Abstract
There is uncertainty about whether respiratory sinus arrhythmia (RSA), a cardiac marker of adaptive emotion regulation, is involved in relatively low or high executive function performance. In the present study, we investigated: (1) whether RSA during rest and tasks predict both relatively low and high executive function within a larger quadratic association among the two variables, and (2) the extent to which this quadratic trend was moderated by individual differences in emotion regulation. To achieve these aims, a sample of ethnically and socioeconomically diverse women self-reported reappraisal and emotion suppression. They next experienced a two-minute resting period during which ECG was continually assessed. In the next phase, the women completed an array of executive function and non-executive cognitive tasks while ECG was measured throughout. As anticipated, resting RSA showed a quadratic association with executive function that was strongest for high suppression. These results suggest that relatively high resting RSA may predict poor executive function ability when emotion regulation consumes executive control resources needed for ongoing cognitive performance.
Keywords: respiratory sinus arrhythmia, executive function, emotion suppression
Respiratory sinus arrhythmia (RSA) reflects the tendency of heart rate (HR) to accelerate during inspiration and decelerate during expiration (Berntson et al., 1993). RSA is thought to represent cardiac vagal control and is often quantified by high-frequency variability in the HR time series (HF-HRV; Malliani et al., 1991). The Neurovisceral Integration Model posits that RSA is a functional output of a central network in which the prefrontal cortex (PFC) tonically inhibits limbic structures and subcortical autonomic centers important for emotion (Thayer & Lane, 2000). Thus, when comparing individuals, relatively higher levels of RSA are said to communicate better control over negative emotion arising from ongoing stressors, such as difficult cognitive tasks (Thayer et al., 2009; Lane et al., 2009). Successful regulation over emotional circuits, and hence high RSA, are seen as beneficial to cognitive performance; however, emotion regulation has also been shown to damage concurrent cognitive performance by draining away limited cognitive resources from the cognitive task at hand. In the present study, we explore whether RSA is related to emotion regulation processes that are costly to effortful cognitive performance. This was tested by investigating the extent to which trait emotion regulation moderated nonlinear associations between RSA and executive function.
Executive function (EF) is a stable individual difference that underlies performance on many difficult cognitive tasks (Miyake & Friedman, 2008). EF involves a variety of PFC structures and describes cognitive processes needed for complex goal-directed behavior requiring use of relevant information and dismissal of non-relevant information (Miyake et al., 2000; Yuan & Raz, 2014). Although EF is measured in a variety of ways, an emerging consensus focuses on the covarying capacities of inhibition, updating, and shifting (Miyake & Friedman, 2012). Inhibition refers to one’s ability to prevent a dominant or automatic response. Updating describes continual maintenance and manipulation of working memory contents. Lastly, shifting gets at moving the focus of attention from one mental set to another. These three EFs, together, are employed in more complex cognitive operations, such as planning and problem-solving (Miyake & Friedman, 2012). EF is consistently linked to between-subjects differences in RSA. RSA, either at rest or during task performance, tends to have positive linear associations with performance on inhibition, working memory, and shifting tasks (Johnsen et al., 2003; Hansen et al., 2003; Hansen et al., 2009; Beaumont et al., 2012; Hovland et al., 2012).
EF is considered critical for effective emotion regulation (i.e., modulation of emotional experience and/or expression; Gross & Thompson, 2007; Hofmann, Schmeichel, & Baddeley, 2012). This idea is supported by work on child temperament that grounds emotion regulation development in the maturation of executive control abilities, as well as by studies that link high working memory capacity to successful emotion regulation in adults (Posner, Rothbart, Sheese, & Voelker, 2014; Schmeichel, Volokhov, & Demaree, 2008; Schmeichel & Demaree, 2010). Consistent with this research, it has been demonstrated that the PFC architecture used in EF substantially overlaps with structures involved in affective control, such that common emotion regulation strategies are thought to be employ executive resources (e.g. working memory) to achieve their effects (Ochsner & Gross, 2005). In effect, high RSA is suggested to index cognitive control over emotional circuits (Thayer & Lane, 2009; Friedman, 2007). Resting RSA is conceived as emotion regulation capacity, which can be conceived as control of negative emotion at the trait level (Thayer et al., 2012; Lane et al., 2009). In contrast, “task” increases in RSA are thought to reflect phasic PFC inhibition over limbic circuits, thus implicating state emotion regulation efforts (Thayer et al. 2012; Butler et al., 2006). Deployment of common emotion regulation strategies, such as reappraisal (i.e., reinterpretation of an emotion to be less negative or neutral) and emotion suppression (i.e., inhibition of affect in terms of its motor and behavioral components such as facial expressions; Gross, 2002), tend to covary with within-person increases in RSA (Butler et al., 2006; Denson et al., 2011). High resting vagal activity also predicts an increased likelihood to engage in both suppression and reappraisal (Pu et al., 2010; Volokhov & Demaree, 2010).
Many have suggested that resting RSA relates to individual differences in EF performance because resting RSA reflects emotion regulatory capacity that supports complex cognition (Thayer et al., 2009; 2012). That is, EF tasks are difficult and can be stressful to complete, with strong anxiety during a complex task harming performance (Al’Abisi et al., 1997; Egloff et al., 2006). Emotion regulation capacity, which may be indexed by resting RSA, has been suggested to limit deleterious influences of anxiety on EF performance across situations (Thayer et al., 2009; Ursache et al., 2013; Dennis et al., 2009). This notion is supported by a number of research domains. First, high trait emotion regulation in childhood and interventions that enhance emotional regulatory skills have been highlighted as key predictors of EF capacity (Ursache, Blair, & Raver, 2012). Second, high resting RSA predicts relatively better EF when presented with a performance-harming emotional stimulus (e.g. phobic imagery, threat of shock; Johnsen et al., 2003; Hansen et al., 2009). As such, higher values of resting RSA are thought to reflect greater tonic inhibition of frontal cortical regions on neural threat circuits. If these circuits were left unrestrained, they would mobilize “fight or flight” reflexes that interfere with complex cognition (Arnsten, 2009; Thayer & Lane, 2009). Consequently, this work implies that resting RSA linearly characterizes the extent of PFC inhibition over emotion, with greater inhibition being conducive to EF ability. Although less emphasized, phasic increases in RSA (i.e., task RSA) have been tied to “state” emotion regulation in the service of concurrent EF performance (Elliot et al., 2012; Butler et al., 2006).
The Cost of Emotion Regulation
Higher RSA as an index of the degree of emotion regulation may not always predict optimal EF. This is because emotion regulation can sometimes be costly to simultaneous cognitive performance (Richards & Gross, 2000; Egloff et al., 2006). Performance on EF tasks and common emotion regulation strategies (e.g. reappraisal, suppression) have been shown to rely on a common pool of limited cognitive control resources akin to EF (Ochsner & Gross, 2005; Kanske et al., 2010; Van Dillen et al., 2009). In cognitive load theory, EF capacity is a limited resource, such that placing high demand on (i.e. loading) working memory diminishes EF performance requiring cognitive inhibition (de Fockert et al., 2001). Emotion regulation, by draining working memory and other EF resources, may impair complex cognitive processes needed for ongoing tasks. In support of this notion, recent studies have shown that compared to not controlling affect at all, attempts to regulate emotion during (or close in time to) an attentional challenge are associated with poorer performance on that attentional task (Ortner et al., 2013; Friese et al., 2013).
As an index of degree of emotion regulation in EF contexts, higher RSA may predict a tradeoff between emotion regulation and cognitive performance on tasks that place high demand on central executive regulatory capacities. In support of this notion, Pu et al. (2010) found that high resting RSA predicted both an increased likelihood to suppress film-induced negative emotion and poor performance on a subsequent working memory task. This study offers indirect evidence that resting RSA is an index of trait emotion regulation processes that negatively impact EF abilities.
