Abstract
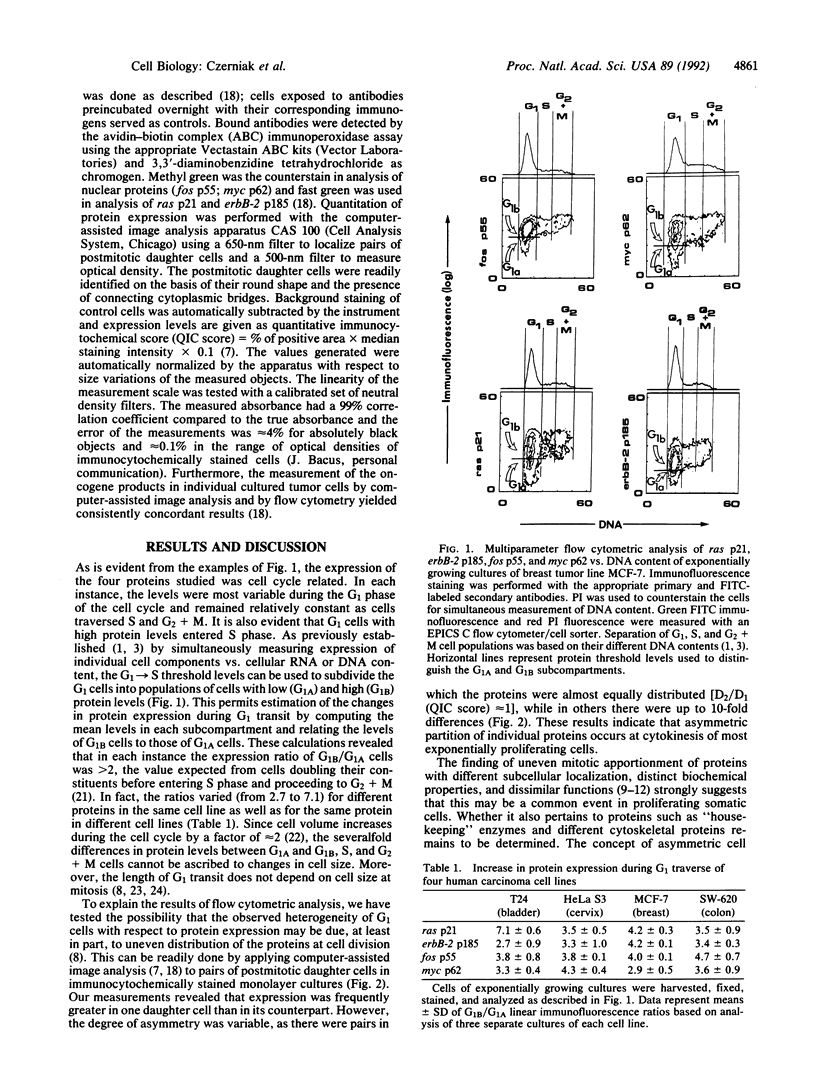
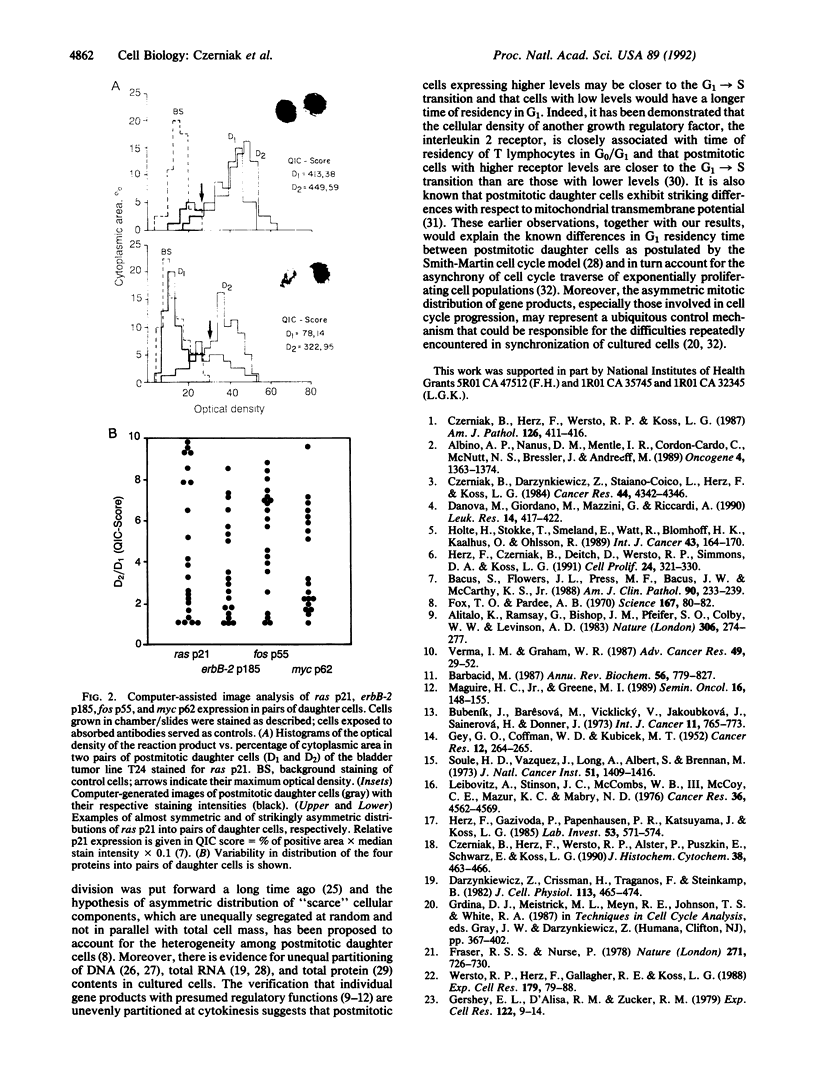
Computer-assisted image analysis was used to demonstrate in exponentially proliferating human tumor cells the uneven postmitotic apportionment of several oncogene-encoded proteins (ras p21; erbB-2 p185; fos p55; myc p62). This observation may provide the explanation for the high degree of heterogeneity of postmitotic cells and the asynchrony in cell cycle traverse of cultured cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Nanus D. M., Mentle I. R., Cordon-Cardo C., McNutt N. S., Bressler J., Andreeff M. Analysis of ras oncogenes in malignant melanoma and precursor lesions: correlation of point mutations with differentiation phenotype. Oncogene. 1989 Nov;4(11):1363–1374. [PubMed] [Google Scholar]
- Alitalo K., Ramsay G., Bishop J. M., Pfeifer S. O., Colby W. W., Levinson A. D. Identification of nuclear proteins encoded by viral and cellular myc oncogenes. Nature. 1983 Nov 17;306(5940):274–277. doi: 10.1038/306274a0. [DOI] [PubMed] [Google Scholar]
- BARKA T. Mitotic distribution of Feulgen material in three ascites tumors. J Natl Cancer Inst. 1959 Feb;22(2):243–257. [PubMed] [Google Scholar]
- Bacus S., Flowers J. L., Press M. F., Bacus J. W., McCarty K. S., Jr The evaluation of estrogen receptor in primary breast carcinoma by computer-assisted image analysis. Am J Clin Pathol. 1988 Sep;90(3):233–239. doi: 10.1093/ajcp/90.3.233. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Baserga R. Growth in size and cell DNA replication. Exp Cell Res. 1984 Mar;151(1):1–5. doi: 10.1007/978-3-642-67986-5_1. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Baresová M., Viklický V., Jakoubková J., Sainerová H., Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973 May;11(3):765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Darzynkiewicz Z., Tobey R. A., Steinkamp J. A. Correlated measurements of DNA, RNA, and protein in individual cells by flow cytometry. Science. 1985 Jun 14;228(4705):1321–1324. doi: 10.1126/science.2408339. [DOI] [PubMed] [Google Scholar]
