Abstract
Background
Cannabinoids have been traditionally used for the treatment of gastrointestinal (GI) symptoms, but the associated central effects, through cannabinoid-1 receptors (CB1R), constitute an important drawback. Our aims were to characterize the effects of the recently developed highly potent long-acting megagonist AM841 on GI motor function and to determine its central effects in rats.
Methods
Male Wistar rats were used for in vitro and in vivo studies. The effect of AM841 was tested on electrically-induced twitch contractions of GI preparations (in vitro) and on GI motility measured radiographically after contrast administration (in vivo). Central effects of AM841 were evaluated using the cannabinoid tetrad. The non-selective cannabinoid agonist WIN 55,212-2 (WIN) was used for comparison. The CB1R (AM251) and CB2R (AM630) antagonists were used to characterize cannabinoid receptor-mediated effects of AM841.
Key results
AM841 dose-dependently reduced in vitro contractile activity of rat GI preparations via CB1R, but not CB2R or opioid receptors. In vivo, AM841 acutely and potently reduced gastric emptying and intestinal transit in a dose-dependent and AM251-sensitive manner. The in vivo GI effects of AM841 at 0.1 mg kg−1 were comparable to those induced by WIN at 5 mg kg−1. However, at this dose, AM841 did not induce any sign of the cannabinoid tetrad, whereas WIN induced significant central effects.
Conclusions & Inferences
The CB1R megagonist AM841 may potently depress GI motor function in the absence of central effects. This effect may be mediated peripherally and may be useful in the treatment of GI motility disorders.
Keywords: AM841, cannabinoid, cannabinoid-1 receptor, gastrointestinal motility, pain, radiographic analysis
Cannabinoid agonists might be useful to cure or relieve symptoms associated with gastrointestinal (GI) disorders (1,2) because they reduce GI motor function (1,2,3,4,5), secretion (6,7), gastroesophageal reflux (8), pain (9) and inflammation (10). Although many of these actions are achieved by direct interaction with peripheral receptors, some of them might also be mediated, at least partially, by their effects in the central nervous system (CNS), in which cannabinoids modify neurotransmission. Thus, cannabinoids may act upon the dorsal vagal complex (11) or the nucleus tractus solitarius (12,13). However, it is also well known from in vitro experiments that cannabinoids decrease excitatory neurotransmitter release from the myenteric neurons (14,15,16), which control GI motor function locally.
Two cannabinoid receptors have been described (see 17 for review). Cannabinoid-1 receptors (CB1R) are classically thought to mediate neuronal actions since they are mainly localized to neurons. In contrast, cannabinoid-2 receptors (CB2R) are mainly expressed by immune cells, although they have also been found in neurons (18), including those of the myenteric plexus (19). However, even though CB2R might be involved in some of the GI cannabinoid effects (19,20), those affecting motility in non-pathological states seem to involve only CB1R (2). Interestingly, activation of CB1R also contributed to normalization of enhanced motility in experimental pathologic models (21) and play a role in control of colonic transit and sensation in humans with irritable bowel syndrome (IBS; 22).
The therapeutic potential (either for GI or other pathologies) of many cannabinoid drugs, particularly those acting upon CB1R, is reduced due to the likely induction of central effects, and thus peripherally-acting cannabinoid agonists, devoid of central actions, would be a preferable approach (23). In addition, in the GI field, these agonists would help better study the involvement of peripheral CB1R in physiology and pathology of GI motor function.
The compound AM841 ((−)-7′-Isothiocyanato-11-hydroxy-1′,1′-dimethylheptylhexahydrocannabinol) is a cannabinoid megagonist (24) developed in Dr Makriyannis’ laboratory (25); it is a high-affinity electrophilic ligand that interacts covalently with a cysteine in helix six of CB1R and CB2R, through distinct binding motifs (24,25,26). In the GI tract, AM841 protected from and healed experimental colitis in mice (27). Additionally, it depressed upper GI transit and fecal pellet output in both control and stressed mice in a peripherally restricted manner (28). To consider AM841’s potential usefulness for treatment of diarrhea-associated conditions, its effects should also be confirmed in other laboratory animals, and through the use of other in vivo methods, preferably non-invasive. Furthermore, in vitro studies are needed to better localize the effects of AM841 throughout the GI tract.
Thus, in the present study, using rats, our aims were: 1- To characterize the effects of the highly potent agonist AM841 on GI motor function in vivo (using non-invasive radiographic techniques) and in vitro; 2- To determine the central effects of AM841; 3- To compare the GI and central effects of AM841 with those of WIN 55,212-2 (WIN), a weaker CB1R/CB2R non-selective cannabinoid agonist (17).
METHODS
Animals
All animal care and experimental procedures complied with the guidelines established by the Canadian Council of Animal Care and the EU (Directive 2010/63/EU for animal experiments) and Spanish regulations (R.D. 53/2013) for care and use of experimental animals, and were approved by the Animal Care Committees at both the University of Calgary and the Universidad Rey Juan Carlos. A total of 170 rats were used in these experiments (54 in the in vitro studies; 90 in the radiographic studies; 26 in the study of central effects). The procedures used were as humane as possible, and every effort was made to avoid or reduce animal suffering, and to reduce the number of animals used.
Male Wistar rats were obtained from Charles River (Senneville, QC, Canada) and Harlan Iberica (Barcelona, Spain), housed under environmentally controlled conditions (temperature = 22°C; humidity = 60%) and kept at a constant photoperiod (12:12 h light-dark cycle; lighting increased in steps from 7:00 to 8:00 and reduced in steps from 19:00 to 20:00) in plastic cages on standard sawdust bedding (4–6 animals per cage) with access to standard laboratory chow and tap water ad libitum. Home cages were cleaned twice weekly.
In vitro study
Rats (200–250 g) were killed by inhalation of isoflurane. Segments of gastric fundus, gastric antrum, ileum and distal colon were removed and kept in ice-cold oxygenated Krebs-Ringer solution (NaCl 115 mM, KCl 8.0 mM, KH2PO4 2.0 mM, NaHCO3 25 mM, MgCl2 2.4 mM, CaCl2 1.3 mM and glucose 10 mM). Full thickness strips (0.8–1 cm long) were prepared from the gastric fundus and antrum. Luminal contents of ileum and colon were gently flushed, and 1 cm long segments were prepared. All strips were longitudinal.
The preparations were mounted between two platinum electrodes, 1 cm apart, and placed in separate organ baths (25 mL; 37 °C; oxygenated with 95% O2 and 5% CO2). Using a silk thread, one end of each preparation was attached to the bottom of the organ bath, while the other end was vertically connected to a FT03 force displacement transducer (Grass Technologies, West Warwick, RI, USA). A tension of 1 gram was applied and the preparations were allowed to equilibrate for 20 min. Changes in tension were amplified by a P11T amplifier (Grass Technologies, West Warwick, RI, USA) and recorded on a personal computer using the POLYVIEW software (Polybytes Inc., Cedar Rapids, IA, USA). Electrical field stimulation (EFS: 8 Hz; 24 V; stimulus duration 1.5 ms; train duration 10 s) was applied by a S88X stimulator (Grass Technologies, West Warwick, RI, USA).
All experiments lasted less than 3 h and each preparation was used for a single experiment only.
AM841 and WIN (both 10−12 – 10−6M) were added cumulatively into the organ baths and effects on the EFS-induced contractions were recorded. Each concentration was allowed to incubate for 15 min. In separate experiments the CB1R antagonist AM251 (10−7M), the CB2 antagonist AM630 (10−7M) and the opioid receptor antagonist naloxone (10−6M) were added 15 min prior to AM841 and WIN. The mean of the last 3 successive contractions of every concentration was analyzed. At the beginning and the end of the experiment, the tissue was exposed to the cholinergic agonist bethanechol (10−5M) to test whether AM841 or WIN acted at a smooth muscular site.
In vivo experiments
Four sets of experiments were performed on rats weighing 240–300 g. In the first one, rats received an intraperitoneal (i.p.) injection of vehicle (30 μl kg−1 of Tocrisolve® in saline solution, see below), AM841 (0.1 or 1 mg kg−1) or WIN (5 mg kg−1), and alterations in GI motor function were radiographically recorded (see below). Doses of AM841 were selected based on previous studies (29, 30) and own pilot experiments.
