Abstract
The local abundance of Culex mosquitoes is a central factor adding to the risk of West Nile virus transmission, and vector abundance data influence public health decisions. This study evaluated differences in abundance estimates from mosquitoes trapped using two common methods: CO2-baited CDC light traps and infusion-baited gravid traps in suburban, Chicago, Illinois. On a weekly basis, the two methods were modestly correlated (r = 0.219) across 71 weeks over 4 years. Lagged weather conditions of up to four weeks were associated with the number of mosquitoes collected in light and gravid traps. Collections in light traps were higher with higher temperature in the same week, higher precipitation one, two, and four weeks before the week of trapping, and lower maximum average wind speed. Collections in gravid traps were higher with higher temperature in the same week and one week earlier, lower temperature four weeks earlier, and with higher precipitation two and four weeks earlier. Culex abundance estimates from light traps were significantly higher in semi-natural areas compared to residential areas, but abundance estimates from gravid traps did not vary by the landscape type. These results highlight the importance of the surveillance methods used in the assessment of local Culex abundance estimates. Measures of risk of exposure to West Nile virus should assess carefully how mosquito abundance has been estimated and integrated into assessments of transmission risk.
Keywords: abundance, Culex, gravid traps, landscape, light traps, weather, West Nile virus, arbovirus
Introduction
The World Health Organization has estimated that vector-borne diseases account for nearly 17% of the global burden of all infectious diseases.1 Mosquitoes are one of the most important disease vectors and can transmit many pathogens including those that cause malaria, dengue, West Nile fever and encephalitis, St. Louis encephalitis, yellow fever, Japanese encephalitis, Zika, and chikungunya.2 The risk of exposure to mosquito-borne pathogens is often estimated using indices that include estimates of vector abundance. Vectorial capacity, for example, takes into account the vector, host, and vector–host interaction, and is a commonly used risk index for assessing the prevalence of malaria and dengue.3–5 Similarly, dengue transmission risk is often measured using the house index, which is the percentage of houses infested with the larvae or pupae of Aedes mosquitoes.6
Estimates of interactions between vectors and hosts are also commonly used to estimate the risk of West Nile virus (WNV) transmission.7 Due to the multi-host transmission cycle of WNV, Kilpatrick et al developed an index combining vector abundance, the fraction of blood meals taken from mammals, WNV infection prevalence, and a vector competence index for alternative vector species to assess WNV transmission risk.8 A simpler vector index, measured as the product of mosquito abundance and the WNV infection rate in mosquitoes, has also been used for WNV transmission risk estimation.9–11 The advantage of the later method is that it is simple to calculate, and data are more readily collected; however, it does not consider vector competence. Because mosquitoes of different species transmit different pathogens, vector-pathogen-host interactions differ across systems, so system-specific abundance and infection risk estimates must be tailored for particular mosquito-borne diseases and particular areas.12
In the United States, mosquitoes of the genus Culex are the vectors for WNV transmission, with nearly 96% of the WNV positive pools obtained from just a few Culex species.13 The proportional distribution of these species varied by region, with Culex tarsalis, Culex quinquefasciatus, and Culex pipiens pipiens and Culex restuans being dominant in the western, southern, and north central regions, respectively.13 In the Chicago, Illinois, region, which is the focus of this study, Culex pipiens complex and C. restuans have played the most important role in the enzootic as well as the epidemic cycle of WNV transmission.14 The C. pipiens form molestus has been detected in this region as well, but data are limited.15 The life history of Culex mosquitoes includes aquatic stages (eggs, larvae, and pupae) and the adult terrestrial stage. Females lay rafts of eggs on the surface of standing water, with a preference for water rich in organic content. Depending upon weather and food availability, adults emerge in about 10–14 days.16 Adult gravid female C. pipiens enter diapause in the fall to overwinter.16,17
Temperature and precipitation play an important role in the life history and population dynamics of mosquitoes. In temperate climates, warmer weather in the spring accelerates mosquito activity, and higher temperatures generally shorten the time between blood meal acquisition and oviposition.12,18 The lifespan of adult Culex spp. varies considerably depending on the temperature.19,20 Adult female mosquitoes raised over a range of constant temperatures had an average longevity ranging from 11 days to over 92 days.20 The shortest observed life span for both sexes was less than two weeks at 30 °C, while they survived more than 90 days at 15 °C.20 This suggests that mosquito survival is higher when the temperature is between 15 °C and 30 °C, and lower when it exceeds 30 °C. In natural systems, weather conditions in the weeks prior to mosquito capture (lagged weather) can predict the number of mosquitoes captured.21–23 Even off-season meteorological conditions can affect mosquito abundance due to diapause conditions24 and because prior year drought conditions may reduce the number of predators, resulting in increased mosquito populations.25
Many other factors can affect the local abundance of mosquitoes. For example, the availability and distribution of larval habitats depend on both weather and local landscape features. Suitable larval habitats, such as natural water bodies, catch basins, and containers, are required to maintain the mosquito population in an area26 and rainfall plays a key role in maintaining these habitats. Culex mosquitoes need wet conditions to reproduce, but heavy rainfall can reduce the survival rate of Culex vectors both at the adult stage and during larval development.27,28 Local vegetation influences mosquito abundance by providing resting sites and sugars to mosquitoes, and different species of vegetation can promote or reduce the emergence rates of adult Culex mosquitoes.29,30 In urban areas of Connecticut, significantly higher numbers of C. pipiens and C. restuans were found in areas with moderate vegetation as measured from imagery using a vegetation index.31 In other studies, orchard habitat,32 forested areas33 and medium height trees were associated with higher Culex abundance.34 In a study conducted in Amherst, Erie County, NY, Culex abundance increased with more mixed urban land use, grass and agriculture land cover, and industrial and recreational areas.35
The abundance of Culex spp. mosquitoes in an area is estimated by counting the number of Culex spp. mosquitoes collected per trap and then adjusting that for the number of nights in which traps were actually set. The most commonly used traps to collect Culex mosquitoes are CO2-baited CDC light traps and infusion-baited gravid traps. Light traps baited with CO2 attract nocturnal phototactic mosquitoes and rely on chemotaxis by host-seeking females, whereas gravid traps attract ovipositing mosquitoes by providing breeding habitat.36 Light traps attract host-seeking female mosquitoes that may be parous, but are more often unfed and nulliparous36 and are useful to capture a diversity of mosquito species37, whereas gravid traps primarily collect ovipositing female Culex mosquitoes.38 Mosquitoes collected in gravid traps are especially suitable for WNV surveillance if detection of infection status is included in the surveillance plan because gravid mosquitoes have had at least one gonotropic cycle and are thus more likely to be infected.39,40 Underlying weather conditions and trap locations might affect the number of Culex being captured in light and gravid traps; however, the differences in these relationships are not well documented.
