Abstract
The purpose of this study was to investigate the acute physiological stress response to an emergency alarm and mobilization during the day and at night. Sixteen healthy males aged 25 ± 4 years (mean ± SD) spent four consecutive days and nights in a sleep laboratory. This research used a within-participants design with repeated measures for time, alarm condition (alarm or control), and trial (day or night). When an alarm sounded, participants were required to mobilize immediately. Saliva samples for cortisol analysis were collected 0 min, 15 min, 30 min, 45 min, 60 min, 90 min, and 120 min after mobilization, and at corresponding times in control conditions. Heart rate was measured continuously throughout the study. Heart rate was higher in the day (F20,442 = 9.140, P < 0.001) and night (F23,459 = 8.356, P < 0.001) alarm conditions compared to the respective control conditions. There was no difference in saliva cortisol between day alarm and day control conditions. Cortisol was higher (F6,183 = 2.450, P < 0.001) following the night alarm and mobilization compared to the night control condition. The magnitude of difference in cortisol between night control and night alarm conditions was greater (F6,174 = 4.071, P < 0.001) than the magnitude of difference between the day control and day alarm conditions. The augmented heart rate response to the day and night alarms supports previous observations in field settings. Variations in the cortisol responses between conditions across the day and night may relate to differences in participants’ ability to interpret the alarm when sleeping versus when awake.
Keywords: Abrupt awakening, emergency services, firefighters, hypothalamus-pituitary-adrenal axis, sympatho-adrenal medullary system
Introduction
Emergency alarms may occur at any time of the day or night and act as precursors to potentially traumatic events.[1,2] The purpose of an emergency alarm is to prepare emergency personnel (e.g. police, firefighters, and paramedics) to respond to a critical incident, such as a fire or rescue task.[3,4] For example, when an in-station fire alarm sounds, firefighters must immediately cease their current activities, change into their protective clothing, and be out of the station and fully functioning within 90 s of the alarm.[5,6] Consequently, exposure to these alarms and the subsequent mobilization is an unavoidable occupational challenge for emergency personnel.[5]
The emergency alarm has been implicated in the high number of adverse cardiovascular events and coronary heart disease related deaths observed in United States firefighters.[4,7,8,9] Geibe and colleagues[7] found that 18% of non-fatal coronary heart disease related events occurred during the alarm response, while Holder et al.[8] observed a 6.4-fold increase in the relative risk of an adverse cardiovascular event during the alarm response compared to non-emergency duties. It has been hypothesized that the sudden nature of an emergency alarm may evoke a physiological stress response.[2,3,10] Despite assertions about the “stress” of an alarm,[3,10,11,12] previous research has limited its focus to the sympatho-adrenal medullary (SAM) system and not the hypothalamus-pituitary-adrenal (HPA) axis.[3,10,11,12] These studies observed increases in heart rate of between ~20 beats·min-1 and 66 beats·min-1 in response to an emergency alarm.[3,10,11,12] However, these studies either did not test for statistical significance, or they only comprised the aggregate response to multiple alarms.
Although several studies have investigated the heart rate response to emergency alarms, these studies have not compared the heart rate response to a day alarm to when woken by an alarm stimulus at night.[3,10,11,12,13] Furthermore, no study to date has investigated the differences in HPA axis response between the day and the night. This is important because firefighters display a circadian distribution of coronary heart disease related deaths, which differs to that of the general population, with a peak in these deaths is between 1200 h and 2400 h in firefighters and 0600 h and 1200 h in the general population.[14] This study aims to establish whether there is a SAM system or HPA axis response to an emergency alarm and mobilization during the day, or when woken at night, and formally compare the SAM system and HPA axis response to an emergency alarm and mobilization during the day to the response evoked by an emergency alarm and mobilization at night.
Methods
Participants
Sixteen healthy males aged 25 ± 4 years (mean ± SD), with an average mass of 71.9 ± 7.7 kg, and body mass index of 23.1 ± 2.2 kg·m-2 were recruited for this study. Potential participants were only recruited if they met the inclusion criteria (male, aged 18-40 years, non-smokers, non-shift workers, no transmeridian travel within the month prior to participation, no heart conditions, not currently on any medication). Written informed consent was given by participants prior to the study, which was approved by the appropriate Human Research Ethics Committees.
