatment (P<0.01).
Effects on ApcMin/+ mice life spans
Fig. 5 shows the Kaplan-Meier survival curve which demonstrated the dose-related effects of palmatine on life span. Before week 19, all ApcMin/+ mice in the model group had died. The curve shows that both low-dose and high-dose palmatine significantly increased mouse survival and dose-related effects were observed (P<0.01 compared with the model group).
Figure 5.

Kaplan-Meier survival curve showing dose-related effects of palmatine. Before week 19, all ApcMin/+ mice in the model group had died. The curve shows both low-dose and high-dose palmatine administration significantly increased mice survival. Lo-dose, low-dose of palmatine (10 mg/kg/day); Hi-dose, high-dose of palmatine (20 mg/kg/day).
Effects on gut histopathological changes
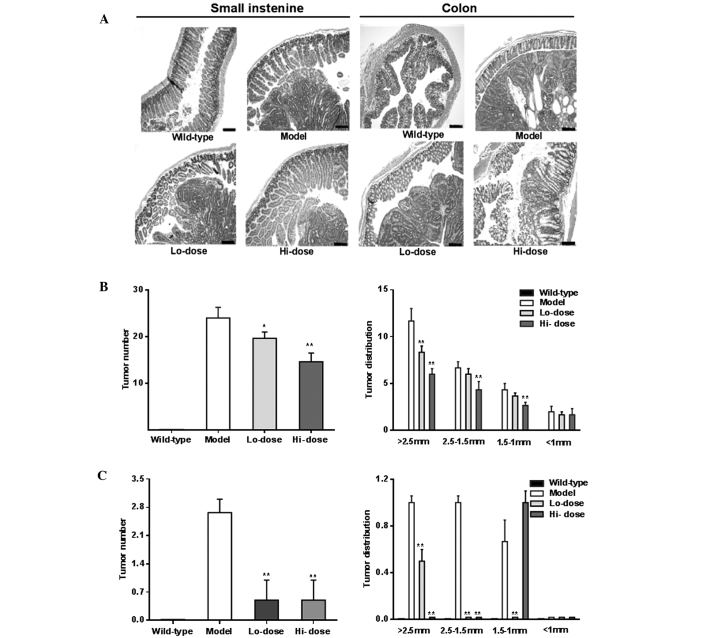
Fig. 6A shows representative H&E staining histological sections of experimental animals with different groups. The histology of the model group showed prominent adenomatous changes along with inflammatory lesions, such as neutrophil infiltration, and focal adenomatous changes were also observed in the ApcMin/+ mice. In the palmatine treatment groups, however, the dysplastic changes were reduced in the small intestine and colon sections.
Figure 6.
Effects of palmatine on gut inflammation and tumorigenesis. (A) Representative hematoxylin and eosin staining histological sections showing inflammatory changes in the small intestine and colon in different groups. The scales in the lower right corner are 100 µm. Tumor number and tumor distribution in (B) small intestine and (C) colon. Palmatine significantly reduced the tumor numbers in the small intestine and colon. *P<0.05 and **P<0.01, compared with the model group. Lo-dose, low-dose of palmatine (10 mg/kg/day); Hi-dose, high-dose of palmatine (20 mg/kg/day).
Effects on tumor multiplicity changes
Compared with the model group, following palmatine treatment, tumor numbers were significantly reduced in the small intestine and colon as shown in Fig. 6B and C (P<0.01). Tumor distribution data of the gut, particularly from the colon, showed a significant decrease in the number of large tumors and an increase in the number of small tumors, supporting the effects of palmatine in the attenuation of the tumorigenesis.
Effects on inflammatory cytokine expression in gut tissue and serum
Fig. 7A and B show the mRNA levels of data of IL-1α, IL1-β, IL-8, granulocyte-colony stimulating factor (G-CSF), and granulocyte-colony stimulating factor (GM-CSF) in the gut tissue from the model group and palmatine treated groups as determined by RT-qPCR. The levels of all of these inflammatory cytokines were reduced significantly following treatment (all P<0.01 compared with the model group). Fig. 7C illustrates the significant reductions of IL-8, IL-10 and G-CSF in serum after palmatine treatment (P<0.01 compared with the model group).
Figure 7.