The second set of experiments was designed to detect long-lasting effects of AM841 and WIN. For this, rats received vehicle, AM841 (0.1 mg kg−1: AM841 0.1) or WIN (5 mg kg−1: WIN 5) and GI motility was radiographically analyzed for 10 h, starting 14 hours after drug administration.
In the third set of experiments, antagonists were used to determine the role of CB1R and CB2R on the effect of AM841 on GI motility. Thus, the CB1R antagonist AM251 (1 mg kg−1, i.p.) or the CB2 antagonist AM630 (1 mg kg−1, i.p.) were administered 30 min prior to AM841 (0.1 mg kg−1, i.p.). The effect of each antagonist was also analyzed in vehicle-treated rats.
Finally, in the fourth set of experiments, the central effects of AM841 (at 0.1 and 0.5 mg kg−1, i.p.) were analyzed using the cannabinoid tetrad, as described below. Additionally, mechanical sensitivity to non-noxious stimuli was measured (see below).
In all in vivo experiments, drug volumes were adjusted to a maximum of 4 mLkg−1.
Radiographic evaluation of GI motor function
GI motor function was studied by radiographic methods (3,4,5,31) at the facilities of the Unidad de Medicina y Cirugía Experimental, Hospital General Universitario Gregorio Marañón (UMCE-HGUGM, Madrid, Spain). Immediately after drug injection (or 14 hours later in the second set of experiments), 2.5 mL of a suspension of barium sulfate (Barigraph® AD, Juste SAQF, Madrid, Spain; 2gmL−1, t°= 22°C) were administered per os. Radiographs of the GI tract were obtained using a Digital X-Ray apparatus (Siemens, Madrid, Spain; 60kV, 7mA) and captured with NPG Real DVD Studio II software (NPG, Madrid, Spain). Exposure time was adjusted to 0.06 s. Immobilization of the rats in prone position was achieved by placing them inside adjustable hand-made transparent plastic tubes, so that they could not move. Habituation to the recording chamber prior to commencement of the study did not significantly alter GI motility (31). To further reduce stress, rats were released immediately after each shot (immobilization lasted for less than 2 min). X-rays were recorded at different times (immediately and 1, 2, 4, 6, 8 and 10h: T0–T10) after administration of the contrast medium. While taking the radiographs, the qualified investigator remained at least 2 m away from the X-ray source, where radioactivity while shooting was not different from environmental readings. Analysis of the radiographs was performed by a trained investigator blind to the drug administered. Alterations in GI motility were semiquantitatively determined from the images by assigning a compounded value to each region of the GI tract considering the following parameters: percentage of the GI region filled with contrast (0–4); intensity of contrast (0–4); homogeneity of contrast (0–2); and sharpness of the GI region profile (0–2). Each of these parameters was scored and a sum (0–12 points) was made. Typical motility curves obtained from control animals in 24 hour-long experiments are shown in Fig. 1-Supplementary material.
In addition, in order to quantitatively analyze the size of the stomach and caecum, images were processed with the aid of an image analysis system (Image J 1.38 for Windows, National Institute of Health, USA, free software: http://rsb.info.nih.gov/ij/). Qualitative differences in intestinal shape, size, tone, and peristaltic activity were also recorded.
Central effects of AM841 (cannabinoid tetrad)
The classical cannabinoid tetrad consists of the combination of four tests that evaluate temperature, antinociception, catalepsy, and locomotor activity in the same animal after cannabinoid administration (32), and was used to identify non-psychoactive and psychoactive doses of AM841. The test values were recorded by an observer unaware of the treatments, as previously reported (3,4,33).
Heat-antinociception was tested 20 min after drug administration using a 37370 plantar test apparatus (Ugo Basile, Comerio VA, Italy). The withdrawal latency from a focused beam of radiant heat applied to the mid plantar surface of the hindpaws was recorded. The intensity of the light was adjusted at the beginning of the experiment so that the control average baseline latencies were about 8 s and a cut-off latency of 25 s was imposed. The withdrawal latency of each paw was measured during three trials separated by 2 min intervals, and the mean of the three readings was used for data analysis.
To measure catalepsy, the rats were hung by their front paws from a rubber-coated metal ring (12 cm diameter) fixed horizontally at a height that allowed their hindpaws to just touch the bench. The time taken for the rat to move off the ring was measured with a cut-off limit of 30 s. Latencies were measured 25 min after drug or vehicle administration.
Rectal temperature was recorded 30 min after drug administration using a P6 thermometer and a lubricated rectal probe (Cibertec S.A., Madrid, Spain) inserted into the rectum to a constant depth of 5 cm.
Spontaneous locomotor activity was evaluated using individual photocell activity chambers (Cibertec S.A.). Rats were placed in the recording chambers (55 × 40 cm, with a 3 cm spacing between beams) 40 min after drug administration; the number of interruptions of photocell beams was recorded over a 10-min period. The mean number of crossings of the photocell beams was used for comparison.
Assessment of mechanical sensitivity to non-noxious stimuli
For this additional behavioral study, rats were placed individually on an elevated iron mesh in a clear plastic cage and were allowed to adapt to the testing environment for at least 10 minutes. Habituation to this environment was also performed on the two days before assessment. Mechanical sensitivity was assessed using an electronic Von Frey apparatus (EVF3, Bioseb, BP89, ChavilleCedez, France). The Von Frey test was applied to the plantar surface of each hindpaw, through the mesh floor. The test was performed four times with an interstimulus interval of approximately 30 s. The mean of the four trials was used for data analysis. In this test, a significant decrease in Von Frey hairs withdrawal threshold evoked by mechanical stimuli is suggestive of mechanical allodynia (increased sensitivity to non-noxious stimuli). The apparatus has an upper cut-off limit for testing of 50 g. This test was performed prior to the cannabinoid tetrad, 20 min after drug administration.
Compounds and drugs
AM841 was synthesized in Dr Makriyannis’ laboratory, at the Center for Drug Discovery, Northeastern University as previously described (24,25,26).
In vitro studies were performed at the University of Calgary, Canada. AM251, AM630, WIN and naloxone were obtained from Tocris Bioscience (Tocris, Ellisville, USA). All drugs were dissolved in dimethyl sulfoxide.
In vivo studies were performed at UMCE-HGUGM and Universidad Rey Juan Carlos, Spain. Barium sulfate (Barigraf® AD; Juste SAQF, Madrid, Spain) was suspended in tap water and continuously hand-stirred until administration. WIN and AM630 were obtained from Tocris Cookson (Bristol, UK). AM251 was obtained from Ascent Scientific (North Somerset, UK). All drugs were dissolved in Tocrisolve® (a commercially available water soluble emulsion composed of a 1:4 ratio of soya oil/water that is emulsified with the block co-polymer Pluronic F68; Tocris, Cookson, Bristol, UK).
The vehicles in the used concentrations and doses had no significant effects on the observed parameters.
Statistical analysis
Data are expressed as the mean ± SEM. PRISM 4.0 (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical and curve-fitting analyses. Differences between groups were analyzed using unpaired Student’s t-test, with Welch’s correction where appropriate, or one- or two-way ANOVA followed by post-hoc Student-Newman-Keuls or Bonferroni multiple comparison tests. P values < 0.05 were considered significant. N refers to the number of animals used (in vivo experiments); n refers to the number of preparations from 3 different animals (in vitro experiments).
