Abstract
The acute effects after exposure to different styles of music on cardiac autonomic modulation assessed through heart rate variability (HRV) analysis have not yet been well elucidated. We aimed to investigate the recovery response of cardiac autonomic modulation in women after exposure to musical auditory stimulation of different styles. The study was conducted on 30 healthy women aged between 18 years and 30 years. We did not include subjects having previous experience with musical instruments and those who had an affinity for music styles. The volunteers remained at rest for 10 min and were exposed to classical baroque (64-84 dB) and heavy metal (75-84 dB) music for 10 min, and their HRV was evaluated for 30 min after music cessation. We analyzed the following HRV indices: Standard deviation of normal-to-normal (SDNN) intervals, root mean square of successive differences (RMSSD), percentage of normal-to-normal 50 (pNN50), low frequency (LF), high frequency (HF), and LF/HF ratio. SDNN, LF in absolute units (ms2) and normalized (nu), and LF/HF ratio increased while HF index (nu) decreased after exposure to classical baroque music. Regarding the heavy metal music style, it was observed that there were increases in SDNN, RMSSD, pNN50, and LF (ms2) after the musical stimulation. In conclusion, the recovery response of cardiac autonomic modulation after exposure to auditory stimulation with music featured an increased global activity of both systems for the two musical styles, with a cardiac sympathetic modulation for classical baroque music and a cardiac vagal tone for the heavy metal style.
Keywords: Autonomic nervous system (ANS), cardiovascular system, music
Introduction
Recently, the literature has shown that music can influence the autonomic nervous system (ANS)[1] and consequently, the cardiovascular system,[2,3] which is controlled partially by the ANS through afferent and efferent nerves that connect the heart at the sympathetic and parasympathetic terminals.[4,5] Heart rate variability (HRV) analysis is a noninvasive tool to evaluate the cardiac autonomic control in humans through an assessment of the fluctuations of intervals between consecutive heart beats, namely, R wave-to-R wave (RR) intervals or inter-beat intervals (IBS). These are related to the control of the ANS on the sinus node.[4,5,6]
Among the indices used to analyze HRV, the time domain indices are included that consist of the standard deviation (SD) of all normal RR intervals recorded in a time interval [SD of normal-to-normal (SDNN) intervals], which indicate the global variability of HRV, the root mean square of successive differences (RMSSD) between adjacent normal RR intervals, and the percentage of adjacent RR intervals (pNN50) with a difference of duration greater than 50 ms representing the parasympathetic cardiac modulation.[4,5,6] The frequency domain indices of HRV are composed of the high frequency (HF) component that corresponds to the respiratory modulation and is an indicator of the performance of the vagus nerve on the heart, the low frequency (LF) component that corresponds to the joint action of the vagal and sympathetic components on the heart with a predominance of the sympathetic one, and the LF/HF ratio that indicates sympathovagal balance.[4,5,6,7]
Changes in HRV through musical auditory stimulation have been reported by some authors who used linear indices to analyze the effect of baroque music on cardiac autonomic control in healthy young males at rest, during musical stimulus, and recovery.[8] The results showed reduced cardiac sympathetic modulation and an increased cardiac parasympathetic modulation following musical stimulation. Another study conducted with students under three different conditions: Relaxing music, exciting music, and no music found that the power of the LF and HF bands of the heart rate (HR) spectrum increased during relaxing and exciting music, with higher values of HF during the relaxing style compared with the exciting music.[9]
HRV analysis was also used to show that high intensity music and noises induced changes in cardiac autonomic modulation, and musical styles such as some classical styles of music result in suppression of the sympathetic component and some exciting styles induce the same reaction.[10]
Despite reports showing the influence of music on HRV indices, the effects of different styles of music on the recovery of the cardiac autonomic control are still unclear. In this context, we raised the hypothesis that acute exposure to auditory stimulation with heavy metal music increases the modulation of the sympathetic nervous system on the heart while exposure to classical styles cause opposite responses.
Elucidating the physiological responses involved in acute musical auditory stimulation is important for the development of future therapies to help treat cardiovascular disorders. Thus, we aimed to investigate the recovery response of cardiac autonomic modulation after exposure to musical auditory stimulation of different styles in women.
Materials and Methods
Study population
We analyzed 30 apparently healthy women aged between 18 years and 30 years. All the volunteers were informed about the procedures and objectives of the study and after agreeing, signed a consent form. All the study procedures were approved by the Research Ethics Committee (REC) of the institution (case number 2011/382) and followed the Resolution 196/96 of the National Health Council.