Study protocol
Participants spent four consecutive days and nights in a sleep laboratory. Upon arrival, participants underwent familiarization with the sampling methods and study protocol. They were then informed that they may receive an alarm at any time of the day or night on Day 1, 3, or 4 and that only one alarm would sound per 24 h. That afternoon, participants received the “day” alarm stimulus at 1558 h from a 105 dB, 15-W megaphone equipped with siren switch (ER-12215S, TOA megaphone, Kobe, Japan). This stimulus was chosen because the audible component of an emergency alarm is typically 95-105 dB(A).[15] Upon hearing the alarm, participants were required to immediately mobilize. This required each to don their shoes and their protective jacket and briskly walk approximately 15-20 m to the testing room. Saliva samples were collected at 1600 h (T = 0 min) and at 15 min, 30 min, 45 min, 60 min, 90 min, and 120 min after mobilization. On the second day, time-matched samples were collected as the control day, where no alarm sounded and participants were not required to mobilize. The alarm and control conditions were time-matched to account for the circadian variation of heart rate[16] and cortisol.[17] The 1558 h alarm was chosen as this falls within the period when there is the highest number of emergency alarms and highest number of coronary heart disease related deaths in emergency service workers.[14] The 120-min sampling window was chosen to gain a comprehensive representation of the cortisol response to the alarm and mobilisation.[18]
On the third night, eight of the 16 participants were woken at 0358 h by the auditory emergency alarm stimulus, followed by the control condition on the fourth night. This time was chosen because it was exactly 12 h out of phase with the day alarm stimulus. As participants did not return to bed following the night testing (0358-0600 h), a crossover design was used for the third and fourth days to minimize the effects of the sleep deprivation from the third night that could have on the cortisol response on the fourth night.[19] Therefore, the other eight participants were exposed to the control condition on the third night and were woken by the auditory emergency alarm on the fourth night. As with the day conditions, the alarm and control conditions were time-matched to account for the circadian variation of heart rate[16] and cortisol.[17] Consistent with the day alarm condition, the night alarm stimulus required mobilization to the testing room. On the control night, the lights in participants’ bedrooms were turned on externally, and participants woken gently by a knock on their door from the researchers. No mobilization was required for this condition; quiet verbal instructions were provided to the participants, asking them to stay in bed but slowly come to a seated position, where they provided their first saliva sample. Participants then slowly walked 3 m to sit at a desk in their room to complete the remaining samples time-matched to the night alarm condition. Baseline (1400 h) saliva samples were also collected to test for drift across the days as a result of sleep deprivation or the stress associated with the study.
Participants’ food and fluid intake was standardized and controlled across the study. Participants did not consume any food during the 90 min prior to any saliva sample collection or any fluid for 10 min prior to collection, to reduce possible confounding effects on salivary cortisol levels.[20] In addition, participants were not permitted any caffeinated beverages, sunlight, or physical exertion throughout the study in an attempt to regulate emotions, arousal and stress levels.
Experimental procedures
Heart rate
Heart rate was recorded continuously for the duration of the study using Polar Team2 Pro heart rate monitors (Polar Electro Oy, Kempele, Finland) sampling at 5-s intervals, and processed using Polar Team2 Pro System software (Polar Electro Oy, Kempele, Finland). Heart rate data from the day and night alarm conditions were analyzed as 15-s averages from 2 min prior to the time of the alarm until 2 min following the completion of mobilization. This collection period aligns with that observed in the literature, which suggests that heart rate rises within 90 s of an alarm,[3,10,11,12] and with operational contexts, which require firefighters to be mobilized and out of the station within 90 s.[6] Control conditions were time-matched to the alarm conditions to account for the circadian variation in heart rate.[16]
Salivary cortisol
Saliva samples were collected using Salivettes (Sarstedt, Nurnbrect, Germany). Participants were instructed to roll the swabs around in their mouth for 2 min. Samples were then immediately placed on ice until the testing block was completed. Thereafter, samples were centrifuged at 5000 rev. min-1 for 5 min, and stored at –80°C until sample analysis was conducted.
Saliva concentrations of cortisol were analyzed in duplicate using an enzyme-linked immunosorbent assay (ELISA; SLV-2930, DRG International, Inc., Hamburg) according to the manufacturer's instructions. To reduce error variance, all samples from a single participant were analyzed in a single assay. The mean intra-assay coefficient of variation was 8.0% and the mean inter-assay coefficient of variation was 10.9%.
