Abstract
Environmental noise causes cognitive impairment, particularly in executive function and episodic memory domains, in healthy populations. However, the possible moderating influences on this relationship are less clear. This study assessed 54 healthy participants (24 men) on a cognitive battery (measuring psychomotor speed, attention, executive function, working memory, and verbal learning and memory) under three (quiet, urban, and social) noise conditions. IQ, subjective noise sensitivity, sleep, personality, paranoia, depression, anxiety, stress, and schizotypy were assessed on a single occasion. We found significantly slower psychomotor speed (urban), reduced working memory and episodic memory (urban and social), and more cautious decision-making (executive function, urban) under noise conditions. There was no effect of sex. Variance in urban noise-induced changes in psychomotor speed, attention, Trail Making B-A (executive function), and immediate recall and social noise-induced changes in verbal fluency (executive function) and immediate recall were explained by a combination of baseline cognition and paranoia, noise sensitivity, sleep, or cognitive disorganization. Higher baseline cognition (but not IQ) predicted greater impairment under urban and social noise for most cognitive variables. Paranoia predicted psychomotor speed, attention, and executive function impairment. Subjective noise sensitivity predicted executive function and memory impairment. Poor sleep quality predicted less memory impairment. Finally, lower levels of cognitive disorganization predicted slower psychomotor speed and greater memory impairment. The identified moderators should be considered in studies aiming to reduce the detrimental effects of occupational and residential noise. These results highlight the importance of studying noise effects in clinical populations characterized by high levels of the paranoia, sleep disturbances, noise sensitivity, and cognitive disorganization.
Keywords: Cognitive performance, individual differences, social noise, urban noise
Introduction
Environmental noise (e.g., transportation and workplace noise) has been documented to impair a range of cognitive domains in healthy populations, e.g.[1,2] Despite methodological inconsistencies between studies, i.e., noise content and loudness, cognitive task examined, or moderating variables considered,[2] robust noise effects are found for selective attention, working memory, and episodic memory.[3] While it is possible to form a cohesive account of average noise effects across studies with varying noise intensities and/or cognitive tasks, it is much harder to draw conclusions concerning the influence of individual differences on noise effects (due to a lack of studies examining their moderating effects). Furthermore, although environmental noise with and without a social component appears to similarly disrupt cognitive performance in healthy adults, this needs to be established using a within-participant design.[2] The present study aimed to elucidate the possible moderators of both social and nonsocial noise-induced changes in cognitive performance. The following variables were selected as targets based on recommendations of a recent review:[2] Sex, age, IQ, subjective noise sensitivity, sleep quality, extraversion, neuroticism, paranoia, depression, anxiety, stress, schizotypy, and baseline cognitive performance.
The evidence of an interaction between noise and sex effects (poorer performance under noise in women, relative to men) is limited to simple cognitive tasks in healthy adults’ arithmetic performance,[4] and not present in children.[5] In addition, research into the potential moderating effect of IQ on noise-induced changes in cognitive performance is sparse[2] and studies examining the influence of age focus on subjective self-report measures of noise sensitivity, e.g.[6] This study will be the first to examine the influence of sex, IQ, and age in noise effects on a variety of cognitive domains. In line with previous literature,[4] a sex and noise interaction may be present for simple cognitive tasks (i.e., psychomotor speed and tasks not involving working memory). Such findings may be underpinned by sex differences in stress hormone production in response to noise, although the evidence for this is unclear,[7] or by differences in levels of neuroticism in men and women, on average women score higher.[8] It is unclear how IQ will moderate the noise-cognitive performance relationship: Having a higher IQ may be protective against noise stress, conversely, having a lower IQ could mean there is less room for performance to reduce under noise conditions. Based on the previously reported association with subjective noise sensitivity,[6] older age is predicted to be associated with greater noise-induced cognitive disruption.
Higher levels of negative appraisal or subjective noise sensitivity have been found to be related to reduced cognitive function in terms of reduced work ability and attention,[9] under noise, though self-reported measures of noise sensitivity do not always correlate with objective impairment of performance under noise.[10,11] Indeed levels of negative affect (i.e., annoyance and irritation), rather than subjective noise sensitivity,[10] could cause increased disruption on certain (complex and arousing) cognitive tasks under noise, potentially by diminishing attentional capacity due to over-arousal.[12]
Unmeasured sleep quality may also moderate noise effects in cognitive performance. Noise may improve performance on simple tasks by increasing arousal levels in low aroused, moderately sleep-deprived individuals to reach the optimum task arousal.[13] Conversely, as sleep disturbances are known to independently impair performance on complex cognitive tasks,[14,15,16,17] the addition of noise may have no effect (floor effect) or still further disrupt cognitive performance.
The most (although still sparsely) studied moderator of noise-induced changes to cognitive performance is personality traits. Introverts, relative to extraverts, have been found to display more pronounced disruption of concentration and impaired logical reasoning performance under noise conditions,[18,19,20] while neuroticism has been shown to be positively related to subjective noise sensitivity and annoyance during noise.[21] However, not all studies demonstrate an interaction between personality and noise, e.g., on information processing.[22] When considering this literature it is important to be mindful that some differences between neurotic introverts and stable extraverts may be underpinned by trait anxiety and distractibility.[20]
The investigation of the moderating effects of negative affect (depression, anxiety, and stress) on the noise-cognitive performance relationship is limited.[2] However, in accordance with the previous finding that noise effects on attention are greater in high anxious, relative to low anxious, individuals,[23] and the finding that long-term exposure to environmental noise is associated with elevated levels of anxiety, depression, and stress responses, reviewed in,[24] negative affect is predicted to be associated with greater noise-induced cognitive disruption. Paranoia is also predicted to be associated with greater impairment under noise in accordance with previous studies showing that individual differences in noise sensitivity are moderated by paranoia, e.g.[25] In addition, schizotypy may also be related to greater noise-induced cognitive impairment, in line with evidence that schizotypy is positively related to increased stress reactivity (as measured by increased heart rate), and that individuals with high levels of schizotypy display diminished cognitive performance (i.e., spatial working memory) under stressors compared to individuals with low levels of schizotypy.[26]
Another possible, but surprisingly little explored, moderating factor is baseline cognitive capacity. Noise-induced impairments may be moderated by differences in baseline cognitive performance, similarly to IQ. Baseline performance on executive function, working memory, and sustained attention tasks may be particularly relevant in this context given the sensitivity of these tasks to noise stress in healthy humans.[2]
The present study is the first, to our knowledge, to comprehensively investigate a range of potential moderators (sex, age, IQ, subjective noise sensitivity, sleep quality, extraversion, neuroticism, paranoia, depression, anxiety, stress, schizotypy, and baseline cognitive performance) of the facilitation or impairing effects of different types of environmental noise (social and nonsocial) on a variety of cognitive domains. Older age, higher levels of subjective noise sensitivity, poor sleep quality, low extraversion and higher levels of neuroticism, paranoia, depression, anxiety, stress, and schizotypy are predicted to be associated with greater noise-induced cognitive impairment. It is unclear whether sex will exert any effects or if lower baseline cognitive performance will be associated with exacerbated impairment under noise.
Methods
Participants
The study involved 54 healthy participants (initial sample was 58, 4 participants were excluded as they did not complete all three testing sessions) recruited via King's College London circulars to staff and students, online posts on the East Dulwich forum and the Experimatch website, and the Unusual Experiences Inquiry Studies (UNIQUE);[27] research register. Participants recruited from the UNIQUE register (N = 12) were characterized by having unusual experience (i.e., hallucinations or out of body experiences) without a need for contact with psychiatric services and were targeted in order to capture the high and low extremes of schizotypal traits that occur in the general population. These participants were included in our healthy sample as noise effects were not different in 12 people recruited from UNIQUE register and the rest of the sample recruited through other means we normally use to recruit healthy participants [Appendix 1].
Appendix 1.