RESULTS
AM841 reduces contractility of isolated GI preparations via CB1 receptors
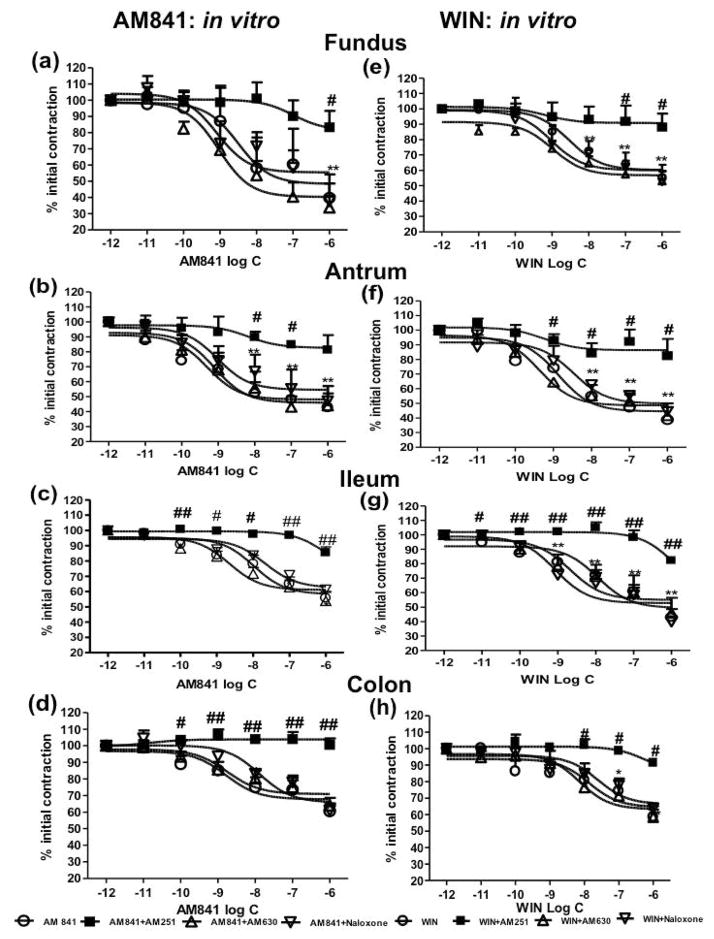
AM841 (10−12M -10−6M) reduced the EFS-induced twitch contractions in rat gastric fundus (Fig. 1a), antrum (Fig. 1b), ileum (Fig. 1c) and colon (Fig. 1d) in a concentration-dependent manner. The maximum inhibitory effect of AM841 was ~50% in fundus and antrum, ~40% in ileum and ~30% in colon. The effects of AM841 in isolated organ baths were similar to those produced by the established cannabinoid agonist, WIN (10−12M -10−6M) (Fig. 1 e–h). No statistically significant difference was found when the potencies of both agonists were compared for each preparation (see the Log EC50 in Table 1-Supplementary Material).
Figure 1.
In vitro effects of the cannabinoid agonists AM841 and WIN 55,212-2 in rat gastrointestinal tissues. AM841 reduced in vitro smooth muscular twitch contractions in rat fundus (a), antrum (b), ileum (c) and colon (d), and was equipotent to WIN (e~h). AM251 (10−7 M), but not AM630 (10−7 M) or the opioid receptor antagonist naloxone (10−6 M) abolished the effects of AM841 and WIN. Data represent mean ± SEM (n=5–6 preparations from 3 different animals). * p<0.05, ** p<0.01, AM841 (or WIN) (10−12 M) group vs. other concentration groups. #p<0.05, ## p<0.01, AM841(or WIN) groups vs. AM841+AM251 (or WIN +AM251) groups (two-way ANOVA followed by Student-Newman-Keuls post-hoc test).
The CB1R antagonist AM251 (10−7M), but not the CB2R antagonist AM630 (10−7M) or the opioid receptor antagonist naloxone (10−6M) abolished the effects of AM841 (Fig. 1 a–d) or WIN (Fig. 1e–h). The antagonists alone, at the concentrations used, had no effects on EFS-induced contractions (data reported earlier).
AM841 reduces GI motility in vivo via CB1 receptors with higher potency than the conventional non-selective cannabinoid agonist WIN 55,212-2
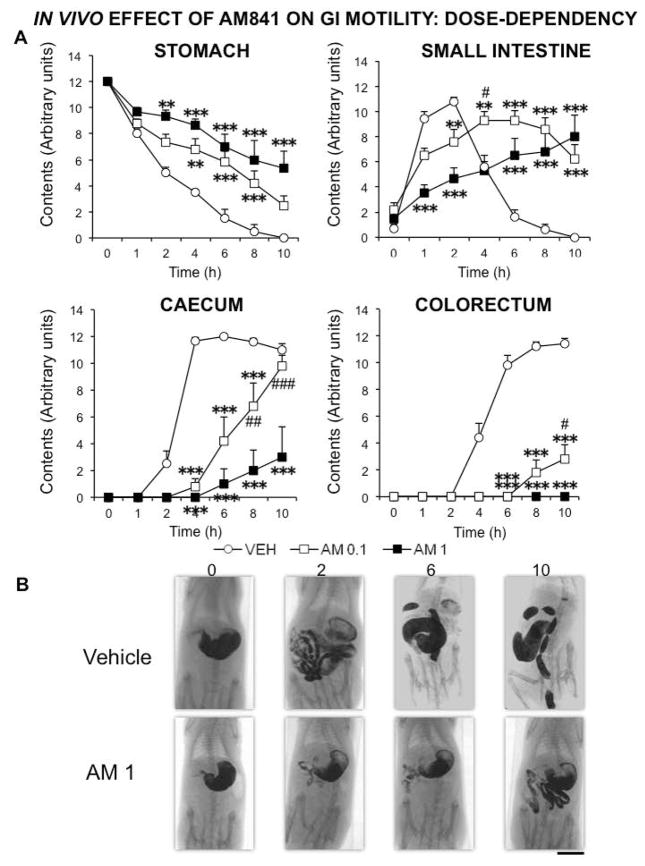
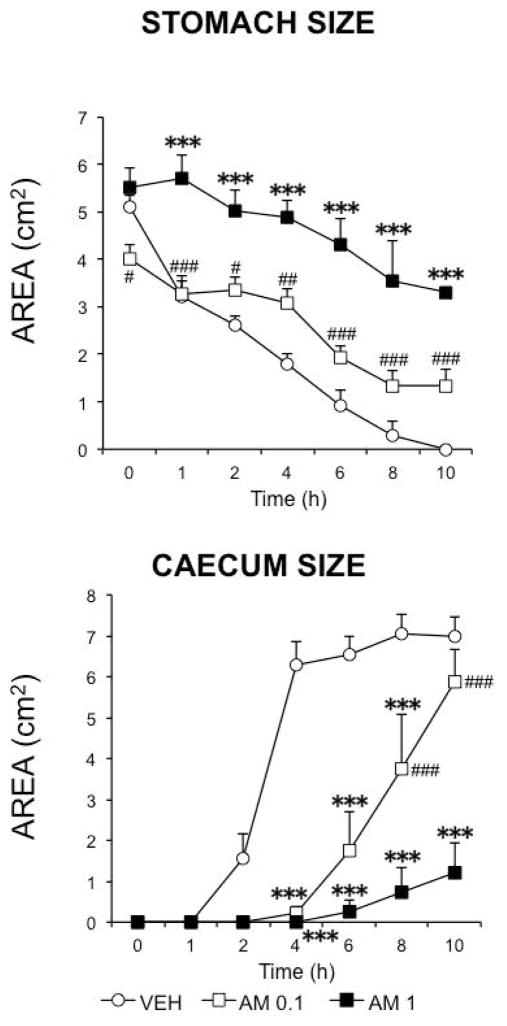
We first analyzed the effects of different doses of AM841. When contrast medium was given immediately after drugs, AM841 reduced gastric emptying and intestinal transit already at 0.1 mg kg−1, but this difference was enhanced when the drug was tested at 1 mg kg−1. This was particularly evident in the intestinal regions, whereas in the stomach the difference between the corresponding curves for the two doses of AM841 did not reach statistical significance at any time-point (Fig. 2). However, when the stomach area was measured, the difference between treatments did reach statistical significance (Fig. 3A). In contrast, the morphometric analysis of the caecum revealed similar results to those obtained semiquantitatively (compare the corresponding curves in Fig. 2A and Fig. 3B).
Figure 2.