A negative association between RSA and EF performance is surprising, given the wealth of studies reporting a positive association between them (e.g. Hovland et al, 2012; Johnsen et al, 2009). However, both a positive and negative association between EF and RSA may exist within a larger nonlinear function, whereby the direction of the EF-RSA association changes across levels of RSA. The existence of a quadratic curvilinear function between RSA and EF is supported by the results of Marcovitch and colleagues (2010), in which moderate levels of RSA during EF tasks (indexed by RSA reactivity) were associated with the best EF performance in children. These effects were attributed to moderate RSA indexing an optimal level of arousal during EF performance (Keeley et al., 2008).
As an additional explanation for Marcovitch et al.’s effects, it is reasonable that task RSA reflected not just affective processes but also distinct emotion regulatory mechanisms that influenced performance as well (Blair & Ursache, 2011; Rothbart, Ahadi, & Evans, 2000). Accordingly, relatively high levels of RSA in their quadratic function may have reflected greater use of central executive resources for emotion regulation. In this view, moderate RSA may have been adaptive for performance through reducing negative emotion with only minimal load-related costs to EF. At very high levels of RSA, as EF capacity is heavily loaded, relatively higher levels of regulation (i.e. higher RSA) may have excessively drained executive resources from the concurrent EF task.
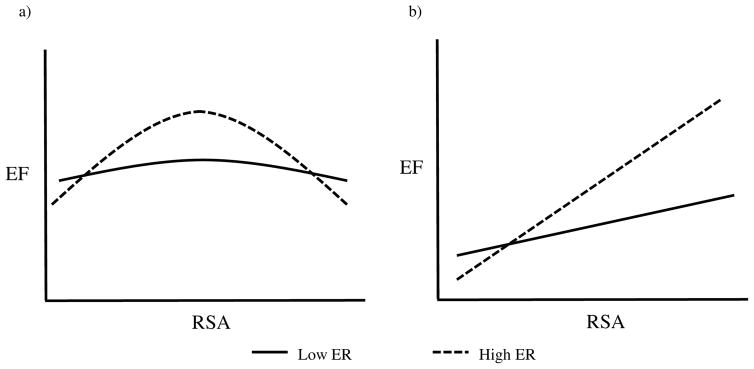
Therefore, studies showing positive associations between RSA and EF may have been capturing an effect that is operating typically at low to moderate levels of RSA—a linear approximation of the “left half” of the full curvilinear function (see hypothesized function in Figure 1). Since it is common for linear and curvilinear effects both to be significant in the same model, the function representing the link between RSA and EF may often be mistakenly represented as strictly linear without additional testing for quadratic effects (Cohen, Cohen, West, & Aiken, 2003). In particular, testing of a potential shift in the function at very high levels of RSA—where the cognitive costs of potentially excessive regulation of emotion should be most acute—has been almost completely overlooked in the literature.
Figure 1. Functions between RSA and EF performance.
a) Hypothesized quadratic functions: Curvilinearity is most present for high ER. The linear function diminishes across RSA such that it is no different from zero at moderate RSA. The relation somewhat reverses (becomes negative) at very high RSA. Moderate RSA is related to the best EF performance. b) Linear characterization of EF-RSA function. The steepness of the positive association is augmented with increases in ER. These functions are considered to be incomplete in capturing the full relation among EF and RSA.
The Current Study
In finding quadratic effects between performance and task vagal control (i.e. RSA reactivity), Marcovitch et al. (2010) were likely studying state emotion regulation effects on children’ concurrent cognitive performance. However, very little work has tested for these functions in adults and whether similar associations are present for resting vagal control, a trait measure of emotion regulation. As mentioned above, emotion regulation capacity and resting RSA are considered influential on individual differences in EF. It is possible that the hypothesized interplay between emotional control and EF occurs at a trait-level, such that resting RSA shows quadratic relations to EF ability as well.
To our knowledge, no one has investigated how a nonlinear association between EF and RSA may be related to the potential costs of emotion regulation on EF performance. In the present study, we included measures of affective control in an effort to substantiate emotion regulation as a factor that helps explain the potential curvilinear function between RSA (at rest and during tasks) and EF performance. Emotion regulation strategy use was measured by individual differences in emotion suppression and reappraisal (Gross, 2002). Individual differences in these emotion regulation strategies are quite stable and predictive of actual use of these strategies in the laboratory (Gross & John, 2003; Drabant et al., 2009). Under conditions of frequent emotion regulation (high suppression/reappraisal), it is expected that physiological emotion regulation capacity captured by resting RSA will be increasingly actualized and thus influential on EF performance. Thus, the identification of a relatively strong quadratic EF-RSA relation amongst high trait emotion regulation compared to that of lower levels would support the decelerating trend in the EF-RSA function (i.e., curvilinearity) as being somewhat dependent on trait emotion regulation. This is also important because resting RSA has been argued to reflect affective processes apart from emotion regulation; e.g. positive emotionality and trait anxiety (Oveis et al., 2009; Miu, Heilman, & Miclea, 2009). However, high RSA among frequent regulation (either high suppression or high reappraisal) may more effectively tap into this construct’s PFC-mediated emotional control, when actual emotion regulation is more likely.
The central aim of the current study was to test whether there is a negative quadratic function between EF and resting RSA in adults and then to test for a similar function between EF and concurrent task levels of RSA in adults, controlling for resting levels of RSA. Most importantly, we examined whether the strength of the hypothesized quadratic effects depended on individual differences in reappraisal and suppression. In particular, we predicted that the presence of quadratic functions between resting RSA and EF would be most pronounced in individuals who frequently regulate their emotions. This pattern of results would highlight emotion regulation capacity as a potential agent that drains executive resources and causes EF to worsen at higher levels of RSA. We expected that state emotion regulation would operate similarly such that the quadratic relation between task RSA and EF would be particularly strong amongst both frequent suppressors and reappraisers. In regard to the specific nature of these quadratic functions, we expected a positive relation between RSA and EF at low levels of resting and task RSA. We also hypothesized that this positive association would attenuate at higher levels of RSA and eventually plateau at more moderate RSA (see Figure 1). At even higher levels of RSA, we anticipated that the flattened function between RSA and EF would shift to a negative association as the costs of excessive regulation on EF performance begin to outweigh emotion regulation’s parallel benefits to performance (i.e., through negative affect reduction). As seen in the different curvilinear functions plotted in Figure 1, the degree of curvature in the EF-RSA relation is expected to be increasingly prominent at high rather than low emotion regulation levels. Note that traditional views of RSA’s effects on EF would characterize EF-RSA functions as positive linear and more prominent at higher levels of emotion regulation (See Figure 1b). In our view, this competing model is incomplete. Positive relations are hypothesized to exist in our current sample (especially at high emotion regulation), but due to increasing use of limited executive resources, they are expected to decelerate across levels of RSA and then exhibit an accelerating negative trend at relatively higher RSA.
We expected that the curvilinear interaction with emotion regulation would be present only for highly demanding tasks (e.g., executive function tasks). In contrast, there would not be evidence for the hypothesized effects in performance of less demanding non-executive cognitive tasks. Non-executive tasks often tap into crystallized abilities, as well as passive memory processes related to retrieval and rehearsal (Kane, Conway, Hambrick, & Engle, 2007). To test our hypotheses, a socioeconomically diverse community sample of women completed an array cognitive tasks while HR and RSA were continuously assessed. In particular, they completed a battery of tasks to assess EF ability as a unitary construct, along with separate tasks to address non-executive processes (Friedman et al., 2008). Women also self-reported reappraisal and suppression.