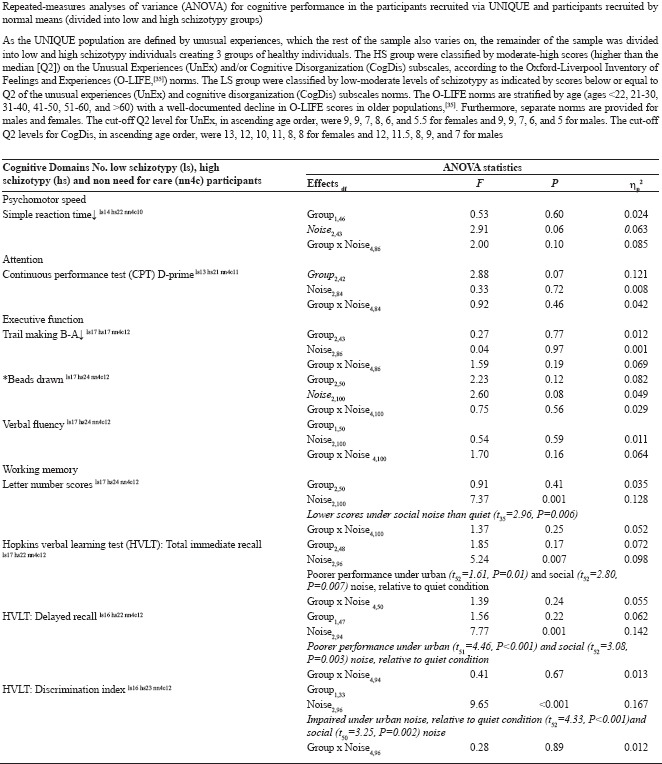
All participants were aged 18-64 years, with no hearing impairment, normal, or corrected to normal, vision, and fluent English. The general exclusion criteria for all participants were: A history of organic brain disorder, predicted IQ <80 as assessed by the two subtest versions of the Wechsler Abbreviated Scale of Intelligence,[28] or primary ICD-10 diagnosis of substance abuse disorder, schizophrenia or schizophrenia spectrum disorder.
The study was approved by the NHS Camden and Islington Research Ethics Committee (12/LO/0626). Written informed consent was obtained from all participants and they were compensated for their time (£15/occasion) and travel.
Assessment of sample characteristics
For sample and individual difference characterization purposes [Table 1], all participants completed the Edinburgh Handedness Inventory,[29] the Noise Sensitivity Questionnaire,[30] the Pittsburgh Quality of Sleep Inventory,[31] the short-scale Eysenck Personality Questionnaire-Revised,[32] the Paranoia checklist,[33] the Depression, Anxiety and Stress symptoms -21 item version,[34] and the Oxford-Liverpool Inventory of Feelings and Experiences.[35]
Table 1.
Sample characteristics
| Demographic characteristics | Male (N = 24) | Female (N = 30) | Entire sample (N = 54) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Age (years) | 37.50 (11.58) | 19-64 | 38.77 (10.97) | 22-59 | 38.20 (11.16) | 19-64 |
| Current full scale IQ (aWASI) | 119.50 (13.43) | 94-139 | 116.10 (12.41) | 87-135 | 117.61 (12.86) | 87-139 |
| Noise sensitivity (bNoiSeQ) ↓ | 51.46 (11.33) | 25-79 | 44.44 (13.39) N1 | 14-67 N1 | 47.62 (13.18) N1 | 14-79 N1 |
| Sleep quality (cPSQI Total) ↑ | 5.04 (3.67) N1 | 0-16 N1 | 5.67 (4.56) | 1-17 | 5.40 (4.17) N1 | 0-17 N1 |
| Extraversion (dEPQ-R) | 7.13 (3.65) | 1-12 | 6.44 (3.48) N 1 | 1-12 N1 | 6.75 (3.4) N1 | 1-12 N1 |
| Neuroticism (dEPQ-R) | 4.13 (3.08) | 0-9 | 5.21 (3.77) N1 | 0-12 N 1 | 4.72 (3.49) N1 | 0-12 N1 |
| Paranoia checklist (eoccurrence) | 5.71 (8.50) | 0-28 | 5.13 (12.14) | 0-63 | 5.39 (10.59) | 0-63 |
| Depression (fDASS-21) | 8.17 (6.18) N1 | 0-20 N1 | 10.27 (8.08) | 0-38 | 9.36 (7.33) N1 | 0-38 N1 |
| Anxiety (fDASS-21) | 3.74 (3.92) N1 | 0-14 N1 | 4.93 (6.53) | 0-28 | 4.42 (5.54) N1 | 0-28 N1 |
| Stress (fDASS-21) | 5.57 (5.04) N1 | 0-18 N 1 | 8.53 (7.66) | 0-24 | 7.25 (6.76) N1 | 0-24 N1 |
| UnEx (gO-LIFE) | 7.21 (6.58) | 0-23 | 8.87 (5.48) | 1-20 | 8.13 (6.00) | 0-23 |
| CogDis (gO-LIFE) | 9.33 (6.01) | 0-22 | 9.17 (5.90) | 0-22 | 9.24 (5.90) | 0-22 |
| IntAn (9O-LIFE) | 7.46 (5.27) | 1-21 | 6.83 (4.26) | 1-16 | 7.11 (4.70) | 1-21 |
| ImpNon (gO-LIFE) | 7.83 (4.71) | 2-18 | 6.17 (3.74) | 0-14 | 6.91 (4.24) | 0-18 |
aWechsler abbreviated scale of intelligence,[28] bNoise sensitivity questionnaire,[30] cPittsburgh sleep quality index,[31] dEysenck personality questionnaire-revised,[32] eParanoia checklist,[28] fDepression, anxiety and stress -21 item version,[34] and gThe Oxford-Liverpool inventory of feelings and experiences,[35] UnEx (unusual experiences), CogDis (cognitive disorganization), IntAn (introverted anhedonia), and ImpNon (impulsive nonconformity) scales, ↓ = Higher scores indicate lower noise sensitivity, ↑ = Higher score indicate poorer overall sleep quality, N1 = data missing from one participant
Experimental design and noise conditions
All participants completed a cognitive battery under three experimental (noise) conditions, separated by a 1-2 week interval. The sound generating equipment was hidden from participants’ view in an adjunct soundproof room (connecting door kept open). The speakers (also hidden from participant's view) were kept in the sound proof testing room.
Development of the noise stimuli and chosen noise intensity were guided by those used in previous studies, e.g.[36] The three noise conditions were: Quiet (no) noise that took place in a quiet (~30 dB) sound proofed laboratory, urban noise that comprised building-site noise (with no elements of social noise), and social noise that consisted of background babble (with no distinguishable speech) and footsteps from a crowded hall. The urban and social noise had a background level of 68 dB with louder peaks of 78 dB urban/social stimuli superimposed on top. The presentation of mean energy level (dBA Leq), time profile, and number of and duration of peaks of noise stimuli were matched. The order of noise conditions was counterbalanced across participants to one of six orders: Quiet-social-urban, quiet-urban-social, urban-social-quiet, urban-quiet-social, social-quiet-urban, and social-urban-quiet (nine participants per order).
The cognitive battery [detailed in Table 2] comprised measures of psychomotor speed, attention, executive functioning, working memory, and verbal learning and memory. Selection of cognitive domains and tasks was based on known noise-induced cognitive disruption in healthy adults,[2] ease and practicality of admission, high test-retest reliability, and lack of practice effects or availability of alternate forms [for Verbal Fluency VF,[40] and Hopkin's Verbal Learning Task revised HVLT].[42]
Table 2.