In vivo dose-dependent effect of AM841 on rat gastrointestinal motor function. Motor function was measured by radiological methods (see text). Vehicle (30 μl kg−1 of Tocrisolve® in saline solution) or AM841 (AM, 0.1 or 1 mg kg−1) were intraperitoneally injected, and barium sulfate (2.5 mL, 2 g mL−1) was intragastrically administered immediately afterwards. X-rays were taken 0, 1, 2, 4, 6, 8 and 10 h after barium administration. Data represent mean ± SEM for motor function in stomach, small intestine, caecum and colorectum (panel A). N ≥ 6, each group. **p <0.01, ***p <0.001 vs vehicle, #p<0.05, ##p<0.01, ###p<0.001 vs AM at 1 mg kg−1 (two-way ANOVA followed by Bonferroni post-hoc test). Panel B: Representative X-rays of rats treated with vehicle or AM (1 mg kg−1), obtained 0, 2, 6 and 10 h after barium. Scale bar: 4 cm.
Figure 3.
Morphometric analysis of stomach and caecum size radiographically imaged. Radiographic methods (see text) were applied to visualize the gastrointestinal regions. The temporal changes in the size of the stomach and the caecum after treatment with vehicle (30 μl kg−1 of Tocrisolve® in saline solution) or AM841 (AM, 0.1 or 1 mg kg−1) were analyzed using an image processor (Image J, NIH). N ≥ 6, each group. ***p <0.001 vs vehicle; #p<0.05, ##p<0.01, ###p<0.001vs AM 0.1 (two-way ANOVA followed by Bonferroni post-hoc test).
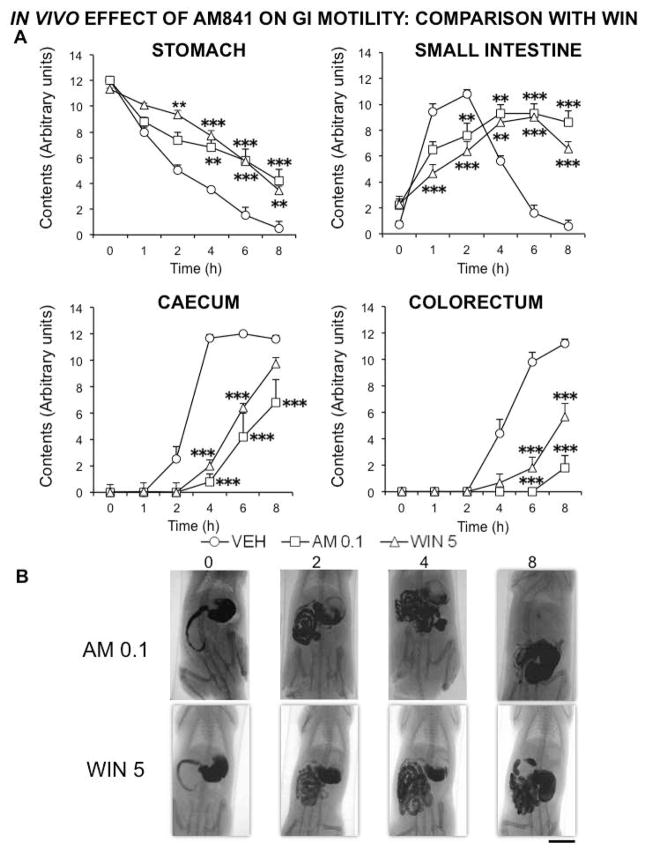
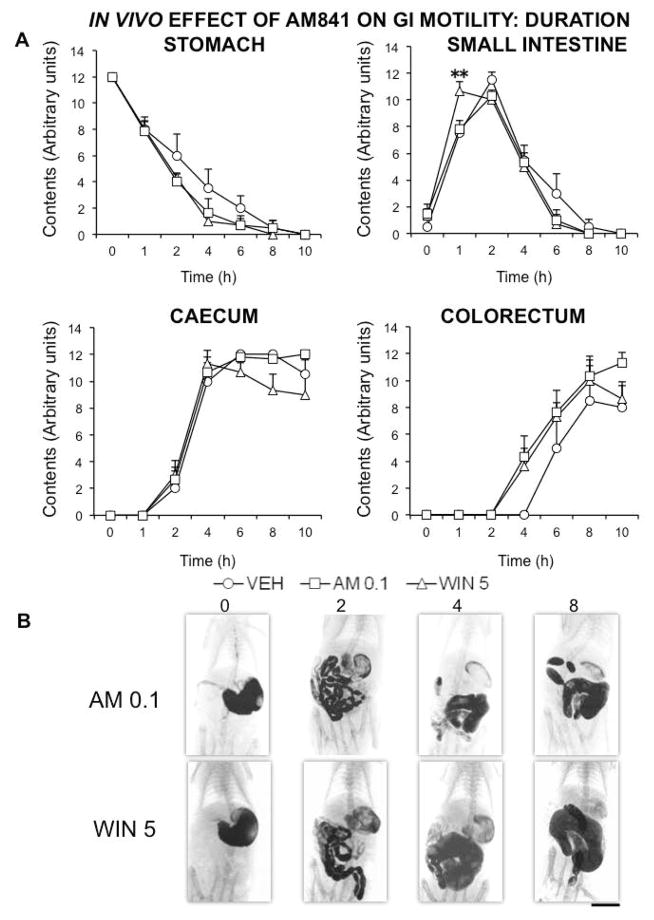
Then, we compared the effects of AM841 with those of WIN, and gave the contrast medium both immediately after administration and 14 hours later. When contrast was given immediately after drugs, the effect of AM841 at the lowest dose tested (0.1 mg kg−1) was practically identical to that induced by the classical non-selective cannabinoid agonist WIN at 5 mg kg−1 (Fig. 4). However, when the contrast was given 14 h after drugs, no significant and meaningful difference was detected between AM841 (0.1 mg kg−1) and WIN (5 mg kg−1) in comparison with vehicle-treated rats (Fig. 5).
Figure 4.
Comparison of the in vivo effects of the cannabinoid agonists AM841 and WIN 55,212-2 on rat gastrointestinal motor function. Motor function was measured by radiological methods (see text). Vehicle (30 μl kg−1 of Tocrisolve® in saline solution), AM841 (AM, 0.1 mg kg−1) or WIN 55,212-2 (WIN, 5 mg kg−1) were intraperitoneally injected, and barium sulfate (2.5 mL, 2 g mL−1) was intragastrically administered immediately afterwards. X-rays were taken 0, 1, 2, 4, 6 and 8 h after barium administration. Data represent mean ± SEM for motor function in stomach, small intestine, caecum and colorectum (panel A). N ≥ 6, each group. **p <0.01, ***p <0.001 vs vehicle (two-way ANOVA followed by Bonferroni post-hoc test). Panel B: Representative X-rays of rats treated with AM (0.1 mg kg−1) or WIN (5 mg kg−1), obtained 0, 2, 4 and 8 h after barium. Scale bar: 4 cm.
Figure 5.
In vivo effects of AM841 and WIN on rat gastrointestinal motor function several hours after administration. Motor function was measured by radiological methods (see text). Vehicle (30 μl kg−1 of Tocrisolve® in saline solution), AM841 (AM, 0.1 mg kg−1) or WIN 55,212-2 (WIN, 5 mg kg−1) were intraperitoneally injected, and barium sulfate (2.5 mL, 2 g mL−1) was intragastrically administered 14 hours afterwards. X-rays were taken 0, 1, 2, 4, 6, 8 and 10 h after barium administration. Data represent mean ± SEM for motor function in stomach, small intestine, caecum and colorectum (panel A). N = 8, each group. **p <0.01 vs vehicle (two-way ANOVA followed by Bonferroni post-hoc test). Panel B: Representative X-rays of rats treated with AM (0.1 mg kg−1) or WIN (5 mg kg−1), obtained 0, 2, 4 and 8 h after barium. Scale bar: 4 cm.