Noninclusion criteria
We did not include women with the following conditions: Body mass index (BMI) >35 kg/m2; systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg (at rest); cardiac arrhythmias (atrial flutter or fibrillation, multiple ventricular or atrial ectopy, second or third degree atrioventricular block); smoking; left ventricular dysfunction; neurological or respiratory disorders; and serious postural deviation in the chest such as severe scoliosis, kyphosis, or hyperlordosis that could influence the respiratory pattern and auditory disorders. The study did not include volunteers who practiced dance for at least 1 year and those who had attended classes in any musical instrument for at least 1 year, considering that cardiovascular responses are suggested to be different in musicians.[11] In order to avoid the effects related to sexual hormones, we did not include women on the 11th-15th days and 21st-25th days after the first day of their menstrual cycle.[12]
Initial assessment
The volunteers were identified by collecting the following information: Age, weight, height, and BMI. Anthropometric measurements were obtained according to the recommendations described in the literature.[13] Weight was measured using a digital scale (W 200/5, Welmy, São Paulo, Brazil) (Svantek, Helsinki, Finland) with a precision of 0.1 kg. Height was determined using a stadiometer (ES 2020, Sanny, São Paulo, Brazil) with a precision of 0.1 cm and was 2.20 m long. The BMI was calculated using the following formula: Weight (kg)/height (m2). HR was evaluated by the Polar RS800CX Heart Rate monitor (Polar Electro, Finland) and blood pressure (BP) was indirectly measured by auscultation through calibrated aneroid sphygmomanometer (Welch Allyn Tycos, New York City, USA), and stethoscope (Littmann, Saint Paul, USA) with the subjects seated.[14]
Measurement of auditory stimulation
The measurements of equivalent sound levels were performed in a soundproof room, using an audio dosimeter SV 102 (Svantek, Warsaw, Poland). It was programmed measuring circuit 7 in A-weighting, slow response.[15]
The auditory stimulation measurement lasted 5 min and 15 s for the heavy metal music, and 4 min and 50 s for the classical music. We used a type of microphone insert [microphone in real ear (MIRE)] that was placed inside the ear canal of the subject, just below the microphone, connected to the personal stereo.
Before each measurement, the microphones were calibrated with the acoustic calibrator CR: Model 514 (Cirrus Research plc, Berlin, Deutschland).
This tool was used to analyze the Leq (A), which is defined as the equivalent sound pressure level and the sound level corresponds to the same constant time interval. It contains the same total sound energy, which also analyzed the spectrum of sound stimulation (eighth track) frequency[16] [Figures 1 and 2].
Figure 1.
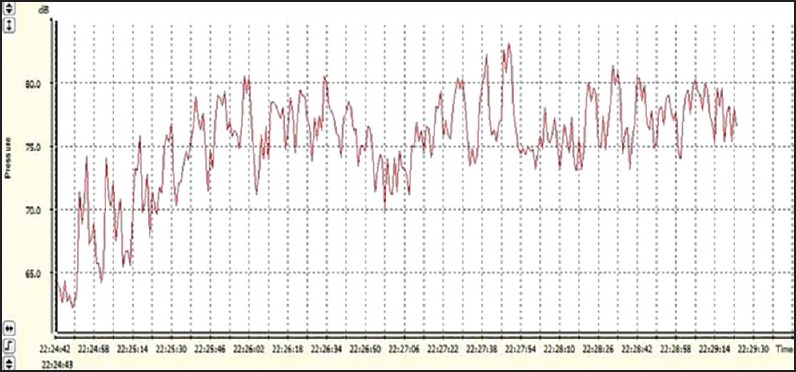
Equivalent sound level of auditory musical stimulation in the classical style
Figure 2.
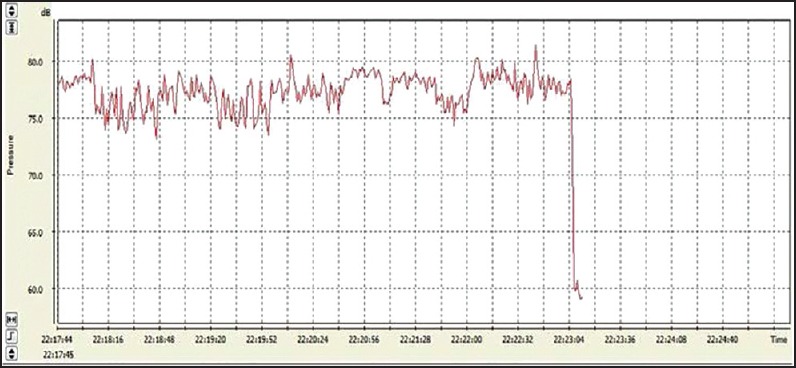
Equivalent sound level of auditory musical stimulation in the heavy metal style
Experimental protocol
Data collection was performed at a room temperature between 21°C and 25°C with humidity between 50% and 60%. The volunteers were instructed to not ingest alcohol and caffeine for 24 h prior to evaluation. The collection was made individually between 8 AM and 11 AM, and the volunteers were instructed to remain at rest, avoiding talking during the experiment.
After the initial evaluation, the heart monitor belt was then placed over the thorax, aligned with the distal third of the sternum and the Polar RS800CX Heart Rate receiver (Polar Electro, Finland) was placed on the wrist. Subsequently, the volunteers remained at seated rest for 10 min with the headset off.
Then, the volunteers were exposed to musical auditory stimulation with heavy metal (Gamma Ray's “Heavy Metal Universe”) and classic baroque styles (Pachelbel's “Canon in D Major”) for a period of 10 min for each style on different days, and after the music exposure the volunteers remained at rest with the headset turned off for 30 min. The full protocol lasted 50 min each day, performed on two consecutive days.