Details of the cognitive battery
| Cognitive domain | Tests | Dependent variables |
|---|---|---|
| Psychomotor speed | Computerized simple reaction time (SRT) | Average RT |
| Attention | Continuous performance test-identical pairs version (CPT-IP)[37] | D-prime (signal detection) |
| Executive function | Trail making (TM) part B minus A[38] | TM B-A (cost of switching between two tasks, RT) |
| ºBeads (60:40)[39] — Alternate versions: Red and blue beads; green and black beads; yellow and black beads | Total number of beads selected | |
| Phonemic verbal fluency[40] - Alternate forms: PRW, CFL, TAG | Total correct number of words produced in 60 s (sum of three letters) | |
| Working Memory | Letter number[41] | Total number of correct letter- number strings |
| Verbal Learning and Memory | Hopkins verbal learning test — Revised[42] — Alternate forms 1, 2 and 4 | Immediate recall (total number words recalled) |
| Delayed recall (total number words recalled) | ||
| Discriminative index (number words correctly recognized minus the number of words falsely recognized) |
ºThe beads task was included as an executive function task due to evidence of significant associations between beads performance and working memory and cognitive flexibility (i.e.,[43])
General procedure
Participants were informed that the study aimed to investigate the effects of stress on cognitive function under “real life” environments. All participants were requested to abstain from alcohol for at least 24 h and participants who smoked (N = 3) were asked to not smoke 30 min prior to each scheduled testing session.
All sessions began with two or more of the sample characteristic assessments (lasting >30 min on each occasion) in quiet surroundings. Following completion of the questionnaires, the experimenter (BW) activated the noise stimuli (in the social and urban conditions). Participants were (implicitly) acclimatized to the noise for five minutes prior to completing the cognitive tasks. During this 5-min break, which was also present in the quiet condition, the experimenter engaged the participants in general conversation. The cognitive assessment session lasted approximately 40 min.
The order of tests in the three experimental conditions was pseudorandomized across participants, with HVLT (immediate) always in position 1-3 to allow delayed recall testing 25 min later, and the remaining tests presented in random order. The tasks were presented in the same order during all three sessions for individual participants, with alternate test forms occurring equally often in the three experimental conditions across participants.
Data analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) (Windows, Version 22.0) (SPSS-Inc., Chicago, IL) and significance determined using a significance level of P < 0.05 in all analyses.
Effects of noise on cognitive performance
All cognitive variables were first examined for their distribution properties and sphericity. Mauchly's test of sphericity was significant for the Simple Reaction Time (SRT) task (W = 0.84, P = 0.02) so results were Greenhouse-Geisser corrected. Missing data and outliers across the three sessions reduced the sample size for CPT D-prime (N = 45), SRT (N = 48), and Trail Making B-A (N = 51). Noise effects across all participants were determined using Sex (between-participant factor: Men, Women) x Noise (within-participant factor: Quiet, Urban, Social) analysis of variance (ANOVA) performed separately for each cognitive variable, followed by post hoc mean comparisons as appropriate.
Associations between individual difference variables, baseline cognitive performance, and noise-induced cognitive change scores
Pearson's correlation analyses were conducted to examine the relationships between individual difference variables (sample characteristics and baseline cognitive performance under the quiet condition) and urban and social cognitive change scores. Change scores were computed by subtracting performance in the quiet condition from performance under noise. Correlations were also run between sample characteristics and cognitive performance under quiet condition to understand whether noise exaggerated any preexisting associations under quiet conditions. Correlation graphs were referred to when interpreting significant associations. Correlations for each cognitive noise index (no. of correlations =15) were not Bonferroni corrected due to their purpose as determiners of inclusion into the regression analysis.
When two or more individual difference variables were significantly associated with urban or social noise change scores, regression analyses were conducted to examine their unique and shared contribution. Linear regressions were employed, with all predictors entered in one block and a backward removal method applied to nonsignificant predictors (removal criterion was probability of F-to-removal ≥0.100). The decision to enter all predictors in one block was chosen as there were no specific hypotheses concerning which variables would be the strongest predictors.
Results
Sample characteristics
Table 1 presents means, standard deviations, and ranges for sample characteristics. There was a good range of scores on most variables.
Effects of noise on cognitive performance
Means and standard deviations under each noise condition and the results of the Noise x Sex ANOVAs and post hoc analyses are presented in Table 3.
Table 3.
Cognitive performance [mean, standard deviation (SD), analysis of variance (ANOVA) statistics, and linear contrasts] under quiet, urban, and social noise conditions
| Cognitive domains | Male Mean (SD) | Female Mean (SD) | ANOVA statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Numbers of participants (n) | Quiet | Urban | Social | Quiet | Urban | Social | Effects df | F | P | ηp2 |
| Psychomotor speed | ||||||||||
| Simple reaction timem22 f26 | 290.90 (50.66) | 328.54 (84.57) | 309.07 (64.00) | 283.86 (54.11) | 299.01 (82.43) | 293.78 (53.82) | Noise2,92 | 3.55 | 0.03 | 0.072 |
| Urban RT slower than quiet RT (t47=2.19, P=0.03) | ||||||||||
| Sex1,46 | 1.26 | 0.27 | 0.027 | |||||||
| Noise x Sex2,92 | 0.66 | 0.52 | 0.014 | |||||||
| Attention | ||||||||||
| Continuous performance test (CPT) D-primem20 f25 | 0.50 (0.16) | 0.48 (0.15) | 0.46 (0.10) | 0.46 (0.13) | 0.48 (0.17) | 0.52 (0.17) | Noise 2,86 | 0.24 | 0.79 | 0.005 |
| Sex1,86 | 2.14 | 0.12 | 0.047 | |||||||
| Noise x Sex2,92 | 0.04 | 0.85 | 0.001 | |||||||
| Executive function | ||||||||||
| Trail making B-A↓m22 f29 | 26.11 (16.13) | 27.04 (18.92) | 26.23 (14.88) | 29.25 (15.91) | 32.63 (20.82) | 34.17 (23.23) | Noise2,98 | 0.48 | 0.62 | 0.010 |
| Sex1,49 | 1.73 | 0.20 | 0.034 | |||||||
| Noise x Sex2,98 | 0.37 | 0.69 | 0.007 | |||||||
| Beads (60:40)m24 f30 | 7.25 (5.23) | 8.96 (5.37) | 7.58 (4.97) | 6.60 (4.93) | 7.40 (5.34) | 7.60 (5.51) | Noise2,104 | 3.93 | 0.02 | 0.070 |
| More Beads selected under urban noise compared to quiet condition (t53=2.83, P=0.007) | ||||||||||
| Sex1,52 | 0.30 | 0.59 | 0.006 | |||||||
| Noise x Sex2,104 | 1.56 | 0.22 | 0.029 | |||||||
| Verbal fluencym24 f30 | 49.50 (12.56) | 48.71 (10.39) | 47.25 (11.43) | 47.40 (10.81) | 46.47 (13.29) | 47.97 (11.56) | Noise 2,104 | 0.37 | 0.69 | 0.007 |
| Sex1,52 | 0.17 | 0.68 | 0.003 | |||||||
| Noise x Sex2,104 | 1.08 | 0.35 | 0.020 | |||||||
| Working memory | ||||||||||
| Letter number scoresm24 f30 | 16.75 (4.15) | 16.21 (3.40) | 15.58 (4.36) | 17.57 (3.39) | 16.07 (3.51) | 16.47 (3.65) | Noise2,104 | 6.55 | 0.002 | 0.112 |
| Less letter number strings completed under urban (t53=3.12, P=0.003) and social (t53=3.59. P=0.001) noise compared to quiet condition | ||||||||||
| Sex1,52 | 0.30 | 0.58 | 0.006 | |||||||
| Noise x Sex2,104 | 1.38 | 0.26 | 0.026 | |||||||
| Verbal learning and memory | ||||||||||
| Hopkins verbal learning test (HVLT): Total immediate recall m24 f30 | 26.33 (4.94) | 24.88 (4.15) | 25.42 (4.90) | 26.40 (4.94) | 25.33 (5.23) | 24.83 (4.56) | Noise2,104 | 3.25 | 0.04 | 0.059 |
| Less words recalled under urban (t53=2.17, P=0.03) and social (t53=2.29. P=0.03) noise compared to quiet condition | ||||||||||
| Sex1,52 | 0.00 | 0.99 | 0.000 | |||||||
| Noise x Sex2,104 | 0.43 | 0.65 | 0.008 | |||||||
| HVLT: Delayed recallm24 f30 | 9.63 (1.97) | 8.08 (2.57) | 8.17 (2.50) | 9.47 (1.74) | 8.40 (2.65) | 8.87 (2.47) | Noise2,102 | 10.89 | <0.0001 | 0.173 |
| Less words recalled under urban (t53=4.33, P<.0001) and social (t53=3.40. P=.001) noise compared to quiet condition | ||||||||||
| Sex1,52 | 0.28 | 0.60 | 0.005 | |||||||
| Noise x Sex2,104 | 1.07 | 0.35 | 0.020 | |||||||
| HVLT: Discrimination indexm24 f30 | 11.00 (1.18) | 9.54 (2.41) | 10.50 (1.32) | 11.00 (1.20) | 10.20 (1.73) | 10.77 (1.59) | Noise2,104 | 10.61 | <0.001 | 0.169 |
| Less words discriminated under urban noise compared to social noise (t53=2.79, P=0.007) and quiet (t53=4.19. P<0.001) | ||||||||||
| Sex1,52 | 0.84 | 0.36 | 0.016 | |||||||
| Noise x Sex2,104 | 0.88 | 0.42 | 0.017 | |||||||
Note, bold P levels indicates significance at P < 0.05
Significant main effects of Noise were found for psychomotor speed (SRT), executive function (Beads), working memory [Letter Number (LN) task], and all verbal learning and memory indices (HVLT). Post hoc analyses of the significant Noise effects demonstrated:
Slower SRT and more Beads selected (a cautious response style) under urban noise, relative to quiet noise,
Poorer LN scores and immediate and delayed verbal recall on HVLT in urban and social noise compared to the quiet condition, and
Poorer HVLT discriminative index (recognition) under urban noise compared to social noise and the quiet condition.
