Abstract
Multiple chemical sensitivity (MCS) is a chronic disorder characterized by a variety of symptoms associated with the exposure to chemicals at a concentration below the toxic level. Previous studies have demonstrated peculiar responses in brain activity in these patients with respect to sensory stimuli while the association between chemical sensitivity and other environmental intolerances such as noise sensitivity has been questioned by researchers. In this study, a cohort of 18 MCS patients underwent transient-evoked otoacoustic emission (TEOAE) testing with and without contralateral suppression to evaluate the functionality of the medial olivocochlear (MOC) reflex involved in speech-in-noise sensitivity. Results were compared with an age- and gender-matched control group (n = 20) and correlation analysis with disease onset and quick environmental exposure sensitivity inventory (qEESI) symptom severity scale was performed. Subjects affected by MCS showed statistically significant impairment of MOC reflex, and the onset of the disease and several symptom subscales showed to be correlated to such reduction in some of the frequencies tested. These data suggest that alterations of MOC reflex could be part of the complex features of this disease although more studies are needed to further explore auditory perception disorders in environmental intolerances.
Keywords: Medial olivocochlear efferent system, multiple chemical sensitivity (MCS), otoacoustic emissions (OAEs)
Introduction
Multiple chemical sensitivity (MCS) is a chronic disorder characterized by a variety of physical and psychological symptoms associated with repeated exposure to nontoxic concentrations of odorous chemicals. Symptoms affect several organs, especially the central nervous system (CNS), most frequently with patients complaining of headache, irritability, and cognitive dysfunctions; the musculoskeletal, respiratory, and digestive systems are also frequently involved.[1] To correctly screen populations for MCS, Miller and Prihoda[2] developed the environmental exposure sensitivity inventory (EESI) and its quick form (qEESI), which are considered to be the most reliable tools for research on patients with a complaint of chemical sensitivity.
Several studies in the last few years showed cerebral blood flow distribution abnormalities in patients with MCS, especially while processing odorous substances.[3,4,5] In particular, MCS sufferers were demonstrated to peculiarly react to sensory stimuli, with activation of such brain areas connected with motivational and emotional processing of the information such as the amygdala and the hippocampus.[3,4,5,6]
In parallel, the same brain circuits seem to be important in auditory processing, given the fact that the limbic system receives neural input from the auditory cortex; tinnitus (a false sound perception) and hyperacusis (the perception of a sound well-tolerated by the majority of hearers as excessively loud or even unbearable) were found to be related to those clinical hearing alterations frequently linked to this neural connections.[7,8] For instance, researchers have investigated the association between chemical sensitivity and other kinds of environmental intolerances including noise sensitivity. This has been done principally by means of questionnaire surveys in clusters of healthy subjects (i.e., teenage students, twins) and no strong association between intolerance to chemicals and to noise was found, raising the hypothesis that they should be considered as separate entities.[9,10,11]
The medial olivocochlear efferent system (MOC) is a widely studied group of neurons involved in auditory function. Outer hair cells (OHCs) receive a rich efferent innervation from MOC neurons, which results, when MOC reflex is activated, in an inhibition of OHC activity. While MOC activation reduces the gain of the cochlear amplifier, its function has been extensively related to ameliorating speech perception in a noisy environment. Another effect related to MOC reflex is to protect the auditory organs from exposure to extremely loud sounds although this seems to be just an epiphenomenon of MOC activity and not its primary function.[12]
MOC reflex activity is studied clinically by means of the amplitude change (usually reduction) in otoacoustic emissions (OAEs) after contralateral suppression (CS-OAE) with broadband noise. Reductions in the contralateral suppression of OAEs, linked to alterations in auditory efferent activity, have been found in several experiments.[13,14] The main purpose of the present study was to evaluate MOC reflex in a cohort of MCS patients without a history of audiological conditions by contralateral suppression of OAEs. In addition, we explore the relationship of MOC reflex functionality with the extent and gravity of MCS symptoms, by means of a correlation analysis of our results with qEESI scores. To our knowledge, this is the first attempt at evaluating the presence of alterations of the auditory function in MCS patients.