Method
Sample
The sample consisted of 151 women (age, M = 32.79 yrs, SD = 6.39 yrs) who exhibited substantial variability in ethnicity and socioeconomic status (75% Caucasian, 13% African American, 2% Asian, 6% multiple races, 5% other; and 4% Hispanic). Roughly one-third of the women were single. Their education level was highly variable and evenly distributed into the following categories: 22% had a high school diploma/GED or less, 58% had at least two years of college, and 20% had some kind of a post-graduate degree. Data collected from these women were a part of a larger family study focused on associations among the women’s EF performance, emotion regulation, and their parenting behaviors and attitudes (Deater-Deckard, Li, & Bell, in press; Deater-Deckard, Chen, Wang, & Bell, 2012; Deater-Deckard, Wang, Chen, & Bell, 2012).
Procedure
Two-thirds of the sample was recruited via community agencies and advertisements (e.g., flyers, university website and email announcements). Women who contacted us by email or telephone were provided with a study description, and if eligible, were consented by a telephone call. The group recruited in this manner participated in a laboratory that was located in a small urban area. The remaining third of our sample was recruited from families in a pre-existing longitudinal study, and participated in a rural university laboratory. All women completed self-report questionnaires before the in-lab session. Once in the laboratory, signed consent was attained from each woman before electrocardiography (ECG) leads were applied. During the electrode application process, participants completed the Peabody Picture Vocabulary Test (PPVT), an assessment of non-executive verbal intelligence. Following a subsequent 2-minute resting ECG recording during which the women sat quietly with their eyes open, each woman completed a battery of cognitive tasks, EF and non-EF, while ECG was continuously assessed. EF tasks (Stroop, Wisconsin Card Sorting Test, Tower of Hanoi, Backward Digit Span) were presented in counter balanced order. After EF measures, women completed a non-executive task, the Corsi-Milner, such that this task occurred at the end of the session. With one exception (backward digit span), all cognitive tasks were presented and completed on a computer and required finger-typed keyboard responses. Task descriptions are given below.
Cognitive Tasks
EF
In order to index levels on a general EF factor, we used four laboratory tasks to measure inhibition, working memory, and shifting. These tasks were counterbalanced and yielded distributions that are typical of adults up to middle age (see Deater-Deckard, Wang, Chen, & Bell, 2012). EF tasks were not of a fixed length, as their duration was dependent on the participant response time. In the Tower of Hanoi, there was a maximum time limit imposed (60 s). Time to complete tasks was recorded and the duration of each cognitive task is given below.
A “mixed” color-word Stroop task was used in which participants named the ink of 20 “color” words that were either congruent (e.g., “red” written in red ink) or incongruent (e.g., “red” written in yellow ink; Stroop, 1935). The mixed condition occurred after three other conditions during which participants read the color of the ink of a series of Xs, read color words for which the ink color was congruent, and then read color words for which the ink color was incongruent. This latter condition required participants to name the color of the ink, not read the word. The mixed condition of interest to our analyses consisted of 20 incongruent and congruent stimuli. This mixed arrangement is thought to be more challenging and hence stressful, as it has been shown to impose a cost on performance resulting from switching between response categories (incongruent vs. congruent; Wylie & Allport, 2000). The average duration of the mixed Stroop was 34 s, and performance on trials was operationalized as the number of correct responses (0–20).
The Wisconsin Card Sorting Test (WCST) incorporated four types of stimulus cards that either varied by color, quantity, or shapes (Heaton, PAR Staff, & Goldin, 2003). The average duration of this task was five and a half minutes. Women were shown a set of “original” cards and then tried to match a new stack of 64 (at the rural university lab) or 128 (at the urban lab) cards to the original ones according to an implicit categorization rule (i.e. by color, quantity or shape). The matching rule changed throughout the task and women had to infer new rules based on computerized feedback about whether a match was correct or not. Performance on this task was scored as the number of perseverative errors. For the women who completed the 128-card version of the task, we divided the number of perseverative errors by 2, in order to account for the increased likelihood of error in the 128 vs. 64 card task. Perseverative errors signify the number of trials in which the participant continued to use a formerly appropriate but now incorrect matching rule after receiving feedback that the given rule was wrong. Perseverative error scores did not differ between the rural (M = 6.6, SD = 5.4 ) and urban (M = 6.8, SD = 4.1) sites, t(149) = .172, p = .864.
Problem solving abilities grounded in all three EFs (i.e. planning) were gauged with a Tower of Hanoi (TOH) task (Davis & Keller, 1998). The task consisted of moving three disks of varying sizes to a target peg while adhering to two rules: 1) only one disk can be moved during a single turn, and 2) larger disks cannot be placed on top of smaller ones. Task performance was measured as time to complete (60 seconds max). Women who took longer to finish had their score converted to 60 seconds. Average duration for the TOH was 43 s (SD = 44.9).
A backward digit span task with a mean duration of 33.4 s (SD = 36.6) was administered to women by an experimenter who read a pseudo-random series of single-digit numbers (4–10). The participant was then asked to recreate the series in reverse with verbal responses. Each woman first completed a practice trial in which there were two sets of two digits. The first trial consisted of a series of four digits and following trials were modified by the addition of one more digit. Women were given only two chances to correctly reverse each series. The entire task ended when the participant incorrectly responded during both chances in a single trial. Performance on the task was represented as the length of the series (# of digits) in the last correct trial. This task was adapted from the Stanford-Binet Intelligence Scale (5th edition) and is comparable to digit span tasks used in other studies (Roid, 2003; Gregoire & Van der Linden, 1997).
Due to the unitary nature of EF, we computed a composite score that combined the metrics of the four EF tasks above, which together represented shifting, inhibition, and working memory (Miyake & Friedman, 2012). Note that WCST and TOH scores were reverse coded to be consistent with metrics of other tasks. Accordingly, the first principal component for the four scores explained 46% of the variance (λ = .62 to .74). To create the composite, the four performance scores were first standardized and then averaged. This mean score was again standardized to create a composite score in standard deviation (SD) units for each woman, such that higher scores represented better EF performance. This overall EF value was the dependent measure in primary analyses.
Non-EF
Like EF measures, length of non-executive tasks varied between persons, and average durations are given below. The Peabody Picture Vocabulary Test (PPVT-III; Dunn & Dunn, 1997; or PPVT-IV; Dunn & Dunn, 2007) was administered to the participants during the laboratory visit, to assess receptive vocabulary as an indicator of verbal intelligence. Non-normed raw scores were used to index performance on this task. As previously noted, the PPVT was administered during EEG and ECG electrode application, a procedure we use in our research lab for all participants from age 4 through adulthood. Length of the assessment is determined by performance on the task and ranged from 10 to 20 minutes.
An adaptation of the Corsi-Milner (CM; Milner, Corsi, & Leonard, 1991) temporal order task was used to assess memory. Forty abstract paintings were presented for 3 seconds each with no inter-trial interval. The sequence was periodically interrupted with questions regarding either recency (“which of these 2 paintings have you seen most recently?”) or recognition (when paired with a novel painting: “which painting have you seen?”). There were 5 recency and 5 recognition questions; performance was total number correct. After about a 45-minute delay, participants were presented with the same recency and recognition questions, but this time without the presentation of the 40 abstract paintings. No feedback was given for either the immediate or the delayed versions of the memory task. The task took an average of 122 s (SD = 15.7).
Measures
Self-report
Women self-reported their emotion regulation on the Emotion Regulation Questionnaire (Gross & John, 2003). This questionnaire is comprised of two separate subscales measuring suppression and reappraisal (α = .81). Suppression and reappraisal items relate to characteristic individual differences in the tendencies to inhibit and re-evaluate affective behaviors/experience, respectively. Items were rated on a 7-point Likert scale (1 = strongly disagree to 7 = strongly agree) and then averaged to yield an overall score for trait suppression as well as a separate score for reappraisal.