The main effect of Sex and all interactions involving Sex were nonsignificant.
Associations between individual difference variables and noise-induced cognitive change scores
Correlations between noise-induced cognitive changes and age, IQ, subjective noise sensitivity, sleep quality, extraversion, neuroticism, paranoia, depression, anxiety, stress, schizotypy and baseline (quiet condition) cognitive performance are provided in Table 4. Figures to aid with the interpretation of significant noise change correlations are provided in Appendices 2 and 3. Significant correlations are summarized later in this article.
Table 4.
Correlations between baseline and noise-change cognitive indices and individual difference variables
| Cognitive domains | Noise index | Age (df) | IQa(df) | NoiSeQ ↓ (df) | PSQI (df) ↑ | Personality | Paranoia (df) | Depression(df) | Anxiety(df) | Stress(df) | Schizotypy | Same task performance under no noise(df) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E(df) | N(df) | UnEx(df) | CogDis(df) | IntAn(df) | ImNon(df) | |||||||||||
| Psychomotor speed | ||||||||||||||||
| Simple reaction time ↓ | Quiet | 0.2146 | −0.34*46 | −0.1845 | 0.1545 | 0.0745 | 0.0045 | 0.0746 | 0.35*45 | 0.0745 | 0.0745 | 0.2446 | 0.1746 | 0.2746 | 0.0146 | — |
| Urban noise change | 0.0246 | 0.0346 | −0.0145 | −0.0345 | −0.0445 | −0.2245 | 0.44**46 | −0.0545 | 0.1845 | −0.0245 | 0.1046 | −0.2146 | −0.0946 | 0.2046 | −0.45**46 | |
| Social noise change | 0.0246 | −0.1746 | 0.1245 | −0.0945 | 0.1045 | −0.35*45 | 0.2746 | −0.1045 | 0.32*45 | −0.0845 | 0.0946 | −0.35*46 | −0.2246 | −0.2346 | −0.2646 | |
| Attention | ||||||||||||||||
| CPT D-prime | Quiet | −0.34*43 | 0.2843 | 0.1142 | 0.2642 | 0.1242 | 0.0142 | 0.30*43 | 0.0642 | −0.0542 | −0.2342 | −0.1143 | 0.0043 | −0.1143 | 0.1743 | — |
| Urban noise change | 0.1543 | 0.0443 | 0.0742 | −0.43**42 | −0.1342 | −0.0842 | −0.42**43 | −0.1542 | −0.0342 | 0.1942 | −0.1543 | −0.0743 | 0.0743 | −0.2643 | −0.59**43 | |
| Social noise change | −0.0343 | 0.12943 | −0.0442 | −0.1542 | −0.0142 | −0.0642 | −0.2943 | −0.1442 | −0.0942 | 0.1942 | −0.1743 | 0.0443 | −0.1943 | −0.1443 | −0.56**43 | |
| Executive function | ||||||||||||||||
| Trail making B-A↓ | Quiet | 0.2149 | −0.59**49 | −0.1148 | 0.2148 | −0.0748 | 0.1148 | 0.2349 | 0.0148 | −0.0448 | 0.0148 | 0.0749 | −0.0749 | 0.1349 | −0.0349 | — |
| Urban noise change | −0.0749 | 0.0349 | −0.30*48 | −0.0648 | 0.0348 | 0.1348 | −0.2049 | 0.0948 | 0.1048 | 0.1248 | 0.1249 | 0.32*49 | 0.1649 | 0.0145 | −0.38**49 | |
| Social noise change | −0.0449 | −0.0349 | −0.2044 | −0.1848 | −0.0648 | 0.1148 | −0.1449 | 0.1248 | −0.0648 | 0.0548 | −0.1249 | 0.2449 | 0.2149 | −0.1345 | −0.34*49 | |
| Beads (60:40) | Quiet | −0.39**52 | 0.39**52 | 0.0351 | 0.0751 | 0.0951 | 0.0551 | 0.28*52 | −0.0551 | −0.2351 | −0.1351 | −0.0452 | 0.1152 | −0.1152 | 0.1652 | — |
| Urban noise change | 0.2052 | −0.1552 | 0.0851 | 0.0551 | 0.1151 | −0.1351 | 0.1352 | 0.1151 | −0.0651 | −0.1351 | 0.0252 | −0.1252 | 0.1752 | −0.0652 | −0.2052 | |
| Social noise change | 0.27*52 | −0.1152 | 0.0151 | 0.1051 | −0.0251 | −0.0351 | 0.0452 | 0.1351 | −0.1051 | 0.1051 | 0.0452 | 0.0552 | 0.2252 | 0.0552 | −0.30*52 | |