Statistical analysis
Normality of saliva cortisol concentrations and heart rate data were confirmed using the Kolmogorov-Smirnov test.[21] Baseline heart rate and cortisol data were analyzed using two-way analysis of variance (ANOVA) with day (day vs. night) and condition (alarm vs. control) as repeat measures factors were used to test for drift across the days as a result of sleep deprivation or the stress associated with the study. Two-way ANOVA were performed with condition and sample time as the repeated measures factors for heart rate and cortisol. Where appropriate, all significant interactions between alarm and time were analyzed post hoc using paired t-tests, with Bonferroni correction, to identify where the significant differences lay.[21] Pre-treatment heart rate was calculated as the mean heart rate 15-s prior to the onset of the alarm and mobilization.
Heart rate reactivity was calculated as the difference between mean pre-treatment heart rate and the peak heart rate during mobilization, or the peak during the same period in the time-matched control condition. Cortisol reactivity was calculated as the difference between the saliva cortisol concentration at the end of mobilization (T = 0 min) and the highest saliva cortisol concentration during the 2 h following the alarm and mobilization. Cortisol reactivity was also assessed as area under the curve with respect to increase (AUCi) for each participant using the trapezoidal method.[22] Normality of peak heart rate, peak cortisol, reactivity of each and AUCi of cortisol were confirmed by non-significant Kolmogorov-Smirnov tests.[21] These data were analyzed using one-way ANOVAs.
Due to the circadian variation of both heart rate[16] and cortisol,[17] the absolute differences between day and night conditions could not be compared. Instead, the magnitude of difference in heart rate and salivary cortisol between the alarm and control conditions during the day and during the night were compared. Since heart rate and cortisol data were both normally distributed, two-way ANOVAs, with trial (magnitude of difference between the day conditions or magnitude of difference between the night conditions) and time as the repeat measures factors, were conducted. All data were presented as mean ± SEM with 95% confidence intervals (CI) unless otherwise indicated. The accepted level of significance was P < 0.05. Outliers were removed if they were two or more standard deviations from the mean.
Results
Heart rate
Day heart rate
Day alarm mobilization took participants an average of 68 ± 3 s to complete [Figure 1a]. Day alarm heart rate data were shown in Figure 1a and Table 1. There was a significant interaction between condition and time for average heart rate (F20,442 = 9.140, P < 0.001). Furthermore, peak heart rate (F1,23 = 34.216; 95% CI 19 to 43; P < 0.001) and reactivity (F1,21 = 44.192, P < 0.001) were higher in the day alarm condition compared to the day control condition [Table 1].
Figure 1.
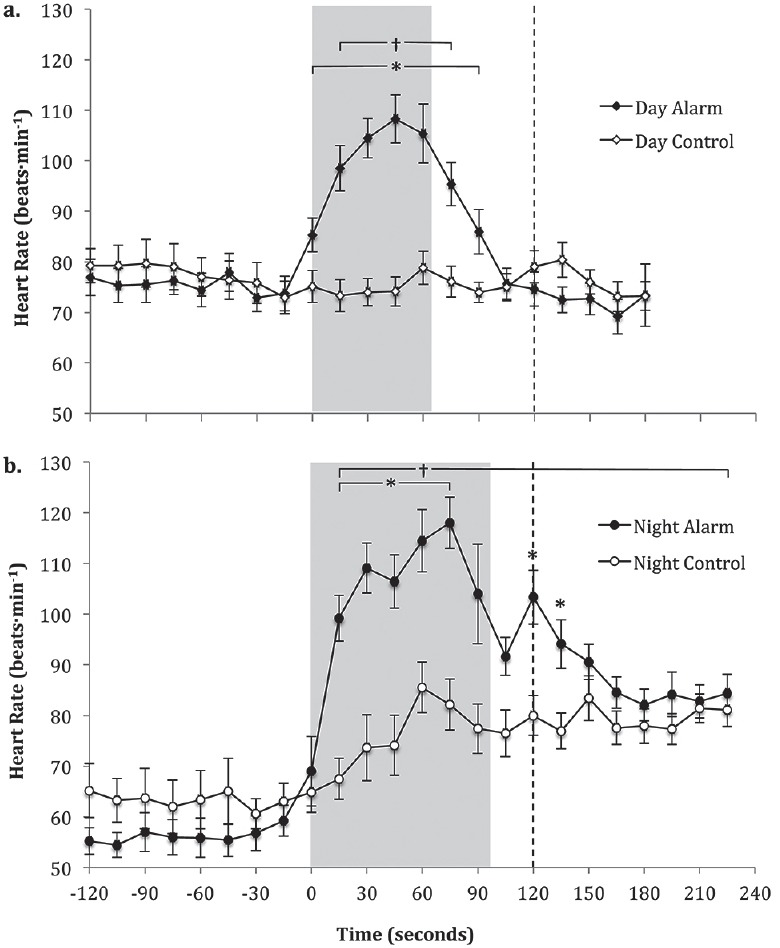
Heart rate 2 min pre alarm to 2 min post alarm and mobilization in the day alarm and day control conditions (a) and night alarm and night control conditions (b)
Note: An original figure. Data displayed as mean ± SEM. Time 0 = 15 s average from 0 s to 15 s post alarm; * significant difference (P < 0.05) between alarm and control conditions; † alarm condition significantly different (P < 0.001) compared to 15 s pre alarm; shaded area denotes average mobilization period; ---- start of saliva sampling
Table 1.