The main objective of this study was to compare abundance measures and factors associated with differences between two common methods for trapping Culex vectors in Illinois: CDC light traps baited with dry ice and gravid traps containing a liquid oviposition attractant. We evaluated the effects of the trapping method on estimates of Culex abundance, taking into account weekly weather conditions and landscape features. We conducted the study between 2009 and 2012 in a suburban Chicago, Illinois, a region with significant WNV activity. Secondarily, we evaluated the relationship between Culex abundance and WNV mosquito infection rate during the same period.
Methods
Study area and data sources
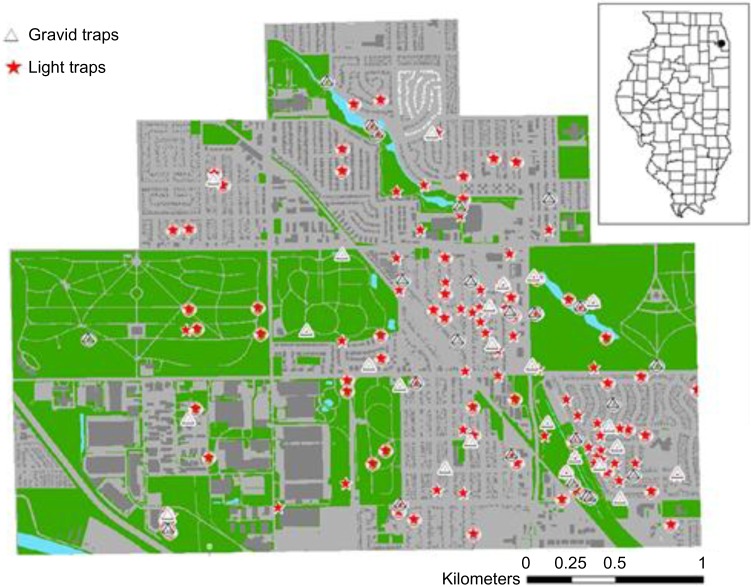
The study area was in south Cook County, Illinois, located in the near suburbs of the city of Chicago (Fig. 1). The study area is approximately 17 km2 with a population of around 20,000 people in several municipalities. This study was part of a broader investigation of WNV transmission ecology.41,42
Figure 1.
Light and gravid trap sampling locations in suburban Chicago. Stars represent CDC light trap locations, triangles represent gravid traps. A circle around the shape indicates multiyear locations. Green indicates semi-natural areas, and the gray color indicates urban residential or commercial areas. The inset shows the state of Illinois, USA with the black dot indicating the neighborhoods where mosquito collections occurred (Alsip, Evergreen Park, Oak Lawn) between 2009 and 2012.
Mosquitoes were collected for 18 weeks from late May to early October each year (weeks 22–39) from 2009 to 2011 and for 17 weeks (weeks 23–39) in 2012, using both CDC miniature light traps and infusion-baited gravid traps. Light traps were hung from a tree or any other structure at a height of about 1.5 m above the ground with a cooler containing dry ice attached each night. Gravid trap oviposition attractant was made by placing a half cup of alfalfa pellets in five gallons of water in a carboy that was placed in the sun for about seven days prior to use (to allow fermentation of organic matter) and changed every two to four weeks. A portion of both types of traps was set up in the evening and collected the following morning each collection day during each week of collection. Traps were distributed throughout the study area in different representative landscapes, including semi-natural sites (cemeteries, parks, and rights of way), and residential areas. The basis for the specific site selections has been described elsewhere.14,41–43 Over the four-year study period, the number of light trap locations ranged from 29 to 76 per year, with 119 unique locations; and the number of gravid trap locations ranged from 11 to 31 per year, with 48 unique locations. Any trap locations with coordinates recorded less than 20 m apart were treated as a single location. Within a single year, data from these traps were combined. Nearly 99% of the traps were located 50 m away from each other in the same year, with a few exceptions when separated by physical barriers such as a road or thick vegetation. Some, but not all, of the trap locations were used in all four years. Among the light traps, 55, 42, 16, and 6 trap locations were used for one, two, three, or four years, respectively. Among the gravid traps, 24, 11, 10, and 3 trap locations were used for one, two, three, or four years, respectively.
After collection, trained personnel determined the mosquito species and sex, and then pools of up to 50 female Culex spp. (C. pipiens complex and C. restuans) were tested for WNV using quantitative rt-PCR.44 Given the difficulties in morphologically differentiating C. pipiens complex and C. restuans, these two species were pooled together and hereafter are referred to as Culex spp.45 Culex spp. are considered the primary enzootic and bridge vectors of WNV in this area.13,45 The average number of Culex spp. per trap night was calculated for each trap location and for the 71 collection weeks across 2009–2012. The minimum infection rate (MIR) of WNV in mosquitoes was estimated using the maximum likelihood method implemented in the program PooledInfRate version 4.0.46 In addition to calculating the MIR for the local study-related mosquito collections, we obtained and calculated the MIR of Culex spp. from WNV testing reported to the Illinois Department of Public Health for the broader region (Cook County) during the same time period.
Daily weather records from January 2009 to December 2012 were obtained from the nearest NOAA weather station at the Midway International Airport, Chicago, Illinois, nine miles north of the study area. Weather records included daily minimum and maximum temperature, precipitation, average humidity, and average and maximum wind speed. Average weekly temperature (°C) was calculated as the mean of the daily minimum and the maximum temperatures for each week. Weekly averages of daily humidity, average wind speed, and maximum wind speed were calculated as the mean of their respective seven readings from that week, and weekly precipitation was calculated as the sum of the precipitation during that week.
Statistical Analysis
The weekly abundance estimates from light trap collections were compared with the abundance measured from gravid traps. Then, temporal and spatial analyses were performed separately for light and gravid traps. The response variable for all analyses was the average number of Culex per trap night. Data from traps, where a trap failed on a given night, were removed from analyses to avoid the artifact of pseudo-negative catches. Predictor variables for temporal analyses included weekly average temperature, total precipitation, average humidity, average wind speed, and average maximum wind speed of the same week. In addition, we assessed each of these predictor variables at one to four week lags. In total, there were 25 weather variables: five each for temperature, precipitation, average humidity, average wind speed, and average maximum wind speed (Table 1).
Table 1.