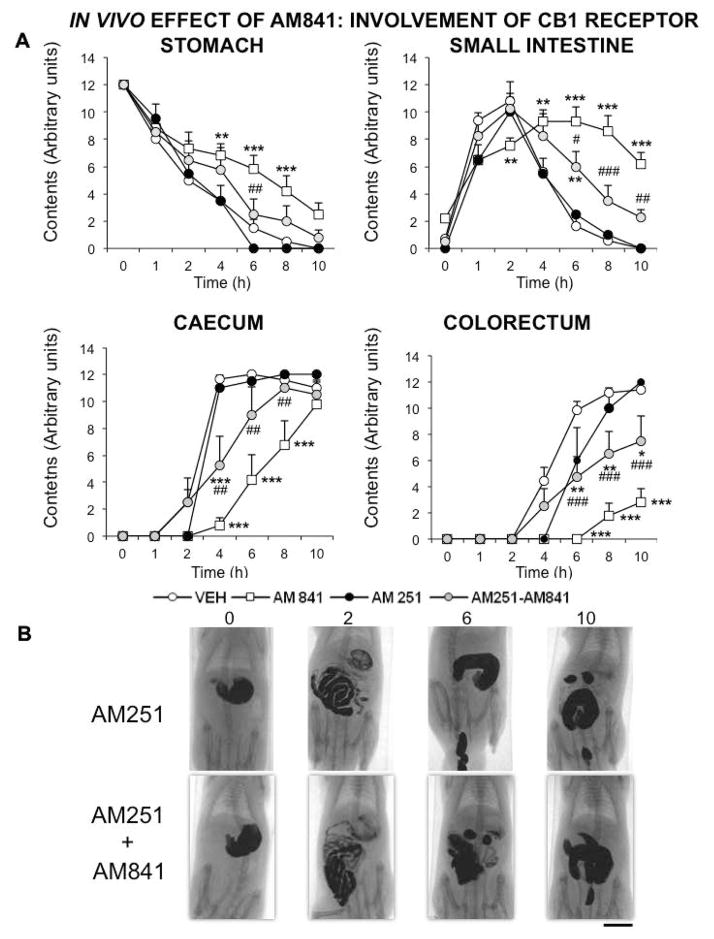
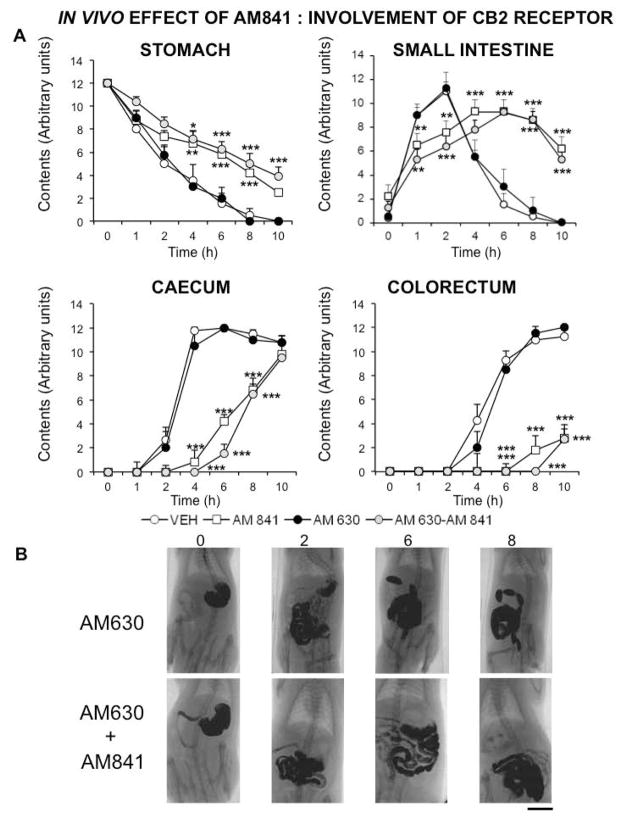
Finally, we investigated the role of CB1R and CB2R in AM841 effects on GI motility. The CB1R selective antagonist AM251 (1 mg kg−1) was able to block the effect of AM841 in the stomach and the corresponding curve was not significantly different from that obtained in control animals. However, in the small and large intestine its blocking effect was only partial and the control values were not reached, suggesting a much longer time of action for AM841 (Fig. 6). The CB2 antagonist AM630 (1 mg kg−1) did not significantly block AM841 effect in any of the GI regions (Fig. 7). Neither AM251 nor AM630 alone induced any significant and meaningful effect in any of the regions considered (Fig. 6, 7).
Figure 6.
Involvement of CB1 receptor on AM841 in vivo effect on gastrointestinal motor function. Motor function was measured by radiological methods (see text). Vehicle (30 μl kg−1 of Tocrisolve® in saline solution), AM841 (0.1 mg kg−1), AM251 (1 mg kg−1) or both (AM251+AM841) were intraperitoneally injected (the CB1R antagonist, AM251, was injected 30 min prior to the agonist AM841), and barium sulfate (2.5 mL, 2 g mL−1) was intragastrically administered immediately afterwards. X-rays were taken 0, 1, 2, 4, 6, 8 and 10 h after barium administration. Data represent mean ± SEM for motor function in stomach, small intestine, caecum and colorectum (panel A). N = 8, each group. *p<0.05, **p <0.01, ***p<0.001 vs vehicle, ##p<0.01, ###p<0.001 vs AM841 (two-way ANOVA followed by Bonferroni post-hoc test). Panel B: Representative X-rays of rats treated with AM251 (1 mg kg−1) alone or with AM841 (0.1 mg kg−1), obtained 0, 2, 6 and 10 h after barium. Scale bar: 4 cm.
Figure 7.
Involvement of CB2 receptor on AM841 in vivo effect on gastrointestinal motor function. Motor function was measured by radiological methods (see text). Vehicle (30 μl kg−1 of Tocrisolve® in saline solution), AM841 (0.1 mg kg−1), AM630 (1 mg kg−1) or both (AM630+AM841) were intraperitoneally injected (the CB2 antagonist, AM630, was injected 30 min prior to the agonist AM841), and barium sulfate (2.5 mL, 2 g mL−1) was intragastrically administered immediately afterwards. X-rays were taken 0, 1, 2, 4, 6, 8 and 10 h after barium administration. Data represent mean ± SEM for motor function in stomach, small intestine, caecum and colorectum (panel A). N = 8, each group. *p<0.05, **p <0.01, ***p<0.001 vs vehicle (two-way ANOVA followed by Bonferroni post-hoc test). Panel B: Representative X-rays of rats treated with AM630 (1 mg kg−1) alone or with AM841 (0.1 mg kg−1), obtained 0, 2, 6 and 8 h after barium. Scale bar: 4 cm.
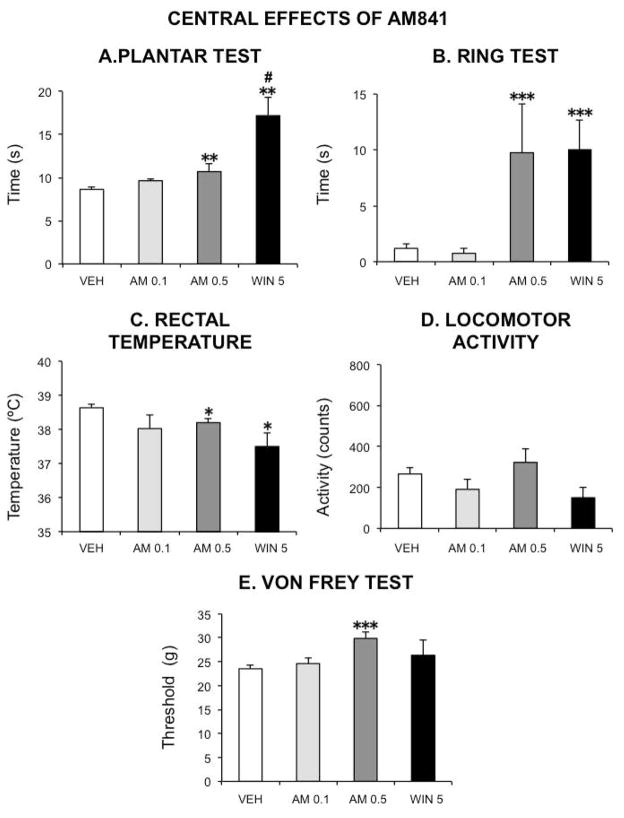
Central effects of AM841 are negligible at doses that potently reduce GI motor function
In the cannabinoid tetrad both WIN (5 mg kg−1) and AM841 at the highest dose tested in this case (0.5 mg kg−1) induced significant antinociception (plantar test), catalepsy (ring test) and hypothermia (rectal temperature recording), whereas locomotor activity was not significantly modified by any drug. In contrast, AM841 at 0.1 mg kg−1, the dose that exerted the potent depression of GI motor function described above, did not exert any significant effect in the cannabinoid tetrad (Fig. 8). Finally, in the Von Frey test, AM841 at 0.5 mg kg−1 significantly increased the threshold response to non-noxious stimuli, whereas neither WIN at 5 mg kg−1 nor AM841 at 0.1 mg kg−1 modified it in a significant manner (Fig. 8).