Day alarm compared to day control and night alarm compared to night control for pre-treatment heart rate, peak heart rate, and heart rate reactivity measures
| Heart rate variable | Day | Night | ||
|---|---|---|---|---|
| Alarm | Control | Alarm | Control | |
| Pre-treatment (beats·min-1) | 74±3 | 73±3 | 59±3 | 63±4 |
| Peak during mobilization (beats·min-1) | 111±5 | 80±3* | 122±5 | 93±6† |
| Reactivity (beats·min-1) | 38±5 | 7±2* | 56±4 | 30±5† |
Note: Data presented as mean ± SEM; * denotes day control significantly different to day alarm condition; † denotes night control significantly different to night alarm condition; day conditions N = 12, night conditions: N = 10
Night heart rate
Night alarm mobilization took participants 97 ± 4 s to complete [Figure 1b]. Night alarm heart rate data were shown in Figure 1b and Table 1. There was a significant interaction between condition and time for average heart rate (F23,459 = 8.356, P < 0.001). Peak heart rate (95% CI 17 to 41; F1,23 = 14.401, P = 0.001) and reactivity (F1,20 = 14.589, P = 0.001) were shown to be higher in the night alarm condition compared to the night control condition [Table 1].
Day alarm compared to night alarm heart rate
There was a significant main effect for time (F23,320 = 9.953, P < 0.001) on absolute average heart rate difference; however, no main effect for trial (night or day; F1,320 = 0.314, P = 0.576) was found. There was also no interaction effect for trial and time (F20,320 = 1.044, P = 0.409) indicating that the heart rate response did not differ significantly between day and night.
Cortisol
There were no significant changes in baseline (1400 h) saliva cortisol across the four days (P = 0.333; data not shown). Day and night cortisol data are shown in Figure 2 and Table 2.
Figure 2.