| Verbal fluency | Quiet | 0.0952 | 0.1952 | −0.0051 | 0.9651 | 0.1251 | −0.0151 | 0.27*52 | 0.0051 | −0.1451 | 0.0551 | 0.37**52 | 0.1152 | 0.1052 | 0.37**52 | — |
| Urban noise change | −0.1652 | −0.1352 | 0.28*51 | −0.0951 | 0.0351 | −0.0951 | 0.0452 | −0.0651 | 0.0751 | −0.1652 | −0.1452 | −0.2252 | −0.0952 | −0.1452 | −0.2652 | |
| Social noise change | 0.1552 | −0.0752 | −0.28*51 | −0.1051 | −0.1251 | 0.1251 | −0.36**52 | −0.1051 | −0.0751 | −0.1651 | −0.1852 | 0.0852 | 0.0052 | −0.1052 | −0.36*52 | |
| Working memory | ||||||||||||||||
| Letter number scores | Quiet | −0.28*52 | 0.43**52 | 0.1351 | 0.0951 | 0.0151 | 0.0651 | 0.2152 | 0.0151 | −0.0051 | 0.1351 | −0.1051 | −0.0552 | −0.2552 | 0.0352 | — |
| Urban noise change | 0.0752 | −0.0652 | −0.2551 | 0.1251 | −0.0451 | 0.0351 | 0.0052 | 0.0851 | −0.0851 | −0.0751 | 0.2452 | 0.1652 | 0.2652 | 0.2052 | −0.45**52 | |
| Social noise change | 0.0952 | 0.1452 | −0.0251 | −0.1251 | 0.1551 | −0.0551 | −0.1552 | −0.1051 | −0.0551 | −0.30*51 | −0.0752 | 0.0452 | −0.0452 | −0.0752 | −0.2152 | |
| Verbal learning and memory | ||||||||||||||||
| HVLT: Total immediate recall | Quiet | −0.2152 | 0.48**52 | −0.0551 | 0.0451 | 0.0551 | −0.1551 | 0.1552 | 0.0851 | 0.0051 | −0.1751 | −0.0452 | −0.1752 | −0.1852 | −0.0452 | |
| Urban noise change | −0.0652 | 0.0052 | 0.30*51 | 0.0251 | 0.0151 | 0.1951 | 0.0352 | 0.0651 | 0.0751 | 0.1451 | 0.1252 | 0.1652 | −0.0752 | 0.2352 | −0.46**52 | |
| Social noise change | 0.0652 | −0.0752 | −0.0351 | 0.0551 | −0.2251 | 0.1851 | 0.0352 | −0.0251 | −0.1351 | 0.0251 | 0.0152 | 0.34*52 | 0.35*52 | 0.2752 | −0.47**52 | |
| HVLT: delayed recall | Quiet | −0.2452 | 0.45**54 | −0.0151 | −0.1551 | 0.0451 | −0.1051 | 0.0252 | 0.0451 | −0.1651 | −0.2151 | −0.2152 | −0.1452 | −0.2152 | −0.0552 | — |
| Urban noise change | 0.0252 | 0.1252 | 0.1551 | 0.2151 | −0.1151 | 0.1151 | 0.2352 | 0.1551 | 0.1151 | 0.0751 | 0.0552 | 0.0452 | 0.0052 | 0.0952 | 0.1552 | |
| Social noise change | 0.0652 | 0.1552 | −0.1251 | 0.43**51 | 0.1851 | −0.0451 | 0.27*52 | 0.1351 | 0.1451 | 0.0951 | 0.0552 | 0.1452 | −0.1152 | 0.0952 | 0.2252 | |
| HVLT: Discrimination index | Quiet | −0.29*52 | 0.31*52 | −0.1751 | 0.0751 | −0.0651 | −0.0951 | 0.2452 | 0.1451 | 0.0251 | −0.0951 | −0.0552 | 0.0652 | 0.0252 | −0.0652 | — |
| Urban noise change | −0.0052 | 0.2052 | 0.1751 | 0.1051 | 0.0351 | 0.1251 | 0.0652 | −0.0451 | −0.1151 | 0.0251 | 0.0052 | 0.0852 | −0.0652 | 0.0852 | 0.1852 | |
| Social noise change | 0.1852 | 0.0252 | 0.0052 | 0.1351 | 0.0151 | 0.0451 | −0.0352 | −0.0651 | −0.0851 | 0.1651 | −0.0352 | −0.1052 | −0.0552 | 0.0052 | −0.47**52 | |
*Indicates significance at P < 0.05; **Indicate significance at P < 0.001; ↓Indicates reversed interpretation; a higher baseline score for SRT and TM B-A = Poorer performance, a lower NoiSeQ score indicates greater noise sensitivity, and for SRT and TM B-A a positive change score = Poorer performance under noise and negative change score = Better performance under noise; ↑Higher scores indicate poorer overall sleep quality
Appendix 2.
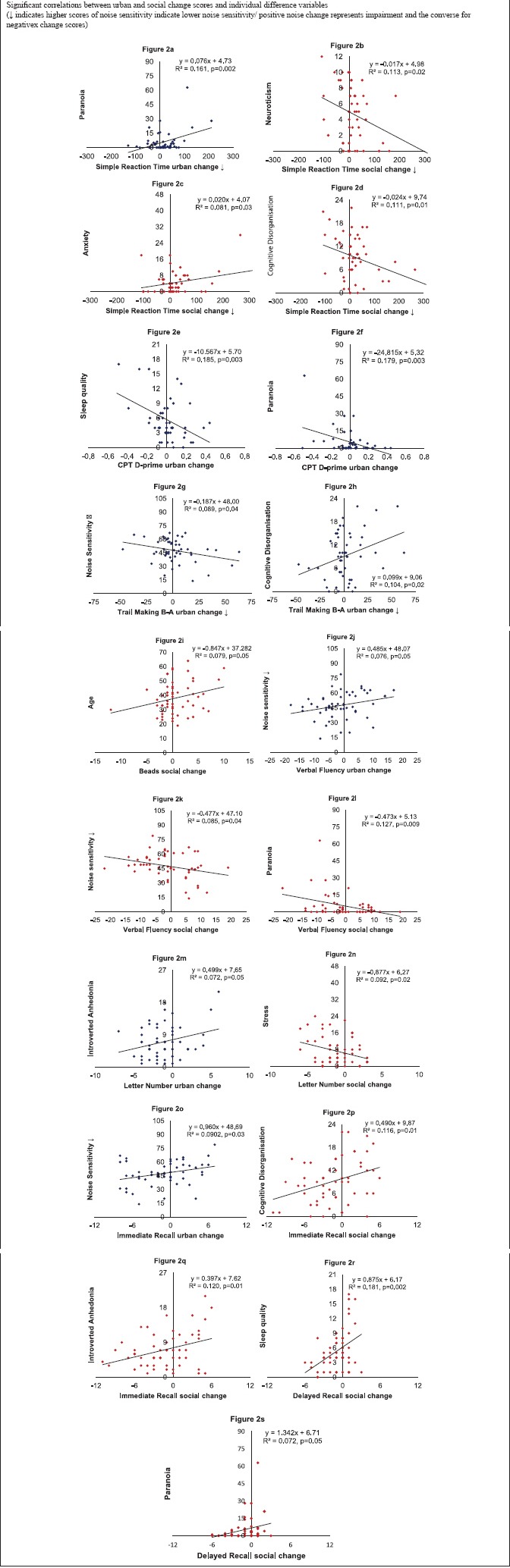
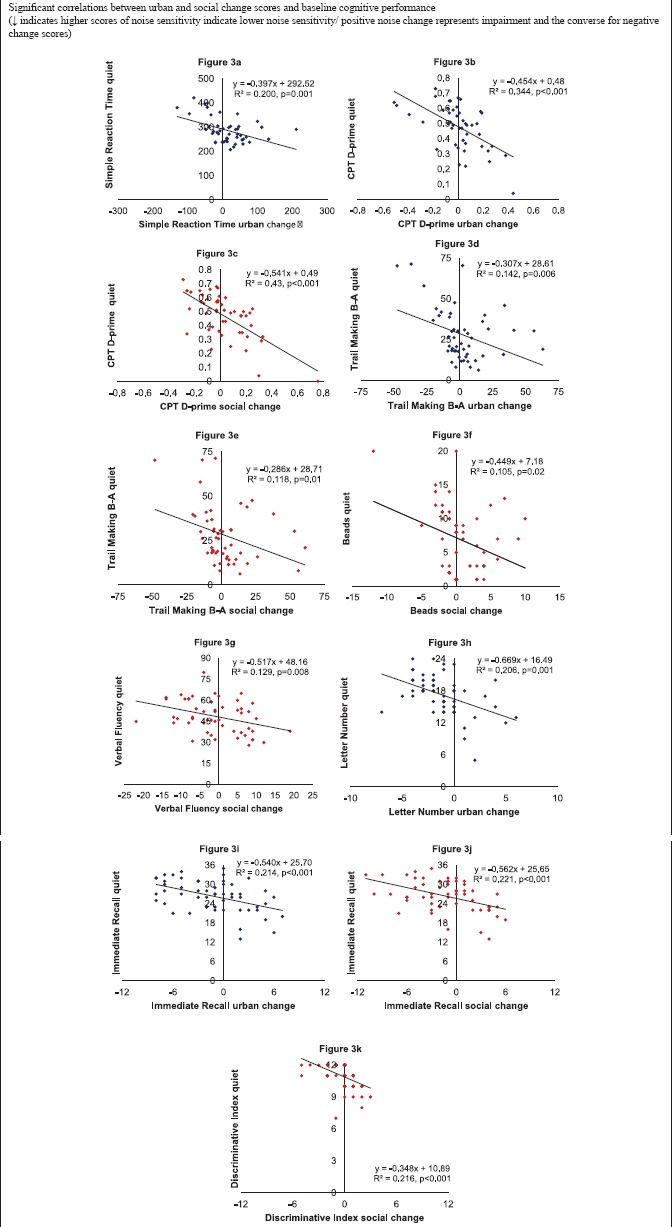
Appendix 3.