Methods
Participants and study design
We included in the study MCS patients admitted to the Regional Center for Diagnosis, Prevention and Treatment of MCS and evaluated at our institution for those symptoms related to ear-nose-throat complaints. Diagnosis of MCS was achieved according to the US Consensus Criteria for MCS and the revisions suggested by Lacour et al,.[15] which were operationalized as follows:
Symptoms are present for at least 6 months;
Symptoms occur in response to exposure to at least two of 11 common volatile chemicals;
Co-occurrence of at least one symptom from the CNS and one symptom from another organ system;
Symptoms cause significant lifestyle changes,
Symptoms occur when exposed and lessen or resolve when the symptom-triggering agent is removed;
Symptoms triggered by exposure levels do not induce symptoms in other individuals who are exposed to the same levels.
We also enrolled as the control group a population of gender- and age-matched healthy controls (HCs).
Both eligible MCS patients and HCs were required to meet the following entry criteria: subjects with diabetes, oncologic or human immunodeficiency virus (HIV) history, neurological and psychiatric or mood disorders, history of surgery, radiation, and trauma to the brain were excluded from the study. No patient showed liver or renal abnormalities or was pregnant or breastfeeding. The peripheral blood of MCS and HCs was tested for the usual parameters. A detailed case history was collected for all subjects who underwent a full ear-nose-throat examination. Neurological diseases were excluded with the mini-mental state examination and magnetic resonance imaging. All those conditions that could potentially develop an auditory dysfunction were considered as exclusion criteria. Thus, patients with hearing disorders or surgery history, head trauma, neuropsychiatric disorders (Parkinson's disease, Alzheimer's disease, schizophrenia, multiple sclerosis, and depression), lower airways and/or lung diseases, active hepatitis, cirrhosis, chronic renal failure, vitamin B12 deficiency, alcohol, tobacco, or drug abuse, cerebral vascular accidents, insulin-dependent diabetes mellitus, hypothyroidism, and Cushing syndrome were not included in the study. Finally, we excluded all subjects taking drugs that could possibly impact auditory functions.
The Ethics Committee of our university approved the protocol research. The study adhered to the principles of the Declaration of Helsinki and all of the participants provided written informed consent after receiving a detailed explanation of the study.
Audiologic testing
Patients and controls underwent pure tone audiometry (PTA) testing (GSI 61 clinical audiometer, Grason-Stadler, Eden Prairie, USA). Subjective auditory thresholds for 500 Hz, 1,000 Hz, 2,000 Hz, and 4,000 Hz were included in the statistical analysis to ensure that there was no difference in hearing level between the groups. Impedance audiometry (GSI Tympstar, Grason-Stadler, United States) was performed, both to rule out asymptomatic chronic otitis media and to assess the cocleostapedial reflex threshold [or middle ear muscle reflex (MEMR)] for the aforementioned frequencies. The utility of measuring the MEMR threshold is to avoid stimulating such reflex while performing contralateral suppression of OAEs with broadband noise.[16]
Otoacoustic emissions
Transient-evoked OAEs (TEOAEs) were recorded in a soundproof room, using the Otodynamics ILO system, calculating sound pressure variations in the external auditory canal in response to acoustic stimulation. Stimuli were administered with a probe sealed in the auditory canal while the subjects were instructed to remain still and relaxed. Both ears were analyzed. TEOAEs were evoked with a “nonlinear” ipsilateral 80-dB sound pressure level (SPL) click stimulation consisting of three in-phase clicks followed by an out-of-phase click with a 10-dB higher intensity. TEOAEs were considered for data analysis if a response 3 dB above noise floor was present for all frequencies tested. By means of the ILO software, 260 samples per condition were recorded; data were included in the analysis if the “stability” of the stimulus was above 80% and the “whole wave reproducibility” was above 60% as calculated by the software. Contralateral suppression TEOAEs (CS-TEOAEs) were recorded with the same setting described above, by adding contralateral 60 dB SPL broadband noise (500-8,000 Hz) administered by earphones connected to a GSI 61 audiometer (see above). The order of experimental testing (left/right ear, with or without CS) was randomized for each patient. Both TEOAE and CS-TEOAE values were calculated for the frequencies 1,000 Hz, 1,500 Hz, 2,000 Hz, 3,000 Hz, and 4,000 Hz for each ear. The suppression value (Δ) was obtained by subtracting CS-TEOAE values to the correspondent TEOAE stimulation.