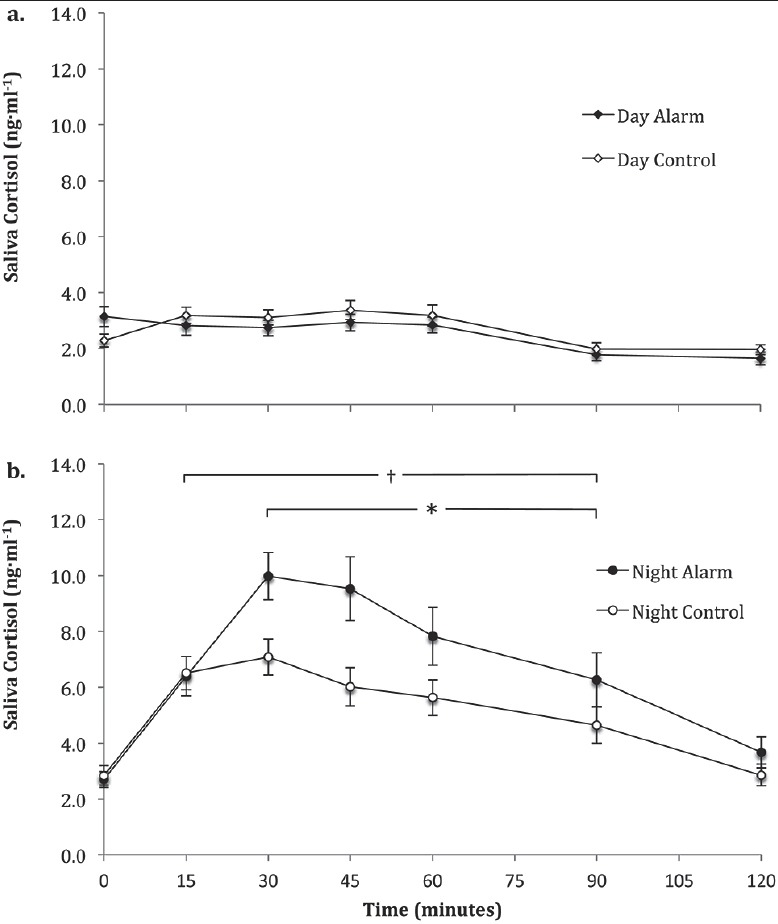
Saliva cortisol concentrations during 2 h post alarm and mobilization in the day alarm and time-matched day control condition (a) and night alarm and time-matched night control condition (b)
Note: An original figure. Data presented as mean ± SEM. * significant (P < 0.05) difference between the night alarm and control; † significantly (P < 0.05) different to 0 min in the night alarm condition
Table 2.
Day alarm compared to day control and night alarm compared to night control for initial cortisol, peak cortisol, cortisol reactivity and cortisol area under the curve with respect to increase measures
| Cortisol variable | Day | Night | ||
|---|---|---|---|---|
| Alarm | Control | Alarm | Control | |
| Initial (T = 0 min) (ng·mL-1) | 3.14±0.36 | 2.44±0.18 | 2.70±0.29 | 2.85±0.35 |
| Peak (ng·mL-1) | 4.00±0.28 | 3.72±0.35 | 11.48±0.90 | 8.73±0.50* |
| Reactivity (ng·mL-1) | 0.86±0.24 | 1.28±0.29 | 8.78±0.93 | 5.88±0.59* |
| AUCi (ng·mL·min-1) | –52.0±43.5 | 38.4±22.0 | 506.8±76.3 | 309.5±52.8* |
Note: Data are presented as mean ± SEM, * denotes night control significantly different (P < 0.05) to night alarm; AUCi denotes area under curve with respect to increase; N = 15
Day salivary cortisol
There was no main effect for condition on day saliva cortisol concentration (P = 0.409; Figure 2a). However, there was a significant main effect for time (F6,185 = 12.87, P < 0.001). There was no interaction between condition and time (P = 0.217) on saliva cortisol concentration during the day [Figure 2a]. Furthermore, there was no significant difference in peak saliva cortisol concentrations (P = 0.525), saliva cortisol reactivity (P = 0.266), or cortisol AUCi (P = 0.108) between the day alarm and day control conditions [Table 2].
Night saliva cortisol concentrations
There was a significant interaction (F6,183 = 2.450, P = 0.027) between condition and time for saliva cortisol concentrations at night [Figure 2b]. Peak saliva cortisol concentrations (F1,30 = 7.176, P = 0.012), cortisol reactivity (F1,30 = 6.915, P = 0.013) and AUCi (F1,15 = 5.666, P = 0.031) were higher in the night alarm condition compared to the night control condition [Table 2].
Day alarm compared to night alarm cortisol concentrations
There was a significant interaction (F6,174 = 4.071, P < 0.001) between trial and time. Post hoc analysis showed that the magnitude of difference in saliva cortisol concentrations was higher (P ≤ 0.011) from 30 min to 120 min post-mobilization in the night trial compared to the day trial.
Discussion
The impacts of a single emergency alarm and mobilization during the day and at night on both the SAM system and HPA axis were investigated in this study. A SAM system stress response to the emergency alarm and mobilization was evident during the day and when woken at night. However, there was no difference in the magnitude of the SAM system response to an emergency alarm and mobilization during the day compared to when woken at night. The results also showed that there was a HPA axis stress response to an emergency alarm and mobilization when woken at night, but no HPA axis response during the day.