- Czerniak B., Darzynkiewicz Z., Staiano-Coico L., Herz F., Koss L. G. Expression of Ca antigen in relation to cell cycle in cultured human tumor cells. Cancer Res. 1984 Oct;44(10):4342–4346. [PubMed] [Google Scholar]
- Czerniak B., Herz F., Wersto R. P., Alster P., Puszkin E., Schwarz E., Koss L. G. Quantitation of oncogene products by computer-assisted image analysis and flow cytometry. J Histochem Cytochem. 1990 Apr;38(4):463–466. doi: 10.1177/38.4.1969431. [DOI] [PubMed] [Google Scholar]
- Czerniak B., Herz F., Wersto R. P., Koss L. G. Expression of Ha-ras oncogene p21 protein in relation to the cell cycle of cultured human tumor cells. Am J Pathol. 1987 Mar;126(3):411–416. [PMC free article] [PubMed] [Google Scholar]
- Danova M., Giordano M., Mazzini G., Riccardi A. Expression of p53 protein during the cell cycle measured by flow cytometry in human leukemia. Leuk Res. 1990;14(5):417–422. doi: 10.1016/0145-2126(90)90027-7. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Crissman H., Traganos F., Steinkamp J. Cell heterogeneity during the cell cycle. J Cell Physiol. 1982 Dec;113(3):465–474. doi: 10.1002/jcp.1041130316. [DOI] [PubMed] [Google Scholar]
- Fox T. O., Pardee A. B. Animal cells: noncorrelation of length of G1 phase with size after mitosis. Science. 1970 Jan 2;167(3914):80–82. doi: 10.1126/science.167.3914.80. [DOI] [PubMed] [Google Scholar]
- Fraser R. S., Nurse P. Novel cell cycle control of RNA synthesis in yeast. Nature. 1978 Feb 23;271(5647):726–730. doi: 10.1038/271726a0. [DOI] [PubMed] [Google Scholar]
- Gershey E. L., D'Alisa R. M., Zucker R. M. Characterization of a CV-1 cell cycle. IV. No critical size for S phase entry. Exp Cell Res. 1979 Aug;122(1):9–14. doi: 10.1016/0014-4827(79)90554-8. [DOI] [PubMed] [Google Scholar]