Cardiac physiology
High-frequency heart rate variability (HF-HRV) corresponding to the rate of normal respiration (i.e. RSA) was used to measure cardiac vagal control. HF-HRV was derived from the electrocardiography (ECG) signal, which was collected using disposable spot electrodes arranged at a modified lead II configuration (Stern, Ray, & Guigley, 2001). An additional ground electrode was used at the scalp near site Fz. Women applied their own ECG electrodes, per the instruction of research assistants. Resting ECG was collected for 2 minutes (Task Force of the European Society of Cardiology, 1996), during which women were asked to relax and remain as still as possible. ECG was continuously assessed during all cognitive tasks except the PPVT (non-EF), as this task was completed during electrode application. The lengths of ECG recordings during a single cognitive task varied among participants, with average times being consistent with respective task completion times provided above.
The analog ECG signals were first amplified using a SA Instrumentation Bioamp (San Diego, CA) and bandpass filtered (0.1 to 100 Hz). The signal was then digitally sampled (512 events per second) before being collected and recorded using Snapshot-Snapstream software (HEM Data Corp.; Southfield, MI). Detection of R-waves, along with the calculation of both interbeat intervals (IBI; the time between heart beats) and HF-HRV were conducted offline, using the IBI Analysis System software’s peak detection algorithm (James Long Company, Caroga Lake, NY). If an epoch evidenced movement artifact, it was removed from further analyses. Movement artifact was operationalized as at least three consecutive, missing R-waves. Cleaned ECG data were used to calculate consecutive IBIs, which were then submitted to spectral analysis to derive HF-HRV (i.e. RSA). Employing a discrete Fourier Transform with a 16-second Hanning window and 50% overlap, power spectral density (ms2) was quantified in the domain of normal respiration (.12–.40 Hz; Berntson, Quigley, & Lozano, 2007). HF power during rest and EF tasks were each normalized with a natural logarithm transformation, and these HF-HRV values were used as indicators of RSA; i.e. cardiac vagal control. In particular, resting RSA was indexed by average levels of HF-HRV during a two-minute resting baseline period. HF-HRV was derived from the ECG signal during each cognitive task (EF and non-EF) except the PPVT and the backward digit span. The backward digit span was excluded from HRV analysis because it involved verbal responses, which have been shown to impose respiratory confound on RSA estimates (Sloan, Korten, & Myers, 1991). The PPVT was excluded because it had no concurrent ECG data (see above). To reflect task RSA, or RSA during the EF tasks accounting for resting levels, reactivity difference scores were calculated by subtracting resting HF-HRV from that of each task (Llabre et al., 1991). Given the high concordance of HF-HRV reactivity among the 3 EF tasks (i.e. Stroop, Wisconsin Card Sorting, Tower of Hanoi; see below), a task RSA composite score was computed in the same manner as the EF performance composite above (Marcovitch et al., 2010). RSA during non-EF cognitive tasks was computed as reactivity scores in the same way as EF. A composite measure was not computed in the non-EF case due to their low covariance. For the Corsi-Milner, however, task HF-HRV from its immediate and delayed trials were aggregated, in order to boost reliability of spectral estimates for RSA.
Data Analysis
Hypotheses were tested using a multiple regression approach in which both linear and quadratic effects in RSA, reappraisal/suppression, and their interactions were used to predict composite EF performance. Reappraisal and suppression scores were entered as separate predictors in each model. Resting and composite task RSA were examined in separate equations to avoid excessive residualizing of resting RSA variance from that of RSA reactivity (Cronbach & Furby, 1970). Furthermore, entry of resting RSA and RSA reactivity (i.e., task RSA) in the same model would constitute residualized difference scores, which in comparison to raw difference scores, have been criticized for their poor reliability and lack of cross-situational generalizability (Malgady & Colon-Malgady, 1991; Llabre et al., 1991). Quadratic terms were built for RSA variables to test curvilinear relations between RSA and performance. This was done by squaring RSA terms in each model. To investigate whether main effects were moderated by self-reported emotion regulation, we included curvilinear (RSA) by linear (emotion regulation: reappraisal, or suppression) interactions. Analyses were conducted in accordance with the recommendations of Cohen, Cohen, West, and Aiken (2003). In each equation, linear and quadratic effects for RSA and performance were probed to determine the extent to which they were moderated by emotion suppression and reappraisal. Simple slopes were tested at one standard deviation (SD) above and below the mean of moderator variables (Aiken & West, 1991). Significant quadratic simple slopes that were deemed significant (p<.05) were graphically inspected to further to understand curvilinear trends at a given level of the emotion regulation score and to detect the extent to which linear relations between EF and RSA changed as a function of RSA. The testing of quadratic by linear interactions in the prediction of non-EF cognitive performance was handled with the same approach as above, with resting RSA and task RSA (i.e., RSA reactivity) being entered into separate models.
Results
Preliminary Analyses
See Table 1 for means and standard deviations of cardiac and cognitive variables. As indicated by a one-way ANOVA on RSA, there was a main effect of Task (rest, WCST, Stroop, TOH, CM), F (4, 144) = 18.17, p < .001. Post-hoc comparisons using Tukey’s Least Significant Difference (LSD) tests indicated that RSA significantly (p<.05) decreased from rest to the cognitive tasks that had RSA estimates. The mean response on the ERQ scales for suppression and reappraisal were 3.31 (SD = 1.20; range = 1–6.5) and 5.01 (SD = 1.05; range = 2–7: ), respectively. In the current sample, means and spread for suppression and reappraisal are similar to that of other studies (e.g. Gross & John, 2003; Moore, Zoellner, & Mollenholt, 2008).
Table 1.
Means (and Standard Deviations) of Cardiac, Performance, and Emotion Regulation Measures
| Rest | WCST | Stroop | TOH | BDS | CM (imm) | CM (del) | PPVT | |
|---|---|---|---|---|---|---|---|---|
| Performance | - | 6.70 (5.01) | 18.03 (3.6) | 40.01 (16.09) | 5.09 (2.17) | 7.52 (1.8) | 6.24 (1.6) | 204.64 (19.1) |
| Heart rate | 72.47 (10.6) | 72.49 (17.9) | 71.71 (20.1) | 71.04 (18.8) | - | 73.16 (18.2) | - | |
| RSA | 6.43 (1.3) | 6.04 (1.3) | 5.80 (1.4) | 5.84 (1.4) | - | 5.97 (1.4) | - | |
Units: WCST = # perseverative errors; Stroop = # accurate trials; TOH= minutes to complete; BDS= # digits in last correct trial span; CM= # correctly recalled/recognized; PPVT= raw score; HR = beats per minute; RSA = natural log of ms2.
Note: Heart rate and RSA for CM are averaged across immediate and delayed trials.