List of explanatory variables used in the temporal analysis to show their relationship with weekly Culex abundance in light and gravid traps.
| S.N. | VARIABLES | ABBREVIATION |
|---|---|---|
| A | Temperature (Degrees celsius) | |
| 1 | Average temperature of the same week | Temp_samewk |
| 2 | Average temperature one to four weeks before | Templagwk1, Templagwk2, Templagwk3, Templagwk4, |
| B | Precipitation (Centimeters) | |
| 1 | Average precipitation of the same week | Preci_samewk |
| 2 | Average precipitation one to four weeks before | Precilagwk1, Precilagwk2, Precilagwk3, Precilagwk4 |
| C | Humidity (Percentage) | |
| 1 | Average humidity of the same week | Avghumidity_samewk |
| 2 | Average humidity one to four weeks before | Humiditylagwk1, Humiditylagwk2, Humiditylagwk3, Humiditylagwk4 |
| D | Average wind speed (Kilometer per hour) | |
| 1 | Average wind speed of the same week | Avgwind_samewk |
| 2 | Average wind speed one to four weeks before | Avgwindlagwk1, Avgwindlagwk2, Avgwindlagwk3, Avgwindlagwk4 |
| E | Average maximum wind speed (Kilometer per hour) | |
| 1 | Average maximum wind speed of the same week | Avgmaxwind_samewk |
| 2 | Average maximum wind speed one to four weeks before | Avgmaxwindlagwk1, Avgmaxwindlagwk2, Avgmaxwindlagwk3, Avgmaxwindlagwk4 |
Descriptive statistics were calculated using the command PROC UNIVARIATE in SAS 9.4 (SAS Institute Inc.). The Shapiro–Wilk W statistic (>0.9) was used to test the normality of outcome variables. In the original dataset, the Culex spp. per trap night in both light and gravid traps was not normally distributed (W < 0.9). We subsequently identified as outliers the Culex spp. per trap night data above the 95th percentile, and captures higher than that value were assigned the value of the 95th percentile, after which data were normally distributed (W > 0.9). Pearson’s product-moment correlation coefficient was used to measure the association between weekly average Culex spp. per trap night by trap type. Bivariate correlation analyses of individual predictor variables were conducted, and variables with P < 0.2 were selected for inclusion in a multivariable regression model. Multiple linear regression analysis was performed to explore the relationship between the response variable and the selected predictor variables using the command PROC GLM in SAS. The variance inflation criterion (VIF < 10) was used to evaluate multicollinearity among explanatory variables. The Akaike Information Criterion (AIC) was used to evaluate candidate models. The model with the lowest AIC value was selected as the model that best fit the data.47 The variable selection criterion for the final multivariate regression was P < 0.05.
For spatial analysis, light and gravid trap locations from 2009 to 2012 were classified as being either in residential areas or in semi-natural areas. Analysis of variance (ANOVA) tests were performed to evaluate whether Culex spp. abundance in light and gravid traps differed by the land cover type. The dependent variable was the average number of Culex spp. per trap night in gravid traps and light traps. The independent variable was the landscape type (whether the traps were located in residential or semi-natural areas).
Results
Light traps captured 18,978 Culex spp. mosquitoes from 3,444 trap nights with 14–76 light traps set per week during 71 weeks from 2009 to 2012. Across all light trap collections, abundance estimates ranged from 0 to 266 Culex spp. per trap night, with an overall mean of 5.5 (±12.9 SD). Over the 71 weeks, the weekly light trap collections with all locations combined averaged between 0.4 and 24.3 Culex spp. per trap night, with a mean of 5.1 (±3.9 SD). Annually, for light traps, 2010 had the highest overall average Culex spp. per trap night measure, with a value of 7.2 (±5.5 SD). The next highest average abundance estimate per trap night was 4.5 (±3.1 SD) in 2009, followed by 4.4 (±3.4 SD) in 2012, and 4.1 (±2.8 SD) in 2011.
Gravid traps captured a total of 22,345 Culex spp. from 1,561 trap nights with 6–31 gravid traps set per week during 71 weeks from 2009 to 2012. Across all gravid trap collections, abundance estimates ranged from 0 to 533 Culex spp. per trap night with a mean of 14.3 (±33.2 SD). Over the 71 weeks, the weekly gravid trap collections with all locations combined averaged from 0.1 to 192.6 Culex spp. per trap night with a mean of 16.1 (±24.6 SD). For gravid traps, the highest overall average Culex per trap night was in 2009, with a value of 26.2 (±44.5 SD). The next highest average abundance estimate per trap night was 18.6 (±13.1 SD) in 2010, followed by 10.1 (±9.1 SD) in 2012, and 8.4 (±7.0 SD) in 2011. The highest annual MIR for the small study area alone was 15.7 in 2009, followed by 11.8 in 2010, 9.2 in 2012, and 0.6 in 2011. County-level MIR was 1.0 in 2009, 5.3 in 2010, 9.8 in 2012, and 3.5 in 2011.
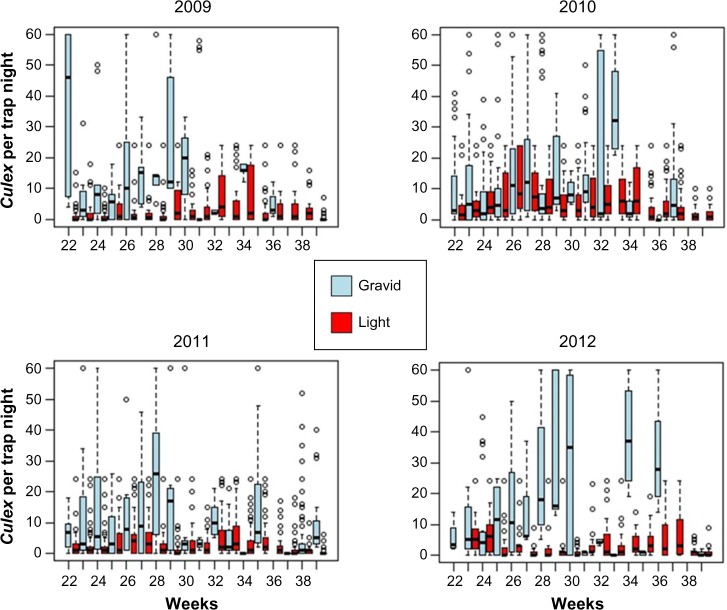
Gravid traps usually captured more Culex spp. each week than light traps (Fig. 2). The exception was during the weeks 35–39 in 2011 and weeks 36–38 in 2012, when the light traps had higher capture rates. This happened when weather conditions during those weeks were within the average range for those weeks. Additional weeks of data would be needed to better explain these patterns. The weekly Culex spp. abundance estimates (Fig. 3) averaged across the four years demonstrated that there was greater variance in Culex spp. abundance collected in gravid traps compared to light traps, and there was a slight peak in gravid trap collections around weeks 26–29 and again at weeks 32–34. In light traps, a bimodal distribution was observed with a first peak at week 26 and a second peak at weeks 32–34 (Fig. 3). After week 33 (about mid-August), abundance was generally low and decreasing in both trap types, and most of the anomalous Culex spp. collections occurred earlier in the season. Of the more than 5,000 collections, 95 data points (individual collections) were removed from the analysis in four years from light traps and gravid traps due to trap failures.