Analysis of HRV
The RR intervals recorded by the Polar RS800CX Heart Rate monitor (with a sampling rate of 1,000 Hz) were transferred to the Polar Precision Performance software (v. 3.0, Polar Electro, Finland). The software allowed visualization of the HR and the extraction of a file relating to a cardiac period (RR interval) in a “txt” file. In the digital filtering, the Polar Trainer program identified artefacts and ectopic beats through moderate grade analysis. Manual filtering was performed using Microsoft Office Excel 2013 through visual analysis. After digital filtering was performed, the Polar Trainer program was supplemented with manual filtering to eliminate artefacts and premature ectopic beats; 256 RR intervals were used for data analysis. Only series with more than 95% of sinus beats were included in the study. Time-frequency domain analysis was performed with HRV analysis software (HRV Kubios v.1.1 for Windows, Biomedical Signal Analysis Group, University of Kuopio, Finland).[17]
Linear indices of HRV
The analysis in the time domain was performed by means of SDANN (SD of the average normal RR intervals), RMSSD between adjacent normal RR intervals, and pNN50 (percentage of adjacent RR intervals with a difference of duration greater than 50 ms).[5]
Cardiac interval variability was evaluated into the frequency domain by means of spectral analysis. Interpolated series of cardiac interval values were split into half-overlapping segments of 256 points and a unique window was used to attenuate the side effects. Using a fast Fourier transform algorithm, spectra were calculated and integrated into LF (0.04-0.15 Hz) and HF (0.15-0.40 Hz) bands. LF and HF power are showed in absolute (ms2) and normalized units (nu) as well as the LF/HF ratio.[5]
Statistical analysis
Standard statistical methods were used to calculate the mean and standard deviations. The normal (or Gaussian) distribution of the data was verified by the Shapiro-Wilk goodness-of-fit test (z value >1.0). For parametric distributions, we applied analysis of variance (ANOVA) for repeated measures test followed by the Bonferroni post-test. For nonparametric distributions, we used the Friedman test followed by the Dunn's post test. We compared the HRV indices between the eight moments (rest vs. music vs. 0-5 min vs. 5-10 min vs. 10-15 min vs. 15-20 min vs. 20-25 min vs. 25-30 min after music exposure). Differences were considered significant when the probability of a Type I error was less than 5% (P < 0.05). We used the software GraphPad StatMate version 2.00 for Windows (GraphPad Software, San Diego, California, USA).
Results
Table 1 shows the values for DBP and SBP, HR, mean RR intervals, weight, height, and BMI of the volunteers.
Table 1.
DBP and SBP, HR, mean RR intervals, weight, height, and BMI of the volunteers
| Variable | Value |
|---|---|
| Age (years) | 20.85±2.11 |
| Weight (kg) | 58.51±8.71 |
| Height (m) | 1.63±0.06 |
| BMI (kg/m2) | 21.91±2.97 |
| HR (bpm) | 79.78±10.78 |
| Mean RR (ms) | 769.02±115.54 |
| SBP (mmHg) | 102.22±7.51 |
| DBP (mmHg) | 63.33±8.32 |
Mean ± standard-deviation, m = Meters, ms = Millisecond, kg = Kilogram, BPM = Beats per minute, mmHg = Millimeter of mercury
In relation to the time domain indices during exposure to classical music, there was a significant increase in SDNN 5-10 min and 15-20 min after exposure to music vs. rest and 15-20 min after exposure to the musical auditory stimulation vs. the period of exposure to music [Figure 3].
Figure 3.
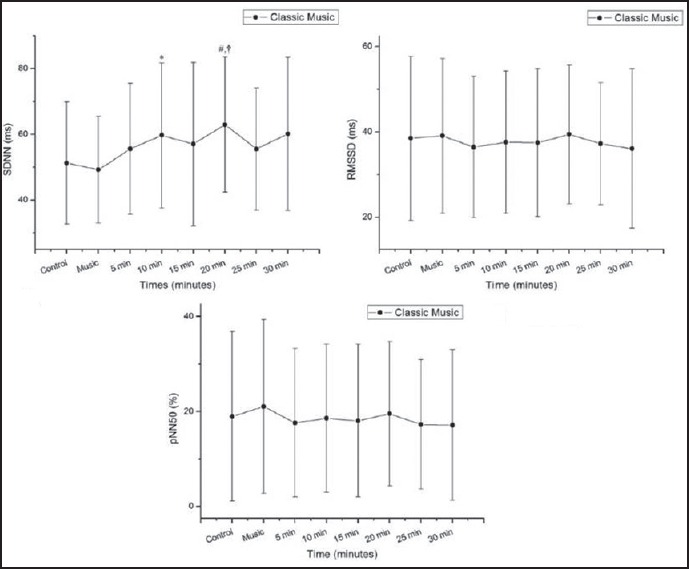
Standard deviation of normal-to-normal cardiac intervals (Panel A; SDNN), root mean square of successive differences between adjacent cardiac intervals (Panel B; RMSSD), and percentage of adjacent cardiac intervals with difference exceeding 50 ms (Panel C; pNN50). Response to classical music ms = milliseconds. *P < 0.05 vs. control, **P < 0.05 vs. music, †P < 0.01 vs. music, #P < 0.001 vs. control
Figure 4 shows the values for the comparisons between the rest, heavy metal music, and the recovery period regarding the time domain indices of HRV. The SDNN (ms) index increased 5-25 min after exposure to the music vs. rest and 5-30 min after exposure to the music vs. the music period. The RMSSD (ms) index showed a significant increase 20-25 min after exposure to the music vs. rest, and 20-25 min after exposure to the music vs. the music period. The pNN50 index increased 20-25 min after exposure to the music vs. rest.