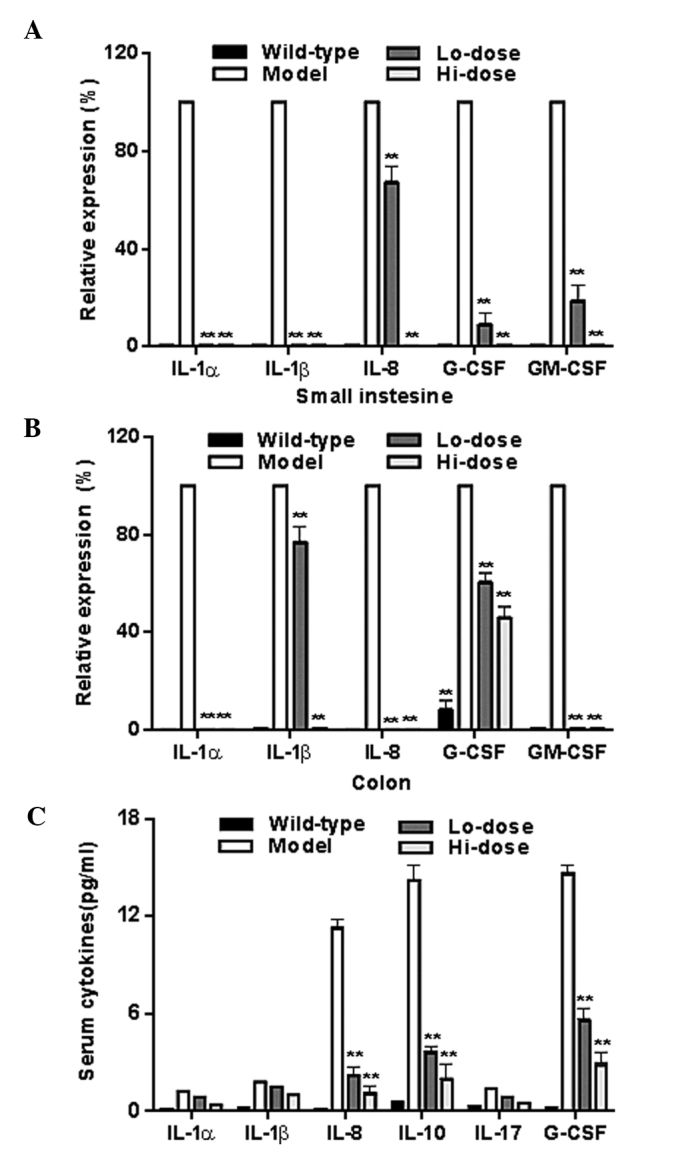
Effects of palmatine on the expression of inflammatory cytokines in the (A) small intestine, (B) colon and (C) serum. **P<0.01, compared with the model group. IL, interleukin; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-colony stimulating factor; Lo-dose, low-dose of palmatine (10 mg/kg/day); Hi-dose, high-dose of palmatine (20 mg/kg/day).
Discussion
Gut inflammation can initiate and promote stimuli and mediators, generating a tumor-prone microenvironment. Chronic inflammation is a risk factor for tumor development, including colorectal cancer (3), and the inhibition of the inflammatory pathway is proven to be effective in preventing the initiation and progression of colon cancer (2). The use of non-steroidal anti-inflammatory drugs (NSAIDS) can reduce colon tumorigenesis, but concerns and long-term risks of NSAIDs render this form of prevention unsuitable as a general recommendation in clinical practice (24). Thus, there is a strong motivation for exploring alternative strategies, including the use of herbal medicines, in the management of malignancies in the gastrointestinal system (25–27).
It was hypothesized that the natural compound palmatine may have anti-colorectal cancer potential, and actions of the compound were at least in part mediated by anti-inflammatory activity. To test this hypothesis, the anti-inflammation and anti-colorectal cancer activities were evaluated using different experimental paradigms. The in vitro effects of palmatine on HT-29 and SW-480 colorectal cancer cell proliferation were observed. As expected, the compound showed concentration-dependent inhibitory effects on cancer cell growth. In addition, the levels of IL-8 in these two types of cancer cell were detected, and palmatine was observed to significantly reduce the IL-8 production of HT-29 cells. In vitro cell models can be used to screen natural compounds with anti-inflammatory activity. In this study, the IL-8 level was tested first using two cancer cell lines following treatment with LPS. Since HT-29 cells were sensitive to LPS stimulation compared with SW-480 cells, data suggested that HT-29 is a suitable cell model to quickly screen for anti-inflammatory effects of natural compounds.