Psychomotor speed
Under quiet, slower SRT was associated with lower IQ and higher levels of depression. Slower SRT under urban noise was associated with higher levels of paranoia and faster baseline SRT. Slower SRT under social noise was associated with lower levels of neuroticism, higher levels of anxiety, and lower levels of CogDis.
Attention
Under quiet, better signal detection (CPT D-prime) was associated with younger age, higher levels of paranoia, and superior baseline CPT D-prime. Impairment under urban noise was associated with poorer sleep quality, higher levels of paranoia, and superior baseline CPT D-prime. Impairment under social noise was associated with superior baseline CPT D-prime.
Executive function
Under quiet, higher TM B-A was associated with lower IQ. Greater TM B-A under urban noise was associated with higher subjective noise sensitivity, higher CogDis, and a smaller baseline TM B-A. Greater TM B-A under social noise was associated with a smaller baseline TM B-A. More beads selected under quiet was associated with younger age, higher IQ, and higher levels of paranoia. No associations were present between the beads urban change score and any individual difference variable. More Beads selected under social noise was associated with older age, and fewer beads selected under quiet conditions. Under quiet, higher VF was associated with higher levels of paranoia. Impairment under urban noise was associated with greater subjective noise sensitivity. Impairment under social noise was associated with lower IntAn, greater subjective noise sensitivity, and superior baseline VF.
Working memory
Under quiet, superior LN was associated with younger age and higher IQ. Impairment under urban noise was associated with superior baseline LN. Impairment under social noise was associated with higher levels of stress.
Verbal Learning and memory
Under quiet, the following associations were present:
Superior immediate and delayed recall with higher IQ, and
Superior discrimination with younger age and higher IQ.
Impairment under urban noise was associated with
Greater subjective noise sensitivity and superior baseline performance (for immediate recall) and
No individual difference variables for delayed recall and the discriminative index.
Impairment under social noise was associated with
Higher stress, lower CogDis, lower IntAn, and superior baseline performance (for immediate recall),
Better sleep quality and lower paranoia (for delayed recall), and
Superior baseline performance for the discriminative index.
Information regarding the predictors entered in each regression analysis is provided in Table 5.
Table 5.
Predictors entered in each regression analysis and significant statistics
| Noise change index | Entered | Statistics for significant predictors |
|---|---|---|
| Psychomotor speed | ||
| Simple Reaction Time urban | Paranoia | beta=0.472, t45=4.15, P<0.002 |
| Baseline SRT | beta=0.480, t45=4.22, P<0.001 | |
| SRT social | Neuroticism | N/A |
| Anxiety | N/A | |
| CogDis | beta=−0.357, t44=−2.53, P=0.015 | |
| Attention | ||
| CPT D-prime urban | Sleep quality↑ | N/A |
| Paranoia | beta=−0.350, t42=−2.88, P=0.006 | |
| Baseline CPT D-prime | beta=−0.466, t42=−3.83, P<0.001 | |
| CPT D-prime social | Baseline CPT D-prime | N/A |
| Executive function | ||
| TMB B-A urban | Noise sensitivity↓ | beta=−0.344, t47=−2.72, P=0.009 |
| CogDis | N/A | |
| Baseline TM B-A | beta=−0.415, t47=−3.29, P=0.002 | |
| TM B-A social | Baseline TM B-A | N/A |
| Beads urban | N/A | |
| Beads social | Age | N/A |
| Baseline beads | beta=−0.32, t49=−2.40, P=0.02 | |
| VF urban | Noise sensitivity↓ | N/A |
| VF social | Noise sensitivity↓ | beta=−0.28, t49=−2.33, P=0.02 |
| Paranoia | beta=−0.28, t49=−2.21, P=0.03 | |
| Baseline VF | beta=−0.29, t49=2.33, P=0.02 | |
| Working memory | ||
| LN urban | IntAn | |
| Baseline LN | beta=−0.45, t52=−3.67, P=0.001 | |
| LN Social | Stress | N/A |
| Verbal learning and memory | ||
| Immediate recall urban | Noise sensitivity↓ | beta=0.278, t50=2.36, P=0.02 |
| Baseline immediate recall | beta=−0.471, t50=4.01, P<0.001 | |
| Immediate recall social | CogDis | beta=0.270, t51=2.26, P=0.03 |
| IntAn | N/A | |
| Baseline immediate recall | beta=−0.426, t51=−3.57, P=0.001 | |
| Delayed recall urban | N/A | |
| Delayed recall social | Sleep quality↑ | beta=0.425, t51=3.35, P=0.002 |
| Paranoia | N/A | |
| Discriminative index urban | N/A | |
| Discriminative index social | Baseline discriminative index | N/A |
↓Indicates reversed interpretation; a higher baseline score for SRT and TM B-A = Poorer performance, a lower NoiSeQ score indicates greater noise sensitivity, and for SRT and TM B-A a positive change score = Poorer performance under noise and negative change score = better performance under noise, ↑Higher score indicate poorer overall sleep quality
Psychomotor speed
Paranoia [Appendix 2 Figure a] and baseline SRT [Appendix 3 Figure a] were entered as predictors for the urban noise change score. Both variables predicted SRT urban noise change scores and explained 42.1% of the variance (F2,45 = 18.21, P < 0.001). Neuroticism [Appendix 2 Figure b], anxiety [Appendix 2 Figure c], and CogDis [Appendix 2 Figure d] were entered as predictors for the SRT social noise change score. Only CogDis predicted SRT social noise change and explained 10.8% of the variance (F2,44 = 6.42, P < 0.001).
Attention
Sleep [Appendix 2 Figure e], paranoia [Appendix 2 Figure f], and baseline CPT D-prime [Appendix 3 Figure b] were entered as predictors for the CPT D-prime urban noise change score. Paranoia and baseline CPT D-prime predicted CPT D-prime urban noise change scores and explained 44.3% of the variance (F2,42 = 16.71, P < 0.001). Only baseline CPT D-prime correlated with the CPT D-prime social noise change scores so no regression was run [Appendix 3 Figure c].
Executive function
TM B-A: Subjective noise sensitivity [Appendix 2 Figure g] and TM B-A baseline performance [Appendix 3 Figure d] were entered as predictors for the TM B-A urban noise change scores. Both were significant predictors and explained 26.0% of the variance (F2,47 = 8.24, P = 0.001). Only baseline TM B-A correlated with the TM B-A social noise change scores so no regression was run [Appendix 3 Figure e].
Beads: No variables correlated with the Beads urban noise change scores so no regression was run. Age [Appendix 2 Figure i] and baseline beads performance [Appendix 3 Figure f] were entered as predictors for the beads social noise change score. Only baseline performance predicted Beads social noise change scores and explained 10.5% of the variance (F1,49 = 12.18, P = 0.02).
VF: Only subjective noise sensitivity correlated with the VF urban noise change score so no regression was run [Appendix 2 Figure j]. Subjective noise sensitivity [Appendix 2 Figure k], paranoia [Appendix 2 Figure l], and baseline VF [Appendix 3 Figure g] were entered as predictors for the VF social change score. All three predicted VF social noise change scores and explained 28.6% of the variance (F3,49 = 6.53, P = 0.001).