qEESI
In order to fulfill the exploratory approach to the intensity of MCS-related symptoms, all MCS subjects filled in a modified version of qEESI symptom severity (SS) scale.[17] In this subtest, patients scored from 1 (low) to 3 (severe) the intensity of head-related (HEAD), cognitive-related (COG), affective-related (AFF), neuromuscular-related (NM), musculoskeletal-related (MS), skin-related (SKIN), genitourinary-related (GU), gastrointestinal-related (GI), heart/chest-related (COR), and airway or mucous membrane-related (AIR/MM) symptoms.
Data handling and statistical analysis
Means and standards deviations (SDs) of TEOAEs and CS-TEOAEs per frequency were calculated in both HC and MCS groups as well as means and SDs of qEESI subscale scores only in the latter.
In order to assess that data were of Gaussian distribution, D’Agostino K squared normality test was applied (where the null hypothesis is that the data are normally distributed).
A “between-groups” analysis of variance (ANOVA) was performed for each frequency PTA measurements and TEOAEs and CS-TEOAEs. Further, statistical differences of each frequency TEOAEs and CS-TEOAEs were calculated by means of a “within-subjects” ANOVA in both groups. Age, disease duration (in months), and gender were treated as continuous and categorical predictors. Significant cutoff level (α) was set at a P value of 0.01.
Bonferroni correction for multiple comparisons was used to test post hoc of significant main effects.
Then Spearman's rank correlation was performed between disease duration, qEESI subitems scores, and Δ.
A significant cutoff level (α) was set at a P value of 0.01. In order to avoid familywise error, a Bonferroni correction for multiple comparisons was applied (STATISTICA 7 package for Windows, StatSoft, Tulsa, USA).
Results
Subjects
Twenty-three consecutive MCS patients were enrolled. Among them, three were using antidepressant drugs, one reported history of alcohol abuse, one of hypothyroidism, and were excluded.
Therefore, 18 MCS patients (11 women and 7 men, mean age 49.5 ± 9.3 years) met the eligibility criteria and were included in the study. The control group (HC) consisted of 20 right-handed healthy individuals (12 women and 8 men; mean age 48.6 ± 11.4 years).
Audiological data
Pure tone audiometry
Analysis of PTA measurements shows that the auditory threshold for both groups was above (i.e., better) 20 dB at all frequencies tested, with a mean threshold of 14.56 ± 4.43 dB for the controls and of 12.29 ± 2.02 dB for MCS patients.
Otoacoustic emissions
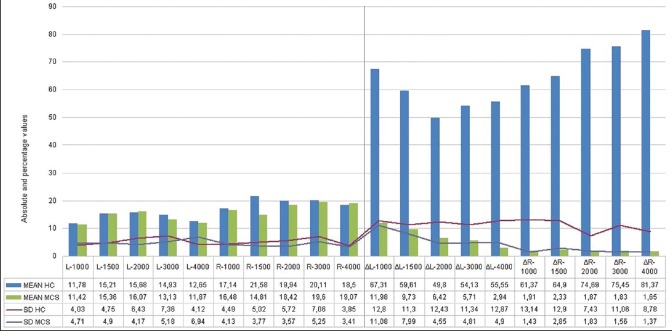
For detailed means and SDs of TEOAEs and CS-TEOAEs, see Figure 1.
Figure 1.