The role of IL-8 in cancer cells and within the tumor microenvironment has been presented, and the IL-8 activity appeared to be associated with tumor progression through its potential function in the regulation of angiogenesis, cancer cell growth and migration, leukocyte infiltration and modification of immune responses (28). The aforementioned functions of IL-8 may be crucial for the in vitro palmatine effects observed in the present study, which were subsequently evaluated in in vivo studies.
Two gut-specific animal models, AOM/DSS mice and ApcMin/+ mice, have been employed in a number of research studies. AOM/DSS is a chemical compound-induced gut disease model, characterized by early inflammation followed by tumor development (29,30). This chemically induced murine model has been used frequently to investigate inflammation-related colon tumorigenesis in the last decade. Ginseng is a commonly used herbal medicine worldwide and has been highly investigated for its chemopreventive effects (31–33). Our group previously used the AOM/DSS mouse model in our ginseng research (30). The ApcMin/+ mouse model is another commonly used gut-specific carcinogenesis model. This mouse model has a mutant in the Apc gene resulting in the growth of small intestine polyps and colon tumors, and the association of inflammation with tumorigenesis has been reported (34). Using the anti-inflammatory agent celecoxib, a cyclooxygenase-2 inhibitor belonging to the NSAID family, tumor preventive and therapeutic effects in ApcMin/+ mice have been demonstrated (34). To verify the in vitro antiproliferation and anti-inflammation effects of palmatine, an in vivo evaluation was performed using the ApcMin/+ mouse model.
Like a number of other herbal medicines, M. bealei is orally administered. In the present study, oral administration of palmatine, an active constituent in M. bealei, was observed to significantly attenuate the progression of high-fat diet enhanced inflammation and tumorigenesis in ApcMin/+ mice. Body weight changes and mice survival data suggested that palmatine administration resulted in intestinal homeostasis and improved general condition. Based on a previous study, the median lethal dose (LD50) of palmatine was 1,533.68 mg/kg (35). In our study, the high dose of palmatine was 20 mg/kg, only ~1.5% of its reported LD50. Thus, the palmatine doses in this study should be safe and without toxicity to the animals. Further safety and pharmacokinetic observations of palmatine will be conducted in our future studies.
Our gut histological observations showed that, compared with the model group, the inflammatory lesions in the small intestine and colon in the palmatine treatment groups were markedly reduced, with less neutrophil infiltration and focal adenomatous change. In addition, the dysplastic changes were greatly reduced, supporting the effects of palmatine on gut inflammation and tumorigenesis. Furthermore, RT-qPCR analysis data showed that, compared with the model group, palmatine significantly reduced the gene expression of several inflammatory cytokines, IL-1α, IL1-β, IL-8, G-CSF and GM-CSF, in the small intestine and colon. In addition, levels of IL-8, IL-10 and G-CSF in the serum were also significantly reduced following palmatine administration. As an anti-inflammatory compound, palmatine likely suppresses the overexpression of inflammatory cytokines in cancerous and non-cancerous tissues.
In conclusion, using a gut-specific inflammation and tumorigenesis ApcMin/+ mouse model, it was reported that palmatine significantly reduced gut inflammation, tumor initiation and tumor progression. The observed effects were supported by the body weight changes, organ index results, mice survival results, gut tissue histology, and gut inflammation cytokine data. Results obtained from the current study suggest that palmatine may have a therapeutic role in colorectal cancer chemoprevention.
Acknowledgments
This study was supported in part by a grant (grant no. BK20131309) from The Natural Science Foundation of Jiangsu Province (China), and NIH/NCCAM grants (grant nos. AT 004418 and AT005362).