Figure 4.
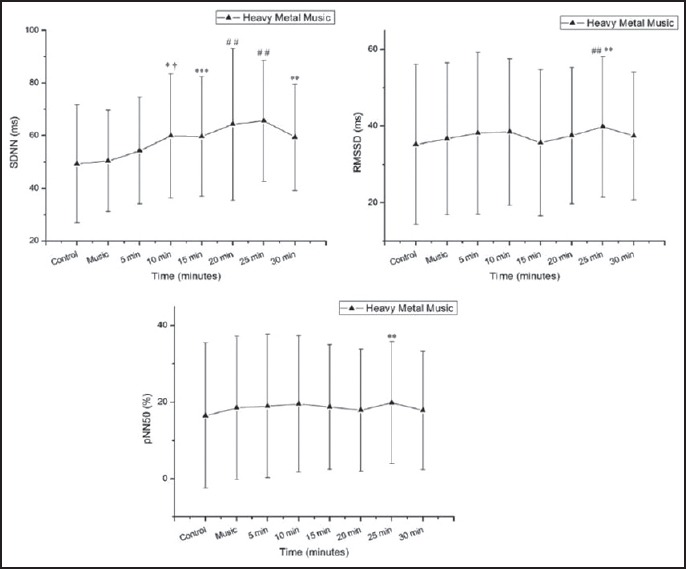
Standard deviation of normal-to-normal cardiac intervals (Panel A; SDNN), root mean square of differences between adjacent cardiac intervals (Panel B; RMSSD) and percentage of adjacent cardiac intervals with difference exceeding 50 ms (Panel C; pNN50). Response to heavy metal music ms = milliseconds. *P < 0.05 - control vs. 10 min, **P < 0.05 vs. music, †P < 0.01 vs. music, ††P < 0.01 vs. control, #P < 0.001 vs. rest, ##P < 0.001 - music vs
Regarding the frequency domain indices in the classical music protocol [Figure 5], we observed an increase in the power of the LF band of the cardiac interval spectrum (ms2) 15-20 min after exposure to the classical music vs. rest and 15-20 min after exposure to the musical auditory stimulation vs. classical music. There was also a significant increase in the LF index in normalized units (nu) 20-25 min and 25-30 min after exposure to the music vs. the music period, while we noted a significant decrease 25-30 min after exposure to music vs. rest and 25-30 min after exposure to the music vs. the period of exposure to music for the HF (nu) index. The LF/HF ratio increased 25-30 min after exposure to music vs. rest and 20-25 min after exposure to the music vs. the music period.
Figure 5.
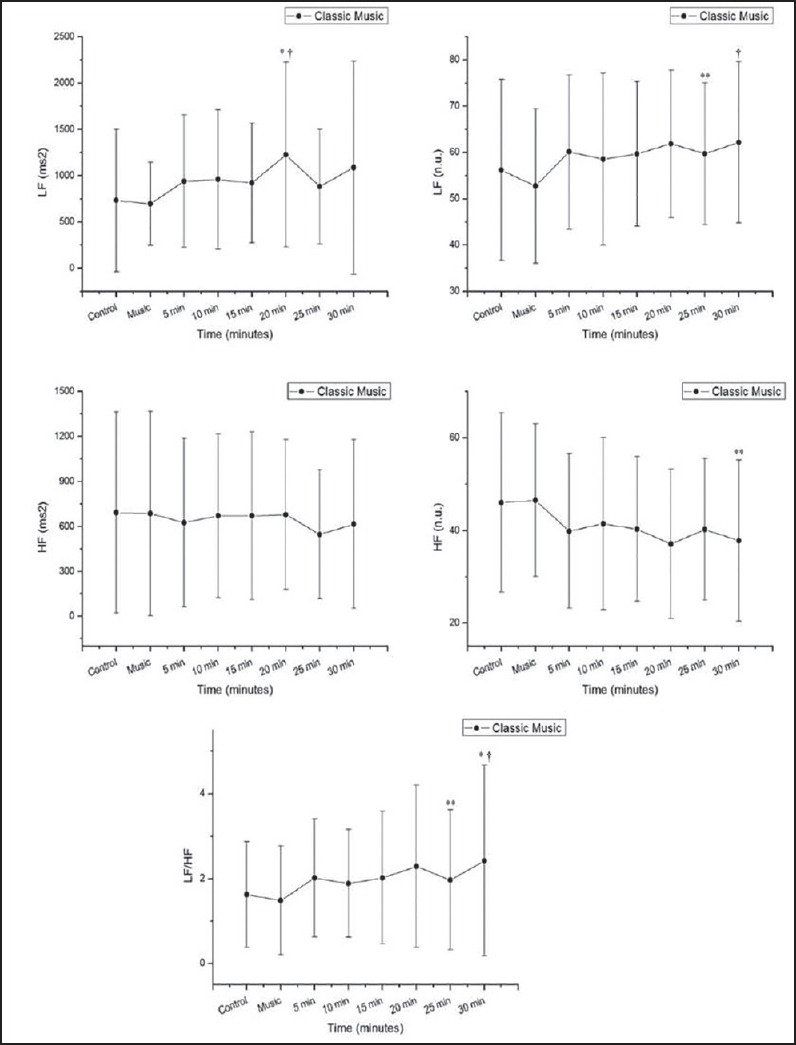
LF = low frequency in absolute (ms2) (Panel A) and normalized units (nu) (Panel B), HF = high frequency in absolute (ms2) (Panel C) and normalized units (nu) (Panel D), and LF/HF ratio (Panel E). Response to classical music ms = milliseconds *P < 0.05 vs. control, **P < 0.05 vs. music, †P < 0.01 vs. music, #P < 0.001 vs. control
In relation to the heavy metal music protocol, the power of the LF band of the cardiac interval spectrum (ms2) increased 20-30 min after exposure to music vs. rest [Figure 6].