On the left side, the mean TEOAE values (in dB) for all frequencies tested in both groups with respective SDs. On the right, CS-TEOAE mean suppression values (Δ) for each frequency tested in both groups
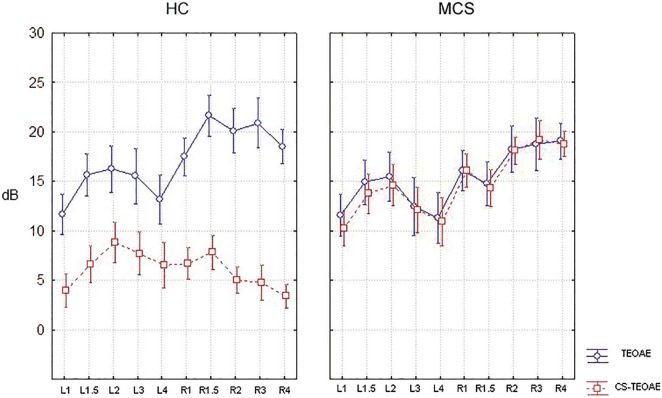
The “within-subject” ANOVA and subsequent post hoc t-test showed a significant (P < 0.01) suppression in HC in all frequencies except for one of the frequencies analysed (left 3,000 Hz). The same model did not find any statistical difference in MCS subjects’ suppression. Moreover, a significant (P < 0.01) “between-groups” effect was found between HC and MCS CS-TEOAE [Figure 2].
Figure 2.
Mean TEOAE and CS-TEOAE values (in dB) for each frequency tested in both groups (on the left healthy controls, HC; on the right multiple chemical sensitivity patients, MCS)
Any statistical difference was found in PTA and TEOAEs when using the between-groups approach.
qEESI
Detailed mean and SDs in Table 1 and Figure 3f.
Table 1.
Means and SDs of qEESI symptom severity data in our MCS cohort
| qEESI subscale/disease onset | Mean | SD |
|---|---|---|
| Disease onset (months) | 186.67 | 54.17 |
| MS | 2.39 | 0.7 |
| AIR | 2.78 | 0.43 |
| COR | 2.11 | 0.68 |
| GI | 1.89 | 0.68 |
| COG | 2.11 | 0.9 |
| AFF | 1.89 | 0.76 |
| NM | 2.22 | 0.81 |
| HEAD | 1.72 | 0.57 |
| SKIN | 1.83 | 0.62 |
| GU | 2 | 0.59 |
Mean time of onset (in months) is showed followed by each symptom subset score (see the Methods section for further explanations)
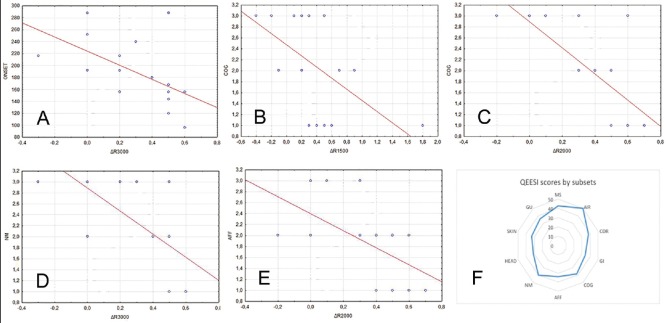
Figure 3.
Spearman's rank correlation analysis for disease onset and ΔR3000 (a) Cognitive symptoms (COG) and ΔR1500 (b) and ΔR2000 (c) Neuromuscular symptoms (NM) and Δ R3000 (d) and affective symptoms (AFF) and ΔR2000 (e). All values showed statistically significant negative correlation (see results). In figure (f), qEESI total scores by subset were obtained by adding single scores of our cohort
Correlation analysis
The Spearman's rank correlation analysis and subsequent correction for multiple comparison found a significant negative correlation between disease onset and ΔR3000 (r = −0.672, P < 0.01, Figure 3a), COG scores and ΔR1500 (r = −0.623, P < 0.01, Figure 3b) and ΔR2000 (r = −0.82, P < 0.01, Figure 3c), NM scores and ΔR3000 (r = −0.745, P < 0.01, Figure 3d) and between AFF scores and ΔR2000 (r = −0.625, P < 0.01, Figure 3e).
Discussion
Auditory efferent activity has been widely studied, both in healthy and diverse diseased populations. Findings of alterations in MOC reflex have been linked to clinical dysfunctions such as troubles identifying speech in noise, or other disorders such as dyslexia or learning disability.[18,19,20]
The first interesting finding in the present study is the general reduction in contralateral suppression of TEOAEs in MCS patients without personal history of auditory and neurological disorders with respect to HC. In particular, it was found a different “within-subjects” behavior in both groups (see Figure 1) and a main “group effect” (P < 0.01) when comparing HC and MCS CS-TEOAE [Figure 2] not depending on differences in age, gender, or in PTA values with respect to HCs. This finding suggests that alterations in the auditory pathway, especially in the cortical–subcortical CNS could exist at a subclinical extent and could thus contribute to the pathological phenotype of MCS sufferers.