- Herz F., Czerniak B., Deitch D., Wersto R. P., Simmons D. A., Koss L. G. Protein expression in relation to the cell cycle of exponentially growing human prostatic epithelial cells. Cell Prolif. 1991 May;24(3):321–330. doi: 10.1111/j.1365-2184.1991.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Herz F., Gazivoda P., Papenhausen P. R., Katsuyama J., Koss L. G. Normal human urothelial cells in culture. Subculture procedure, flow cytometric and chromosomal analyses. Lab Invest. 1985 Nov;53(5):571–574. [PubMed] [Google Scholar]
- Holte H., Stokke T., Smeland E., Watt R., Blomhoff H. K., Kaalhus O., Ohlsson R. Levels of myc protein, as analyzed by flow cytometry, correlate with cell growth potential in malignant B-cell lymphomas. Int J Cancer. 1989 Jan 15;43(1):164–170. doi: 10.1002/ijc.2910430130. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel M., Darzynkiewicz Z., Arino O., Traganos F. Analysis of a cell cycle model based on unequal division of metabolic constituents to daughter cells during cytokinesis. J Theor Biol. 1984 Oct 21;110(4):637–664. doi: 10.1016/s0022-5193(84)80149-6. [DOI] [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- Maguire H. C., Jr, Greene M. I. The neu (c-erbB-2) oncogene. Semin Oncol. 1989 Apr;16(2):148–155. [PubMed] [Google Scholar]
- Sennerstam R., Auer G. Partition of protein and DNA during cytokinesis in human breast cancer cell lines. Cytometry. 1990;11(2):292–299. doi: 10.1002/cyto.990110210. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Graham W. R. The fos oncogene. Adv Cancer Res. 1987;49:29–52. doi: 10.1016/s0065-230x(08)60793-9. [DOI] [PubMed] [Google Scholar]
- Wersto R. P., Herz F., Gallagher R. E., Koss L. G. Cell cycle-dependent reactivity with the monoclonal antibody Ki-67 during myeloid cell differentiation. Exp Cell Res. 1988 Nov;179(1):79–88. doi: 10.1016/0014-4827(88)90350-3. [DOI] [PubMed] [Google Scholar]