Figure 6.
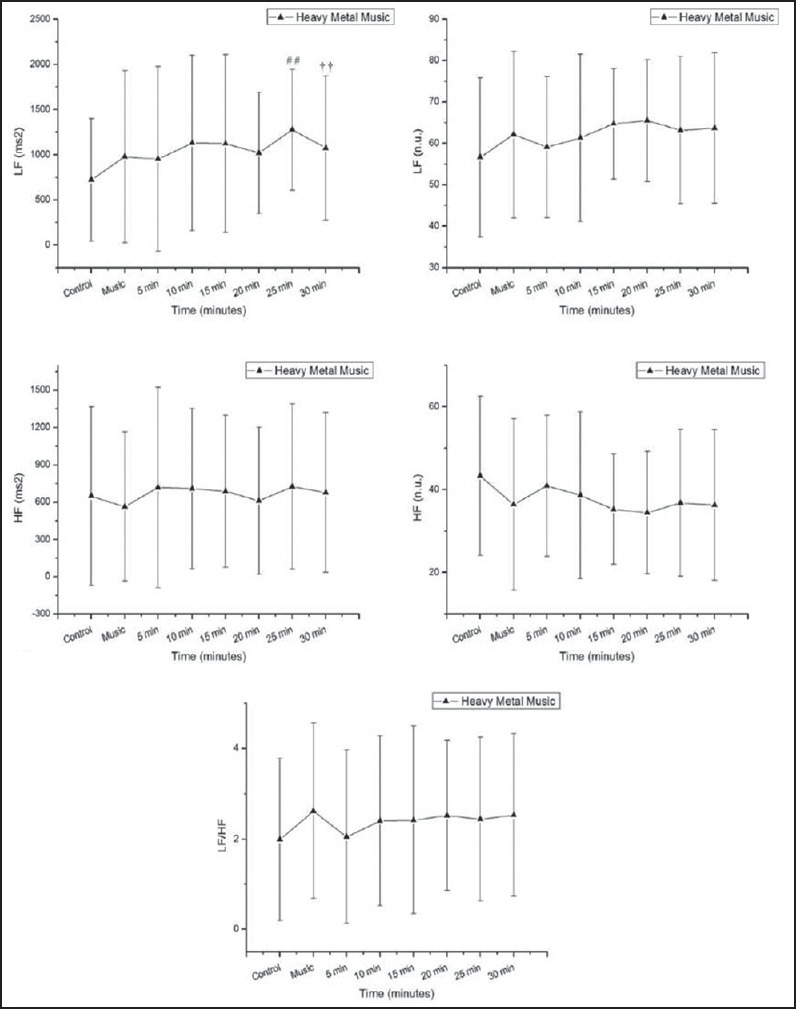
LF = low frequency in absolute (ms2) (Panel A) and normalized units (nu) (Panel B), HF = high frequency in absolute (ms2) (Panel C) and normalized units (nu) (Panel D), and LF/HF ratio (Panel E). Response to heavy metal music. ms = milliseconds *P < 0.05 vs. control vs. 10 min, **P < 0.05 vs. music, †P < 0.01 vs. music, ††P < 0.01 vs. control, #P < 0.001 vs. control, ##P < 0.001 - music vs
Discussion
This study showed that the recovery response of the cardiac autonomic modulation after exposure to auditory stimulation with classic baroque and heavy metal music styles was characterized by an increased sympathetic-vagal balance with a predominant increase in the sympathetic component and decrease in the parasympathetic component of the HRV. In relation to the heavy metal style, there was an increase of both systems after exposure, i.e., sympathetic and parasympathetic components compared to the rest. The increase in the sympathetic modulation of the heart was more pronounced 20-25 min after exposure to the classical and heavy metal music.
Based on our results, the SDNN index and the LF/HF ratio increased after exposure to auditory stimulation with classical baroque music. The SDNN represents the overall autonomic modulation of the heart, while the LF/HF ratio corresponds to the sympathetic-vagal balance.[5] Regarding the musical stimulation of the classical style, a recent study published by our group found a decrease in HRV during exposure to the same classical baroque music used in our investigation through analysis of the geometric indices of HRV.[15] Other authors found that tantric relaxing music therapy for 8 weeks (1 h per day, 2 days per week) tended to relax the body and increase the parasympathetic modulation of the heart.[18] Such evidence is reinforced by a study where this musical style caused an increase in the HF index, which is an indicator of the vagal nerve modulation on the heart.[9] These results were contradictory to our data, where classical music caused an increased sympathetic modulation of the heart. In this context, it was elucidated in the literature that exposure to classical music style has positive effects on the cardiovascular system featured by synchronization of cardiovascular physiology.[19] However, the focus of our investigation was to evaluate the acute autonomic responses after the auditory stimulation. After a careful review of the PubMed/MEDLINE database, we found no previous study that investigated these responses of the ANS.