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy N. Tumorigenesis: All together now. Nat Rev Cancer. 2013;13:148–149. doi: 10.1038/nrc3469. [DOI] [PubMed] [Google Scholar]
- 3.Madka V, Rao CV. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr Cancer Drug Targets. 2013;13:542–557. doi: 10.2174/15680096113139990036. [DOI] [PubMed] [Google Scholar]
- 4.Moreno-Jimenez MR, Trujillo-Esquivel F, Gallegos-Corona MA, Reynoso-Camacho R, González-Laredo RF, Gallegos-Infante JA, Rocha-Guzmán NE, Ramos-Gomez M. Antioxidant, anti-inflammatory and anticarcinogenic activities of edible red oak (Quercus spp.) infusions in rat colon carcinogenesis induced by 1,2-dimethylhydrazine. Food Chem Toxicol. 2015;80:144–153. doi: 10.1016/j.fct.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Yu C, Wen XD, Zhang Z, Zhang CF, Wu XH, Martin A, Du W, He TC, Wang CZ, Yuan CS. American ginseng attenuates azoxymethane/dextran sodium sulfate-induced colon carcinogenesis in mice. J Ginseng Res. 2015;39:14–21. doi: 10.1016/j.jgr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Song ZJ, He X, Zhang RQ, Zhang CF, Li F, Wang CZ, Yuan CS. Antitumor and immunomodulatory activity of genkwanin on colorectal cancer in the APC(Min/+) mice. Int Immunopharmacol. 2015;29:701–707. doi: 10.1016/j.intimp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Chinese Pharmacopoeia Commission . Pharmacopoeia of the People's Republic of China (Part 1) China. Medical Science Press; Beijing: 2010. [Google Scholar]
- 9.Bhadra K, Kumar GS. Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: Binding aspects and implications for drug design. Med Res Rev. 2011;31:821–862. doi: 10.1002/med.20202. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Li J, Ma F, Yao S, Li N, Wang J, Wang Y, Wang X, Yao Q. Synthesis and cytotoxicity evaluation of 13-n-alkyl berberine and palmatine analogues as anticancer agents. Molecules. 2012;17:11294–11302. doi: 10.3390/molecules171011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung J, Choi JS, Jeong CS. Inhibitory activities of palmatine from coptis chinensis against helicobactor pylori and gastric damage. Toxicol Res. 2014;30:45–48. doi: 10.5487/TR.2014.30.1.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CZ, Du GJ, Zhang Z, Wen XD, Calway T, Zhen Z, Musch MW, Bissonnette M, Chang EB, Yuan CS. Ginsenoside compound K, not Rb1, possesses potential chemo-preventive activities in human colorectal cancer. Int J Oncol. 2012;40:1970–1976. doi: 10.3892/ijo.2012.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CZ, Zhang Z, Huang WH, Du GJ, Wen XD, Calway T, Yu C, Nass R, Zhao J, Du W, et al. Identification of potential anticancer compounds from Oplopanax horridus. Phytomedicine. 2013;20:999–1006. doi: 10.1016/j.phymed.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fichera A, Guo Y, Romero L, Michelassi F, Arenas RB. Quantitation of in vivo gene delivery by restriction enzyme PCR generated polymorphism. J Surg Res. 1997;69:188–192. doi: 10.1006/jsre.1997.5073. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed A, Janakiram NB, Li Q, Choi CI, Zhang Y, Steele VE, Rao CV. Chemoprevention of colon and small intestinal tumorigenesis in APC (Min/+) mice by licofelone, a novel dual 5-LOX/COX inhibitor: Potential implications for human colon cancer prevention. Cancer Prev Res (Phila) 2011;4:2015–2026. doi: 10.1158/1940-6207.CAPR-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty U, Mustafi R, Wang Y, Musch MW, Wang CZ, Konda VJ, Kulkarni A, Hart J, Dawson G, Kim KE, et al. American ginseng suppresses Western diet-promoted tumorigenesis in model of inflammation-associated colon cancer: Role of EGFR. BMC Complement Altern Med. 2011;11:111. doi: 10.1186/1472-6882-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Meer R, Lapré JA, Govers MJAP, Kleibeuker JH. Mechanisms of the intestinal effects of dietary fats and milk products on colon carcinogenesis. Cancer Lett. 1997;114:75–83. doi: 10.1016/S0304-3835(97)04629-6. [DOI] [PubMed] [Google Scholar]