Heart rate was significantly elevated following the day and night alarm stimuli and during mobilization. These findings are comparable to previous results in field research, where multiple alarms were analyzed together and/or not tested for statistical significance.[3,10,11,12,13] For example, previous research observed a mean increase of 47 beats·min-1 and 61 beats·min-1, respectively, within 30 s of alarm stimuli across a 24-h period in professional firefighters.[3,10] Similar increases of ~20 beats·min-1 were observed in Singaporean firefighters;[12] however, this study analyzed multiple alarms and did not differentiate between day and night shifts. Nevertheless, the increase in heart rate in response to both the day and night emergency alarm and mobilizations were similar to those observed in other occupational alarm studies,[3,10,11,12,13,23] and suggests that there is a SAM system stress response to a single emergency alarm and mobilization occurring during the day and when woken at night.
Due to the preliminary nature of this work, it is unclear as to why the magnitude of the heart rate response to the emergency alarm and mobilizations did not differ between the day and the night. It is possible that the heart rate response caused by an emergency alarm and mobilization masks any rise caused by the abrupt change in posture in the night condition.[24] Due to the standard operational coupling of an emergency alarm and mobilization, this study was not concerned with whether it was the alarm or the subsequent mobilization that resulted in a physiological stress response. Nevertheless, future research could endeavor to ascertain the relative contribution of the alarm and subsequent physical movement to the stress response exhibited when an alarm and mobilization occur, and the role of posture in the heart rate response.
The results of this study demonstrated no significant change in saliva cortisol concentrations following the day alarm and mobilization suggesting that it did not evoke a HPA axis stress response. Had there been a HPA axis stress response to an emergency alarm and mobilisation it is likely to be attributed to the noise and psychological representation of the alarm[25] as the short-term low intensity physical activity required for the mobilisation component of the response is unlikely to evoke a cortisol response.[26,27,28] It is possible that the dB(A) level of the emergency alarm, typically 95-105 dB(A),[15] was not loud enough to elicit a HPA axis stress response,[29] or that the duration of the alarm was not sufficient to evoke a response.[30,31] It is also possible that because this study did not use emergency service personnel, and was not conducted in an emergency setting, the importance of the alarm sounding was lessened, as there was no real world consequence.[5,13,32,33] Further research is required to investigate HPA axis response to both single and multiple emergency alarm and mobilization events using emergency service workers in an emergency context.
This study showed that there was a HPA axis response to an emergency alarm and mobilization when woken at night. Due to the previously established cortisol awakening response,[17,34] it was important to compare the cortisol response to the night alarm and mobilization with a night control condition. A possible explanation for the heightened cortisol response to the night alarm and mobilization is the low predictability and controllability of the alarm stimulus. In general, when an individual is exposed to a stressful situation, the HPA axis is activated,[35,36] particularly in circumstances that involve low predictability, low controllability and novelty.[37,38] Furthermore, during sleep, there is no impact of reasoning on the interpretation of noise.[39] Research suggests that the auditory system is always operating, even during sleep,[40] and that during sleep, organisms are particularly sensitive to noise exposure.[39] The greater increase in cortisol levels observed in the night alarm condition compared to the night control condition post wakening, and at night compared to during the day, may be due to the reduced ability to predict and interpret the sound at night and the greater rise in noise level (above background levels) in the night alarm condition.
It is possible that the results of this study were affected by the variation between people. Previous research suggests that people may be “responders,” those that show a cortisol response, or “non-responders,” individuals who do not show a cortisol response.[41] The current study did not have sufficient pre-treatment samples to identify responders and non-responders. Therefore, future research should attempt to devise a method to investigate whether there are responders and non-responders to an emergency alarm and mobilization. Further research is also required to establish whether emergency service workers respond in the same manner and to ascertain the magnitude of the stress response to multiple alarms and whether there are causal links between acute responses observed in the current study and the association between alarm response and adverse cardiovascular events observed in large-scale population studies.
Conclusion
In conclusion, the current investigation demonstrated that there was a SAM system but no HPA axis stress response to the emergency alarm and mobilization during the day. The results further suggest that an alarm and mobilization when woken at night may be viewed as an acute stressor, which activates the SAM system and the HPA axis. This study also investigated the difference between the acute stress responses during the day compared to at night. The results reveal that there was no difference in the SAM system stress response to an emergency alarm and mobilization during the day compared to at night; in contrast the HPA axis stress response was present at night and non-existant during the day.