Neuropsychological and neuroimaging studies have shown––with a certain degree of debate — Several peculiar features in people affected by MCS and related illnesses. In particular, the prevalence of multiple personality traits as well as CNS hypereactivity and limbic kindling as key nodes in pathophysiological underpinnings of MCS was demonstrated.[21,22] In particular, the onset of MCS has been related to exposure to a “trigger” stimulus that alters CNS response in a way that subsequent stimuli are perceived as excessive and cause symptoms.[22]
Moreover, the relative hyperactivation found in some primary subcortical sensory structures (i.e., amygdala) — not counterbalanced by the same HC cortical activity and the same ability in controlling the arousing stimuli — was interpreted as neural behavioral changes related to low-level chemical exposures before emerging at the mind level.[5,23]
Aligning with these arguments and the supposed physiological role of the MOC system in humans in protecting from excessive sound transmission to the IHC by inhibition of OHC contractile activity,[12] the found MCS reduction in contralateral suppression with respect to HC could be related to a top-down deregulation along auditory perception network. This noteworthy aspect could be more relevant since noise sensitivity and MCS have been shown to overlap to a certain degree.[24]
Alternatively, MOC reflex has been shown to enhance speech perception in a noisy environment;[18] alterations in this pathway could impair, even subclinically, the listening performance of MCS subjects and contribute to the psychological distress, which is peculiar of this condition. It is worthy that MCS sufferers have been shown to have an increased harm avoidance (especially after olfactory stimuli) with respect to controls.[25] However, we can hypothesize that such a mechanism could happen also in the presence of an acoustic stimulation even though speech-in-noise recognition has not been studied — To our knowledge — In MCS.
The second finding of the study demonstrated that contralateral suppression of TEOAEs is negatively correlated (for different frequencies; see Figure 3) both with i) the onset of MCS [Figure 3a] and ii) qEESI subsets [Figures 3b–e]. The first aspect could strengthen the hypothesis that MOC reflex alteration could be interpreted as an indirect CNS top-down deregulation clue related to this condition onset and in particular, it could be useful along MCS disease staging in order to better follow the disease development. Second, we found that CS-TEOAE alterations are clustered to each of the most frequent neural-related symptom subsets of MCS (cognitive, affective, and neuromuscular). We argue that patients with predominance of this spectrum of symptoms may be screened for complaints of speech-in-noise disturbances or noise sensitivity.
More studies are needed to understand the role of this MOC alteration in the wide spectrum of MCS clinical features; for example, further abnormalities of the auditory central pathways can be present, especially in the connection between the auditory cortex and the limbic system. Moreover, other auditory perception disorders such as tinnitus and hyperacusis are highly underestimated and could have a bearing in MCS sufferers as part of a general intolerance to sensory stimuli. Thus, it would be of great interest to evaluate the prevalence of such symptoms in this cohort of patients.
Strengths and limitations of the study
Many uncertainties in the literature involving MCS auditory pathways could clearly be explained by different criteria for patient enrolment, different kinds of questionnaires employed in order to investigate symptoms spectrum, distortion related to the general incidence of personality traits in control subjects, and many nuisance variables biasing the general research in MCS.
In this work, we tried to reduce artifacts that could affect symptoms spectrum outcomes by enrolling and studying — by means of validated questionnaires — only MCS patients with commonly accepted criteria and regularly followed by the local center for diagnosis, treatment and prevention of MCS.
Moreover, we performed a mixed ANOVA model aiming at studying outcomes by using a powerful within/between effect. Thus, we tried to reduce biases related to sex and age ratios, misdiagnosis of MCS, and possible outcome distortions related to the general incidence of personality and social disturbances in the general population.[26,27] On the other hand, a possible limitation of the study could be represented by the Bonferroni multiple correction applied as post hoc test to both ANOVA and Spearman's correlation, possibly increasing the likelihood of type II statistical errors.