Figure 2.
Box plots of the weekly average Culex abundance in light and gravid traps from 2009 to 2012.
Figure 3.
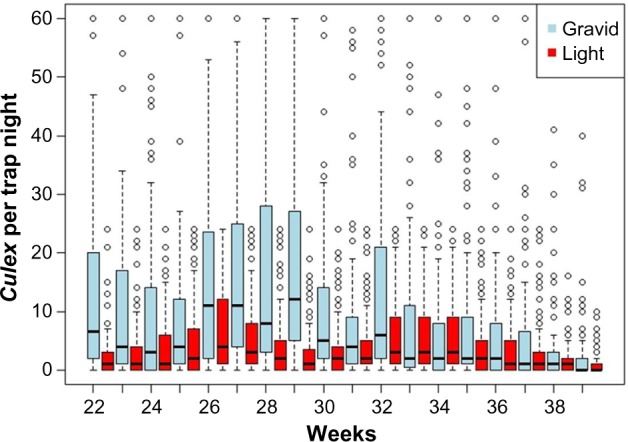
Box plot of overall weekly Culex abundance in light and gravid traps with weeks combined for the years from 2009 to 2012.
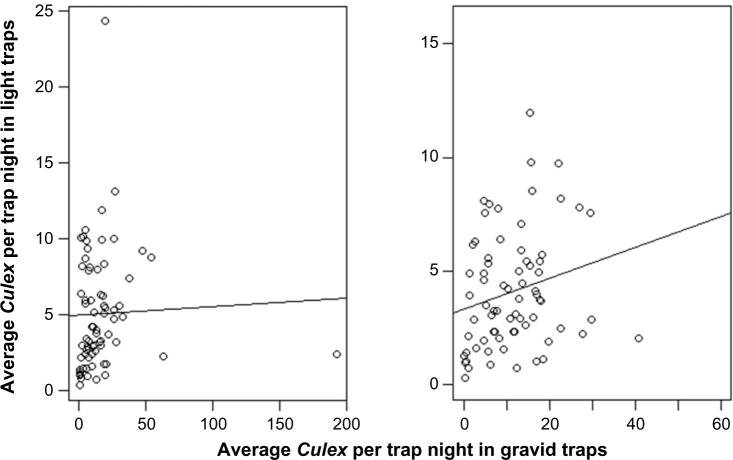
With 71 weeks of data without truncating for outliers, there was no measurable correlation between average abundance in light traps and the average abundance in gravid traps (r = 0.03). However, after truncating the outliers, the correlation was stronger, although still marginal (r = 0.219; P = 0.06; N = 71) (Fig. 4).
Figure 4.
Scatter plots between weekly average Culex per trap night in light and gravid collections with all original numbers (left) and after truncating of outliers (right).
Temporal Analysis
Using AIC, light trap data were best fit with a model that included five weather variables (AIC = 319.6), which explained 28% of the variation in Culex spp. abundance (adjusted R2 = 0.276) (Table 2). For gravid traps, data were best fit with an analogous model including five weather variables (AIC = 484.2), which explained about 30% of the variability of abundance (adjusted R2 = 0.303) (Table 3). In light traps, the Culex spp. abundance was higher with higher temperature in the same week, higher precipitation one, two, and four weeks before, and a lower maximum average wind speed in the same week (Table 4). In gravid traps, the Culex spp. abundance was higher with higher temperature in the same week and one week before, higher precipitation two and four weeks before, and lower temperature four weeks before (Table 5).
Table 2.
Candidate models for predicting the abundance of Culex spp. in light traps.
| MODEL | VARIABLES INCLUDED | K | −2 Log LikeLihood | AIC | ∆AiC |
|---|---|---|---|---|---|
| 1 | Avgtemp_samewk, Precilagwk1–2 and 4, Avgmaxwind_samewk | 6 | 305.6 | 319.6 | 0 |
| 2 | Avgtemp_samewk, Templagwk1, Precilagwk1–2 and 4, Avgmaxwind_samewk | 7 | 304.2 | 320.2 | 0.6 |
| 3 | Avgtemp_samewk, Precilagwk1 and 4, Avgmaxwind_samewk | 5 | 309 | 321.0 | 1.4 |
| 4 | Avgtemp_samewk, Templagwk1, Precilagwk1–4, Avgmaxwind_samewk | 8 | 303.4 | 321.4 | 1.8 |
| 5 | Avgtemp_samewk, Templagwk1, Precilagwk1–4, Avgwindlagwk3, Avgmaxwind_samewk | 9 | 302.5 | 322.5 | 2.9 |
| 6 | Avgtemp_samewk, Templagwk1, Precilagwk1–4, Humiditylagwk2, Avgwindlagwk3, Avgmaxwind_samewk | 10 | 302.2 | 324.2 | 4.6 |
| 7 | Avgtemp_samewk, Templagwk1–2, Precilagwk1–4, Humiditylagwk2, Avgwindlagwk3, Avgmaxwind_samewk | 11 | 302.2 | 326.2 | 6.6 |
| 8 | Null model | 1 | 333.8 | 337.8 | 18.2 |
| 9 | Global (all explanatory variables included) | 26 | 285.1 | 339.1 | 19.5 |
Table 3.
Candidate models for predicting the abundance of Culex spp. in gravid traps.
| MODEL | VARIABLES INCLUDED | K | −2 Log LikeLihood | AIC | ∆AiC |
|---|---|---|---|---|---|
| 1 | Avgtemp_samewk, Templagwk1 and 4, Precilagwk2 and 4 | 6 | 470.2 | 484.2 | 0 |
| 2 | Avgtemp_samewk, Templagwk1 and 4, Precilagwk4 | 5 | 472.5 | 484.5 | 0.3 |
| 3 | Avgtemp_samewk, Templagwk1 and 4, Precilagwk2 and 4, Avgwindlagwk4 | 7 | 470.2 | 486.2 | 2.0 |
| 4 | Global (all explanatory variables included) | 26 | 448.9 | 502.9 | 18.7 |
| 5 | Null | 1 | 501.2 | 505.2 | 21.0 |
Table 4.
Model parameters for the top-ranked model using weather variables to predict the abundance of Culex spp. in light traps.
| VARIABLE | PARAMETER ESTIMATE | F-VALUE | P-VALUE | STANDARDIZED PARAMETER ESTIMATE |
|---|---|---|---|---|
| Average temperature of the same week | 0.219 | 3.04 | 0.003 | 0.332 |
| Precipitation one week before | 0.212 | 2.13 | 0.036 | 0.229 |
| Precipitation two weeks before | 0.183 | 1.80 | 0.076 | 0.199 |
| Precipitation four weeks before | 0.216 | 2.22 | 0.029 | 0.232 |
| Maximum average wind speed of the same week | −0.117 | −1.92 | 0.058 | −0.217 |
Table 5.