Regressions: Hypothesis Testing
EF and resting RSA
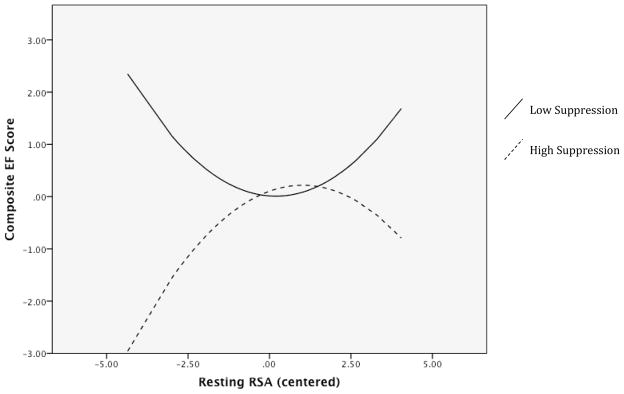
The model examining reappraisal or emotion suppression interactions with resting RSA explained a significant amount of variance in EF composite performance, R2 = .19, F(8, 113) = 3.38, p = .002. The quadratic by linear interaction between RSA and suppression was the only significant predictor in the model, β = −.499, p < .001. Probing the interaction revealed that at low emotion suppression scores, there was no significant linear relation between resting RSA and EF performance β = −.057, p = .693. There was a trend towards a positive quadratic association between these variables, but it did not reach significance, β = .318, p = .08. At high suppression, however, there was a positive association, β = .288, p = .036, and a significant quadratic relation between resting RSA and EF performance, β = −.311, p = .005. High suppression’s simple slopes of RSA on EF performance were graphically inspected at different levels of RSA to better understand the nature of the quadratic effect. As seen in left side of Figure 2, high emotion suppression exhibited a positive relation between resting RSA and EF performance at low levels of RSA. High suppression’s positive association between RSA and EF became weaker at higher levels of resting RSA, This relation became increasingly negative, such that even higher RSA was met with relatively worse EF performance. The quadratic by linear interaction between RSA and reappraisal was not significant, β = .103, p = .455. Regressions were also conducted on each EF task separately, and similar curvilinear by linear interactions between emotion regulation and resting RSA were found for all EF tasks but the backward digit span (i.e. WCST, Stroop, TOH).
Figure 2.
Simple slopes of resting RSA and RSA2 on EF performance at high and low suppression
EF and task RSA
Interaction effects were not found when examining curvilinear trends in task RSA (RSA reactivity) separately. In particular, the model predicting composite EF performance with the composite metric of RSA reactivity and its interactions with suppression and reappraisal was not significant. Regressions were also conducted separately such that a single task’s RSA reactivity was used to predict performance on the corresponding task. None of these analyses yielded significant interactions. See Table 2 for a summary of multiple regression analyses examining EF performance.
Table 2.
EF Tasks: Summary of Regression Models
| EF Compositey | WCST | Stroop | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest | Reactivit | Rest | Reactivity | Rest | Reactivity | |||||||
| B | SE (Tol) | B | SE (Tol) | B | SE (Tol) | B | SE (Tol) | B | SE (Tol) | B | SE | |
| Suppression | .038 | .087 (.72) | −.198 | .123 (.44) | .281 | .452 (.73) | −.234 | .443 (.82) | .025 | .324 (.73) | −.912* | .336 (.60) |
| Reappraisal | −.106 | .113 (.57) | −.070 | .114 (.71) | −1.06 | .595 (.58) | −1.16* | .562 (.70) | .210 | .429 (.59) | .166 | .393 (.70) |
| RSA | .090 | .080 (.68) | −.340 | .239 (.16) | .241 | .408 (.68) | −.346 | .640 (.60) | −.052 | .298 (.68) | .016 | .416 (.80) |
| RSA2 | .001 | .043 (.45) | .002 | .129 (.01) | −.209 | .219 (.49) | −.149 | .428 (.28) | −.003 | .160 (.49) | −.096 | .294 (.70) |
| Supp X RSA | .112 | .062 (.63) | .088 | .331 (.41) | .477 | .320 (.62) | −.929 | .606 (.40) | .297 | .232 (.62) | −.241 | .341 (.90) |
| Supp X RSA2 | −.093** | .026 (.37) | .321 | .426 (.07) | −.302* | .132 (.37) | −.241 | .274 (.20) | −.279** | .096 (.37) | .662 | .226 (.53) |
| Reapp X RSA | −.140 | .075 (.69) | .006 | .197 (.14) | −.396 | .393 (.70) | −.193 | .586 (.65) | −.470 | .285 (.70) | −.090 | .334 (.78) |
| Reapp X RSA2 | .034 | .045 | −.019 | .081 (.02) | .145 | .229 (.38) | .721 | .396 (.26) | .045 | .167 (.38) | .031 | .206 (.60) |
| F | 3.38** | .907 | 2.26* | 1.29 | 2.67* | 1.00 | ||||||
| R2 | .193 | .069 | .142 | .087 | .160 | .069 | ||||||
| TOH | BDS | |||||||
|---|---|---|---|---|---|---|---|---|
| Rest | Reactivity | Rest | Reactivity | |||||
| B | SE (Tol) | B | SE (Tol) | B | SE (Tol) | B | SE | |
| Suppression | 2.50 | .830 (.71) | −1.26 | 1.43 | −.241 | .199 (.74) | - | - |
| Reappraisal | −2.46 | 1.09 (.56) | −.711 | 1.67 | −.079 | .264 (.59) | - | - |
| RSA | 2.55 | .750 (.67) | −4.16* | 1.77 | .101 | .183 (.67) | - | - |
| RSA2 | 1.29 | .403 (.49) | 2.13 | 1.25 | −.077 | .099 (.45) | - | - |
| Supp X RSA | 1.41 | .587 (.62) | .186 | 1.45 | .101 | .144 (.61) | - | - |
| Supp X RSA2 | −1.49** | .243 (.36) | 1.27 | .959 | −.058 | .059 (.37) | - | - |
| Reapp X RSA | −.890 | .721 (.69) | 1.18 | 1.42 | −.169 | .176 (.69) | - | - |
| Reapp X RSA2 | 1.16 | .419 (.37) | .454 | .877 | −.019 | .103 (.38) | - | - |
| F | 2.03* | 2.85** | 1.22 | - | ||||
| R2 | .129 | .187 | .082 | - | ||||
Notes. EF = executive function, WCST = Wisconsin Card Sorting Test, TOH = Tower of Hanoi, BDS = backward digit span, RSA= respirator sinus arrhythmia. Supp = suppression, Reapp = reappraisal. Unstandardized regression coefficients are presented.
p < .01.
p < .05
Non-EF and RSA
Due to the substantial covariance among recency and recognition scores within immediate and also delayed contexts, regressions using the combined recency+recognition outcome measure (separately for immediate and delayed) are presented for both resting and task RSA models. Both models did not explain a significant amount of variance in CM performance. In regard to the prediction of PPVT performance, the model examining resting RSA effects and the model containing task RSA interactions were nonsignificant. Summaries for all non-EF regression analyses appear in Table 3.
Table 3.
Non-Executive Tasks: Summary of Regression Models
| CM- immediate | CM- delayed | PPVT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest | Reactivity | Rest | Reactivity | Rest | Reactivity | |||||||
| B | SE (Tol) | B | SE (Tol) | B | SE (Tol) | B | SE (Tol) | B | SE (Tol) | B | SE | |
| Suppression | .026 | .158 (.73) | .017 | .154 (.75) | −.182 | .150 (.75) | −.084 | .161 (.75) | −2.32 | 1.75 (.72) | - | - |
| Reappraisal | −.164 | .207 (.57) | −.140 | .192 (.65) | .207 | .198 (.58) | .234 | .203 (.65) | .044 | 2.29 (.57) | - | - |
| RSA | −.001 | .146 (.68) | .013 | .207 (.73) | .186 | .139 (.72) | −.097 | .216 (.68) | 5.00** | 1.61 (.68) | - | - |
| RSA2 | −.071 | .079 (.49) | −.193 | .187 (.28) | .012 | .074 (.52) | −.088 | .194 (.26) | −.230 | .869 (.49) | - | - |
| Supp X RSA | .222 | .113 (.63) | .043 | .178 (.56) | .063 | .109 (.72) | .102 | .191 (.55) | 1.39 | 1.25 (.63) | - | - |
| Supp X RSA2 | −.070 | .048 (.37) | −.165 | .127 (.14) | .020 | .045 (.44) | −.063 | .134 (.13) | −.750 | .524 (.37) | - | - |
| Reapp X RSA | −.256 | .138 (.69) | .030 | .196 (.76) | −.203 | .134 (.67) | .129 | .213 (.76) | −1.04 | 1.52 (.69) | - | - |
| Reapp X RSA2 | −.015 | .082 (.38) | .073 | .160 (.27) | −.048 | .080 (.37) | −.002 | .173 (.27) | −.740 | .907 (.38) | - | - |
| F | 1.54 | .371 | 1.19 | .521 | 1.76 | - | ||||||
| R2 | .097 | .025 | .081 | .037 | .111 | - | ||||||
Notes. CM = Corsi-Milner, PPVT = Peabody Picture Vocabulary Test, RSA= respiratory sinus arrhythmia, Supp = suppression, Reapp = reappraisal
p < .01.