Conclusions
In our study, for the first time we found alterations of the auditory efferent system (i.e., MOC reflex) by means of a reduction in the CS-TEOAEs in MCS patients. This finding suggests that subclinical alterations of the auditory pathway can be present, and justifies the need for a thorough evaluation in MCS patients to unveil the presence of auditory perception disorders such as noise sensitivity or speech-in-noise disturbances. Our results were not related to age, gender, or subjective pure-tone auditory thresholds; indeed, we found a correlation between the time of onset of the disease and the absence of contralateral TEOAE suppression at certain frequencies. Furthermore, the reduction in MOC reflex was related, for some studied frequencies, to different symptoms subsets based on qEESI data. These findings suggest that MOC alterations could be part of the complex CNS features of this condition.
Further studies are needed to clarify the extent of auditory perception disorders in MCS patients, and to explore other alterations of the auditory central pathways in these diseases.
Financial support and sponsorship
The authors did not receive any kind of financial support.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Berg ND, Linneberg A, Dirksen A, Elberling J. Prevalence of self-reported symptoms and consequences related to inhalation of airborne chemicals in a Danish general population. Int Arch Occup Environ Health. 2008;81:881–7. doi: 10.1007/s00420-007-0282-0. [DOI] [PubMed] [Google Scholar]
- 2.Miller CS, Prihoda TJ. The environmental exposure and sensitivity inventory (EESI): A standardized approach for measuring chemical intolerances for research and clinical applications. Toxicol Ind Health. 1999;15:370–85. doi: 10.1177/074823379901500311. [DOI] [PubMed] [Google Scholar]
- 3.Hillert L, Musabasic V, Berglund H, Ciumas C, Savic I. Odor processing in multiple chemical sensitivity. Hum Brain Mapp. 2007;28:172–82. doi: 10.1002/hbm.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alessandrini M, Micarelli A, Bruno E, Ottaviani F, Conetta M, Cormano A, et al. Intranasal administration of hyaluronan as a further resource in olfactory performance in multiple chemical sensitivity syndrome. Int J Immunopathol Pharmacol. 2013;26:1019–25. doi: 10.1177/039463201302600424. [DOI] [PubMed] [Google Scholar]
- 5.Chiaravalloti A, Pagani M, Micarelli A, Di Pietro B, Genovesi G, Alessandrini M, et al. Cortical activity during olfactory stimulation in multiple chemical sensitivity: A (18)F-FDG PET/CT study. Eur J Nucl Med Mol Imaging. 2015;42:733–40. doi: 10.1007/s00259-014-2969-2. [DOI] [PubMed] [Google Scholar]
- 6.Azuma K, Uchiyama I, Takano H, Tanigawa M, Azuma M, Bamba I, et al. Changes in cerebral blood flow during olfactory stimulation in patients with multiple chemical sensitivity: A multi-channel near-infrared spectroscopic study. PLoS ONE. 2013;8:e80567. doi: 10.1371/journal.pone.0080567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knipper M, Van Dijk P, Nunes I, Rüttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: Recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Kraus KS, Canlon B. Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res. 2012;288:34–46. doi: 10.1016/j.heares.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Nordin S, Millqvist E, Löwhagen O, Bende M. The chemical sensitivity scale: Psychometric properties and comparison with the noise sensitivity scale. J Environ Psychol. 2003;23:359–67. [Google Scholar]
- 10.Andersson L, Johansson A, Millqvist E, Nordin S, Bende M. Prevalence and risk factors for chemical sensitivity and sensory hyperreactivity in teenagers. Int J Hyg Environ Health. 2008;211:690–7. doi: 10.1016/j.ijheh.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Heinonen-Guzejev M, Koskenvuo M, Mussalo-Rauhamaa H, Vuorinen HS, Heikkilä K, Kaprio J. Noise sensitivity and multiple chemical sensitivity scales: Properties in a population based epidemiological study. Noise Health. 2012;14:215–23. doi: 10.4103/1463-1741.102956. [DOI] [PubMed] [Google Scholar]