Model parameters for the top-ranked model using weather variables to predict the abundance of Culex spp. in gravid traps.
| VARIABLE | PARAMETER ESTIMATE | F-VALUE | P-VALUE | STANDARDIZED PARAMETER ESTIMATE |
|---|---|---|---|---|
| Average temperature of the same week | 0.515 | 1.79 | 0.077 | 0.240 |
| Temperature one week before | 0.746 | 2.25 | 0.028 | 0.313 |
| Temperature four weeks before | −0.721 | −3.59 | 0.0006 | −0.387 |
| Precipitation two weeks before | 0.437 | 1.44 | 0.153 | 0.146 |
| Precipitation four weeks before | 0.665 | 2.21 | 0.031 | 0.221 |
Spatial Analysis
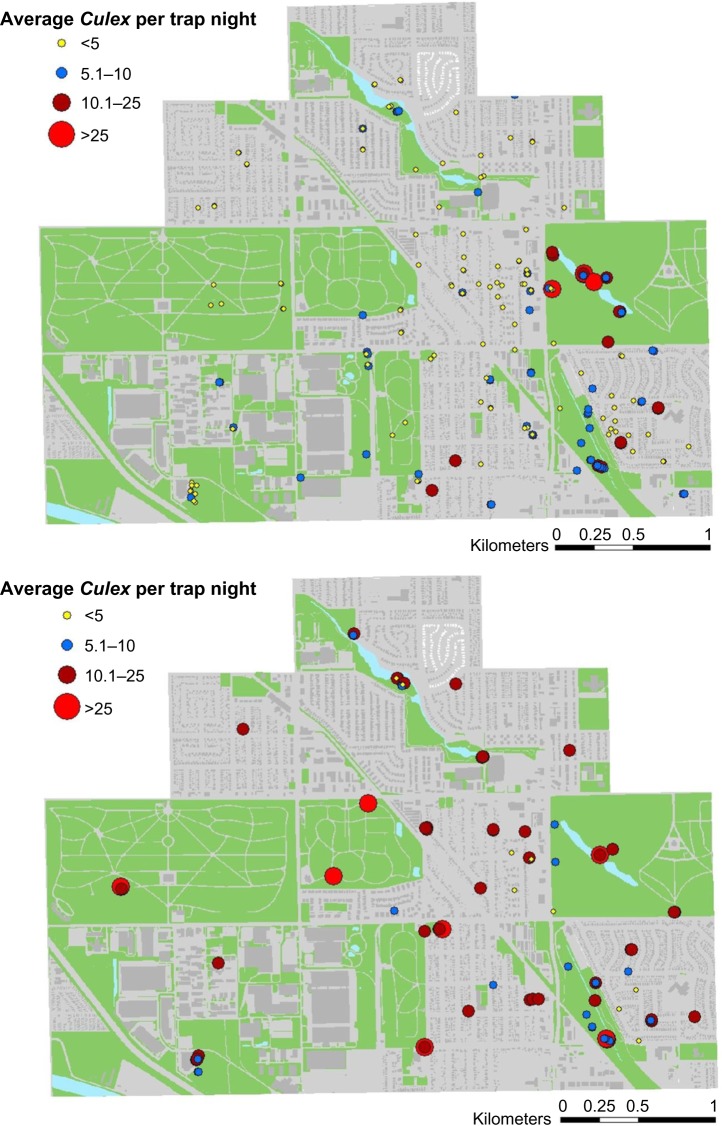
Of the 119 light trap locations, 53 were in semi-natural areas and 66 were in residential areas. From the light traps, the average number of Culex spp. captured in natural areas was 5.3 (±5.0 SD) and was 2.7 (±3.1 SD) in residential areas. Of the 48 gravid trap locations, 29 were in semi-natural areas and 19 were in residential areas. From gravid traps, the average number of Culex spp. captured in natural areas was 12.4 (±8.5 SD), whereas for residential areas it was 11.8 (±7.5 SD). While both light and gravid trap collections resulted in higher numbers of Culex spp. when traps were located in the semi-natural areas (Fig. 5), the difference was statistically significant only for light traps (P = 0.0002).
Figure 5.
Map showing the spatial distribution of Culex per trap night in light (top) and gravid collections (bottom).
Discussion
Our results demonstrated that Culex abundance estimates across urban landscapes vary with the trapping method, and that spatial, temporal, and weather-related factors influence these estimates. In particular, strong winds reduced abundance in light traps but did not noticeably affect gravid trap collections. Abundance estimates were generally higher in semi-natural areas from both trap types, but this difference was stronger for light traps. Both gravid and light traps had higher abundance when set during weeks with warmer temperatures and when conditions were wetter during the previous several weeks. Higher abundance in gravid traps also followed warmer temperatures during the prior week, but this effect was not seen in light traps. Cooler temperatures four weeks prior also increased gravid trap abundance. Gravid traps collected more Culex spp. mosquitoes compared to light traps. The differences observed in the collections from light and gravid traps may be related to their tendency to collect mosquitoes from different life stages. It is also possible that gravid traps attract Culex spp. mosquitoes more than light traps do, and pull in mosquitoes from larger areas.
Temperature is an important factor in mosquito abundance because it affects the life history traits of mosquitoes. The positive association between prior temperature and Culex abundance has been observed in several other studies.21,48–52 Mechanistically, high temperatures may support faster growth of mosquito larvae53 and adult emergence and increase the number captured. However, following emergence, the ambient temperature may either increase or decrease adult mosquito longevity. Lebl et al found that higher temperatures two weeks prior to capture increased Culex abundance in light traps21, while accumulated temperature one to four weeks before capture was negatively correlated with C. pipiens abundance.54 These equivocal findings suggest that there may be a temperature threshold, above or below which mosquito survival and activity markedly decrease. Higher abundance of mosquitoes in gravid traps after cooler temperatures four weeks before suggests that mosquito longevity may increase in cooler temperatures, making a larger pool of adults ready to oviposit with higher temperatures.55
Precipitation also appears to drive Culex abundance. The positive relationship between precipitation at one to four weeks prior and Culex abundance has also been observed in other studies.21,42,45,47 However, the relationship between precipitation and Culex abundance is not necessarily linear. Pecoraro et al found no association between weekly precipitation and Culex abundance,51 but at more protracted temporal scales, correlations were positive in some years and negative in others.48 Lebl et al found that precipitation had a weaker association with abundance than other weather variables, with higher precipitation over 10 weeks associated with higher abundance. In the same Chicago study system, Gardner et al documented that Culex larval abundance was associated with low rainfall, and suggested that precipitation greater than 3.5 cm during a single week may flush immature Culex out of larval habitats.28 Our data with adult mosquitoes demonstrate a similar pattern, where precipitation in the weeks prior to sampling increases adult abundance by providing habitat for adult females to lay egg rafts. Alternatively, this relationship between high Culex abundance following rain events might have more to do with favorable ambient conditions for adults (eg, higher humidity reducing desiccation risk), which could promote activity and increase trapping success. Though the gravid traps can themselves act as a larval habitat because of the water availability in those traps, we found positive relationships between prior rainfall and Culex spp. abundance estimates in both light and gravid traps, without an indication that gravid traps collected more mosquitoes during dry periods.