Working memory
IntAn [Appendix 2 Figure m] and baseline LN [Appendix 3 Figure h] were entered as predictors for LN urban noise change scores. Baseline LN predicted LN urban noise change scores and explained 20.6% of the variance (F1,52 = 13.46, P = 0.001). Only stress correlated with the LN social noise change scores so no regression was run [Appendix 2 Figure n].
Verbal learning and memory
Immediate recall: Subjective noise sensitivity [Appendix 2 Figure o] and baseline immediate recall performance [Appendix 3 Figure i] were entered as predictors for immediate recall urban noise change. Both were significant predictors and explained 28.4% of the variance (F2,50 = 11.30, P < 0.001). CogDis [Appendix 2 Figure p], IntAn [Appendix 2 Figure q] and baseline immediate recall performance [Appendix 3 Figure j] were entered as predictors of immediate recall social noise change. Both were significant predictors and explained 29.3% of the variance (F2,51 = 10.55, P < 0.001).
Delayed recall: No variables were correlated with delayed recall change scores so no regression was run. Sleep quality [Appendix 2 Figure r] and paranoia [Appendix 2 Figure s] were entered as predictors for delayed recall social change. Sleep quality predicted delayed recall social noise change scores and explained 18.0% of the variance (F2,51 = 11.23, P = 0.002).
Discriminative index: No variables and only baseline discriminative index [Appendix 3 Figure k] correlated with urban and social discriminative index change, respectively, so no regressions were run.
Discussion
The present study demonstrated
Similar disruption by urban and social noise to working memory and verbal learning and memory, and slower psychomotor speed and a more cautious (Beads) response under urban noise;
No main effects of Sex or interactions with noise for any cognitive variables;
A variety of moderator variables [age, subjective noise sensitivity, sleep quality, neuroticism, paranoia, anxiety, stress, two specific dimensions of schizotypy [cognitive disorganization (CogDis) and introverted anhedonia (IntAn)], and baseline cognitive performance], and
Noise-induced change in most cognitive variables were predicted by, and had variance explained, by a combination of same task baseline cognitive performance along with paranoia, subjective noise sensitivity, CogDis and sleep quality.
More specifically, baseline cognitive performance predicted urban noise change (in psychomotor speed and attention in combination with paranoia, TM B-A and immediate recall in combination with subjective noise sensitivity, and working memory uniquely) and social noise change (in beads uniquely, VF in combination with paranoia and subjective noise sensitivity, and immediate recall in combination with CogDis). In addition, some individual differences uniquely predicted social noise-induced cognitive change (CogDis for SRT and sleep quality for delayed recall).
The regression analyses findings depict a picture of baseline cognitive performance, paranoia, subjective noise sensitivity, sleep quality, and CogDis being the most prominent moderators. Furthermore, it is clear that individual differences predict the extent of noise-induced changes, largely independent of whether a within-participant Noise effect is found across conditions. The exceptions were for LN (where only stress predicted greater LN disruption under social noise) and delayed recall: Two variables with large noise effects suggesting the noise-cognitive performance relationship in these tasks may be stable irrespective of individual differences.
The most robust associations were between higher baseline cognitive ability and greater noise-induced cognitive impairment. These associations occurred independent of IQ as no moderating influence of IQ was found despite the presence of correlations between higher IQ and cognitive performance under quiet conditions [faster psychomotor speed, better inhibition of prepotent response (TM B-A), more Beads selected, and superior working memory and verbal learning and memory]. One potential explanation for the moderating influence of baseline cognitive performance is that individuals with poorer baseline ability display reduced noise-induced changes in cognitive performance due to cognitive performance having less room to reduce. Individuals with lower baseline performance may counteract their lower cognitive reserves and the addition of stress by adopting a more effortful response strategy. An alternate postulate is that individuals with lower cognitive reserves adopt a strategy of focussing attention on the central aspect of the task, resulting in less noise-disruption and even facilitation as has been previously found in the Stroop task.[44]
Subjective noise sensitivity, paranoia, and sleep quality were the individual differences variables that predicted the most noise change indices. The majority of these associations were noise specific (as no associations were present under quiet conditions, Table 4). The present results partially supported the hypothesis that higher levels of these individual differences variables would be associated with greater noise-induced disruption (e.g., greater paranoia predicted greater urban noise-induced impairment in SRT and VF, and social noise-induced impairment in attention). However, there was also evidence of lower levels of these variables being associated with greater impairment under noise (e.g., poor sleep quality and higher levels of paranoia predicted less impairment of delayed recall under social noise). The unclear picture of the moderating influence of subjective noise sensitivity on noise-induced cognitive change is in accordance with inconsistent results in the literature reviewed in.[2]
CogDis predicted social noise-induced facilitation to SRT and urban noise-induced facilitation to immediate recall, and was associated with disruption to TM B-A. As individuals with CogDis are purported to have lower attention capacity,[35] the facilitation under social noise could be representative of more effortful response style. While it difficult to marry the TM B-A finding with this explanation, it should be noted that TM B-A does not provide us with information regarding a general slowing or quickening of response under noise, only a smaller or larger difference between part A and part B.
Surprisingly, neuroticism and intro-/extraversion did not explain any variance in performance under noise; a finding that contradicts the conclusions drawn in a recent review that these two variables may represent the most consistent moderators.[2] This discrepancy could be due to previous studies’ use of more complex environmental (i.e., multiple urban and social stimuli layered) noise stimuli and different tasks (i.e., logical reasoning) when exploring the moderating role of these variables, e.g.,[45] or the lack of testing of more influential predictors. As there is a close link between neuroticism and trait anxiety, the lack of significant associations between neurotic traits and noise change scores is in agreement with the lack of significant association with anxiety.
Strengths of the present study included the use of a within-participant design, a comprehensive cognitive battery, and exploration of a wide range of individual difference variables. By employing this design we were able to delineate the pattern of noise effects on different cognitive domains and the moderators of this relationship. A potential limitation to the interpretation of results is possible fatigue or habituation to the noise in tasks at the end of the cognitive battery,[46] although efforts were taken to control for this by counterbalancing the order of task presentation. Future studies should recruit a larger sample in order to examine the duration of noise exposure as a confound. Another limitation was that the range of some individual difference variables (i.e., paranoia, stress, and anxiety) was not representative of the higher scores (although this is to be expected in healthy samples). In particular, although robust associations were found for paranoia, replication is needed with a more diverse range of scores. A final limitation was that individual difference variables were only assessed on one occasion and may have fluctuated for sleep quality or variables known to be affected by state (i.e., levels of depression, anxiety, and stress). Future studies could assess such variables at each session.
Conclusion
To conclude, this study replicated previous findings of environmental noise-induced disruption to working memory and episodic memory tasks, demonstrated a noise effect for the beads probabilistic reasoning task and psychomotor speed, and added to the field by elucidating the predictors of this relationship. A novel finding was that same task baseline performance is a robust moderator of noise effects and accounted for variance in performance in combination with other individual differences variables (most notably paranoia, subjective noise sensitivity, sleep, and CogDis). The present results demonstrate that the relationships between noise and cognitive performance are complex, and support previous recommendations[2] for consideration of individual differences, in particular baseline cognitive capacity, as potential moderators. As noise-induced changes to cognitive performance are moderated by individual difference variables (e.g., poor sleep quality and paranoia) that are elevated in clinical populations, it may be fruitful to examine the relationship of environmental noise and cognitive performance in individuals diagnosed with psychiatric disorders such as schizophrenia, depression, and post-traumatic stress disorder.