- 12.Smith DW, Keil A. The biological role of the medial olivocochlear efferents in hearing: Separating evolved function from exaptation. Front Syst Neurosci. 2015;9:12. doi: 10.3389/fnsys.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karabulut H, Karabulut I, Daðli M, Bayazit YA, Bilen S, Aydin Y, et al. Evaluation of outer hair cell function and medial olivocochlear efferent system in patients with type II diabetes mellitus. Turk J Med Sci. 2014;44:150–6. doi: 10.3906/sag-1207-99. [DOI] [PubMed] [Google Scholar]
- 14.Garinis AC, Glattke T, Cone-Wesson BK. TEOAE suppression in adults with learning disabilities. Int J Audiol. 2008;47:607–14. doi: 10.1080/14992020802129402. [DOI] [PubMed] [Google Scholar]
- 15.Lacour M, Zunder T, Schmidtke K, Vaith P, Scheidt C. Multiple chemical sensitivity syndrome (MCS) - Suggestions for an extension of the U.S. MCS-case definition. Int J Hyg Environ Health. 2005;208:141–51. doi: 10.1016/j.ijheh.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Marshall L, Lapsley Miller JA, Guinan JJ, Shera CA, Reed CM, Perez ZD, et al. Otoacoustic-emission-based medial-olivocochlear reflex assays for humans. J Acoust Soc Am. 2014;136:2697–713. doi: 10.1121/1.4896745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnakenberg E, Fabig KR, Stanulla M, Strobl N, Lustig M, Fabig N, et al. A cross-sectional study of self-reported chemical-related sensitivity is associated with gene variants of drug-metabolizing enzymes. Environ Health. 2007;6:6. doi: 10.1186/1476-069X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: Efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci. 2008;28:4929–37. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veuillet E, Magnan A, Ecalle J, Thai-Van H, Collet L. Auditory processing disorder in children with reading disabilities: Effect of audiovisual training. Brain. 2007;130:2915–28. doi: 10.1093/brain/awm235. [DOI] [PubMed] [Google Scholar]
- 20.Yalçinkaya F, Yilmaz ST, Muluk NB. Transient evoked otoacoustic emissions and contralateral suppressions in children with auditory listening problems. Auris Nasus Larynx. 2010;37:47–54. doi: 10.1016/j.anl.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Bascom R, Kesavanathan J. Differential susceptibility to inhaled pollutants: Effects of demographics and diseases. Environ Toxicol Pharmacol. 1997;4:323–30. doi: 10.1016/s1382-6689(97)10033-3. [DOI] [PubMed] [Google Scholar]
- 22.Winder C. Mechanisms of multiple chemical sensitivity. Toxicol Lett. 2002;128:85–97. doi: 10.1016/s0378-4274(01)00536-7. [DOI] [PubMed] [Google Scholar]
- 23.Alessandrini M, Micarelli A, Chiaravalloti A, Bruno E, Danieli R, Pierantozzi M, et al. Involvement of subcortical brain structures during olfactory stimulation in multiple chemical sensitivity. Brain Topogr. 2016;29:243–52. doi: 10.1007/s10548-015-0453-3. [DOI] [PubMed] [Google Scholar]
- 24.Palmquist E, Claeson AS, Neely G, Stenberg B, Nordin S. Overlap in prevalence between various types of environmental intolerance. Int J Hyg Environ Health. 2014;217:427–34. doi: 10.1016/j.ijheh.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Hillert L, Jovanovic H, Åhs F, Savic I. Women with multiple chemical sensitivity have increased harm avoidance and reduced 5-HT(1A) receptor binding potential in the anterior cingulate and amygdala. PLoS One. 2013;8:e54781. doi: 10.1371/journal.pone.0054781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dereboy C, Güzel HS, Dereboy F, Okyay P, Eskin M. Personality disorders in a community sample in Turkey: Prevalence, associated risk factors, temperament and character dimensions. Int J Soc Psychiatry. 2014;60:139–47. doi: 10.1177/0020764012471596. [DOI] [PubMed] [Google Scholar]
- 27.Amad A, Geoffroy PA, Vaiva G, Thomas P. Personality and personality disorders in the elderly: Diagnostic, course and management. Encephale. 2013;39:374–82. doi: 10.1016/j.encep.2012.08.006. [DOI] [PubMed] [Google Scholar]