Wind speed affects mosquito host-seeking activities and flight direction. In this study, the maximum average wind speed of the same week of capture was negatively associated with Culex abundance in light traps but not gravid traps. The reason for this difference may be associated with trap placement. CDC light traps were set approximately 1.5 m above the ground, whereas gravid traps were placed directly on the ground: it is possible that this subtle difference in trap height may have exposed host-seeking and adult females to different wind conditions.56 Indeed, Hamer et al found that Culex spp. may move as far as 2.48 km, but that this dispersal was likely mediated by wind.42 Similarly, Lebl et al found that average wind speed three weeks prior to capture was negatively associated with Culex abundance.21
Landscape features may also affect mosquito production through variation in breeding habitat and resting places for adult mosquitoes. We found that in light traps, more Culex spp. mosquitoes were captured in natural areas than in residential areas, whereas in the gravid traps, this variability was negligible. Availability of hosts and competition with natural container habitats, respectively, may modify these relationships. For example, more birds in natural areas could result in more host-seeking mosquitoes being present and thus available for light trap capture. Lower numbers of Culex mosquitoes in light traps in residential areas may also be related to mosquito abatement practices that target those areas, such as pesticide treatments for adult mosquitoes and the placement of larvicides in urban catch basins.57 In a study conducted in Suffolk County, New York, the highest abundance of C. pipiens was in areas where WNV was mostly prevalent in birds, not in humans, and this may be the case in the current study region as well.58 Further, comparison of our findings to other studies is somewhat confounded by how landscapes are defined. Our study occurred within an urban area, and our semi-natural areas were relatively small patches within a highly urbanized landscape. If we had used the same index of urbanization that was used to assess Culex spp. abundance in New York, for example, all our sites would have been classified as “urban”.59
The estimated abundance of mosquitoes was not clearly correlated with mosquito infection rates or negative public health outcomes. The Illinois Department of Public Health reported only one human WNV case in Cook County Illinois in 2009 when abundance estimates from gravid traps were higher than any of the four years. There were 30 cases of WNV reported in 2010, 22 cases in 2011, and 174 cases in 2012, clearly indicating that 2012 was a locally notable WNV outbreak year.60 During 2012, temperatures were above average (hot) and rainfall was below average (dry), supporting prior patterns of higher MIR observed by Ruiz et al.61 The MIR was also higher in 2012 in Cook County at large, but the local MIR in 2012 in our smaller study area was higher in 2009 and 2010 than in 2012, highlighting the variability of the WNV transmission potential and surveillance outcomes at different scales. Messina et al found that MIR in the Chicago area was not associated spatially with human illness after controlling for other factors in a multivariate regression, and differences in mosquito abundance or a failure to capture temporal and spatial dynamics may have accounted for this.62 It was not possible to compare the relationship between mosquito abundance, MIR, and the reported WNV human illness at a broader level due to the lack of comprehensive mosquito abundance data at that scale. Future work should include the collection of additional abundance estimates and consider alternative spatio-temporal approaches.
Mosquito vector abundance is an important theoretical predictor of human infection, especially for multi-host pathogens. The weak correlation between the fine-scale mosquito abundance estimates and regional measures of human WNV illnesses observed in our study area may be related to the differences in the geographic scale of analysis. Our data demonstrate that in the Cook County, Illinois, gravid and light traps set at the same time in similar conditions do not produce identical abundance estimates. These findings highlight both the importance of local weather and landscape features in combination with the trapping methods for the development of mosquito abundance measures that are relevant to public health. Our findings indicate that abundance estimates obtained from only one type of trap, for a short period of time, and from a limited sampling of landscape types, may not truly represent mosquito abundance. Consequently, investment into long-term surveillance that accounts for different habitats within a control district, and that implements different trapping methods, will give better estimates of mosquito abundance for use in the assessment of arboviral transmission risk in human populations.
Acknowledgments
We thank the Village of Oak Lawn for the use of a space for a field laboratory and the many municipalities, cemeteries, and private homeowners in Cook County for granting us permission to conduct this study. Tim Thompson, Diane Gohde, Patrick Kelly, Carl Hutter, Marija Gorinshteyn, Zach Allison, and Mike Glester provided field assistance.
Footnotes
ACADEMIC EDITOR: Timothy Kelley, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,860 words, excluding any confidential comments to the academic editor.