Ethics
All procedures used in this study were approved by the Deakin University Human Research Ethics Committee (Project number: 2012-338) and by the Central Queensland University Human Research Ethics Committee (Project number: EC00158).
Financial support and sponsorship
This work was supported by a Deakin University Faculty Research Development Grant and the CQUniversity Cooperative Research Grant Scheme.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to acknowledge the efforts of Dr Sarah Jay for her assistance with participant recruitment and project management of this study. We also thank the participants for their time in partaking in this study.
References
- 1.van der Ploeg E, Kleber RJ. Acute and chronic job stressors among ambulance personnel: Predictors of health symptoms. Occup Environ Med. 2003;60(Suppl 1):i40–6. doi: 10.1136/oem.60.suppl_1.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidotti TL, Clough VM. Occupational health concerns of firefighting. Annu Rev Public Health. 1992;13:151–71. doi: 10.1146/annurev.pu.13.050192.001055. [DOI] [PubMed] [Google Scholar]
- 3.Kuorinka I, Korhonen O. Firefighters’ reaction to alarm, an ECG and heart rate study. J Occup Med. 1981;23:762–6. doi: 10.1097/00043764-198111000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Kales SN, Soteriades ES, Christophi CA, Christiani DC. Emergency duties and deaths from heart disease among firefighters in the United States. N Engl J Med. 2007;356:1207–15. doi: 10.1056/NEJMoa060357. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PH. The experience of traumatic stress among urban firefighters. Aust J Emerg Manage. 2000;14:59–60. [Google Scholar]
- 6.Ferguson S, Main L, Aisbett B. East Melbourne, Victoria: Australasian Fire Authorities Council Worker Health and Safety Group; 2012. Alarm Response - Is this a problem for Australian firefighters? [Google Scholar]
- 7.Geibe JR, Holder J, Peeples L, Kinney AM, Burress JW, Kales SN. Predictors of on-duty coronary events in male firefighters in the United States. Am J Cardiol. 2008;101:585–9. doi: 10.1016/j.amjcard.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Holder JD, Stallings LA, Peeples L, Burress JW, Kales SN. Firefighter heart presumption retirements in Massachusetts 1997-2004. J Occup Environ Med. 2006;48:1047–53. doi: 10.1097/01.jom.0000235909.31632.46. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc PR, Fahy RF. Quincy, MA: National Fire Protection Association; 2005. Full Report: Firefighter Fatalities in the United States-2002. [PubMed] [Google Scholar]
- 10.Barnard RJ, Duncan HW. Heart rate and ECG responses of fire fighters. J Occup Med. 1975;17:247–50. [PubMed] [Google Scholar]
- 11.Blimkie C, Rechnitzer P, Cunningham D. Heart rate and catecholamine responses of fire fighters to an alarm. Can J Appl Sport Sci. 1977;2:153–6. [Google Scholar]
- 12.Lim CS, Ong CN, Phoon WO. Work stress of firemen as measured by heart rate and catecholamine. J Hum Ergol (Tokyo) 1987;16:209–18. [PubMed] [Google Scholar]
- 13.Karlsson K, Niemelä P, Jonsson A. Heart rate as a marker of stress in ambulance personnel: A pilot study of the body's response to the ambulance alarm. Prehosp Disaster Med. 2011;26:21–6. doi: 10.1017/s1049023x10000129. [DOI] [PubMed] [Google Scholar]
- 14.Kales SN, Soteriades ES, Christoudias SG, Christiani DC. Firefighters and on-duty deaths from coronary heart disease: A case control study. Environ Health. 2003;2:14. doi: 10.1186/1476-069X-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diel C. Noise exposure in the fire department. Independent Studies and Capstones: Washington University School of Medicine. 2001:1–25. [Google Scholar]
- 16.Huikuri HV, Kessler KM, Terracall E, Castellanos A, Linnaluoto MK, Myerburg RJ. Reproducibility and circadian rhythm of heart rate variability in healthy subjects. Am J Cardiol. 1990;65:391–3. doi: 10.1016/0002-9149(90)90308-n. [DOI] [PubMed] [Google Scholar]
- 17.Hucklebridge F, Clow A, Rahman H, Evans P. The cortisol response to normal and nocturnal awakening. J Psychophys. 2000;14:24–8. [Google Scholar]