- 18.Jung HA, Yoon NY, Bae HJ, Min BS, Choi JS. Inhibitory activities of the alkaloids from Coptidis Rhizoma against aldose reductase. Arch Pharm Res. 2008;31:1405–1412. doi: 10.1007/s12272-001-2124-z. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Yu C, Zhang CF, Wu XH, Wen XD, Anderson S, Du W, Huang WH, Li SP, Wang CZ, Yuan CS. Chemopreventive effects of oplopantriol A, a novel compound isolated from Oplopanax horridus, on colorectal cancer. Nutrients. 2014;6:2668–2680. doi: 10.3390/nu6072668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bin-Hafeez B, Haque R, Parvez S, Pandey S, Sayeed I, Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int Immunopharmacol. 2003;3:257–265. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 21.Zheng YQ, Wei W, Zhu L, Liu JX. Effects and mechanisms of Paeoniflorin, a bioactive glucoside from paeony root, on adjuvant arthritis in rats. Inflamm Res. 2007;56:182–188. doi: 10.1007/s00011-006-6002-5. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Xie Y, Feng Y, Zhang L, Huang X, Shen X, Luo X. (−)-Epigallocatechingallate induces apoptosis in B lymphoma cells via caspase-dependent pathway and Bcl-2 family protein modulation. Int J Oncol. 2015;46:1507–1515. doi: 10.3892/ijo.2015.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimoud J, Durand H, de Souza S, Monsan P, Ouarné F, Theodorou V, Roques C. In vitro screening of probiotics and synbiotics according to anti-inflammatory and anti-proliferative effects. Int J Food Microbiol. 2010;144:42–50. doi: 10.1016/j.ijfoodmicro.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Garcia Rodriguez LA, Cea-Soriano L, Tacconelli S, Patrignani P. Coxibs: Pharmacology, toxicity and efficacy in cancer clinical trials. Recent Results Cancer Res. 2013;191:67–93. doi: 10.1007/978-3-642-30331-9_4. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Qu X, Wan P, Li QW, Wang Z, Guo F, Bai L, Hu Z, Tan W, Li J. Norcantharidin inhibits pre-replicative complexes assembly of HepG2 cells. Am J Chin Med. 2013;41:665–682. doi: 10.1142/S0192415X13500468. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Wang XJ, Liu Y, Cui YF. PI3K/AKT/mTOR signaling is involved in (−)-epigallocatechin-3-gallate-induced apoptosis of human pancreatic carcinoma cells. Am J Chin Med. 2013;41:629–642. doi: 10.1142/S0192415X13500444. [DOI] [PubMed] [Google Scholar]
- 27.Wang CY, Bai XY, Wang CH. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014;42:543–559. doi: 10.1142/S0192415X14500359. [DOI] [PubMed] [Google Scholar]
- 28.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 29.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen XD, Wang CZ, Yu C, Zhang Z, Calway T, Wang Y, Li P, Yuan CS. Salvia miltiorrhiza (dan shen) significantly ameliorates colon inflammation in dextran sulfate sodium induced colitis. Am J Chin Med. 2013;41:1097–1108. doi: 10.1142/S0192415X13500742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu C, Qiao J, Zhu M, Du J, Shang W, Yin W, Wang W, Han M, Lu W. Preliminary evaluation of the interactions of Panax ginseng and Salvia miltiorrhiza Bunge with 5-fluorouracil on pharmacokinetics in rats and pharmacodynamics in human cells. Am J Chin Med. 2013;41:443–458. doi: 10.1142/S0192415X13500328. [DOI] [PubMed] [Google Scholar]
- 32.Park EY, Kim MH, Kim EH, Lee EK, Park IS, Yang DC, Jun HS. Efficacy comparison of Korean ginseng and American ginseng on body temperature and metabolic parameters. Am J Chin Med. 2014;42:173–187. doi: 10.1142/S0192415X14500128. [DOI] [PubMed] [Google Scholar]
- 33.Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry. 2011;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040–5044. [PubMed] [Google Scholar]
- 35.Yi J, Ye X, Wang D, He K, Yang Y, Liu X, Li X. Safety evaluation of main alkaloids from Rhizoma Coptidis. J Ethnopharmacol. 2013;145:303–310. doi: 10.1016/j.jep.2012.10.062. [DOI] [PubMed] [Google Scholar]