We reported that the auditory stimulation with heavy metal musical style resulted in an increase in the overall cardiac autonomic modulation and parasympathetic regulation, i.e., an increase in SDNN, RMSSD, and pNN50, and an increase in sympathetic modulation (LF-ms2) after the music exposure. Previous studies indicated that music genres such as techno, hip-hop, and heavy metal cause physiological arousal.[20,21] It was observed that the techno style increased the levels of cortisol, norepinephrine, and adrenocorticotropic hormone, which is related to the sympathetic nervous system and also increased HR.[20,21] In addition, a recent study found that the heavy metal style decreased the geometric indices of HRV that represent the global modulation of the heart. It was suggested that acute exposure to this style of music can induce acute stress responses and alter cardiac autonomic modulation.[15]
Regarding the sound intensity of the auditory stimulation applied, we opted for sound level between 75 dB and 84 dB for the heavy metal style and between 65 dB and 80 dB for the classical baroque genre. This equivalent sound level was also used in the previous studies published by our group.[16,22] However, it was found that these were the few published studies that reported the sound intensity used, which makes it an important limitation of the previous studies published in the literature, and presents a positive factor in our investigation, once it has been elucidated that the noise level is involved in cardiac autonomic modulation and that exposure to white noise above 50 dB increases sympathetic modulation of the heart observed through the LF/HF ratio in the frequency domain analysis.[23]
When analyzed during the recovery period, the indices that reflect the global and sympathovagal balance, SDNN and LF/HF ratio, respectively, increased with higher intensity 15-20 min after the auditory stimulation with classical baroque music. In relation to the heavy metal music style, the indices that correspond to the cardiac vagal modulation, i.e., RMSSD and pNN50 showed a gradual increase 10-15 min and 15-20 min after musical auditory stimulation, suggesting an increased parasympathetic regulation of the heart after this stimulus.
It is suggested that the mechanism involved in cardiac autonomic responses induced by auditory stimulation occurs through several pathways, which may include the startle reflex response that is regulated by a brainstem circuit.[24] The acoustic startle reflex, an effect known on cardiovascular physiology induced by loud sounds, is cited as a sudden rise in arterial BP and HR to abruptly loud sound stimuli.[24] The intensity used to cause a startle reflex is approximately 110 dB, an intensity that is much higher than that used in our study.
Some possible mechanisms involved in the recovery of the ANS responses to auditory stimulation were elucidated by a study in the interaction of the consumption of drinks during exercise. The authors identified that the maintenance of the volume and plasma osmolality associated with the conservation of body temperature possibly influenced the recovery of HRV indices responses, which was evaluated in the time and frequency domain.[25] Other authors studied the association between HR recovery after exercise test on a treadmill and HRV in 485 subjects of both sexes and observed that there was no relationship between the levels of variability and HR recovery in the first 2 min after the test period in which the parasympathetic modulation is the main determinant of the reduction in HR.[26] The authors mentioned that the conditions of data acquisition within 24 h of HRV analysis may have influenced the results.
In this context, it is known that the postexercise exponential HRV decline is an intrinsic property of the intact circulation regardless of autonomic control. The HR decreases rapidly during the first 1-2 min after cessation of exercise and then gradually recovers.[27] During recovery after moderate and heavy exercise, the HR remains elevated above preexercise level for a relatively long period of time (up to 60 min).
Although we expected an increased parasympathetic modulation after a single exposure to musical auditory stimulation with classical music, we found opposite responses. A different style of relaxant music was reported to chronically increase the parasympathetic modulation of the heart.[18] The authors observed that music therapy during 8 months with popular Taiwanese songs with moderate, pleasant rhythms and tempos improved HRV of the patients treated with an antineoplastic cardiotoxic drug. The therapy proposed by the authors also involved learning how to play musical instruments and featured different instruments in each session. Furthermore, Nakamura et al.[27] investigated the effects of a classic relaxant music (“Träumerei” from Kinderszenen Op.15-7, R. Schumann) on the parasympathetic nerve activity of urethane-anesthetized rats and observed increased responses to this song style. The music applied in our study is not entirely relaxant; “Canon in D” of Pachelbel presents some pieces with high and low equivalent sound pressure. We believe that the style of music used by us was a methodological factor that may explain the HRV responses.
Another point to be raised is that we selected a minimal number of 256 RR intervals. This number is usually reached in only 4-5 min. As the audition process lasted 10 min, there might be some bias since the volunteers eventually heard different musical excerpts. However, our main purpose was to investigate cardiac autonomic responses after a single exposure to music.