FUNDING: Funding was provided by the National Science Foundation and National Institutes of Health Ecology of Infectious Disease program under Award No. 0840403. Additional funding was from the Stormwater and Mosquito Control Theme at the University of Illinois, Institute for Sustainability, Energy, and Environment. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: SK, MOR. Analyzed the data: SK, MOR, TKA, GLH. Wrote the first draft of the manuscript: SK, MOR. Contributed to the writing of the manuscript: SK, MOR, TKA, GLH. Agreed with manuscript results and conclusions: UDK, EDW. Made critical revisions and approved the final version: TLG, BLK. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.World Health Organization Vector-borne diseases. 2015. [Accessed February 15, 2016]. Available at: http://www.who.int/mediacentre/factsheets/fs387/en/
- 2.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85(2):328–45. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JR, Rico-Hesse R. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am J Trop Med Hyg. 2006;75(5):886–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Ceccato P, Vancutsem C, Klaver R, Rowland J, Connor SJ. A vectorial capacity product to monitor changing malaria transmission potential in epidemic regions of Africa. Re Dai Yi Xue Za Zhi. 2012;2012:1–6. doi: 10.1155/2012/595948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrett-Jones C, Shidrawi G. Malaria vectorial capacity of a population of Anopheles gambiae: an exercise in epidemiological entomology. Bull World Health Organ. 1969;40(4):531–45. [PMC free article] [PubMed] [Google Scholar]
- 6.Scott TW, Morrison AC. Aedes aegypti density and the risk of dengue-virus. In: Takken W, Scott TW, editors. Ecological Aspects for Application of Genetically Modified Mosquitoes. Vol. 2. Dordrecht: FRONTIS; 2003. p. 187. [Google Scholar]
- 7.Ciota AT, Kramer LD. Vector-virus interactions and transmission dynamics of West Nile virus. Viruses. 2013;5(12):3021–47. doi: 10.3390/v5123021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 2005;11(3):425–9. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gujral IB, Zielinski-Gutierrez EC, LeBailly A, Nasci R. Behavioral risks for West Nile virus disease, northern Colorado, 2003. Emerg Infect Dis. 2007;13(3):419. doi: 10.3201/eid1303.060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolling BG, Barker CM, Moore CG, Pape WJ, Eisen L. Seasonal patterns for entomological measures of risk for exposure to Culex vectors and West Nile virus in relation to human disease cases in northeastern Colorado. J Med Entomol. 2009;46(6):1519–31. doi: 10.1603/033.046.0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick AM, Pape WJ. Predicting human West Nile virus infections with mosquito surveillance data. Am J Epidemiol. 2013;178(5):829–35. doi: 10.1093/aje/kwt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–83. doi: 10.1146/annurev-ento-112408-085419. [DOI] [PubMed] [Google Scholar]
- 13.Andreadis TG. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. J Am Mosq Control Assoc. 2012;28(4 s):137–51. doi: 10.2987/8756-971X-28.4s.137. [DOI] [PubMed] [Google Scholar]
- 14.Hamer GL, Kitron UD, Brawn JD, et al. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol. 2008;45(1):125–8. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Mutebi JP, Savage HM. Discovery of Culex pipiens pipiens form molestus in Chicago. J Am Mosq Control Assoc. 2009;25(4):500–3. doi: 10.2987/09-5910.1. [DOI] [PubMed] [Google Scholar]
- 16.Crans WJ. A classification system for mosquito life cycles: life cycle types for mosquitoes of the northeastern United States. J Vector Ecol. 2004;29:1–10. [PubMed] [Google Scholar]
- 17.Vinogradova EB. Culex pipiens Pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control. Sofia, Bulgaria: Pensoft Publishers; 2000. [Google Scholar]
- 18.Hartley DM, Barker CM, Le Menach A, Niu T, Gaff HD, Reisen WK. Effects of temperature on emergence and seasonality of West Nile virus in California. Am J Trop Med Hyg. 2012;86(5):884–94. doi: 10.4269/ajtmh.2012.11-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD. The effect of temperature on life history traits of Culex mosquitoes. J Med Entomol. 2014;51(1):55–62. doi: 10.1603/me13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreadis S, Dimotsiou O, Savopoulou-Soultani M. Variation in adult longevity of Culex pipiens f. pipiens, vector of the West Nile Virus. Parasitol Res. 2014;113(11):4315–9. doi: 10.1007/s00436-014-4152-x. [DOI] [PubMed] [Google Scholar]
- 21.Lebl K, Brugger K, Rubel F. Predicting Culex pipiens/restuans population dynamics by interval lagged weather data. Parasit Vectors. 2013;6:129–39. doi: 10.1186/1756-3305-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrieri M, Fariselli P, Maccagnani B, Angelini P, Calzolari M, Bellini R. Weather factors influencing the population dynamics of Culex pipiens (Diptera: Culicidae) in the Po Plain Valley, Italy 1997–2011. Environ Entomol. 2014;43(2):482–90. doi: 10.1603/EN13173. [DOI] [PubMed] [Google Scholar]
- 23.DeGaetano AT. Meteorological effects on adult mosquito (Culex) populations in metropolitan New Jersey. Int J Biometeorol. 2005;49(5):345–53. doi: 10.1007/s00484-004-0242-2. [DOI] [PubMed] [Google Scholar]
- 24.Walsh AS, Glass GE, Lesser CR, Curriero FC. Predicting seasonal abundance of mosquitoes based on off-season meteorological conditions. Environ Ecol Stat. 2008;15(3):279–91. [Google Scholar]
- 25.Chase JM, Knight TM. Drought-induced mosquito outbreaks in wetlands. Ecol Lett. 2003;6(11):1017–24. [Google Scholar]
- 26.Cailly P, Balenghien T, Ezanno P, Fontenille D, Toty C, Tran A. Role of the repartition of wetland breeding sites on the spatial distribution of Anopheles and Culex, human disease vectors in Southern France. Parasit Vectors. 2011;6:65–72. doi: 10.1186/1756-3305-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CE, Lounibos LP, Marra PP, Kilpatrick AM. Rainfall influences survival of Culex pipiens (Diptera: Culicidae) in a residential neighborhood in the mid-Atlantic United States. J Med Entomol. 2012;49(3):467–73. doi: 10.1603/me11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner AM, Hamer GL, Hines AM, Newman CM, Walker ED, Ruiz MO. Weather variability affects abundance of larval Culex (Diptera: Culicidae) in storm water catch basins in suburban Chicago. J Med Entomol. 2012;49(2):270–6. doi: 10.1603/me11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner AM, Anderson TK, Hamer GL, et al. Terrestrial vegetation and aquatic chemistry influence larval mosquito abundance in catch basins, Chicago, USA. Parasit Vectors. 2013;6:9–19. doi: 10.1186/1756-3305-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner AM, Allan BF, Frisbie LA, Muturi EJ. Asymmetric effects of native and exotic invasive shrubs on ecology of the West Nile Virus vector Culex pipiens (Diptera: Culicidae) Parasit Vectors. 2015;16:329–37. doi: 10.1186/s13071-015-0941-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown H, Diuk-Wasser M, Andreadis T, Fish D. Remotely-sensed vegetation indices identify mosquito clusters of West Nile virus vectors in an urban landscape in the northeastern United States. Vector Borne Zoonotic Dis. 2008;8(2):197–206. doi: 10.1089/vbz.2007.0154. [DOI] [PubMed] [Google Scholar]