Financial support and sponsorship
Medical Research Council UK and King's College London Studentship (for BW).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Szalma JL, Hancock PA. Noise effects on human performance: A meta-analytic synthesis. Psychol Bull. 2011;137:682–707. doi: 10.1037/a0023987. [DOI] [PubMed] [Google Scholar]
- 2.Wright B, Peters E, Ettinger U, Kuipers E, Kumari V. Understanding noise stress-induced cognitive impairment in healthy adults and its implications for schizophrenia. Noise Health. 2014;16:166–76. doi: 10.4103/1463-1741.134917. [DOI] [PubMed] [Google Scholar]
- 3.Tzivian L, Winkler A, Dlugaj M, Schikowski T, Vossoughi M, Fuks K, et al. Effect of long-term outdoor air pollution and noise on cognitive and psychological functions in adults. Int J Hyg Environ Health. 2015;218:1–11. doi: 10.1016/j.ijheh.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Gulian E, Thomas JR. The effects of noise, cognitive set and gender on mental arithmetic performance. Br J Psychology. 1986;77:503–11. [Google Scholar]
- 5.Boman E. The effects of noise and gender on children's episodic and semantic memory. Scand J Psychol. 2004;45:407–16. doi: 10.1111/j.1467-9450.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 6.Schreckenberg D, Griefahn B, Meis M. The associations between noise sensitivity, reported physical and mental health, perceived environmental quality, and noise annoyance. Noise Health. 2010;12:7–16. doi: 10.4103/1463-1741.59995. [DOI] [PubMed] [Google Scholar]
- 7.Babisch W. Stress hormones in the research on cardiovascular effects of noise. Noise Health. 2003;5:1–11. [PubMed] [Google Scholar]
- 8.Feingold A. Gender differences in personality: A meta-analysis. Psychol Bull. 1994;116:429–56. doi: 10.1037/0033-2909.116.3.429. [DOI] [PubMed] [Google Scholar]
- 9.Jelínková Z, Picek M, Hyncica V. Psychophysiological factors determining responses to noise load. Act Nerv Super (Praha) 1988;30:146–7. [PubMed] [Google Scholar]
- 10.Dorniè S, Laaksonen T, Ekehammar B. Noise Sensitivity: General Self-Reports vs. Noise Effect in Laboratory Situations: Department of Psychology. Stockholm University. 1990:295–301. [Google Scholar]
- 11.Ellermeier W, Zimmer K. Individual differences in susceptibility to the “irrelevant speech effect”. J Acoust Soc Am. 1997;102:2191–9. doi: 10.1121/1.419596. [DOI] [PubMed] [Google Scholar]
- 12.Gaillard AW. Concentration, stress and performance. Performance under stress. 2008:59–75. [Google Scholar]
- 13.Humphreys MS, Revelle W. Personality, motivation, and performance: A theory of the relationship between individual differences and information processing. Psychol Rev. 1984;91:153–84. [PubMed] [Google Scholar]
- 14.Ohrström E. Sleep disturbances caused by road traffic noise — Studies in laboratory and field. Noise Health. 2000;2:71–8. [PubMed] [Google Scholar]
- 15.Ohrström E. Sleep studies before and after-results and comparison of different methods. Noise Health. 2002;4:65–7. [PubMed] [Google Scholar]
- 16.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–97. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 17.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 18.Baddeley A. A 3 Min reasoning test based on grammatical transformation. Psychon Sci. 1968;10:341–2. [Google Scholar]
- 19.Belojevic G, Jakovljevic B, Slepcevic V. Noise and mental performance: Personality attributes and noise sensitivity. Noise Health. 2003;6:77–89. [PubMed] [Google Scholar]
- 20.Eysenck MW, Graydon J. Susceptibility to distraction as a function of personality. Pers Individ Dif. 1989;10:681–7. [Google Scholar]
- 21.Öhrström E, Björkman M, Rylander R. Noise annoyance with regard to neurophysiological sensitivity, subjective noise sensitivity and personality variables. Psychol Med. 1988;18:605–13. doi: 10.1017/s003329170000828x. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger U, Corr PJ. The Frequency Accrual Speed Test (FAST): Psychometric intelligence and personality correlates. Eur J Pers. 2001;15:143–52. [Google Scholar]
- 23.Ballard JC. Assessing attention: Comparison of response-inhibition and traditional continuous performance tests. J Clin Exp Neuropsychol. 2001;23:331–50. doi: 10.1076/jcen.23.3.331.1188. [DOI] [PubMed] [Google Scholar]
- 24.Van Kamp I, Davies H. Environmental noise and mental health: Five year review and future directions. Noise as a public health problem Proceedings of 9th Congress of the International Commission on the Biological Effects of Noise (ICBEN); 2008 [Google Scholar]
- 25.Lincoln T, Peter N, Schäfer M, Moritz S. Impact of stress on paranoia: An experimental investigation of moderators and mediators. Psychol Med. 2009;39:1129–39. doi: 10.1017/S0033291708004613. [DOI] [PubMed] [Google Scholar]
- 26.Smith NT, Lenzenweger MF. Increased stress responsivity in schizotypy leads to diminished spatial working memory performance. Personal Disord. 2013;4:324–31. doi: 10.1037/per0000014. [DOI] [PubMed] [Google Scholar]
- 27.Peters E, Ward T, Jackson M, Morgan C, Charalambides M, McGuire P, et al. Clinical, socio-demographic and psychological characteristics in individuals with persistent psychotic experiences with and without a ‘need-for-care’. World Psychiatry. 2016;15:41–52. doi: 10.1002/wps.20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler D. Manual for the Wechsler abbreviated intelligence scale (WASI) San Antonio, Tex: The Psychological Corporation; 1999. [Google Scholar]
- 29.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 30.Schutte M, Marks A, Wenning E, Griefahn B. The development of the noise sensitivity questionnaire. Noise Health. 2007;9:15–24. doi: 10.4103/1463-1741.34700. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Eysenck SB, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Pers Individ Dif. 1985;6:21–9. [Google Scholar]
- 33.Freeman D, Garety PA, Bebbington PE, Smith B, Rollinson R, Fowler DG, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186:427–35. doi: 10.1192/bjp.186.5.427. [DOI] [PubMed] [Google Scholar]
- 34.Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335–43. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 35.Mason O, Claridge G. The Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE): Further description and extended norms. Schizophr Res. 2006;82:203–11. doi: 10.1016/j.schres.2005.12.845. [DOI] [PubMed] [Google Scholar]
- 36.Hygge S. Classroom experiments on the effects of different noise sources and sound levels on long-term recall and recognition in children. Appl Cogn Psychol. 2003;17:895–914. [Google Scholar]
- 37.Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version (CPT-IP): I New findings about sustained attention in normal families. Psychiat Res. 1988;26:223–8. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- 38.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 39.Dudley R, John CH, Young A, Over D. The effect of self-referent material on the reasoning of people with delusions. Br J Clin Psychol. 1997;36:575–84. doi: 10.1111/j.2044-8260.1997.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 40.Benton AL, Hamsher KD, Sivan AB. Iowa city, la: AJA Association; 1994. Multilingual Aphasia Examination: Manual of Instructions. [Google Scholar]
- 41.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–65. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 42.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test-revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 43.Lunt L, Bramham J, Morris RG, Bullock PR, Selway RP, Xenitidis K, David AS. Prefrontal cortex dysfunction and ‘jumping to conclusions’: bias or deficit? J Neuropsychol. 2012;6:65–78. doi: 10.1111/j.1748-6653.2011.02005.x. [DOI] [PubMed] [Google Scholar]
- 44.Chajut E, Algom D. Selective attention improves under stress: Implications for theories of social cognition. J Pers Soc Psychol. 2003;85:231–48. doi: 10.1037/0022-3514.85.2.231. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds J, McClelland A, Furnham A. An investigation of cognitive test performance across conditions of silence, background noise and music as a function of neuroticism. Anxiety Stress Coping. 2014;27:401–21. doi: 10.1080/10615806.2013.864388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith A, Waters B, Jones H. Effects of prior exposure to office noise and music on aspects of working memory. Noise Health. 2010;12:235–43. doi: 10.4103/1463-1741.70502. [DOI] [PubMed] [Google Scholar]