p < .05
Discussion
An aim of the current study was to investigate whether RSA, at rest and during cognitive tasks, shows nonlinear associations with EF performance in adults. In particular, we examined the extent to which these relations were moderated by individual differences in reappraisal and suppression in order to substantiate that the curvilinearity in the EF-RSA function was reliant on emotion regulation processes. As hypothesized, quadratic effects between resting RSA, but not task RSA, and EF were most present for individuals scoring high on emotion suppression. Similar interactions between RSA and emotion suppression were not yielded for non-executive tasks. Results suggest that at high suppression of emotion, as resting cardiac vagal control (RSA) became higher, resting RSA less robustly predicted better EF. Our findings also indicate that at even higher levels of resting vagal control in high suppression, relatively larger values of RSA predicted relatively lower EF performance.
This curvilinear association among high scorers on emotion suppression is consistent with a previous quadratic relation between EF and RSA in children (Marcovitch et al., 2010). Whereas work focused on vagal reactivity during “stress”, our study is among the first to show that resting RSA exhibits similar quadratic associations. Since resting RSA is as indicator of trait emotion regulation, this finding may be the result of a tradeoff between high trait emotion regulation and EF ability, as is discussed below.
Identification of this quadratic relation is important because resting cardiac vagal control is typically conceived as being either low or high, with the latter representing a “gold standard”, communicating relatively better cognitive control and bio-behavioral adaptability (Thayer et al., 2009). This view is most evident in the psychometric approach of many studies that use median splits to investigate differences between low and high resting RSA groups (e.g. Hansen et al., 2003; Hansen et al., 2009; Bos et al., 2013). A dichotomous approach to resting RSA inherently forces interpretations of its relations with EF to be linear, such that higher vagal control unilaterally reflects better cognitive control. However, our modeling of quadratic effects indicated that this positive association does not hold equally across all values of resting RSA, and that exacerbated vagal control (i.e. very high RSA) may even predict worse EF in comparison to more moderate RSA values. Unlike the true inverted-U association in Marcovitch et al., we found a quadratic function that mostly consisted of a plateauing positive association between resting RSA and EF, with only modestly worse performance at higher RSA levels.
A particularly novel contribution of our study is that it provides evidence for emotion suppression processes being critical for the curvilinearity in the EF-RSA function. This possibility is bolstered by the fact that hypothesized nonlinear associations were only present for high suppression. It is probable that compared to that of low suppressors resting RSA amongst high suppressors increasingly reflected emotion regulation capacity; i.e., the ability to deploy cognitive (PFC) resources in the service of inhibiting performance-harming negative emotion. As such, this moderation effect suggests that trait emotion suppression is implicated in the attenuation and reversal (i.e. curvilinearity) of linear RSA-EF relations across levels of resting RSA. Trait suppression’s draining of executive resources, more specifically, may play a role in driving this curvilinearity, as is discussed below.
Results are consistent with the hypothesized function that is grounded in a cognitive load-dependent tradeoff between emotion regulation and EF. If findings are interpreted through the lens of this tradeoff account, different pieces of our quadratic relation meaningfully integrate previous and seemingly contradictory effects between affective control and cognitive performance. The first piece of the quadratic relation, at relatively low levels of resting RSA, exhibits a positive EF-RSA association. This lines up with studies that highlight emotion regulation and thus RSA as supporting factors of cognitive performance (e.g. Hansen et al., 2003; Elliot et al., 2011; Arnsten, 2009; Hovland et al., 2012). Tonic regulation over negative affect, as indicated by relatively higher resting RSA, may foster EF ability by reducing distracting influences of negative affective experiences (Vytal et al., 2012; Dolcos & McCarthy, 2006; Thayer et al., 2012). This part of the function is consistent with past studies showing positive relations of EF to both cognitive emotion regulation strategy use and RSA (Ursache et al., 2013; Elliot et al., 2011). However, under high suppression, the relation between resting RSA and EF was shown to attenuate and eventually become negative across levels of RSA. This result may indicate that tonic PFC inhibition of negative emotion, as indexed by high RSA amongst high suppression, confers improvements to complex cognitive ability; however, such increased use of cognitive resources may also increasingly load and thus worsen EF.
In light of shared executive resources between emotion regulation and EF, it is possible that higher resting RSA amongst high emotion suppressors reflected trait-like affective control’s inherent consumption of limited executive resources (Ochsner & Gross, 2005; Schmeichel, Volokhov, & Demaree, 2008; Ortner et al., 2013). A variety of studies have provided strong evidence for cognitive load theory and its notion that increased use of executive resources leaves less capacity for other functions that use (i.e. load) the same resource (Lavie et al., 2004; de Fockert, Rees, Frith, & Lavie, 2001). Hence, relatively higher levels of resting RSA may have predicted lowered EF because high resting RSA indirectly reflected a load-based tradeoff between EF ability and emotion regulation in executive resources. This finding is also consistent with studies that provide direct evidence for the role of emotion regulation in decreasing ongoing EF performance through loading EF (Ortner et al., 2013). In particular, use of emotion suppression has been shown to negatively impact Stroop performance and reduce activity in “executive” brain regions (Friese et al., 2013). Thus, at relatively higher RSA (see right side of Figure 2), the accompanying EF-enhancing effects of emotion regulation may have been increasingly negated and then reversed as affective control drained cognitive resources from EF. It is also beneficial to interpret the function amongst low suppression. In particular, a significant quadratic relation may not exist for these individuals because high RSA amongst infrequent suppressors may instead reflect a tendency to use emotion regulation processes that do not heavily load EF (e.g. distracting oneself from anxiety with mental imagery).
It must be noted that these tradeoffs can operate at a state level such that emotion regulation efforts influence concurrent EF measures. However, the present results are most relevant to tradeoffs among emotion regulation and EF at a trait level, in which very high resting RSA loads EFs and possibly limits cognitive resources that can be used for EF ability (i.e. performance on ongoing EF tasks across time and situations) (Ursache, Blair, & Raver, 2012; Thayer et al., 2012). Interpreting RSA’s nonlinear effects in light of a tradeoff between emotion regulation’s influences on cognition is supported by the fact that hypothesized functions were not found for “easier” (e.g. non-executive) cognitive tasks. These results suggest that high trait emotion regulation, as indexed by resting RSA under high emotion suppression, may only index a spending of shared executive resources when there is an opportunity for the cognitive task to be difficult and demanding of emotion regulation in the first place. In the realm of psychophysiological research, many laboratory EF performance measures can be considered stressful (Al’Absi et al., 1997).