Figure 8.
Central effects of the cannabinoid agonists AM841 and WIN 55,212-2 in the rat. The following tests were used to evaluate the occurrence of the characteristic cannabinoid central effects (cannabinoid tetrad): A–plantar test (for analgesia); Bring test (for catalepsy); C–rectal temperature (for hypothermia); D–spontaneous locomotor activity (for hypolocomotion). The threshold for paw withdrawal in the von Frey test was also measured (E). Vehicle (30 μl kg−1 of Tocrisolve® in saline solution), AM841 (AM, 0.1 or 0.5 mg kg−1) or WIN 55,212-2 (WIN 5, 5 mg kg−1) were intraperitoneally injected. Tests were performed as described in the text. Bars show mean values ± SEM. N ≥ 6 each group. *p <0.05, **p <0.01, ***p<0.001 vs saline (one-way ANOVA followed by Bonferroni post-hoc test). # p<0.05 vs AM 0.5 (unpaired Student’s t-test).
DISCUSSION
In the present study, the cannabinoid megagonist, AM841, dose-dependently inhibited GI motor function in the rat, with higher potency than the reversible non-selective cannabinoid agonist WIN. Furthermore, AM841 exerted these effects both in vivo and in vitro by activating CB1R (but not CB2R) located peripherally, without the need to significantly affect cannabinoid receptors located in the CNS.
AM841 effects in organ bath studies
AM841, similarly to WIN, potently inhibited rat GI contractility in vitro, and the effect was exclusively mediated by CB1R. This agrees with previously published data, demonstrating that cannabinoid agonists act principally on prejunctional CB1R and reduce acetylcholine release (34,35) to inhibit smooth muscle contractility in different regions of the GI tract in different laboratory animals (16, 36). Although CB2R might be involved in the control of normal motility in the rat gastric fundus (36), mouse stomach (37) or rat ileal myenteric plexus preparations (38), in our in vitro study the CB2R selective antagonist AM630 did not block the action of AM841, suggesting a negligible role for these receptors.
AM841 inhibits motility in vivo
Many cannabinoid agonists depress GI motor function in vivo (see reviews in 1, 2). In our non-invasive radiographic study, similarly to an approximately 50-fold higher concentration of WIN, the acute effects of AM841 were dose-dependent with a very high in vivo potency and also more intense in the intestine. Whereas at 1 mg kg−1 AM841 clearly depressed motility both in the stomach and the different intestinal regions, at 0.1 mg kg−1 did not produce such a clear effect in the stomach in which transit (semiquantitatively measured) was significantly reduced, but its size (morphometrically evaluated) was not significantly different from vehicle-treated animals (compare Fig 2A and 3A). Thus, gastric emptying might be to some extent independent from distension. This hypothesis warrants further investigation.
Although in the in vitro experiments both AM841 and WIN appeared to have equivalent potencies in all isolated rat GI tissues, the effects obtained in vivo for AM841 at 0.1 mg kg−1 were similar to those obtained for WIN at 5 mg kg−1, which suggests that the new agonist is much more potent (at least 50x) than WIN as a motility depressor. WIN and AM841 are the only cannabinoid agonists tested so far using X-ray studies, but in invasive motility studies, several cannabinoid agonists have delayed intestinal transit and/or gastric emptying (15) with higher (39,40) or lower potency than WIN (41). Interestingly, in mice, using invasive methods, AM841 was also more potent than WIN in its ability to depress GI motility, particularly in stressed animals, and its effects at 0.1 mg kg−1 were comparable to those of WIN at 3 mg kg−1 (30). It would be very interesting to test AM841 effects in models of GI pathology, particularly diarrhea, in which different cannabinergic drugs were able to reverse or prevent accelerated motility (21,42,43). AM841 reduced inflammation in experimental models of colitis, but motility was not specifically measured (29). Interestingly, CB1R may play a significant role in colonic transit and sensation in IBS patients (22), suggesting potential clinical importance of AM841 for the treatment of lower functional GI disorders.
AM841 interacts covalently with CB1R (24,25,26) and, therefore, it might exert a long-lasting effect on motility. In the previous study in mice, the effects of AM841, at either 0.1 or 1 mg kg−1, and those of WIN, at 3 mg kg−1 (but not at 0.3 mg kg−1) on colonic bead expulsion lasted for at least 3.5 hours. In our previous studies, 24 hours after administration of WIN at 5 mg kg−1, no effect was induced on motility (4). Here, 14 hours after WIN administration, no effect on GI motility of WIN was detected either, and we did not find any significant and meaningful effect of AM841 (0.1 mg kg−1), suggesting that the duration of action on GI motility in vivo of both agonists is lower than 14 hours, although the length of the effect may be increased at higher AM841 doses. Further research should determine the pharmacokinetic effects of AM841 on intestinal motility, and whether or not this happens as well in pathological situations (i.e., models of diarrhea).
Like in our previous radiographic studies using WIN (3,4,5), the in vivo effects of AM841 on GI motility were dependent on the activation of CB1R but not CB2R. This is also similar to results from invasive studies using WIN and other cannabinoid agonists in healthy animals (15,44) and agrees with our in vitro results and those from other laboratories (15,34,35). Thus, AM251 (at 1 mg kg−1) completely blocked AM841 effect in the stomach, but only partially in the intestinal regions. It is not known whether other receptors are involved in AM841 intestinal effects, although, as mentioned above, this probably excludes CB2R since AM630 was completely ineffective to block AM841 effect (either in vitro or in vivo). Although the possible involvement of opioid receptors in the effect of AM841 was not tested in vivo, our in vitro results (see above) and the results obtained by others in vivo suggest that opioid receptors do not mediate cannabinoid effects on GI motor function (44). Other possibilities, such as the involvement of prostanoid release (45), should be investigated.
AM841 may exert its effects on motility in the absence of central effects
We compared the effects of AM841 at 0.1 mg kg−1 and WIN at 5 mg kg−1 also in the CNS, using the cannabinoid tetrad. Mechanical sensitivity was likewise evaluated, as an additional test for nociception assessment. Moreover, AM841 was used at a higher dose, 0.5 mg kg−1.
As expected, WIN at 5 mg kg−1 produced significant analgesia in the plantar test, catalepsy in the ring test, and hypothermia. It tended to reduce also spontaneous locomotor activity, but the difference did not reach statistical significance in this particular study, although it did in previous research from our laboratory (3). This parameter seems to be less reliable than the others in demonstrating a cannabinoid central effect. In fact, some researchers use other tests for motor activity (46), or check only for catalepsy (47), which seems to be much more reliable as well as characteristic for a central cannabinoid effect.
In mice, contrary to WIN at 1 mg kg-1, AM841 at 0.1 mg kg-1 did not reduce locomotor activity, body temperature or the latency for paw withdrawal in the hot plate (28). However, in that study higher doses of AM841 (1 mg kg−1) were only tested in the locomotor activity test (with no significant effects compared to vehicle-treated animals), whereas the ring test (for detecting catalepsy), was not applied. In the present study in rats, AM841 at 0.1 mg kg−1, which exerted comparable effects on GI motility to those induced by WIN at 5 mg kg−1, did not significantly alter any of the parameters in the tetrad, further suggesting that AM841 is safer (with regards to central effects) than the non-selective cannabinoid agonist. However, its safety window could be somewhat narrower than was earlier suggested, since at the dose of 0.5 mg kg−1 it did induce significant analgesia, catalepsy and hypothermia. In addition, it increased the withdrawal threshold in the Von Frey test, a finding not produced by WIN. Although analgesia and the effects on the Von Frey test might be due to activation of peripheral CB1R (33, 48), catalepsy and hypothermia most likely involve central CB1R. Thus, these results suggest that, at high enough doses, AM841 may cross the blood brain barrier and reach the CNS and that its effects are not necessarily confined to the periphery, at least in the rat. More research is needed to determine the mechanism by which AM841 is excluded from the brain at low doses and why high doses are capable of reaching central CB1R.