Considering that the brain is an important regulator of the cardiovascular system,[28,29] neurophysiological hypotheses concerning the effects of music on cardiac autonomic control based on previous studies may help to explain our findings. HRV was analyzed in students listening to a story.[30] The authors reported that the intense parts of a story caused changes in the LF/HF ratio and suggested that it is a similar pattern of brain regions related to the processing of conditioned responses to auditory stimuli. Another notable study evaluated the effects of auditory stimulation with the relaxing music “Träumerei” from Kinderszenen Op. 15-7 on the ANS in rats anesthetized with urethane.[31] Even under anesthesia, this style of music caused a reduction of renal sympathetic nerve activity and blood pressure. Moreover, the authors found that this effect depended on an intact cochlea and auditory cortex and the autonomic response was regulated by the hypothalamic suprachiasmatic nucleus and H3 histamine receptors. The same study group also noted that the classical music “Träumerei” from Kinderszenen Op.15-7 caused an increased parasympathetic activity in anesthetized rats by analysis of gastric vagal nerve activity.[31] An important point that is to be raised is the style of music used by the authors and invasive method used to investigate the sympathetic and parasympathetic activity in animals.
Another highlight was the choice of females for this study that was made based on evidence from the literature showing differences in cardiac autonomic modulation between genders, demonstrating a greater parasympathetic drive in women than in men at rest based on analyses of HRV,[26,32] and differences in physiological responses before auditory stimulation musical, where women presented a higher increase in sympathetic nervous system responses compared to men.[20] In the study by Nater et al.,[20] physiological measurements were made through salivary alpha-amylase and cortisol, finger temperature, and skin conductance level. No significant changes were reported for skin conductance level, however, significant results were found for salivary alpha-amylase, i.e., a higher intensity increase in salivary alpha-amylase in females compared to males. Considering that salivary alpha-amylase is related to digestion and due its secretion by the sympathetic and parasympathetic nerves,[33] the measurement method reflects autonomic activity and reactivity.
Conclusion
The acute recovery response of cardiac autonomic modulation after exposure to auditory stimulation with different musical styles featured an increased global activity of both systems for the two musical styles, with a cardiac sympathetic modulation for classical baroque music and a cardiac vagal tone in relation to heavy metal music.
Acknowledgment
We thank the Pró-Reitoria de Pesquisa e Pós-Graduação (Pro-PG) of Universidade Estadual Paulista (UNESP) for their financial support for this research and Proof-Reading-Service.com for English grammar and spelling review.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Valenti VE, Guida HL, Frizzo AC, Cardoso AC, Vanderlei LC, Abreu LC. Auditory stimulation and cardiac autonomic regulation. Clinics (Sao Paulo) 2012;67:955–8. doi: 10.6061/clinics/2012(08)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutoo D, Akiyama K. Music improves dopaminergic neurotransmission: Demonstration based on the effect of music on blood pressure regulation. Brain Res. 2004;1016:255–62. doi: 10.1016/j.brainres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Cervellin G, Lippi G. A journey with Euterpe. Sinfonia concertante for music, heart and brain. Recenti Prog Med. 2011;102:352–8. doi: 10.1701/948.10377. [DOI] [PubMed] [Google Scholar]
- 4.Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med. 2003;33:889–919. doi: 10.2165/00007256-200333120-00003. [DOI] [PubMed] [Google Scholar]
- 5.Vanderlei LC, Pastre CM, Hoshi RA, Carvalho TD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc. 2009;24:205–17. doi: 10.1590/s0102-76382009000200018. [DOI] [PubMed] [Google Scholar]
- 6.Rajendra Acharya U, Paulo Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: A review. Med Biol Eng Comput. 2006;44:1031–51. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 7.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 8.Corrêa AP, Tavares JN, Santos MF, Fagundes AA, Barbosa DG, Meneguetti CA, et al. Efeito do estímulo musical no controle autonômico da frequência cardíaca. In: Corrêa AP, Tavares JN, Santos MF, Fagundes AA, Barbosa DG, Meneguetti CA, et al., editors. XII Encontro Latino Americano de Iniciação Científicae VIII Encontro Latino Americano de Pós-Graduação. São José dos Campos: Universidade do Vale do Paraíba; 2009. pp. 1–4. [Google Scholar]
- 9.Iwanaga M, Kobayashi A, Kawasaki C. Heart rate variability with repetitive exposure to music. Biol Psychol. 2005;70:61–6. doi: 10.1016/j.biopsycho.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Umemura M, Honda K. Influence of music on heart rate variability and comfort — A consideration through comparison of music and noise. J Hum Ergol (Tokyo) 1998;27:30–8. [PubMed] [Google Scholar]
- 11.Jausovec N, Habe K. The “Mozart effect”: An electroencephalographic analysis employing the methods of induced event-related desynchronization/synchronization and event-related coherence. Brain Topogr. 2003;16:73–84. doi: 10.1023/b:brat.0000006331.10425.4b. [DOI] [PubMed] [Google Scholar]