Unexpected Findings
Lack of interactions between RSA and reappraisal were unanticipated given that RSA has been shown to reflect emotion regulation more generally, including reappraisal (Butler et al., 2006). Lack of these findings may have been due to reappraisal being less costly to cognitive resources than is emotion suppression. For instance, it has been shown that individuals who score high in suppression, but not high in reappraisal, tend to have worse non-executive memory performance than those who score low in the dimension (Richards & Gross, 2000). Furthermore, neuroimaging studies indicate that suppression requires more sustained effort and PFC inhibitory control than that of reappraisal, while reappraisal uses brain regions typical of more automatic cognition (Goldin et al., 2008; Kalisch et al., 2005). In a study by Ortner and colleagues, (2013), emotion suppression was associated with worse concurrent attentional performance than was reappraisal. Therefore, it is possible that reappraisal did not use enough resources to lower performance. In contrast, high emotion suppression may have had a marked expenditure of cognitive control resources, thereby leading to decelerations in EF benefits and eventual acceleration in EF costs as RSA values increased.
Contrary to expectations, task RSA, as indexed by RSA reactivity, did not exhibit quadratic relations with EF performance. Such a finding is not concordant with the nonlinear association found by Marcovitch et al. (2010). This may have been due to the unreliable nature of RSA collected during mental stressors, as even minimal motoric demands of such tasks (i.e., keyboard strokes) have been claimed to obscure the psychological relations being tested (Lehrer et al., 2010). Lack of findings for task RSA may alternatively indicate that trait emotion regulatory processes are more influential on performance than that of state regulation. This interpretation is conceptually similar to studies showing that trait, but not state, negative emotion negatively impacts cognitive performance (for review, see Eysenck, Derakshan, Santos, & Calvo, 2007). Our null finding should be replicated before substantive conclusions are made regarding the role of task RSA in the proposed tradeoff model.
Although high suppression’s resting RSA-EF relation was negative quadratic, as hypothesized, its precise shape was slightly different from that which was predicted (see Figure 1a). In accord with Marcovitch and colleagues, the theorized function was an inverted-U. In this function, moderate values of RSA were expected to predict the best performance, with EF decrements at higher RSA mirroring low EF levels at lower RSA. However, it may be that our sample of women was incomplete in that it did not include participants with large enough resting RSA values to evidence heavy decreases in performance. It is possible that such EF decrements would occur at extremely high levels of RSA. On the other hand, it is possible that the yielded shape of the resting RSA-EF relation for high emotion regulation is the true function, such that the point at which the function reverses and curves downward exists at levels of RSA that are higher than anticipated.
It is also important to note unexpected trends towards a positive quadratic relation between resting RSA and EF performance at low emotion suppression. Although not statistically significant, this trend had an effect size (regression coefficient) comparable in magnitude to same relation at high suppression. Consequently, this result may point to the importance of nonlinear effects within RSA that rely on emotion regulation mechanisms unrelated to cognitive resource depletion and/or affective arousal.
Future Directions and Conclusions
It is feasible that quadratic relations may have been a function of resting RSA reflecting emotional activation that influences performance. In particular, negative affect’s arousal is a potent controller of EF quality, such that moderate arousal optimizes performance (Ursache et al., 2013; Vytal et al., 2012). In the realm of physiology, RSA tends to be inversely correlated with the intensity of emotional activation and the presence of anxiety (Frazier et al., 2004). Therefore, high resting RSA among frequent emotion suppressors may have indicated reduced negative affect that resulted from their ongoing emotion regulation efforts. This blunted affect may push arousal to a suboptimal level for EF. We believe that this explanation is less likely than the one focused on competition for PFC resources, because suppression has been shown to be ineffective at reducing subjective negative affect (Gross, 2002). The lack of interaction between resting RSA and reappraisal may also be explained by the tendency of reappraisal to be more effective at reducing negative emotion than is suppression. In effect, reappraisal may have aptly decreased performance-harming negative emotion, thereby cancelling out the negative impacts of reappraisal on EF at high resting RSA. Future studies can help rule out these possibilities by including measures of emotion experienced during the EF tasks. In addition, the dependence of nonlinear relations on affective control might be better substantiated by an experiment that manipulates emotion regulation strategies in the laboratory (Gross, 2002). Future studies with this design would not only better test the causal role of emotion regulation processes in quadratic EF-RSA associations, but they would better probe emotion regulation states that actively use resources during the cognitive tasks. We only collected trait emotion regulation measures, which may or may not have translated into discrete use of emotion suppression during the EF tasks. In this regard, forthcoming studies should also pinpoint which emotion regulation strategies are spontaneously used during EF tasks. For instance, EF tasks might naturally elicit use of suppression but not reappraisal, which may have accounted for this strategy’s lack of interactions with RSA in the current study. In addition, future studies should test whether quadratic functions generalize to men, as our sample consisted of only women and gender effects on RSA have been noted (Healy, Treadwell, & Reagan, 2011).
Despite these limitations, the current study provides evidence for the nonlinear aspects of resting RSA, which cannot be captured with approaches that dichotomize RSA. Further, our data suggest that high cardiac vagal control may not always be a protective factor for cognitive ability, which is contrary to traditional views in which all domains of self-regulation operate in parallel (Thayer & Lane, 2000). Our study expands on the applicability of the Neurovisceral Integration model by showing that resting vagal activity additionally marks use of emotion regulation in situations where affective self-regulation is costly to ongoing cognitive function. The status of high resting RSA as adaptive indicator may be subject to the type of emotion regulation strategy used.
Acknowledgments
The authors would like to acknowledge Zhe Wang for her assistance in various aspects of this manuscript. This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants HD57319 and HD60110. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or National Institutes of Health. We are grateful to the women for their participation in our studies and to our research teams for their assistance with data collection and coding.
Contributor Information
Derek P. Spangler, Email: dpspang@gmail.com, Department of Psychology, Virginia Tech (phone: 540/892-3488)
Martha Ann Bell, Email: mabell@vt.edu, Department of Psychology, Virginia Tech (phone: 540/231-2546).
Kirby Deater-Deckard, Email: kirbydd@vt.edu, Department of Psychology, Virginia Tech (phone: 540/231-6581).