- 18.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biol Psychol. 2005;69:113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–70. [PubMed] [Google Scholar]
- 20.Hjortskov N, Garde AH, Ørbæk P, Hansen ÅM. Evaluation of salivary cortisol as a biomarker of self-reported mental stress in field studies. Stress Health. 2004;20:91–8. [Google Scholar]
- 21.Field A. 4th ed. London: SAGE Publications; 2013. Discovering statistics using IMB SPSS statistics. [Google Scholar]
- 22.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 23.Toivonen L, Helenius K, Viitasalo M. Electrocardiographic repolarization during stress from awakening on alarm call. J Am Coll Cardiol. 1997;30:774–9. doi: 10.1016/s0735-1097(97)00222-2. [DOI] [PubMed] [Google Scholar]
- 24.Yeragani VK, Pohl R, Berger R, Balon R, Srinivasan K. Relationship between age and heart rate variability in supine and standing postures: A study of spectral analysis of heart rate. Pediatr Cardiol. 1994;15:14–20. doi: 10.1007/BF00797000. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 26.Jacks DE, Sowash J, Anning J, McGloughlin T, Andres F. Effect of exercise at three exercise intensities on salivary cortisol. J Strength Cond Res. 2002;16:286–9. [PubMed] [Google Scholar]
- 27.Ortega E, Collazos M, Maynar M, Barriga C, De la Fuente M. Stimulation of the phagocytic function of neutrophils in sedentary men after acute moderate exercise. Eur J Appl Physiol Occup Physiol. 1993;66:60–4. doi: 10.1007/BF00863401. [DOI] [PubMed] [Google Scholar]
- 28.Rojas Vega S, Strüder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 29.Ising H, Rebentisch E, Babisch W, Curio I, Sharp D, Baumgärtner H. Medically relevant effects of noise from military low-altitude flights-results of an interdisciplinary pilot study. Environ Int. 1990;16:411–23. [Google Scholar]
- 30.Follenius M, Brandenberger G, Lecornu C, Simeoni M, Reinhardt B. Plasma catecholamines and pituitary adrenal hormones in response to noise exposure. Eur J Appl Physiol Occup Physiol. 1980;43:253–61. doi: 10.1007/BF00421839. [DOI] [PubMed] [Google Scholar]
- 31.Yamamura K, Maehara N, Sadamoto T, Harabuchi I. Effect of intermittent (traffic) noise on man-Temporary threshold shift, and change in urinary 17-OHCS and saliva cortisol levels. Eur J Appl Physiol Occup Physiol. 1982;48:303–14. doi: 10.1007/BF00430220. [DOI] [PubMed] [Google Scholar]
- 32.Burt JL, Bartolome DS, Burdette DW, Comstock JR., Jr A psychophysiological evaluation of the perceived urgency of auditory warning signals. Ergonomics. 1995;38:2327–40. doi: 10.1080/00140139508925271. [DOI] [PubMed] [Google Scholar]
- 33.Fagerlönn J. Pitea, Sweden: IET Intelligent Transport Systems; 2011. Urgent Alarms in Trucks: Effects on Annoyance and Subsequent Driving Performance; pp. 252–8. [Google Scholar]
- 34.Wüst S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response-normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]
- 35.Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM. Hormones and the auditory system: A review of physiology and pathophysiology. Neuroscience. 2008;153:881–900. doi: 10.1016/j.neuroscience.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 36.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses. Integrating permissive, suppressive, stimulatory, and preparative actions? Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 37.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–33. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 38.Breier A, Albus M, Pickar D, Zahn TP, Wolkowitz OM, Paul SM. Controllable and uncontrollable stress in humans: Alterations in mood and neuroendocrine and psychophysiological function. Am J Psychiatry. 1987;144:1419–25. doi: 10.1176/ajp.144.11.1419. [DOI] [PubMed] [Google Scholar]
- 39.Ising H, Braun C. Acute and chronic endocrine effects of noise: Review of the research conducted at the Institute for Water, Soil and Air Hygiene. Noise Health. 2000;2:7–24. [PubMed] [Google Scholar]
- 40.Spreng M. Possible health effects of noise induced cortisol increase. Noise Health. 2000;2:59–64. [PubMed] [Google Scholar]
- 41.Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav Immun. 2003;17:373–83. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]