- 12.Bai X, Li J, Zhou L, Li X. Influence of the menstrual cycle on nonlinear properties of heart rate variability in young women. Am J Physiol Heart Circ Physiol. 2009;297:H765–74. doi: 10.1152/ajpheart.01283.2008. [DOI] [PubMed] [Google Scholar]
- 13.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics Books; 1988. [Google Scholar]
- 14.Sociedade Brasileira de Cardiologia; Sociedade Brasileira de Hipertensão; Sociedade Brasileira de Nefrologia. VI Brazilian Guidelines on Hypertension. Arq Bras Cardiol. 2010;95(Suppl):1–51. [PubMed] [Google Scholar]
- 15.Roque AL, Valenti VE, Guida HL, Campos MF, Knap A, Vanderlei LC, et al. The effects of auditory stimulation with music on heart rate variability in healthy women. Clinics (Sao Paulo) 2013;68:960–7. doi: 10.6061/clinics/2013(07)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roque AL, Valenti VE, Guida HL, Campos MF, Knap A, Vanderlei LC, et al. The effects of different styles of musical auditory stimulation on cardiac autonomic regulation in healthy women. Noise Health. 2013;15:281–7. doi: 10.4103/1463-1741.113527. [DOI] [PubMed] [Google Scholar]
- 17.Vanderlei LC, Silva RA, Pastre CM, Azevedo FM, Godoy MF. Comparison of the Polar S810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Braz J Med Biol Res. 2008;41:854–9. doi: 10.1590/s0100-879x2008005000039. [DOI] [PubMed] [Google Scholar]
- 18.Chuang CY, Han WR, Li PC, Song MY, Young ST. Effect of long-term music therapy intervention on autonomic function in anthracycline- treated breast cancer patients. Integr Cancer Ther. 2011;10:312–6. doi: 10.1177/1534735411400311. [DOI] [PubMed] [Google Scholar]
- 19.Bernardi L, Porta C, Casucci G, Balsamo R, Bernardi NF, Fogari R, et al. Dynamic interactions between musical, cardiovascular, and cerebral rhythms in humans. Circulation. 2009;119:3171–80. doi: 10.1161/circulationaha.108.806174. [DOI] [PubMed] [Google Scholar]
- 20.Nater UM, Abbruzzese E, Krebs M, Ehlert U. Sex differences in emotional and psychophysiological responses to musical stimuli. Int J Psychophysiol. 2006;62:300–8. doi: 10.1016/j.ijpsycho.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson U, Unosson M, Rawal N. Stress reduction and analgesia in patients exposed to calming music postoperatively: A randomized controlled trial. Eur J Anaesthesiol. 2005;22:96–102. doi: 10.1017/s0265021505000189. [DOI] [PubMed] [Google Scholar]
- 22.De Castro BC, Guida HL, Roque AL, de Abreu LC, Ferreira LL, Raimundo RD, et al. Previous exposure to musical auditory stimulation immediately influences the cardiac autonomic responses to the postural change maneuver in women. Int Arch Med. 2013;6:32. doi: 10.1186/1755-7682-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee OK, Chung YF, Chan MF, Chan WM. Music and its effect on the physiological responses and anxiety levels of patients receiving mechanical ventilation: A pilot study. J Clin Nurs. 2005;14:609–20. doi: 10.1111/j.1365-2702.2004.01103.x. [DOI] [PubMed] [Google Scholar]
- 24.Valenti VE, Abreu LC, Fonseca FL, Adami F, Sato MA, Vanderlei LC, et al. Effects of the administration of a catalase inhibitor into the fourth cerebral ventricle on cardiovascular responses in spontaneously hypertensive rats exposed to sidestream cigarette smoke. Clinics (Sao Paulo) 2013;68:851–7. doi: 10.6061/clinics/2013(06)21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno IL, Pastre CM, Ferreira C, de Abreu LC, Valenti VE, Vanderlei LC. Effects of an isotonic beverage on autonomic regulation during and after exercise. J Int Soc Sports Nutr. 2013;10:2. doi: 10.1186/1550-2783-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antelmi I, Chuang EY, Grupi CJ, Latorre MR, Mansur AJ. Recuperação da frequência cardíaca após teste de esforço em esteira ergométrica e variabilidade da frequência cardíaca em 24 horas em indivíduos sadios. Arq Bras Cardiol. 2008;90:413–8. [Google Scholar]
- 27.Nakamura T, Tanida M, Niijima A, Hibino H, Shen J, Nagai K. Auditory stimulation affects renal sympathetic nerve activity and blood pressure in rats. Neuroscience Lett. 2007;416:107–12. doi: 10.1016/j.neulet.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 28.Valenti VE, de Abreu LC, Sato MA, Ferreira C, Adami F, Fonseca FL, et al. Sidestream cigarette smoke effects on cardiovascular responses in conscious rats: Involvement of oxidative stress in the fourth cerebral ventricle. BMC Cardiovasc Disord. 2012;12:22. doi: 10.1186/1471-2261-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cisternas JR, Valenti VE, Sato MA, Fonseca FL, Saldiva PH, De Mello Monteiro CB, et al. The effects of catalase inhibition into the fourth cerebral ventricle on the Bezold-Jarisch reflex in spontaneously hypertensive rats. J Integr Neurosci. 2011;10:475–87. doi: 10.1142/S021963521100283X. [DOI] [PubMed] [Google Scholar]
- 30.Wallentin M, Nielsen AH, Vuust P, Dohn A, Roepstorff A, Lund TE. Amygdala and heart rate variability responses from listening to emotionally intense parts of a story. Neuroimage. 2011;58:963–73. doi: 10.1016/j.neuroimage.2011.06.077. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Tanida M, Niijima A, Nagai K. Effect of auditory stimulation on parasympathetic nerve activity in urethane-anesthetized rats. In Vivo. 2009;23:415–9. [PubMed] [Google Scholar]
- 32.Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–5. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 33.Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, et al. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophysiol. 2005;55:333–42. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]