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- Al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34(3):266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of general psychology. 2006;10(3):229. [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont A, Burton AR, Lemon J, Bennett BK, Lloyd A, Vollmer-Conna U. Reduced cardiac vagal modulation impacts on cognitive performance in chronic fatigue syndrome. PloS one. 2012;7(11):e49518. doi: 10.1371/journal.pone.0049518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Lozano D. Handbook of psychophysiology. 3. Cambridge, U.K: Cambridge University Press; 2007. Cardiovascular psychophysiology. [Google Scholar]
- Blair C, Ursache A. A bidirectional model of executive functions and self-regulation. Handbook of self-regulation: Research, theory, and applications. 2011;2:300–320. [Google Scholar]
- Bos MG, Jentgens P, Beckers T, Kindt M. Psychophysiological response patterns to affective film stimuli. PloS one. 2013;8(4) doi: 10.1371/journal.pone.0062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43(6):612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Bell MA, Deater-Deckard K. Maternal Frontal EEG Asymmetry and Chronic Stressors Moderate the Link between Child Conduct Problems and Maternal Negativity. Social Development. 2014 doi: 10.1111/sode.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Routledge; 2003. [Google Scholar]
- Cronbach LJ, Furby L. How we should measure “change”: Or should we? Psychological bulletin. 1970;74(1):68. [Google Scholar]
- Davis HP, Keller FR. Colorado assessment test manual. 1998. Colorado Springs: Colorado Assessment Tests. [Google Scholar]
- Deater–Deckard K, Chen N, Wang Z, Bell MA. Socioeconomic risk moderates the link between household chaos and maternal executive function. Journal of Family Psychology. 2012;26(3):391. doi: 10.1037/a0028331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K, Wang Z, Chen N, Bell MA. Maternal executive function, harsh parenting, and child conduct problems. Journal of Child Psychology and Psychiatry. 2012;53(10):1084–1091. doi: 10.1111/j.1469-7610.2012.02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291(5509):1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Cole PM, Wiggins CN, Cohen LH, Zalewski M. The functional organization of preschool-age children’s emotion expressions and actions in challenging situations. Emotion. 2009;9(4):520–530. doi: 10.1037/a0016514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Grisham JR, Moulds ML. Cognitive reappraisal increases heart rate variability in response to an anger provocation. Motivation and Emotion. 2011;35(1):14–22. [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological psychiatry. 2009;65(5):367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff B, Schmukle SC, Burns LR, Schwerdtfeger A. Spontaneous emotion regulation during evaluated speaking tasks: associations with negative affect, anxiety expression, memory, and physiological responding. Emotion. 2006;6(3):356. doi: 10.1037/1528-3542.6.3.356. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Payen V, Brisswalter J, Cury F, Thayer JF. A subtle threat cue, heart rate variability, and cognitive performance. Psychophysiology. 2011;48(10):1340–1345. doi: 10.1111/j.1469-8986.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- Epstein S. Aggregation and beyond: Some basic issues on the prediction of behavior. Journal of Personality. 1983;51(3):360–392. doi: 10.1111/j.1467-6494.1983.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Strauss ME, Steinhauer SR. Respiratory sinus arrhythmia as an index of emotional response in young adults. Psychophysiology. 2004;41(1):75–83. doi: 10.1046/j.1469-8986.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biological psychology. 2007;74(2):185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biological Psychology. 1998;47(3):243–263. doi: 10.1016/s0301-0511(97)00027-6. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137(2):201. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese M, Binder J, Luechinger R, Boesiger P, Rasch B. Suppressing emotions impairs subsequent stroop performance and reduces prefrontal brain activation. PloS one. 2013;8(4):e60385. doi: 10.1371/journal.pone.0060385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire J, Van Der Linden M. Effect of age on forward and backward digit spans. Aging, neuropsychology, and cognition. 1997;4(2):140–149. [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of personality and social psychology. 2003;85(2):348. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 3–26. [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. International Journal of Psychophysiology. 2003;48(3):263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety, Stress, & Coping. 2009;22(1):77–89. doi: 10.1080/10615800802272251. [DOI] [PubMed] [Google Scholar]
- Healy B, Treadwell A, Reagan M. Measures of RSA suppression, attentional control, and negative affect predict self-ratings of executive functions. Journal of Psychophysiology. 2011;25(4):164. [Google Scholar]
- Heaton RK, Staff PAR, Goldin JN. WCST: CV4 Wisconsin Card Sorting Test: Computer Version 4 research edition user’s manual. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends in cognitive sciences. 2012;16(3):174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hovland A, Pallesen S, Hammar Å, Hansen AL, Thayer JF, Tarvainen MP, Nordhus IH. The relationships among heart rate variability, executive functions, and clinical variables in patients with panic disorder. International Journal of Psychophysiology. 2012;86(3):269–275. doi: 10.1016/j.ijpsycho.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Jennings RJ, Allen B, Gianaros PJ, Thayer JF, Manuck SB. Focusing neurovisceral integration: Cognition, heart rate variability, and cerebral blood flow. Psychophysiology. 2014 doi: 10.1111/psyp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen BH, Thayer JF, Laberg JC, Wormnes B, Raadal M, Skaret E, … Berg E. Attentional and physiological characteristics of patients with dental anxiety. Journal of anxiety disorders. 2003;17(1):75–87. doi: 10.1016/s0887-6185(02)00178-0. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O’doherty JP, Oakley DA, … Dolan RJ. Anxiety reduction through detachment: subjective, physiological, and neural effects. Journal of Cognitive Neuroscience. 2005;17(6):874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway AR, Hambrick DZ, Engle RW. Variation in working memory capacity as variation in executive attention and control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. New York, NY: Oxford University Press; 2007. pp. 21–46. [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2010;21(6):1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Keeley J, Zayac R, Correia C. Curvilinear relationships between statistics anxiety and performance among undergraduate students: Evidence for optimal anxiety. Statistics Education Research Journal. 2008;7(1) [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44(1):213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Lehrer P, Karavidas M, Lu SE, Vaschillo E, Vaschillo B, Cheng A. Cardiac data increase association between self-report and both expert ratings of task load and task performance in flight simulator tasks: An exploratory study. International Journal of Psychophysiology. 2010;76(2):80–87. doi: 10.1016/j.ijpsycho.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Ironson GH, Schneiderman N. The reliability and specificity of delta versus residualized change as measures of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28(6):701–711. doi: 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Malgady RG, Colon-Malgady G. Comparing the reliability of difference scores and residuals in analysis of covariance. Educational and Psychological Measurement. 1991;51(4):803–807. [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84(2):482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerks EM, O’Brien M, Blankson AN. Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Developmental psychobiology. 2010;52(6):603–608. doi: 10.1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contribution to recency judgements. Neuropsychologia. 1991;29:601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Miu AC, Heilman RM, Miclea M. Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Autonomic Neuroscience. 2009;145(1):99–103. doi: 10.1016/j.autneu.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions four general conclusions. Current Directions in Psychological Science. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moore SA, Zoellner LA, Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behaviour research and therapy. 2008;46(9):993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner CN, Zelazo PD, Anderson AK. Effects of emotion regulation on concurrent attentional performance. Motivation and Emotion. 2013;37(2):346–354. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in cognitive sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9(2):265. doi: 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Voelker P. Developing attention: Behavioral and brain mechanisms. Advances in Neuroscience. 2014;2014:9. doi: 10.1155/2014/405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J, Schmeichel BJ, Demaree HA. Cardiac vagal control predicts spontaneous regulation of negative emotional expression and subsequent cognitive performance. Biological Psychology. 2010;84(3):531–540. [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: the cognitive costs of keeping one’s cool. Journal of Personality and Social Psychology. 2000;79(3):410. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Roid GH. Stanford-Binet Intelligence Scales (SB5) Rolling Meadows, IL: Riverside; 2003. [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. Journal of personality and social psychology. 2000;78(1):122. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Demaree HA. Working memory capacity and spontaneous emotion regulation: high capacity predicts self-enhancement in response to negative feedback. Emotion. 2010;10(5):739. doi: 10.1037/a0019355. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. Journal of personality and social psychology. 2008;95(6):1526. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Korten JB, Myers MM. Components of heart rate reactivity during mental arithmetic with and without speaking. Physiology & behavior. 1991;50(5):1039–1045. doi: 10.1016/0031-9384(91)90434-p. [DOI] [PubMed] [Google Scholar]
- Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. Journal of cardiovascular electrophysiology. 2005;16(1):13–20. doi: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. Oxford University Press; 2001. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology. 1935;18(6):643. [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of affective disorders. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Ursache A, Blair C, Stifter C, Voegtline K. Emotional reactivity and regulation in infancy interact to predict executive functioning in early childhood. Developmental psychology. 2013;49(1):127. doi: 10.1037/a0027728. [DOI] [PubMed] [Google Scholar]
- Ursache A, Raver CC. Trait and state anxiety: Relations to executive functioning in an at-risk sample. Cognition & emotion. 2014;28(5):845–855. doi: 10.1080/02699931.2013.855173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: an fMRI study of the effects of cognitive load on the processing of affective images. Neuroimage. 2009;45(4):1212–1219. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Volokhov RN, Demaree HA. Spontaneous emotion regulation to positive and negative stimuli. Brain and cognition. 2010;73(1):1–6. doi: 10.1016/j.bandc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Vytal K, Cornwell B, Arkin N, Grillon C. Describing the interplay between anxiety and cognition: from impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49(6):842–852. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie G, Allport A. Task switching and the measurement of “switch costs”. Psychological research. 2000;63(3–4):212–233. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]
- Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2014;42:180–192. doi: 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]