Concluding remarks
In rats, AM841 potently reduced GI motility at doses that did not produce the classical central effects. The ability to induce central effects is a characteristic feature of cannabinoid agonists acting at CB1R that reduces their therapeutic potential. Thus, AM841 (at relatively low doses) might be used to overcome this problem. Furthermore, it can be used for the development of new drugs with a similar mechanism of action, but also completely free of central effects. Hopefully, such an approach will lead to safer, more selective treatments for patients suffering from GI motility disorders.
Supplementary Material
Figure 1. SUPPLEMENTARY MATERIAL. Typical gastrointestinal motility curves for control animals in radiographic studies. Motor function was measured by radiological methods (see text). Barium sulfate (2.5 mL, 2 g mL−1) was intragastrically administered in control animals and X-rays were taken 0, 1, 2, 4, 6, 8, 10 and 24 h after barium administration. Data represent mean ± SEM for motor function in stomach, small intestine, caecum and colorectum. N = 8. Notice that contrast fills the stomach immediately after its administration, and then it moves progressively from the stomach to the small intestine, caecum and colorectum; therefore, these intestinal regions have a bi-phasic shape, with both a filling phase (the region is reached and filled by contrast previously found in the region located proximally to it) and an emptying phase (the contrast moves from that region to the region located distally to it, or it abandons the body in the case of the colorectum).
Table 1. SUPPLEMENTARY MATERIAL
Log EC50 of AM841 and WIN 55-212,2 (WIN) calculated from the in vitro experiments. Values are mean Log EC50 ± SEM.
Key Messages.
In search for new, more useful and safer therapies for pathologic conditions characterized by accelerated transit and/or diarrhea (i.e., inflammatory bowel disease, irritable bowel syndrome, chemotherapy-induced diarrhea), we analyzed the effects of AM841, a novel highly potent cannabinoid-1 receptor (CB1R) agonist, on gastrointestinal (GI) motor function in the rat.
In vitro studies were performed on gastric (fundus, antrum) and intestinal (ileum, colon) preparations. In vivo studies included both the non-invasive radiographic analysis of the effects of AM841 on GI motility and its effects in the cannabinoid tetrad for determination of central effects. The mixed cannabinoid agonist WIN 55,212-2 (WIN) was used for comparison.
AM841 reduced gastric emptying and intestinal transit with much higher potency than WIN. These effects were sensitive to CB1R but not to CB2R blockade.
At a dose that depressed in vivo GI motility comparably to WIN, AM841 did not produce any central effects, although it did at a dose 5 times higher than that.
Acknowledgments
FUNDING: Funding was obtained from: Ministerio de Ciencia y Tecnología-Spain (SAF2009-12422-C02-01; SAF2012-40075-C02-01); Comunidad de Madrid (S-SAL/0261/2006; S2010/BMD-2308); Crohn’s and Colitis Foundation of Canada (CCFC); NIH Grants DA9158 and DA3801 to AM.
The authors are grateful to Pablo A. Cabezos and María Martínez-Villaluenga for their technical assistance.
Footnotes
Author contributions:
Design of the study by R.A. and M.S.; performance and analysis of in vitro experiments by C.C., M.S. and J.F.; performance and analysis of in vivo experiments by G.V., R.A. and A.E.L.-P.; contribution to interpretation of data and financial support by M.I.M.-F. and M.S.; contribution of essential reagents by G.A.T. and A.M.; writing of the manuscript by R.A. and M.S.
CONFLICTS OF INTEREST
The authors have no conflict of interest.
References
- 1.Schicho R, Storr M. Alternative targets within the endocannabinoid system for future treatment of gastrointestinal diseases. Canadian Journal of Gastroenterology & Hepatology. 2011;25:377–383. doi: 10.1155/2011/953975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abalo R, Vera G, López-Pérez AE, Martínez-Villaluenga M, Martín-Fontelles MI. The gastrointestinal pharmacology of cannabinoids: focus on motility. Pharmacology. 2012;90:1–10. doi: 10.1159/000339072. [DOI] [PubMed] [Google Scholar]
- 3.Abalo R, Cabezos PA, López-Miranda V, Vera G, González C, Castillo M, Fernández-Pujol R, Martín MI. Selective lack of tolerance to delayed gastric emptying after daily administration of WIN 55,212-2 in the rat. Neurogastroenterology and Motility. 2009;21:1002–e80. doi: 10.1111/j.1365-2982.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- 4.Abalo R, Cabezos PA, Vera G, Fernández-Pujol R, Martín MI. The cannabinoid antagonist SR144528 enhances the acute effect of WIN 55,212-2 on gastrointestinal motility in the rat. Neurogastroenterology and Motility. 2010;22:694–e206. doi: 10.1111/j.1365-2982.2009.01466.x. [DOI] [PubMed] [Google Scholar]
- 5.Abalo R, Cabezos PA, Vera G, López-Miranda V, Herradón E, Martín-Fontelles MI. Cannabinoid-induced delayed gastric emptying is selectively increased upon intermittent administration in the rat: role of CB1 receptors. Neurogastroenterology and Motility. 2011;23:457–467. e177. doi: 10.1111/j.1365-2982.2011.01677.x. [DOI] [PubMed] [Google Scholar]
- 6.Coruzzi G, Adami M, Coppelli G, Frati P, Soldani G. Inhibitory effect of the cannabinoid receptor agonist WIN 55,212-2 on pentagastrin-induced gastric acid secretion in the anaesthetized rat. Naunyn Schmiedeberg’s Archives of Pharmacology. 1999;360:715–18. doi: 10.1007/s002109900135. [DOI] [PubMed] [Google Scholar]
- 7.Tyler K, Hillard CJ, Greenwood-Van Meerveld B. Inhibition of small intestinal secretion by cannabinoids is CB1 receptor-mediated in rats. European Journal of Pharmacology. 2000;409:207–11. doi: 10.1016/s0014-2999(00)00843-8. [DOI] [PubMed] [Google Scholar]
- 8.Beaumont H, Jensen J, Carlsson A, Ruth M, Lehmann A, Boeckxstaens G. Effect of delta9-tetrahydrocannabinol, a cannabinoid receptor agonist, on the triggering of transient lower oesophageal sphincter relaxations in dogs and humans. British Journal of Pharmacology. 2009;156:153–62. doi: 10.1111/j.1476-5381.2008.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterology and Motility. 2006;18:949–56. doi: 10.1111/j.1365-2982.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 10.Izzo AA, Camilleri M. Cannabinoids in intestinal inflammation and cancer. Pharmacological Research. 2009;60:117–125. doi: 10.1016/j.phrs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2003;285:G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- 12.Mostafeezur RM, Zakir HM, Takatsuji H, Yamada Y, Yamamura K, Kitagawa J. Cannabinoids facilitate the swallowing reflex elicited by the superior laryngeal nerve stimulation in rats. PLoS One. 2012;7(11):e50703. doi: 10.1371/journal.pone.0050703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vianna CR, Donato J, Jr, Rossi J, Scott M, Economides K, Gautron L, Pierpont S, Elias CF, et al. Cannabinoid receptor 1 in the vagus nerve is dispensable for body weight homeostasis but required for normal gastrointestinal motility. The Journal of Neuroscience. 2012;32:10331–7. doi: 10.1523/JNEUROSCI.4507-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pertwee RG, Stevenson LA, Elrick DB, Mechoulam R, Corbett AD. Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. British Journal of Pharmacology. 1992;105:980–984. doi: 10.1111/j.1476-5381.1992.tb09088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abalo R, Rivera AJ, Vera G, Suardíaz M, Martín MI. Evaluation of the effect of age on cannabinoid receptor functionality and expression in guinea-pig ileum longitudinal muscle-myenteric plexus preparations. Neuroscience Letters. 2005;383:176–81. doi: 10.1016/j.neulet.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB 1 and CB 2. Pharmacological Reviews. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 19.Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. British Journal of Pharmacology. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan M, Mouihate A, Mackie K, Keenan CM, Buckley NE, Davison JS, Patel KD, Pittman QJ, et al. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2008;295:G78–G87. doi: 10.1152/ajpgi.90285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, Esposito G, Mascolo N, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. British Journal of Pharmacology. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camilleri M, Kolar GJ, Vazquez-Roque MI, Carlson P, Burton DD, Zinsmeister AR. Cannabinoid receptor 1 gene and irritable bowel syndrome: phenotype and quantitative traits. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2013;304:G553–G560. doi: 10.1152/ajpgi.00376.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunos G, Osei-Hyiaman D, Bátkai S, Sharkey KA, Makriyannis A. Should peripheral CB1 cannabinoid receptors be selectively targeted for therapeutic gain? Trends in Pharmacological Sciences. 2009;30:1–7. doi: 10.1016/j.tips.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makriyannis A. 2012 Division of Medicinal Chemistry Award Address. Trekking the Cannabinoid Road: A Personal Perspective. Journal of Medicinal Chemistry. 2012;57:3891–3911. doi: 10.1021/jm500220s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makriyannis A, Nikas SP, Khanolkar AD. Patent US7057076 Bicyclic and Tricyclic Cannabinoids. 2006
- 26.Picone RP, Khanolkar AD, Xu W, Ayotte LA, Thakur GA, Hurst DP, Abood ME, Reggio PH, et al. (-)-7′-Isothiocyanato-11-hydroxy-1′,1′-dimethylheptylhexahydrocannabinol (AM841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Molecular Pharmacology. 2005;68:1623–35. doi: 10.1124/mol.105.014407. [DOI] [PubMed] [Google Scholar]
- 27.Pei Y, Mercier RW, Anday JK, Thakur GA, Zvonok AM, Hurst D, Reggio PH, Janero DR, et al. Chemistry and Biology. 2008;15:1207–19. doi: 10.1016/j.chembiol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szymanski DW, Papanastasiou M, Melchior K, Zvonok N, Mercier RW, Janero DR, Thakur GA, Cha S, et al. Mass spectrometry-based proteomics of human cannabinoid receptor 2: covalent cysteine 6. 47(257)-ligand interaction affording megagonist receptor activation. Journal of Proteome Research. 2011;10:4789–98. doi: 10.1021/pr2005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fichna J, Bawa M, Thakur GA, Tichkule R, Makriyannis A, McCafferty DM, Sharkey KA, Storr M. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. PLoS One. 2014;9:e109115. doi: 10.1371/journal.pone.0109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keenan CM, Storr MA, Thakur GA, Wood JT, Wager-Miller J, Straiker A, Eno MR, Nikas SP, et al. AM841, a covalent cannabinoid ligand, powerfully slows gastrointestinal motility in normal and stressed mice in a peripherally restricted manner. British Journal of Pharmacology. 2015;172:2406–18. doi: 10.1111/bph.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabezos PA, Vera G, Castillo M, Fernández-Pujol R, Martín MI, Abalo R. Radiological study of gastrointestinal motor activity after acute cisplatin in the rat. Temporal relationship with pica. Autonomic Neuroscience: Basic and Clinical. 2008;141:54–65. doi: 10.1016/j.autneu.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. Journal of Pharmacology and Experimental Therapeutics. 1993;265:218–26. [PubMed] [Google Scholar]
- 33.Vera G, López-Miranda V, Herradón E, Martín MI, Abalo R. Characterization of cannabinoid-induced relief of neuropathic pain in rat models of type 1 and type 2 diabetes. Pharmacology Biochemistry and Behavior. 2012;102:335–43. doi: 10.1016/j.pbb.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Yüce B, Sibaev A, Broedl UC, Marsicano G, Göke B, Lutz B, Allescher HD, Storr M. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterology and Motility. 2007;19:744–53. doi: 10.1111/j.1365-2982.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- 35.Sibaev A, Yüce B, Kemmer M, Van Nassauw L, Broedl U, Allescher HD, Göke B, Timmermans JP, et al. Cannabinoid -1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2009;296:G119–28. doi: 10.1152/ajpgi.90274.2008. [DOI] [PubMed] [Google Scholar]
- 36.Storr M, Gaffal E, Saur D, Schusdziarra V, Allescher HD. Effect of cannabinoids on neural transmission in rat gastric fundus. Canadian Journal of Physiology and Pharmacology. 2002;80:67–76. doi: 10.1139/y02-005. [DOI] [PubMed] [Google Scholar]
- 37.Mulè F, Amato A, Baldassano S, Serio R. Involvement of CB1 and CB2 receptors in the modulation of cholinergic neurotransmission in mouse gastric preparations. Pharmacological Research. 2007;56:185–92. doi: 10.1016/j.phrs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Kurjak M, Hamel AM, Allescher HD, Schusdziarra V, Storr M. Differential stimulatory effects of cannabinoids on VIP release and NO synthase activity in synaptosomal fractions from rat ileum. Neuropeptides. 2008;42:623–32. doi: 10.1016/j.npep.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Izzo AA, Mascolo N, Pinto L, Capasso R, Capasso F. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. European Journal of Pharmacology. 1999;384:37–42. doi: 10.1016/s0014-2999(99)00673-1. [DOI] [PubMed] [Google Scholar]
- 40.Landi M, Croci T, Rinaldi-Carmona M, Maffrand JP, Le Fur G, Manara L. Modulation of gastric emptying and gastrointestinal transit in rats through intestinal cannabinoid CB(1) receptors. European Journal of Pharmacology. 2002;450:77–83. doi: 10.1016/s0014-2999(02)02053-8. [DOI] [PubMed] [Google Scholar]
- 41.Izzo AA, Mascolo N, Capasso R, Germanò MP, De Pasquale R, Capasso F. Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. Naunyn Schmiedeberg’s Archives of Pharmacology. 1999;360:221–3. doi: 10.1007/s002109900054. [DOI] [PubMed] [Google Scholar]
- 42.Izzo AA, Pinto L, Borrelli F, Capasso R, Mascolo N, Capasso F. Central and peripheral cannabinoid modulation of gastrointestinal transit in physiological states or during the diarrhoea induced by croton oil. British Journal of Pharmacology. 2000;129:1627–32. doi: 10.1038/sj.bjp.0703265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2006;291:G364–71. doi: 10.1152/ajpgi.00407.2005. [DOI] [PubMed] [Google Scholar]
- 44.Carai MAM, Colombo G, Gessa GL, Yalamanchili R, Basavarajppa BS, Hungund BL. Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice. British Journal of Pharmacology. 2006;148:1043–50. doi: 10.1038/sj.bjp.0706824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krowicki ZK. Involvement of hindbrain and peripheral prostanoids in gastric motor and cardiovascular responses to delta-9-tetrahydrocannabinol in the rat. Journal of Physiology and Pharmacology. 2012;63:581–8. [PubMed] [Google Scholar]
- 46.Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 47.Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. British Journal of Pharmacology. 2007;152:765–77. doi: 10.1038/sj.bjp.0707333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vera G, Cabezos PA, Martín MI, Abalo R. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacology Biochemistry and Behavior. 2013;105:205–12. doi: 10.1016/j.pbb.2013.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. SUPPLEMENTARY MATERIAL. Typical gastrointestinal motility curves for control animals in radiographic studies. Motor function was measured by radiological methods (see text). Barium sulfate (2.5 mL, 2 g mL−1) was intragastrically administered in control animals and X-rays were taken 0, 1, 2, 4, 6, 8, 10 and 24 h after barium administration. Data represent mean ± SEM for motor function in stomach, small intestine, caecum and colorectum. N = 8. Notice that contrast fills the stomach immediately after its administration, and then it moves progressively from the stomach to the small intestine, caecum and colorectum; therefore, these intestinal regions have a bi-phasic shape, with both a filling phase (the region is reached and filled by contrast previously found in the region located proximally to it) and an emptying phase (the contrast moves from that region to the region located distally to it, or it abandons the body in the case of the colorectum).
Table 1. SUPPLEMENTARY MATERIAL
Log EC50 of AM841 and WIN 55-212,2 (WIN) calculated from the in vitro experiments. Values are mean Log EC50 ± SEM.