- 32.Crowder DW, Dykstra EA, Brauner JM, et al. West Nile virus prevalence across landscapes is mediated by local effects of agriculture on vector and host communities. PLoS One. 2013;8(1):e55006. doi: 10.1371/journal.pone.0055006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang T-W, Hockett CW, Kightlinger L, Wimberly MC. Landscape-level spatial patterns of West Nile virus risk in the Northern Great Plains. Am J Trop Med Hyg. 2012;86(4):724–31. doi: 10.4269/ajtmh.2012.11-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landau KI, van Leeuwen WJ. Fine scale spatial urban land cover factors associated with adult mosquito abundance and risk in Tucson, Arizona. J Vector Ecol. 2012;37(2):407–18. doi: 10.1111/j.1948-7134.2012.00245.x. [DOI] [PubMed] [Google Scholar]
- 35.Trawinski PR, Mackay DS. Identification of environmental covariates of West Nile virus vector mosquito population abundance. Vector Borne Zoonotic Dis. 2010;10(5):515–26. doi: 10.1089/vbz.2008.0063. [DOI] [PubMed] [Google Scholar]
- 36.Reisen W, Pfuntner A. Effectiveness of five methods for sampling adult Culex mosquitoes in rural and urban habitats in San Bernardino County, California. J Am Mosq Control Assoc. 1987;3(4):601–6. [PubMed] [Google Scholar]
- 37.Dimenna MA, Bueno R, Jr, Parmenter RR, et al. Comparison of mosquito trapping method efficacy for West Nile virus surveillance in New Mexico. J Am Mosq Control Assoc. 2006;22(2):246–53. doi: 10.2987/8756-971x(2006)22[246:comtme]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore CG, McLean R, Mitchell CJ, et al. Guidelines for Arbovirus Surveillance Programs in the United States. Vol. 500. Atlanta, GA: Department of Health and Human Services. Division of Vector-Borne Infectious Diseases; 1993. [Google Scholar]
- 39.Williams GM, Gingrich JB. Comparison of light traps, gravid traps, and resting boxes for West Nile virus surveillance. J Vector Ecol. 2007;32(2):285–91. doi: 10.3376/1081-1710(2007)32[285:coltgt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Hribar LJ, Vlach JJ, Demay DJ, et al. Mosquitoes infected with West Nile virus in the Florida Keys, Monroe County, Florida, USA. J Med Entomol. 2003;40(3):361–3. doi: 10.1603/0022-2585-40.3.361. [DOI] [PubMed] [Google Scholar]
- 41.Krebs BL, Anderson TK, Goldberg TL, et al. Host group formation decreases exposure to vector-borne disease: a field experiment in a ‘hotspot’ of West Nile virus transmission. Proc Biol Sci. 2014;281(1796):20141586. doi: 10.1098/rspb.2014.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamer GL, Anderson TK, Donovan DJ, et al. Dispersal of adult Culex mosquitoes in an urban West Nile virus hotspot: a mark-capture study incorporating stable isotope enrichment of natural larval habitats. PLoS Negl Trop Dis. 2014;8(3):e2768. doi: 10.1371/journal.pntd.0002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamer GL, Walker ED, Brawn JD, et al. Rapid amplification of West Nile virus: the role of hatch-year birds. Vector Borne Zoonotic Dis. 2008;8(1):57–68. doi: 10.1089/vbz.2007.0123. [DOI] [PubMed] [Google Scholar]
- 44.Harrington LC, Poulson RL. Considerations for accurate identification of adult Culex restuans (Diptera: Culicidae) in field studies. J Med Entomol. 2008;45(1):1–8. doi: 10.1603/0022-2585(2008)45[1:cfaioa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 45.Hamer GL, Kitron UD, Goldberg TL, et al. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80(2):268. [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention . Software for mosquito surveillance. Fort Collins, Colorado: 2006. [Accessed January 15, 2015]. Available at: http://www.cdc.gov/ncidod/dvbid/westnile/software.htm. [Google Scholar]
- 47.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):716–23. [Google Scholar]
- 48.Jacob BG, Gu W, Caamano EX, Novak RJ. Developing operational algorithms using linear and non-linear squares estimation in Python® for the identification of Culex pipiens and Culex restuans in a mosquito abatement district (Cook County, Illinois, USA) Geospat Health. 2009;3(2):157–76. doi: 10.4081/gh.2009.218. [DOI] [PubMed] [Google Scholar]
- 49.Mulatti P, Ferguson HM, Bonfanti L, Montarsi F, Capelli G, Marangon S. Determinants of the population growth of the West Nile virus mosquito vector Culex pipiens in a repeatedly affected area in Italy. Parasit Vectors. 2014;7(1):26–36. doi: 10.1186/1756-3305-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paz S, Albersheim I. Influence of warming tendency on Culex pipiens population abundance and on the probability of West Nile Fever outbreaks (Israeli case study: 2001–2005) Ecohealth. 2008;5(1):40–8. doi: 10.1007/s10393-007-0150-0. [DOI] [PubMed] [Google Scholar]
- 51.Pecoraro HL, Day HL, Reineke R, et al. Climatic and landscape correlates for potential West Nile virus mosquito vectors in the Seattle region. J Vector Ecol. 2007;32(1):22–8. doi: 10.3376/1081-1710(2007)32[22:calcfp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Ogden NH, Zhu H. The impact of weather conditions on Culex pipiens and Culex restuans (Diptera: Culicidae) abundance: a case study in Peel region. J Med Entomol. 2011;48(2):468–75. doi: 10.1603/me10117. [DOI] [PubMed] [Google Scholar]
- 53.Hagstrum DW, Workman EB. Interaction of temperature and feeding rate in determining the rate of development of larval Culex tarsalis (Diptera, Culicidae) Ann Entomol Soc Am. 1971;64(3):668–71. [Google Scholar]
- 54.Roiz D, Ruiz S, Soriguer R, Figuerola J. Climatic effects on mosquito abundance in Mediterranean wetlands. Parasit Vectors. 2014;7:333–45. doi: 10.1186/1756-3305-7-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mordecai EA, Paaijmans KP, Johnson LR, et al. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol Lett. 2013;16(1):22–30. doi: 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- 56.Darbro JM, Harrington LC. Bird-baited traps for surveillance of West Nile mosquito vectors: effect of bird species, trap height, and mosquito escape rates. J Med Entomol. 2006;43(1):83–92. doi: 10.1093/jmedent/43.1.83. [DOI] [PubMed] [Google Scholar]
- 57.Harbison JE, Henry M, Xamplas C, Berry R, Bhattacharya D, Dugas LR. A comparison of FourStar™ briquets and Natular™ XRT tablets in a North Shore suburb of Chicago, IL. J Am Mosq Control Assoc. 2014;30(1):68–70. doi: 10.2987/13-6355.1. [DOI] [PubMed] [Google Scholar]
- 58.Rochlin I, Ginsberg HS, Campbell SR. Distribution and abundance of host-seeking Culex species at three proximate locations with different levels of West Nile virus activity. Am J Trop Med Hyg. 2009;80(4):661–8. [PubMed] [Google Scholar]
- 59.Drummond CL, Drobnack J, Backenson PB, Ebel GD, Kramer LD. Impact of trap elevation on estimates of abundance, parity rates, and body size of Culex pipiens and Culex restuans (Diptera: Culicidae) J Med Entomol. 2006;43(2):177–84. doi: 10.1603/0022-2585(2006)043[0177:ioteoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 60.IDPH West Nile Virus. 2015. [Accessed January 15, 2016]. Available at: http://www.dph.illinois.gov/topics-services/diseases-and-conditions/west-nile-virus.
- 61.Ruiz MO, Chaves LF, Hamer GL, et al. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors. 2010;3(1):19–34. doi: 10.1186/1756-3305-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Messina JP, Brown W, Amore G, Kitron UD, Ruiz MO. West Nile virus in the Greater Chicago area: a geographic examination of human illness and risk from 2002 to 2006. URISA J. 2011;23:5–22. [Google Scholar]